Marine Plankton-Derived Whitlockite Powder-Based 3D-Printed Porous Scaffold for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder Preparation and 3D Printing
2.2. Micro-Structural Observation and X-ray Diffraction Analysis
2.3. Determination of Heavy Metal Content and Trace Elements
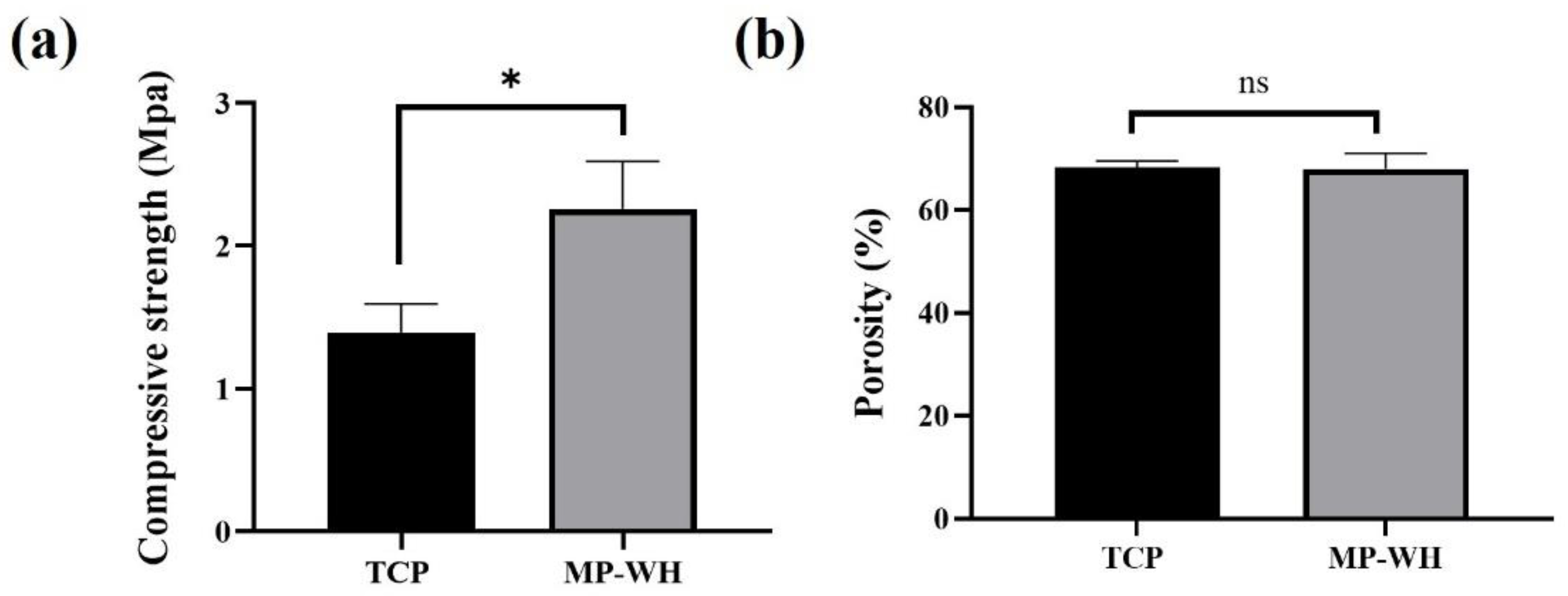
2.4. Compressive Strength and Porosity Analysis
2.5. Cell Culture
2.6. Cell Proliferation Assay
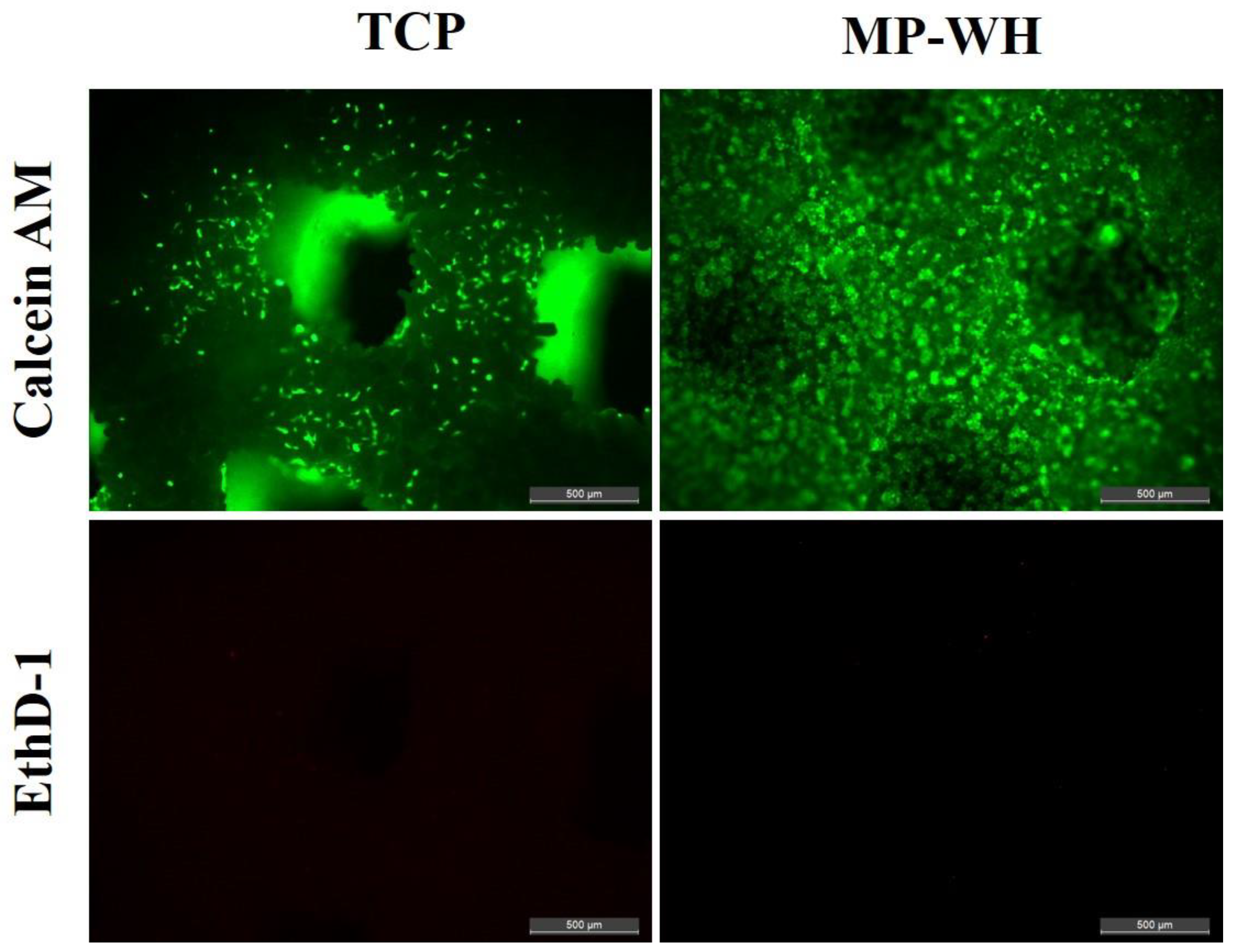
2.7. Viability/Cytotoxicity Assay
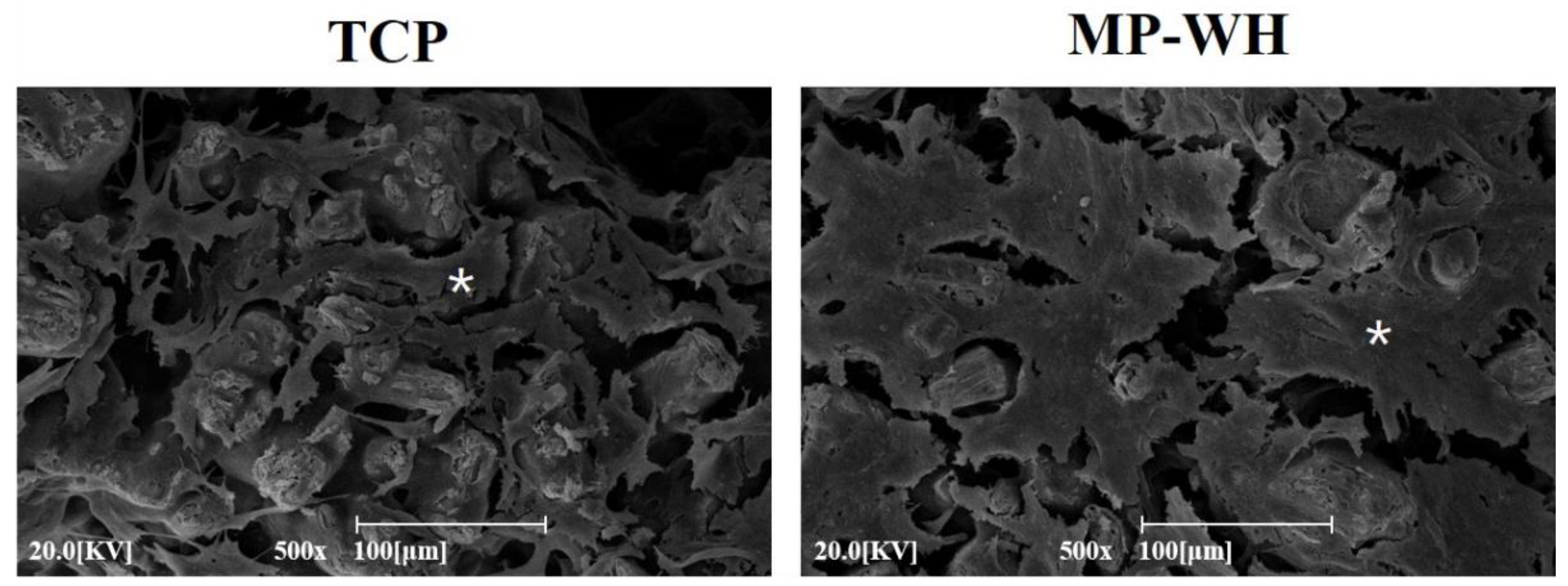
2.8. Observation of Cell Adherence
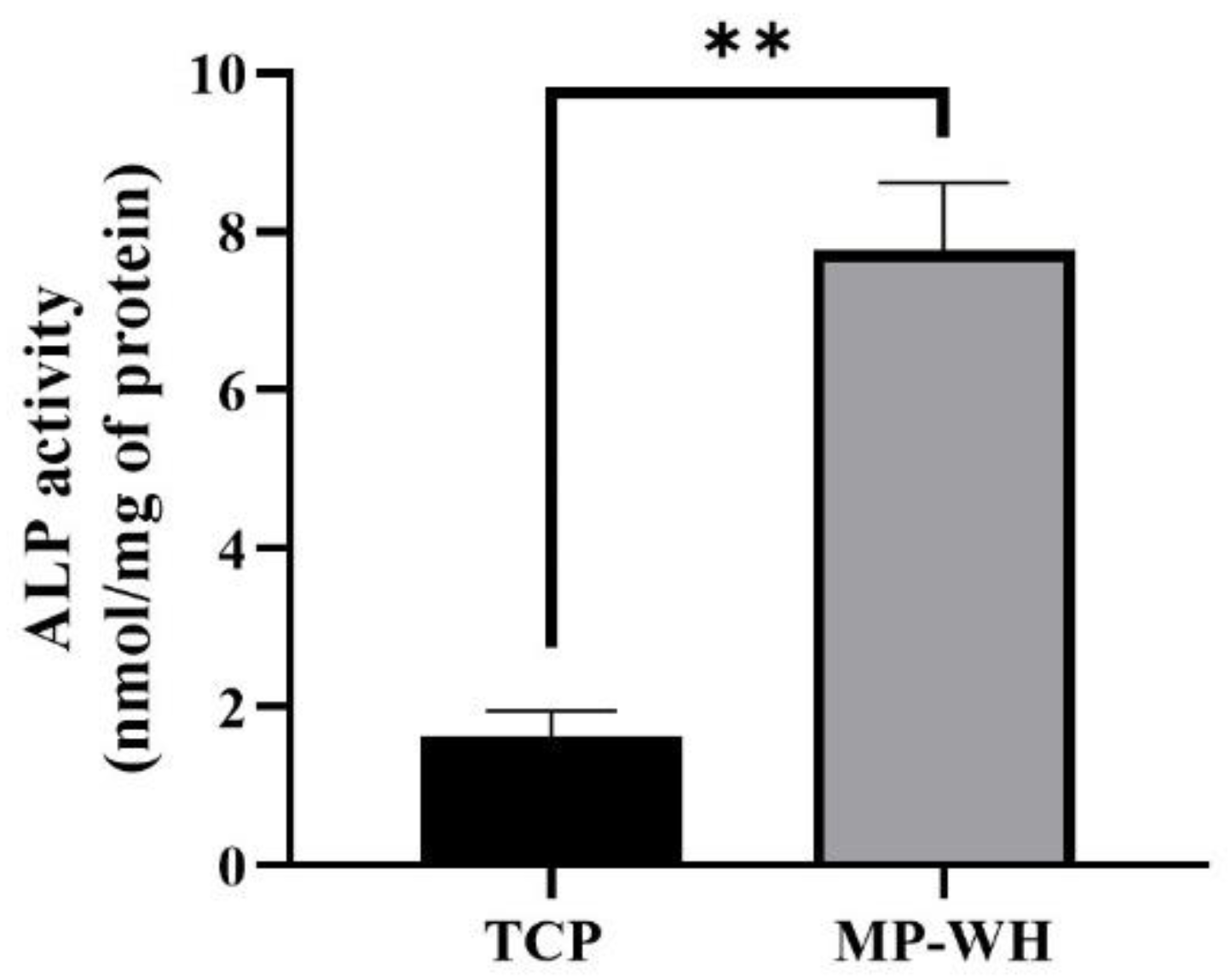
2.9. Alkaline Phosphatase Activity Assay
2.10. Real-Time Polymerase Chain Reaction
2.11. Statistical Analysis
3. Results
3.1. Fabrication of 3D Printing Model
3.2. Compressive Strength and Porosity
3.3. ICP-OES Analysis
3.4. Cell Proliferation Cultured on the 3D Scaffold
3.5. Cell Viability and Cytotoxicity Assay Cultured on the 3D Scaffold
3.6. Cell Adhesion Observation by SEM
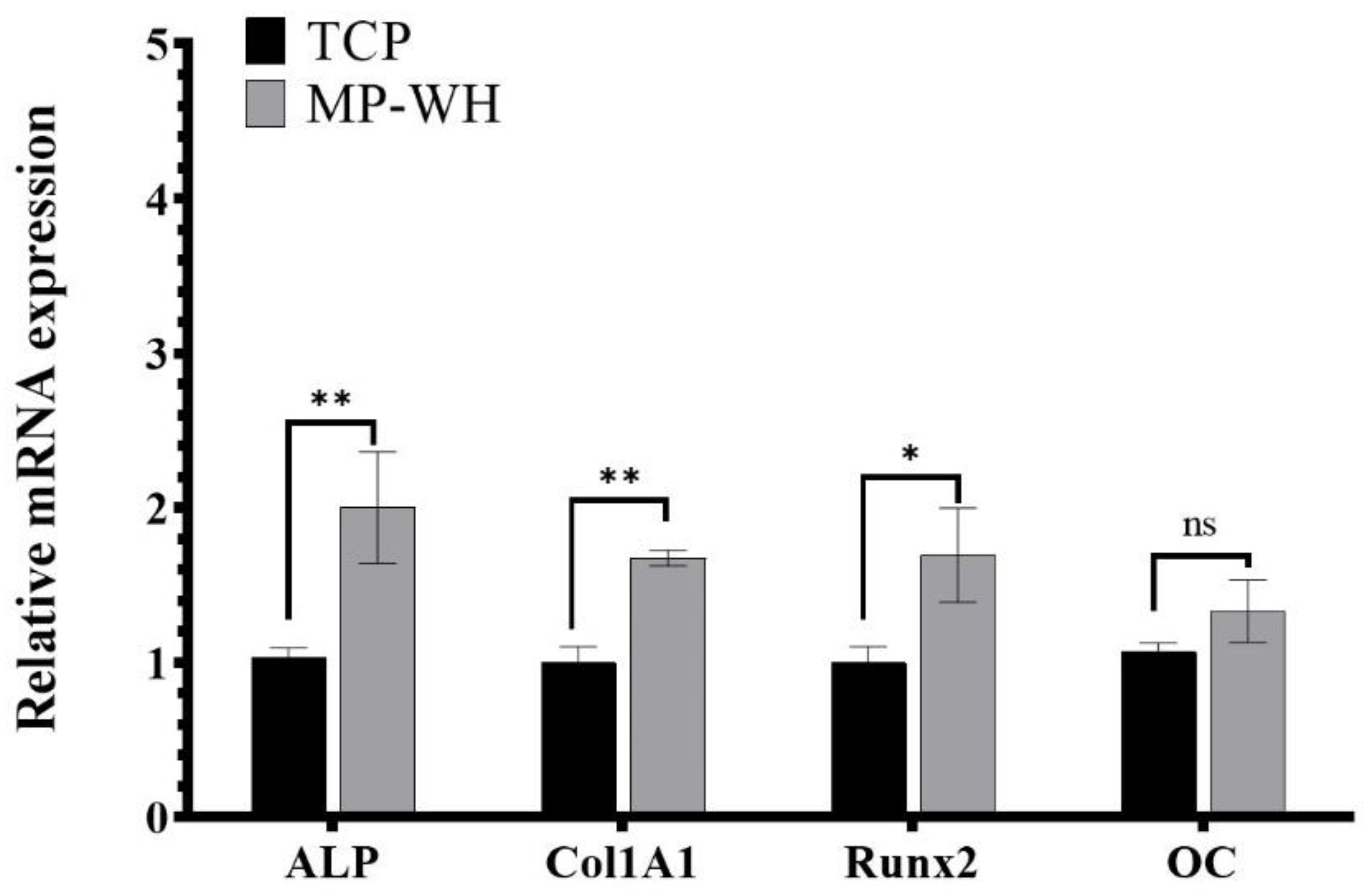
3.7. Measurement of Osteoblast Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schemitsch, E.H. Size matters: Defining critical in bone defect size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E.; Mow, V.C.; Butler, D.; Niklason, L.; Huard, J.; Mao, J.; Yannas, I.; Kaplan, D.; Vunjak-Novakovic, G. Tissue Engineering and Developmental Biology: Going Biomimetic. Tissue Eng. 2006, 12, 3265–3283. [Google Scholar] [CrossRef]
- Vats, A.; Tolley, N.S.; Polak, J.M.; Gough, J.E. Scaffolds and biomaterials for tissue engineering: A review of clinical applications. Clin. Otolaryngol. Allied Sci. 2003, 28, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone Tissue Engineering Using 3D Printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Butscher, A.; Bohner, M.; Hofmann, S.; Gauckler, L.; Müller, R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011, 7, 907–920. [Google Scholar] [CrossRef]
- Tarafder, S.; Balla, V.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J. Tissue Eng. Regen. Med. 2013, 7, 631–641. [Google Scholar] [CrossRef]
- Kasten, P.; Beyen, I.; Niemeyer, P.; Luginbühl, R.; Bohner, M.; Richter, W. Porosity and pore size of β-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: An in vitro and in vivo study. Acta Biomater. 2008, 4, 1904–1915. [Google Scholar] [CrossRef]
- Miranda, P.; Saiz, E.; Gryn, K.; Tomsia, A.P. Sintering and robocasting of β-tricalcium phosphate scaffolds for orthopaedic applications. Acta Biomater. 2006, 2, 457–466. [Google Scholar] [CrossRef]
- Jin, F.; Cai, Q.; Wang, W.; Fan, X.; Lu, X.; He, N.; Ding, J. The Effect of Stromal-Derived Factor 1α on Osteoinduction Properties of Porous β-Tricalcium Phosphate Bioceramics. BioMed Res. Int. 2021, 2021, 8882355. [Google Scholar] [CrossRef] [PubMed]
- Lowmunkong, R.; Sohmura, T.; Suzuki, Y.; Matsuya, S.; Ishikawa, K. Fabrication of freeform bone-filling calcium phosphate ceramics by gypsum 3D printing method. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.L.; Zheng, G.B.; Park, J.; Kim, H.D.; Baek, H.R.; Lee, H.K.; Lee, K.; Han, H.N.; Lee, C.K.; Hwang, N.S.; et al. In Vitro and In Vivo Evaluation of Whitlockite Biocompatibility: Comparative Study with Hydroxyapatite and β-Tricalcium Phosphate. Adv. Healthc. Mater. 2016, 5, 128–136. [Google Scholar] [CrossRef]
- Kim, H.-K.; Han, H.-S.; Lee, K.-S.; Lee, D.-H.; Lee, J.W.; Jeon, H.; Cho, S.-Y.; Roh, H.-J.; Kim, Y.-C.; Seok, H.-K. Comprehensive study on the roles of released ions from biodegradable Mg-5 wt% Ca-1 wt% Zn alloy in bone regeneration. J. Tissue Eng. Regen. Med. 2016, 11, 2710–2724. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, H.L.; Ahn, H.-Y.; Lee, H.K.; Park, J.; Lee, E.-S.; Lee, E.A.; Jeong, Y.-H.; Kim, D.-G.; Nam, K.T.; et al. Biomimetic whitlockite inorganic nanoparticles-mediated in situ remodeling and rapid bone regeneration. Biomaterials 2017, 112, 31–43. [Google Scholar] [CrossRef]
- Leem, Y.-H.; Lee, K.-S.; Kim, J.-H.; Seok, H.-K.; Chang, J.-S.; Lee, D.-H. Magnesium ions facilitate integrin alpha 2- and alpha 3-mediated proliferation and enhance alkaline phosphatase expression and activity in hBMSCs. J. Tissue Eng. Regen. Med. 2016, 10, E527–E536. [Google Scholar] [CrossRef]
- Kim, B.-S.; Kang, H.J.; Yang, S.-S.; Lee, J. Comparison of in vitro and in vivo bioactivity: Cuttlefish-bone-derived hydroxyapatite and synthetic hydroxyapatite granules as a bone graft substitute. Biomed. Mater. 2014, 9, 025004. [Google Scholar] [CrossRef]
- Borden, M. Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair. Biomaterials 2003, 24, 597–609. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.S.; Sung, H.; You, H.; Lee, J. Cellular attachment and osteoblast differentiation of mesenchymal stem cells on natural cuttlefish bone. J. Biomed. Mater. Res. Part A 2012, 100A, 1673–1679. [Google Scholar] [CrossRef] [Green Version]
- Cestari, F.; Agostinacchio, F.; Galotta, A.; Chemello, G.; Motta, A.; Sglavo, V. Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomaterials 2021, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Ibrahim, A.-R.; Li, X.; Zhou, Y.; Huang, Y.; Chen, W.; Wang, H.; Li, J. Synthesis of Spongy-Like Mesoporous Hydroxyapatite from Raw Waste Eggshells for Enhanced Dissolution of Ibuprofen Loaded via Supercritical CO2. Int. J. Mol. Sci. 2015, 16, 7960–7975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, H.Y.; Safwat, N.; Shehata, R.; Althubaiti, E.H.; Kareem, S.; Atef, A.; Qari, S.H.; Aljahani, A.H.; Al-Meshal, A.S.; Youssef, M.; et al. Synthesis of Natural Nano-Hydroxyapatite from Snail Shells and Its Biological Activity: Antimicrobial, Antibiofilm, and Biocompatibility. Membranes 2022, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Lowe, B.; Manivasagan, P.; Kang, K.-H.; Chalisserry, E.P.; Anil, S.; Kim, D.G.; Kim, S.-K. Isolation and Characterization of Nano-Hydroxyapatite from Salmon Fish Bone. Materials 2015, 8, 5426–5439. [Google Scholar] [CrossRef] [PubMed]
- Jinawath, S.; Polchai, D.; Yoshimura, M. Low-temperature, hydrothermal transformation of aragonite to hydroxyapatite. Mater. Sci. Eng. C 2002, 22, 35–39. [Google Scholar] [CrossRef]
- Lee, D.J.; Kwon, J.; Kim, Y.; Wang, X.; Wu, T.; Lee, Y.; Kim, S.; Miguez, P.; Ko, C. Effect of pore size in bone regeneration using polydopamine-laced hydroxyapatite collagen calcium silicate scaffolds fabricated by 3D mould printing technology. Orthod. Craniofacial Res. 2019, 22, 127–133. [Google Scholar] [CrossRef]
- Tang, M.S.; Kadir, A.A.; Ngadiman, N.H.A. Simulation analysis of different bone scaffold porous structures for fused deposition modelling fabrication process. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Scotchford, C.; Vickers, M.; Ali, S.Y. The isolation and characterization of magnesium whitlockite crystals from human articular cartilage. Osteoarthr. Cartil. 1995, 3, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Khorashadizade, F.; Abazari, S.; Rajabi, M.; Bakhsheshi-Rad, H.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Berto, F. Overview of magnesium-ceramic composites: Mechanical, corrosion and biological properties. J. Mater. Res. Technol. 2021, 15, 6034–6066. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Lee, O.K.; Kuo, T.K.; Chen, W.-M.; Lee, K.-D.; Hsieh, S.-L.; Chen, T.-H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Jia, J.; Wu, F.; Wei, S.; Zhou, H.; Zhang, H.; Shin, J.-W.; Liu, C. Hierarchically microporous/macroporous scaffold of magnesium–calcium phosphate for bone tissue regeneration. Biomaterials 2010, 31, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-B.; Wang, C.; Lee, J.-H.; Jeong, K.-J.; Jang, Y.-S.; Park, J.-Y.; Ryu, M.H.; Kim, U.-K.; Lee, J.; Hwang, D.-S. Whitlockite Granules on Bone Regeneration in Defect of Rat Calvaria. ACS Appl. Bio Mater. 2020, 3, 7762–7768. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-Z.; Zheng, G.-B.; Cho, M.; Lee, J.H. Effect of Whitlockite as a new bone substitute for bone formation in spinal fusion and ectopic ossification animal model. Biomater. Res. 2021, 25, 34. [Google Scholar] [CrossRef] [PubMed]
- Majeska, R.J.; Wuthier, R.E. Studies on matrix vesicles isolated from chick epiphyseal cartilage Association of pyrophosphatase and ATPase activities with alkaline phosphatase. Biochim. Biophys. Acta BBA-Enzymol. 1975, 391, 51–60. [Google Scholar] [CrossRef]
- Masi, L.; Franchi, A.; Santucci, M.; Danielli, D.; Arganini, L.; Giannone, V.; Formigli, L.; Benvenuti, S.; Tanini, A.; Beghe, F.; et al. Adhesion, growth, and matrix production by osteoblasts on collagen substrata. Calcif. Tissue Res. 1992, 51, 202–212. [Google Scholar] [CrossRef]
- Banerjee, C.; McCabe, L.R.; Choi, J.Y.; Hiebert, S.W.; Stein, J.L.; Stein, G.S.; Lian, J.B. Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J. Cell. Biochem. 1997, 66, 1–8. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Z.; Yellowley, C.; Donahue, H. Inhibiting gap junctional intercellular communication alters expression of differentiation markers in osteoblastic cells. Bone 1999, 25, 661–666. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- Mouriño, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef] [Green Version]
- Rude, R.; Gruber, H.; Wei, L.; Frausto, A.; Mills, B. Magnesium Deficiency: Effect on Bone and Mineral Metabolism in the Mouse. Calcif. Tissue Res. 2003, 72, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Kim, J.S.; Park, Y.M.; Choi, B.-Y.; Lee, J. Mg ion implantation on SLA-treated titanium surface and its effects on the behavior of mesenchymal stem cell. Mater. Sci. Eng. C 2013, 33, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Sila-Asna, M.; Bunyaratvej, A.; Maeda, S.; Kitaguchi, H.; Bunyaratavej, N. Osteoblast differentiation and bone formation gene expression in strontium-inducing bone marrow mesenchymal stem cell. Kobe J. Med Sci. 2007, 53, 25–35. [Google Scholar] [PubMed]
- Zhu, L.-L.; Zaidi, S.; Peng, Y.; Zhou, H.; Moonga, B.S.; Blesius, A.; Dupin-Roger, I.; Zaidi, M.; Sun, L. Induction of a program gene expression during osteoblast differentiation with strontium ranelate. Biochem. Biophys. Res. Commun. 2007, 355, 307–311. [Google Scholar] [CrossRef]


| Element | As | Cd | Hg | Pb | Ca | K | Mg | P | Si | Sr |
|---|---|---|---|---|---|---|---|---|---|---|
| ND | ND | ND | ND | 346,476 | 3795 | 32,854 | 198,798 | 99 | 1869 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.-W.; Park, H.; Kim, K.-S.; Chun, S.-K.; Kim, B.-S. Marine Plankton-Derived Whitlockite Powder-Based 3D-Printed Porous Scaffold for Bone Tissue Engineering. Materials 2022, 15, 3413. https://doi.org/10.3390/ma15103413
Baek J-W, Park H, Kim K-S, Chun S-K, Kim B-S. Marine Plankton-Derived Whitlockite Powder-Based 3D-Printed Porous Scaffold for Bone Tissue Engineering. Materials. 2022; 15(10):3413. https://doi.org/10.3390/ma15103413
Chicago/Turabian StyleBaek, Ji-Won, Ho Park, Ki-Su Kim, Sung-Kun Chun, and Beom-Su Kim. 2022. "Marine Plankton-Derived Whitlockite Powder-Based 3D-Printed Porous Scaffold for Bone Tissue Engineering" Materials 15, no. 10: 3413. https://doi.org/10.3390/ma15103413
APA StyleBaek, J.-W., Park, H., Kim, K.-S., Chun, S.-K., & Kim, B.-S. (2022). Marine Plankton-Derived Whitlockite Powder-Based 3D-Printed Porous Scaffold for Bone Tissue Engineering. Materials, 15(10), 3413. https://doi.org/10.3390/ma15103413






