Atomic Structure Evaluation of Solution-Processed a-IZO Films and Electrical Behavior of a-IZO TFTs
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
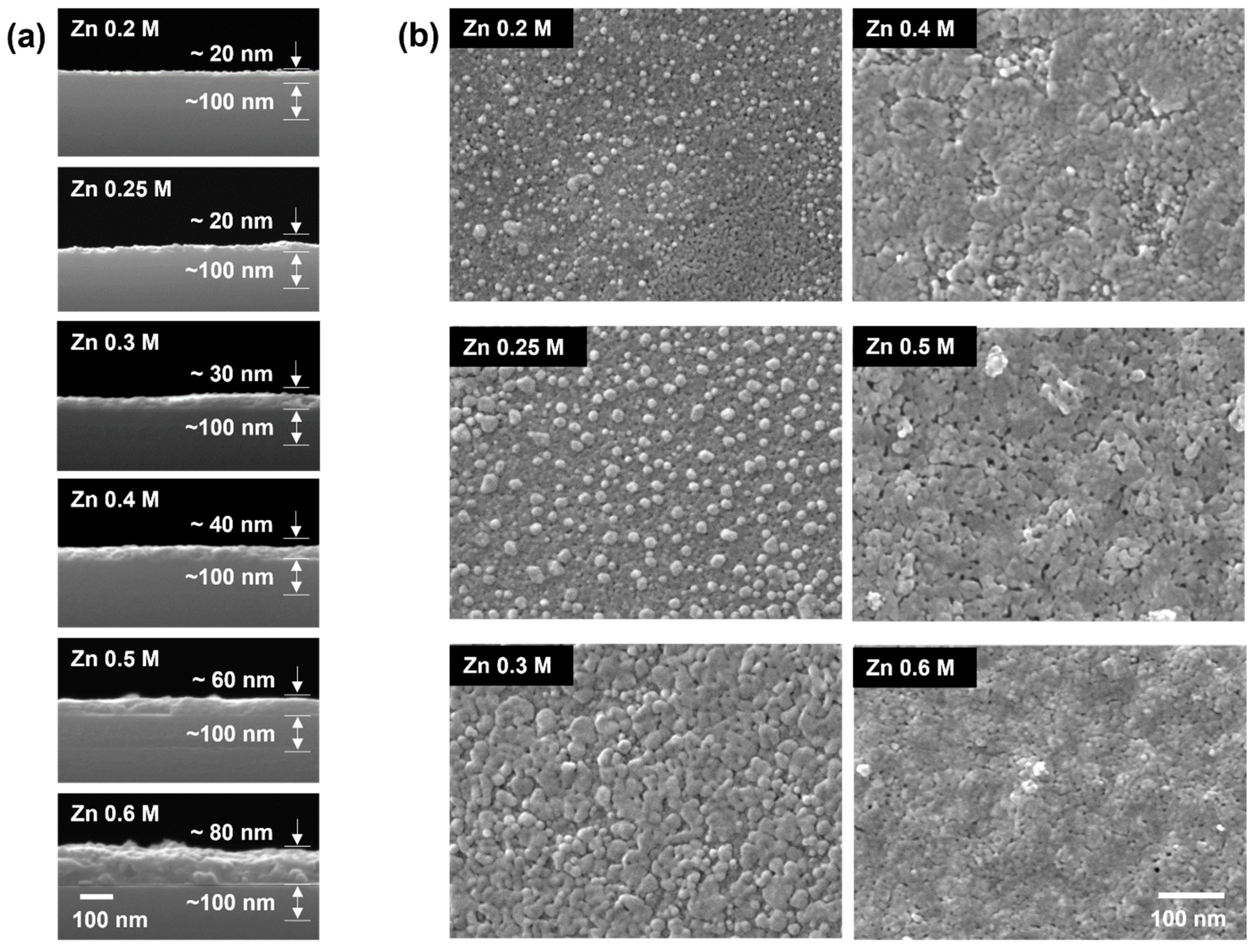
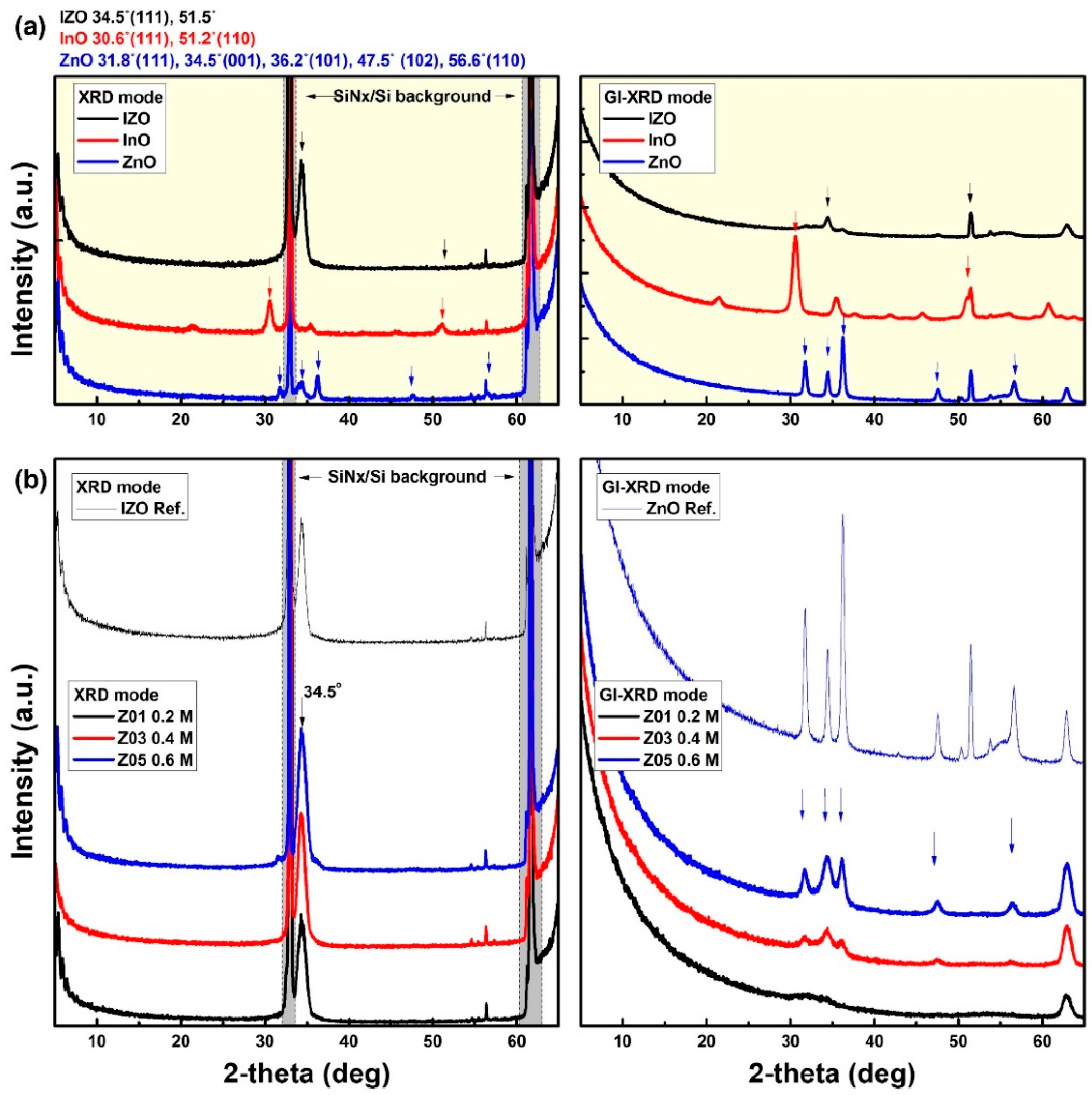
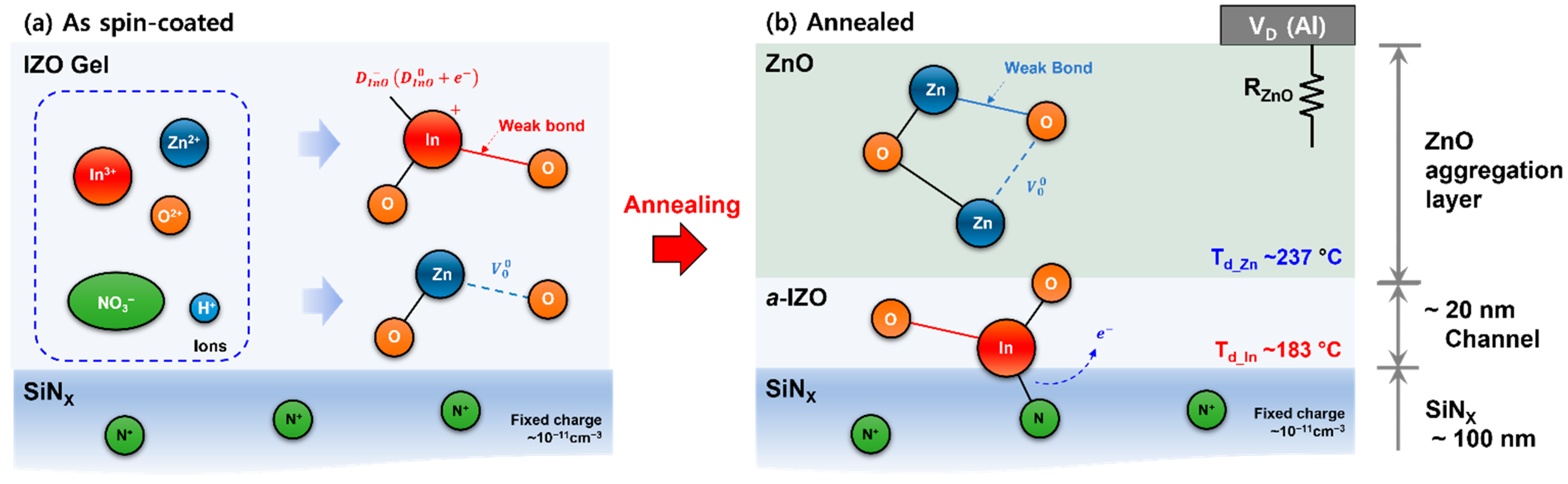
3.1. Structural Properties
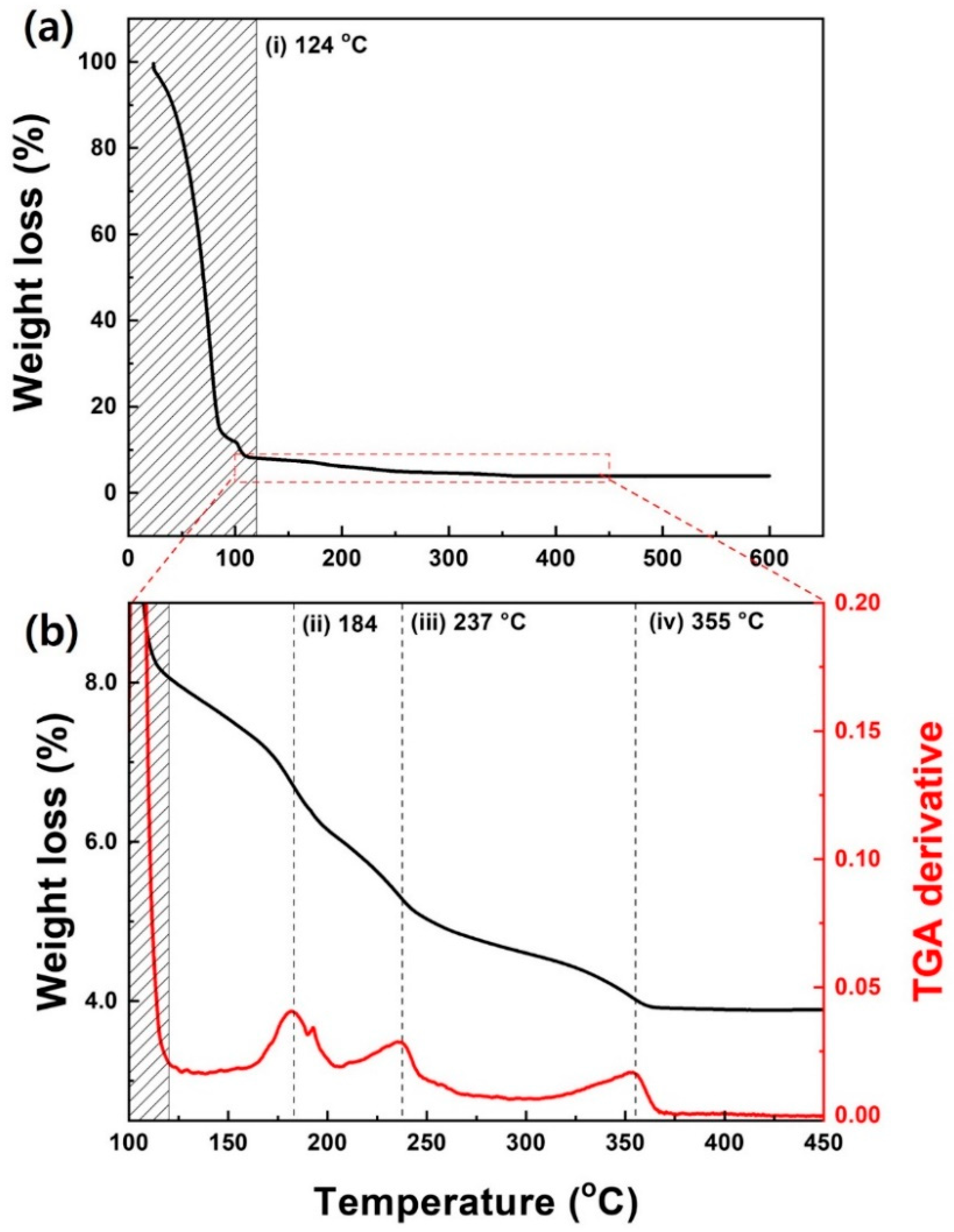
3.2. Growth of a-IZO Film
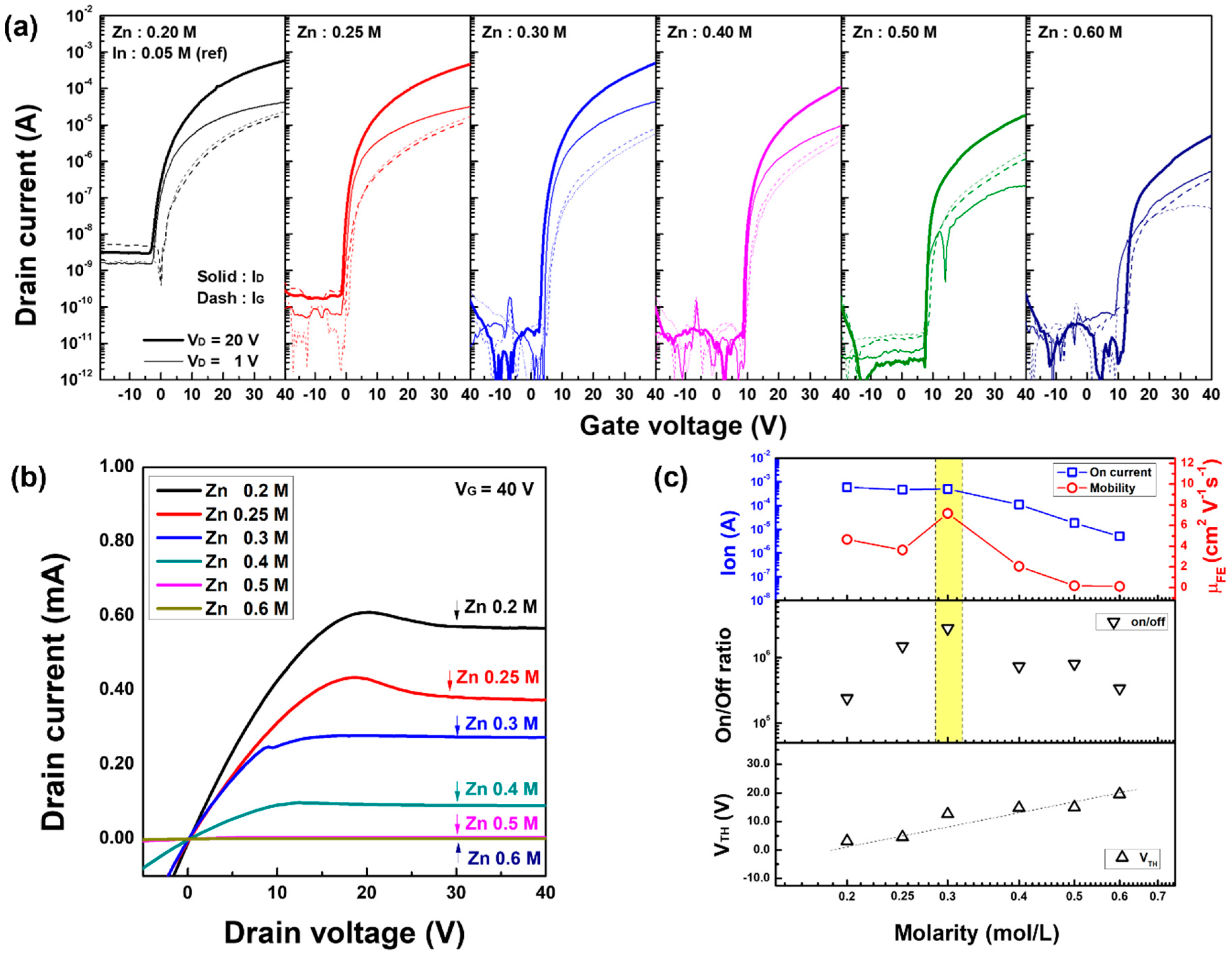
3.3. Electrical Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Lan, Y.; Qiu, S.; Min, S.; Jang, H.; Park, S.; Gong, S.; Ma, Z. Flexible and stretchable microwave electronics: Past, present, and future perspective. Adv. Mater. Technol. 2021, 6, 2000758. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Hong, J.; Nam, S.Y.; Choi, H.H.; Li, X.; Jeong, Y.J.; Kim, S.H. Overview of recent progress in electrohydrodynamic jet printing in practical printed electronics: Focus on the variety of printable materials for each component. Mater. Adv. 2021, 2, 5593–5615. [Google Scholar] [CrossRef]
- Park, J.W.; Kang, B.H.; Kim, H.J. A review of low-temperature solution-processed metal oxide Thin-Film transistors for flexible electronics. Adv. Funct. Mater. 2020, 30, 1904632. [Google Scholar] [CrossRef]
- Carlos, E.; Branquinho, R.; Martins, R.; Kiazadeh, A.; Fortunato, E. Recent progress in solution-based metal oxide resistive switching devices. Adv. Mater. 2021, 33, 2004328. [Google Scholar] [CrossRef]
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide semiconductor thin-film transistor: A review of recent advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef]
- Lu, K.; Li, X.; Sun, Q.; Pang, X.; Chen, J.; Minari, T.; Liu, X.; Song, Y. Solution-processed electronics for artificial synapses. Mater. Horiz. 2021, 8, 447. [Google Scholar] [CrossRef]
- Shi, J.; Zjang, J.; Yang, L.; Qu, M.; Qi, D.-C.; Zhang, K.H.L. Wide bandgap oxide semiconductors: From materials physics to optoelectronic devices. Adv. Mater. 2021, 33, 2006230. [Google Scholar] [CrossRef]
- Ji, D.; Jang, J.; Park, J.H.; Kim, D.; Rim, Y.S.; Hwang, D.K.; Noh, Y.-Y. Recent progress in the development of backplane thin film transistors for information display. J. INF DISP 2021, 22, 1–11. [Google Scholar] [CrossRef]
- Kim, W.-G.; Yak, Y.J.; Yoo, H.; Kim, H.T.; Park, J.W.; Choi, D.H.; Kim, H.J. Photo-induced reactive oxygen species activation for amorphous indium-gallium-zinc oxide thin-film transistors using sodium hypochlorite. ACS Appl. Mater. Interfaces 2021, 13, 44531–44540. [Google Scholar] [CrossRef]
- Min, S.-Y.; Cho, W.-J. High-performance resistive switching in solution-derived IGZO:N memristors by microwave-assisted nitridation. ACS Appl. Mater. Interfaces 2021, 11, 1081. [Google Scholar] [CrossRef]
- Cantarella, G.; Costa, J.; Meister, T.; Ishida, K.; Carta, C.; Ellinger, F.; Lugli, P.; Münzenrieder, N.; Petti, L. Review of recent trends in flexible metal oxide thin-film transistors for analog applications. Flex. Print. Electron. 2020, 5, 033001. [Google Scholar] [CrossRef]
- Kang, Y.H.; Jeong, S.; Ko, J.M.; Lee, J.Y.; Choi, Y.; Lee, C.; Cho, S.Y. Two-component solution processing of oxide semiconductors for thin-film transistors via self-combustion reaction. Mater. Chem. C 2014, 2, 4247. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, J.Y.; Lee, S.S.; Oh, S.W.; Lee, H.H.; Seo, Y.H.; Ryu, B.H.; Choi, Y. Chemically improved high performance printed indium gallium zinc oxide thin-film transistors. J. Mater. Chem. 2011, 21, 17066. [Google Scholar] [CrossRef]
- Sirringhaus, H. 25th anniversary article: Organic field-effect transistors: The path beyond amorphous silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, I.; Eshu, O.V.; Chukwunonso, O.B.; Okoro, U.C. Synthesis and characterization of zinc oxide nanowire. J. Nanomed. Nanotechnol. 2015, 6, 1000321. [Google Scholar]
- Liu, G.; Liu, A.; Zhu, H.; Shin, B.; Fortunato, E.; Martins, R.; Wang, Y.; Shan, F. Low-temperature, nontoxic water-induced metal-oxide thin films and their application in thin-film transistors. Adv. Funct. Mater. 2015, 25, 2564–2572. [Google Scholar] [CrossRef]
- Scheideler, W.J.; Kumar, R.; Zeumault, A.R.; Subramanian, V. Low-temperature-processed printed metal oxide transistors based on pure aqueous inks. Adv. Func. Mater. 2017, 27, 1606062. [Google Scholar] [CrossRef]
- Zhong, G.; Xu, S.; Chen, C.; Kline, D.J.; Giroux, M.; Pei, Y.; Jiao, M.; Liu, D.; Mi, R.; Xie, H.; et al. Synthesis of metal oxide nanoparticles by rapid high-temperature 3D microwave heating. Adv. Funct. Mater. 2019, 29, 1904282. [Google Scholar] [CrossRef]
- Yarali, E.; Koutsiaki, C.; Faber, H.; Tetner, K.; Yengel, E.; Patsalas, P.; Kalfagiannis, N.; Koutsogeorgis, D.C.; Anthopoulos, T.D. Recent progress photonic processing of metal-oxide transistors. Adv. Funct. Mater. 2020, 30, 1906022. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Kang, Y.; Kim, W.; Park, J.B.; Park, S.K.; Kim, Y.H. Optimization of dual-fuel combustion synthesis for rapid formation of solution-processed metal-oxide thin-film transistors. ACS Appl. Elecron. Mater. 2022, 4, 1327–1334. [Google Scholar] [CrossRef]
- Mude, N.N.; Bukke, R.N.; Jang, J. High performance of solution-processed amorphous p-channel copper-tin-sulfur-gallium oxide thin-film transistors by UV/O3 photocuring. ACS Appl. Mater. Interfaces 2021, 13, 20277–20287. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Park, J.; Hwang, J.; Kim, H.D.; Ryu, J.H.; Lee, K.B.; Baek, K.H.; Do, L.M.; Choi, J.S. Rapid curing of solution-processed zinc oxide films by pulse-light annealing for thin-film transistor Applications. Electron. Mater. Lett. 2015, 11, 82–87. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, D.; Shin, H.; Lee, D.K.; Zhang, X.; Park, J.; Choi, J.S. Advanced photo-annealing of indium zinc oxide films for thin-film transistors using pulse UV light. J. Inf. Disp. 2016, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.J.; Yoon, D.H.; Jung, T.S.; Kim, H.J. Recent advances in low-temperature solution-processed oxide backplanes. J. Inf. Disp. 2013, 14, 79–87. [Google Scholar] [CrossRef]
- Nayak, P.; Busani, T.; Elamurugu, E.; Barquinha, P.; Martins, R.; Hong, Y.; Fortunato, E. Zinc concentration dependence study of solution processed amorphous indium gallium zinc oxide thin film transistors using high-k dielectric. Appl. Phys. Lett. 2010, 97, 183504. [Google Scholar] [CrossRef]
- Tak, Y.J.; Rim, Y.S.; Yoon, D.H.; Kim, S.J.; Park, S.P.; Lee, H.; Kim, W.G.; Yang, Y.; Kim, H.J. Modified stoichiometry in homogeneous indium–zinc oxide system as vertically graded oxygen deficiencies by controlling redox reactions. Adv. Mater. Interfaces 2016, 3, 1500606. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, J.H.; Jo, Y.H.; Lee, J.S. Precipitating metal nitrate decomposition of amorphous metal oxyhydroxide electode containing Ni, Fe, and Co for electrocatalytic water oxidation. ACS Catal. 2019, 9, 9650–9662. [Google Scholar] [CrossRef]
- Damma, D.; Boningari, T.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. Direct decomposition of NOx over TiO2 supported transition metal oxides at low temperatures. Ind. Eng. Chem. Res. 2018, 57, 16615–16621. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, J.Y.; Lee, S.S.; Seo, Y.H.; Kim, S.Y.; Park, J.U.; Ryu, B.H.; Yang, W.; Moon, J.; Choi, Y. Metal salt-derived In–Ga–Zn–O semiconductors incorporating formamide as a novel co-solvent for producing solution-processed, electrohydrodynamic-jet printed, high performance oxide transistors. J. Mater. Chem. A 2013, 1, 4236. [Google Scholar] [CrossRef] [Green Version]
- Cochran, E.A.; Woods, K.N.; Johnson, D.W.; Page, C.J.; Boettcher, S.W. Unique chemistries of metal–nitrate precursors to form metal-oxide thin films from solution: Materials for electronic and energy applications. J. Mater. Chem. A 2019, 7, 24124–24129. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Fan-Yuan, L.; Tsong-Huei, C.; Chuin-Tih, V. Thermal decomposition of metal nitrates in air and hydrogen environments. J. Phys. Chem. B 2003, 107, 4. [Google Scholar] [CrossRef]
- Nickel, N.H.; Jackson, W.B. Hydrogen-mediated creation and annihilation of strain in amorphous silicon. Phys. Rev. B 1995, 51, 4872–4881. [Google Scholar] [CrossRef] [PubMed]
- Stutzmann, M.; Biegelsen, D.K.; Street, R.A. Detailed investigation of doping in hydrogenated amorphous silicon and germanium. Phys. Rev. B 1987, 35, 5666–5701. [Google Scholar] [CrossRef] [PubMed]
- Street, R.A. Doping and the Fermi energy in amorphous silicon. Phys. Rev. Lett. 1982, 49, 1187–1190. [Google Scholar] [CrossRef]



| No. | Zn01 | Zn02 | Zn03 | Zn04 | Zn05 | Zn06 | Zn07 | Zn08 |
|---|---|---|---|---|---|---|---|---|
| Zn molarity (M) | 0.1 (failed) | 0.15 (failed) | 0.2 | 0.25 | 0.3 | 0.4 | 0.5 | 0.6 |
| In molarity (M) | 0.05 M (fixed) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Lee, H.; Kim, B.; Zhang, X.; Bae, J.-H.; Choi, J.-S.; Baang, S. Atomic Structure Evaluation of Solution-Processed a-IZO Films and Electrical Behavior of a-IZO TFTs. Materials 2022, 15, 3416. https://doi.org/10.3390/ma15103416
Kim D, Lee H, Kim B, Zhang X, Bae J-H, Choi J-S, Baang S. Atomic Structure Evaluation of Solution-Processed a-IZO Films and Electrical Behavior of a-IZO TFTs. Materials. 2022; 15(10):3416. https://doi.org/10.3390/ma15103416
Chicago/Turabian StyleKim, Dongwook, Hyeonju Lee, Bokyung Kim, Xue Zhang, Jin-Hyuk Bae, Jong-Sun Choi, and Sungkeun Baang. 2022. "Atomic Structure Evaluation of Solution-Processed a-IZO Films and Electrical Behavior of a-IZO TFTs" Materials 15, no. 10: 3416. https://doi.org/10.3390/ma15103416







