The Relationship between Osteoinduction and Vascularization: Comparing the Ectopic Bone Formation of Five Different Calcium Phosphate Biomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. X-ray Diffraction (XRD)
2.2. Scanning Electron Microscopy (SEM)
2.3. Animal Surgery
2.4. Histological Staining
2.5. Immunohistochemistry
2.6. Morphological Analysis
2.7. Statistical Analysis
3. Results
3.1. The XRD Spectra of Materials
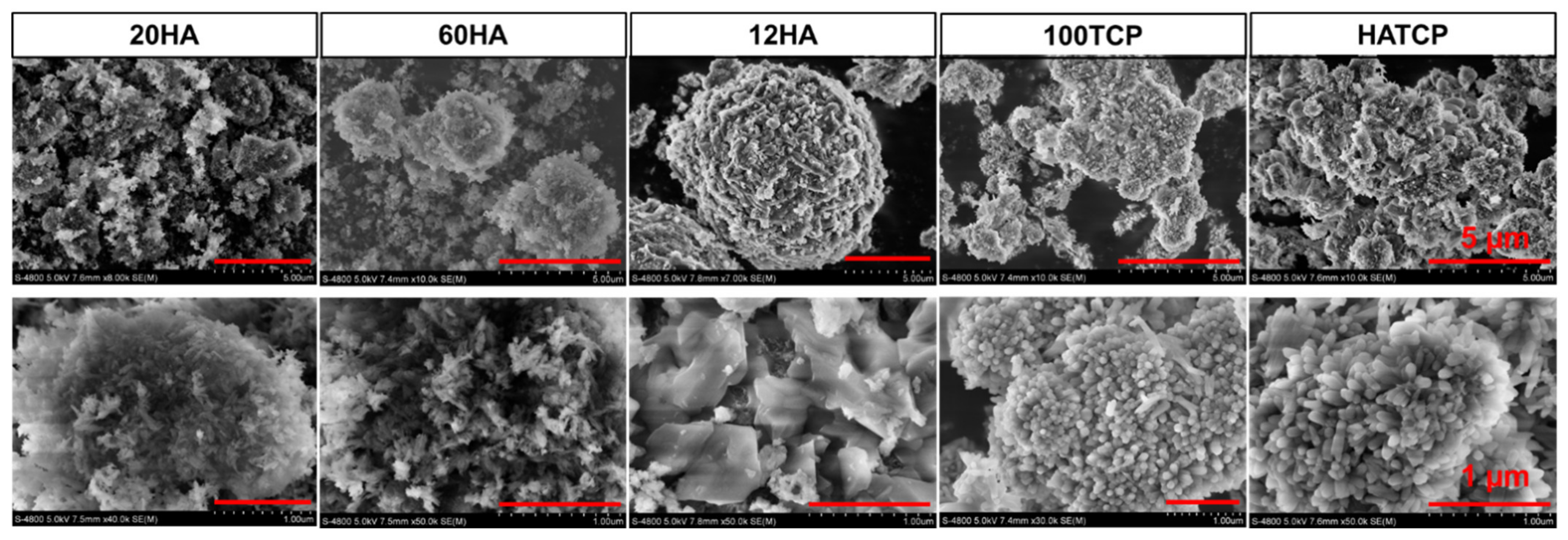
3.2. The Micromorphology of Materials
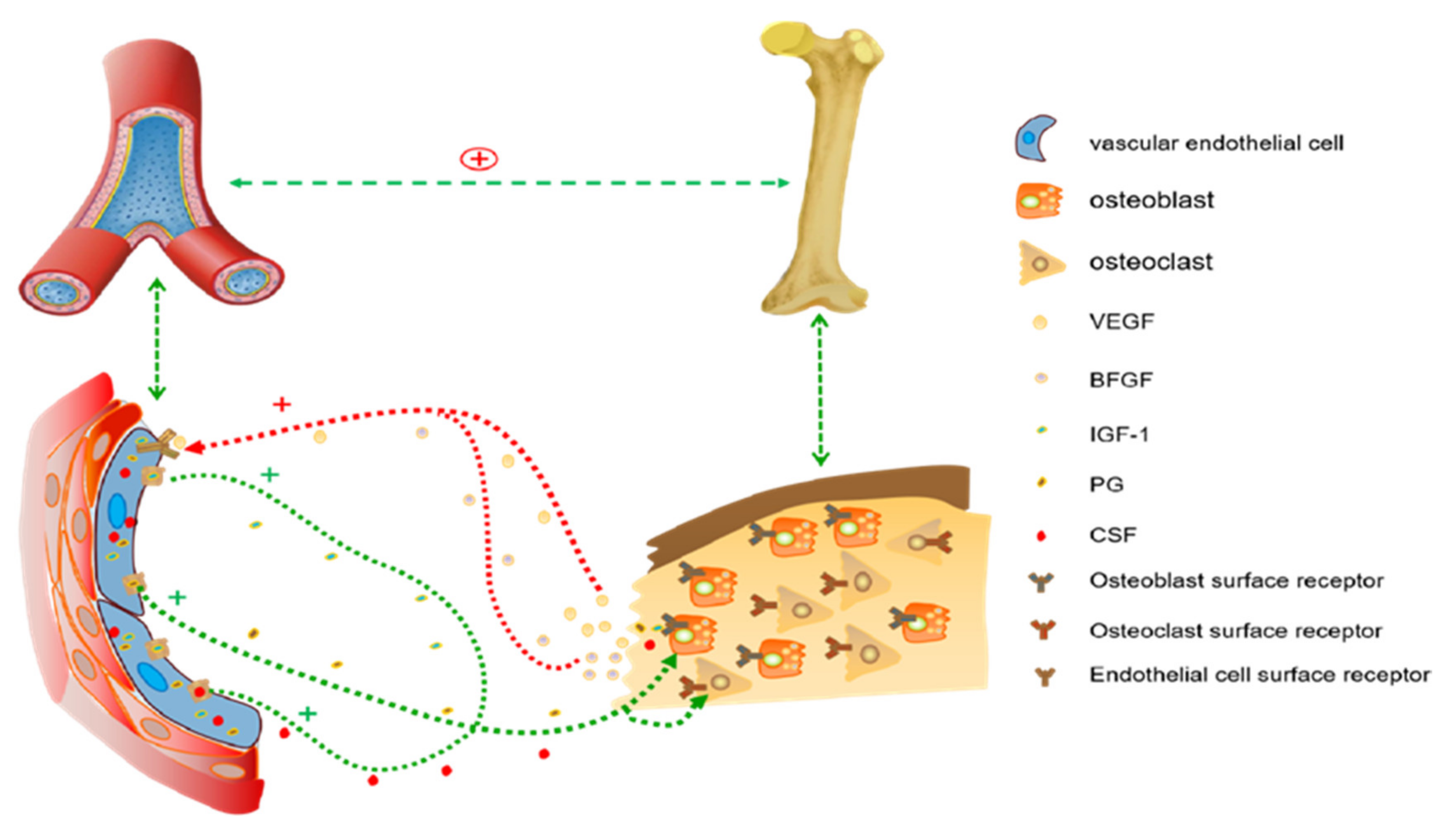
3.3. Angiogenesis Prior to Bone Formation
3.4. Osteoinduction of Ca-P Biomaterials
3.5. Quantitative Analysis of Bone Induction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.D.; Zhou, P.; Wu, C.; Qu, Y.; Zhang, J. A study of porous block HA ceramics and its osteogenesis. In Bioceramics and the Human Body; Ravaglioli, A., Krajewski, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 408–415. [Google Scholar]
- Yamasaki, H.; Sakai, H. Osteogenic response to porous hydroxyapatite ceramics under the skin of dogs. Biomaterials 1992, 13, 308–312. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y.; Ji, L.; Geng, Z.; Wang, J.; Liu, C. Calcium phosphate-based materials regulate osteoclast-mediated osseointegration. Bioact. Mater. 2021, 6, 4517–4530. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Zhang, Y.; van Dijk, L.; Barbieri, D.; van den Beucken, J.; Yuan, H.; de Bruijn, J. Coupling between macro-phage phenotype, angiogenesis and bone formation by calcium phosphates. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, X.; Barbieri, D.; Barradas, A.M.; de Bruijn, J.D.; van Blitterswijk, C.A.; Yuan, H. The size of surface micro-structures as an osteogenic factor in calcium phosphate ceramics. Acta Biomater. 2014, 10, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.J.; Yu, T.; Shi, Z. Osteoinduction Mechanism of Calcium Phosphate Biomaterials In Vivo: A Review. J. Biomater. Tissue Eng. 2017, 7, 911–918. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- Da Silva Brum, I.; Frigo, L.; Goncalo Pinto Dos Santos, P.; Nelson Elias, C.; da Fonseca, G.A.M.D.; Jose de Carvalho, J. Performance of Nano-Hydroxyapatite/Beta-Tricalcium Phosphate and Xenogenic Hydroxyapatite on Bone Regeneration in Rat Calvarial Defects: Histomorphometric, Immunohistochemical and Ultrastructural Analysis. Int. J. Nanomed. 2021, 16, 3473–3485. [Google Scholar] [CrossRef]
- Erdem, U.; Turkoz, M.B. La3+ and F− dual-doped multifunctional hydroxyapatite nanoparticles: Synthesis and characterization. Microsc. Res. Tech. 2021, 84, 3211–3220. [Google Scholar] [CrossRef]
- Bas, M.; Daglilar, S.; Kuskonmaz, N.; Kalkandelen, C.; Erdemir, G.; Kuruca, S.E.; Tulyaganov, D.; Yoshioka, T.; Gunduz, O.; Ficai, D.; et al. Mechanical and Biocompatibility Properties of Calcium Phosphate Bioceramics Derived from Salmon Fish Bone Wastes. Int. J. Mol. Sci. 2020, 21, 8082. [Google Scholar] [CrossRef]
- Sadowska, J.M.; Guillem-Marti, J.; Montufar, E.B.; Espanol, M.; Ginebra, M.P. Biomimetic Versus Sintered Calcium Phosphates: The In Vitro Behavior of Osteoblasts and Mesenchymal Stem Cells. Tissue Eng. Part A 2017, 23, 1297–1309. [Google Scholar] [CrossRef]
- Cheng, L.; Yan, S.; Zhu, J.; Cai, P.; Wang, T.; Shi, Z. Exercise enhance the ectopic bone formation of calcium phosphate biomaterials in muscles of mice. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sha, J.; Kanno, T.; Miyamoto, K.; Hideshima, K.; Matsuzaki, Y. Comparison of the Bone Regenerative Capacity of Three-Dimensional Uncalcined and Unsintered Hydroxyapatite/Poly-d/l-Lactide and Beta-Tricalcium Phosphate Used as Bone Graft Substitutes. J. Investig. Surg. 2021, 34, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wang, N.; Jiang, Q.; Tian, L.; Hu, L.; Zhang, Z. The immunogenic reaction and bone defect repair function of epsilon-poly-L-lysine (EPL)-coated nanoscale PCL/HA scaffold in rabbit calvarial bone defect. J. Mater. Sci. Mater. Med. 2021, 32, 63. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Wang, P.; Yao, Y.; Han, X.; Liang, J.; Jiang, Q.; Sun, Y.; Fan, Y.; Zhang, X. A highly interweaved HA-SS-nHAp/collagen hybrid fibering hydrogel enhances osteoinductivity and mineralization. Nanoscale 2020, 12, 12869–12882. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Rismanchian, M.; Jafari-Pozve, N.; Nosouhian, S. Comparing the Efficacy of Three Different Nano-scale Bone Substitutes: In vivo Study. Adv. Biomed. Res. 2017, 6, 64. [Google Scholar]
- Wang, L.; Zhang, B.; Bao, C.; Habibovic, P.; Hu, J.; Zhang, X. Ectopic osteoid and bone formation by three calcium-phosphate ceramics in rats, rabbits and dogs. PLoS ONE 2014, 9, e107044. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Song, T.; Chen, X.; Wang, M.; Yang, X.; Xiao, Y.; Zhang, X. Osteoinductivity of Porous Biphasic Calcium Phos-phate Ceramic Spheres with Nanocrystalline and Their Efficacy in Guiding Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 3722–3736. [Google Scholar] [CrossRef]
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef]
- Azari, R.; Rezaie, H.R.; Khavandi, A. Effect of titanium dioxide intermediate layer on scratch and corrosion resistance of sol-gel-derived HA coating applied on Ti-6Al-4V substrate. Prog. Biomater. 2021, 10, 259–269. [Google Scholar] [CrossRef]
- Bulina, N.V.; Rybin, D.K.; Makarova, S.V.; Dudina, D.V.; Batraev, I.S.; Utkin, A.V.; Prosanov, I.Y.; Khvostov, M.V.; Ulianitsky, V.Y. Detonation Spraying of Hydroxyapatite on a Titanium Alloy Implant. Materials 2021, 14, 4852. [Google Scholar] [CrossRef]
- Lugovskoy, A.; Lugovskoy, S. Production of hydroxyapatite layers on the plasma electrolytically oxidized surface of titanium alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chen, H.; Yuan, B.; Zhou, Y.; Min, L.; Xiao, Z.; Yang, X.; Zhu, X.; Tu, C.; Zhang, X. The morphological effect of nanostructured hydroxyapatite coatings on the osteoinduction and osteogenic capacity of porous titanium. Nanoscale 2020, 12, 24085–24099. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ye, F.; Lu, X.; Wang, J.; Li, S.; Fan, H.; Bu, H. Tissue Response of an Osteoinductive Bioceramic in Bone Defect Rabbit Model. J. Wuhan Univ.Technol. Mater. Sci. Ed. 2010, 1, 28–31. [Google Scholar] [CrossRef]
- Xing, F.; Chi, Z.; Yang, R.; Xu, D.; Cui, J.; Huang, Y.; Zhou, C.; Liu, C. Chitin-hydroxyapatite-collagen composite scaffolds for bone regeneration. Int. J. Biol. Macromol. 2021, 184, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Amnael Orozco-Díaz, C.; Moorehead, R.; Reilly, G.C.; Gilchrist, F.; Miller, C. Characterization of a composite polylactic acid-hydroxyapatite 3D-printing filament for bone-regeneration. Biomed. Phys. Eng. Express 2020, 6, 025007. [Google Scholar] [CrossRef]
- Liang, T.; Wu, J.; Li, F.; Huang, Z.; Pi, Y.; Miao, G.; Ren, W.; Liu, T.; Jiang, Q.; Guo, L. Drug-loading three-dimensional scaffolds based on hydroxyapatite-sodium alginate for bone regeneration. J. Biomed. Mater. Res. A 2021, 109, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Chatzipetros, E.; Damaskos, S.; Tosios, K.I.; Christopoulos, P.; Donta, C.; Kalogirou, E.M.; Yfanti, Z.; Tsiourvas, D.; Papavasiliou, A.; Tsiklakis, K. The effect of nano-hydroxyapatite/chitosan scaffolds on rat calvarial defects for bone regeneration. Int. J. Implant. Dent. 2021, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dong, X.; Qin, H.; Sui, L.; Wang, J. Three-dimensional porous reduced graphene oxide/hydroxyapatite membrane for guided bone regeneration. Colloids Surf. B Biointerfaces 2021, 208, 112102. [Google Scholar] [CrossRef]
- Negrila, C.C.; Predoi, M.V.; Iconaru, S.L.; Predoi, D. Development of Zinc-Doped Hydroxyapatite by Sol-Gel Method for Medical Applications. Molecules 2018, 23, 2986. [Google Scholar] [CrossRef] [Green Version]
- Song, J.E.; Tian, J.; Kook, Y.J.; Thangavelu, M.; Choi, J.H.; Khang, G. A BMSCs-laden quercetin/duck’s feet collagen/hydroxyapatite sponge for enhanced bone regeneration. J. Biomed. Mater. Res. A 2020, 108, 784–794. [Google Scholar] [CrossRef]
- Bal, Z.; Korkusuz, F.; Ishiguro, H.; Okada, R.; Kushioka, J.; Chijimatsu, R.; Kodama, J.; Tateiwa, D.; Ukon, Y.; Nakagawa, S.; et al. A novel nano-hydroxyapatite/synthetic polymer/bone morphogenetic protein-2 composite for efficient bone regeneration. Spine J. 2021, 21, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lun, D.X. Current application of β-tricalcium phosphate composites in orthopaedics. Orthop. Surg. 2012, 4, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, M.; Chen, F.; Wang, J.; Li, X.; Liang, J.; Fan, Y.; Xiao, Y.; Zhang, X. Correlations between macrophage polarization and osteoinduction of porous calcium phosphate ceramics. Acta Biomater. 2020, 103, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Vishnu Priya, M.; Sivshanmugam, A.; Boccaccini, A.R.; Goudouri, O.M.; Sun, W.; Hwang, N.; Deepthi, S.; Nair, S.V.; Jayakumar, R. Injectable osteogenic and angiogenic nanocomposite hydrogels for irregular bone defects. Biomed Mater. 2016, 11, 035017. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]




| Groups | Number of Blood Vessels | Area Percentage of New Bone Tissue (%) | F | p |
|---|---|---|---|---|
| 20HA | 65 | 3.27 | 13.9327 | 0.0202 |
| 60HA | 78 | 3.43 | ||
| 12HA | 12 | 0 | ||
| 100TCP | 118 | 7.33 | ||
| HATCP | 139 | 8.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Peng, Y.; Liu, L.; Hou, S.; Mu, J.; Lan, L.; Cheng, L.; Shi, Z. The Relationship between Osteoinduction and Vascularization: Comparing the Ectopic Bone Formation of Five Different Calcium Phosphate Biomaterials. Materials 2022, 15, 3440. https://doi.org/10.3390/ma15103440
He Y, Peng Y, Liu L, Hou S, Mu J, Lan L, Cheng L, Shi Z. The Relationship between Osteoinduction and Vascularization: Comparing the Ectopic Bone Formation of Five Different Calcium Phosphate Biomaterials. Materials. 2022; 15(10):3440. https://doi.org/10.3390/ma15103440
Chicago/Turabian StyleHe, Yun, Yu Peng, Lishuang Liu, Sha Hou, Junyu Mu, Liang Lan, Lijia Cheng, and Zheng Shi. 2022. "The Relationship between Osteoinduction and Vascularization: Comparing the Ectopic Bone Formation of Five Different Calcium Phosphate Biomaterials" Materials 15, no. 10: 3440. https://doi.org/10.3390/ma15103440







