The Effect of Magnesium Hydroxide Addition on the Extinguishing Efficiency of Sodium Bicarbonate Powders
Abstract
:1. Introduction
2. Materials and Methods
Characterization of the BC Extinguishing Powders
3. Results and Discussion
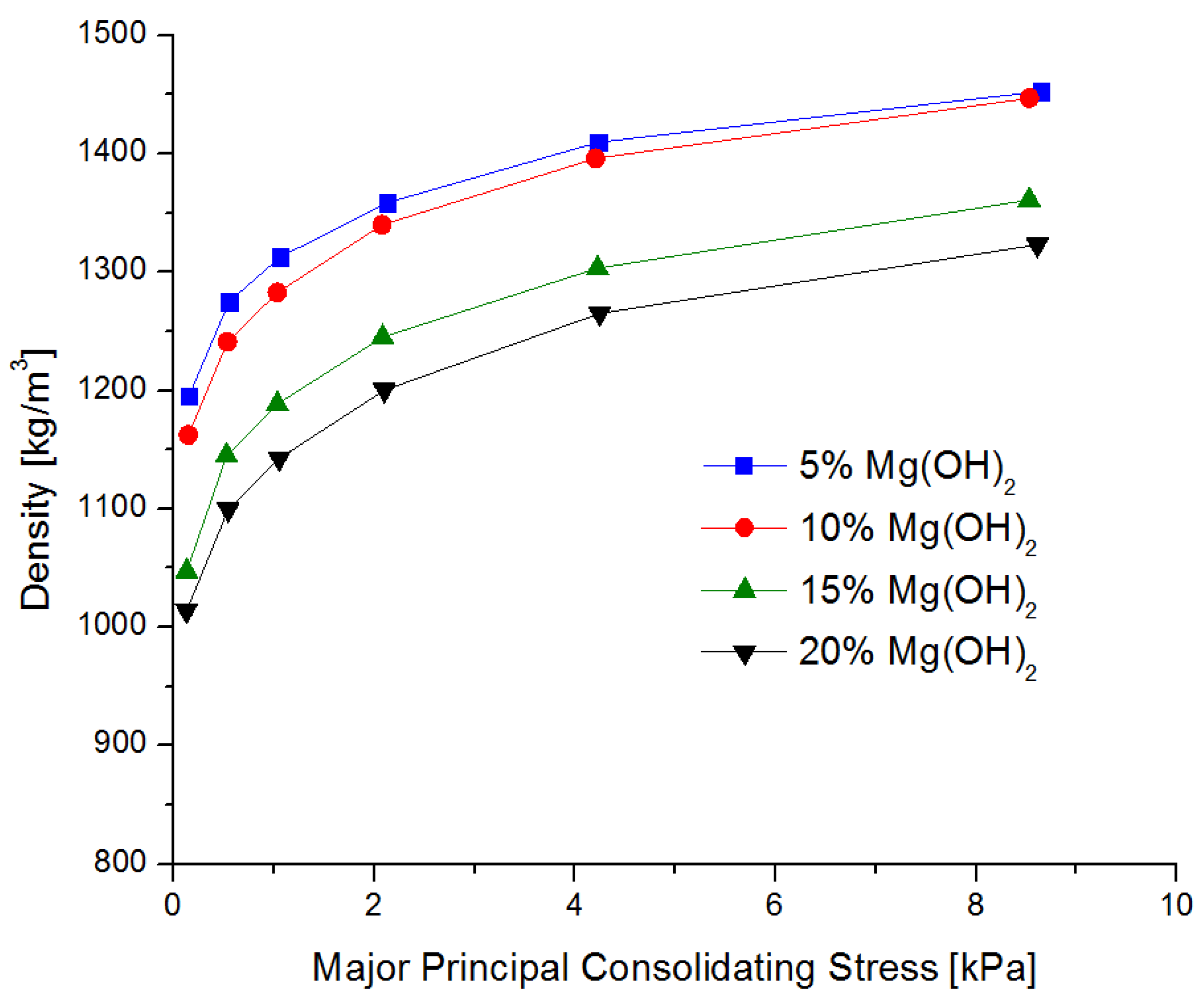
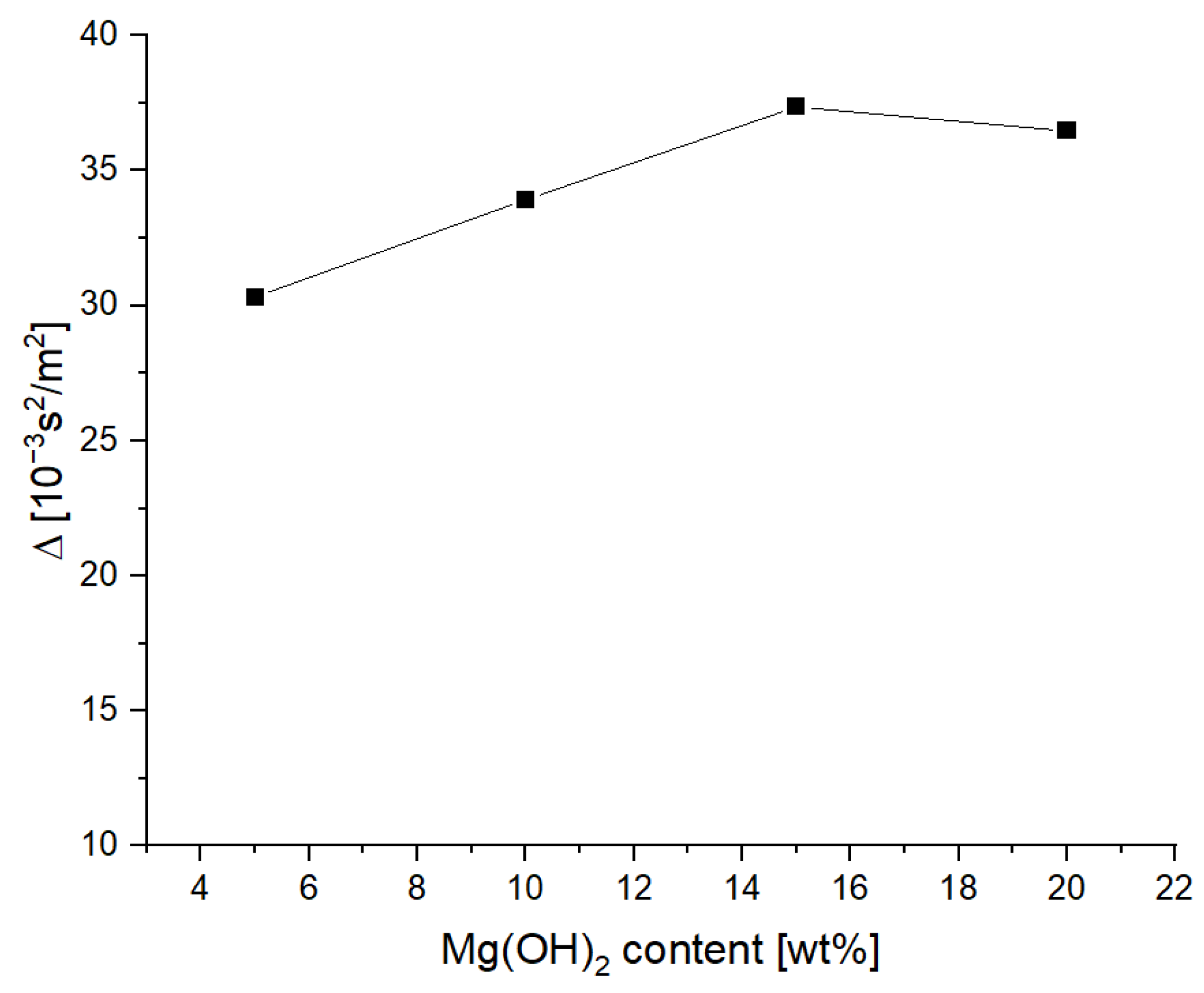
3.1. Rheology of the Extinguishing Powders
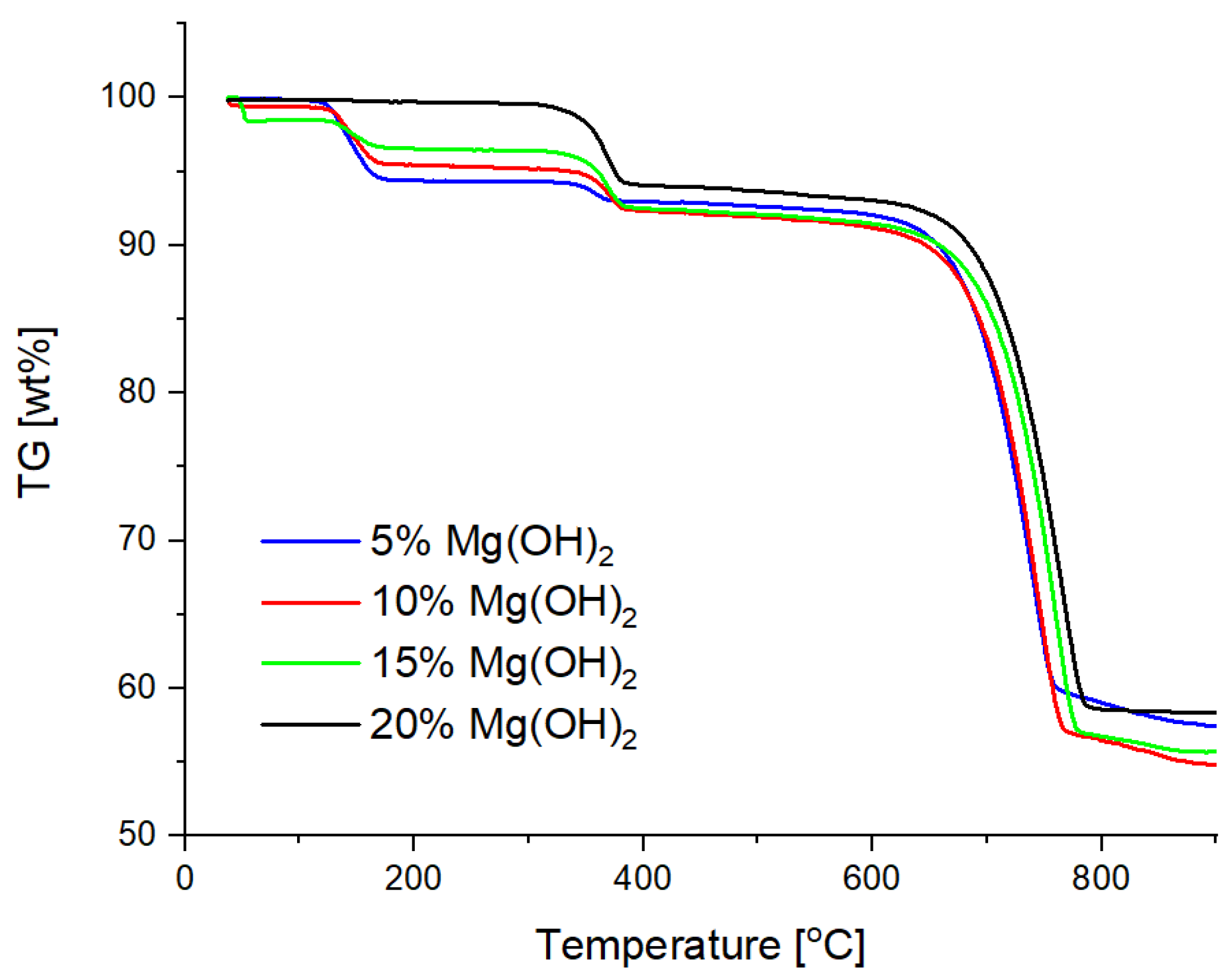
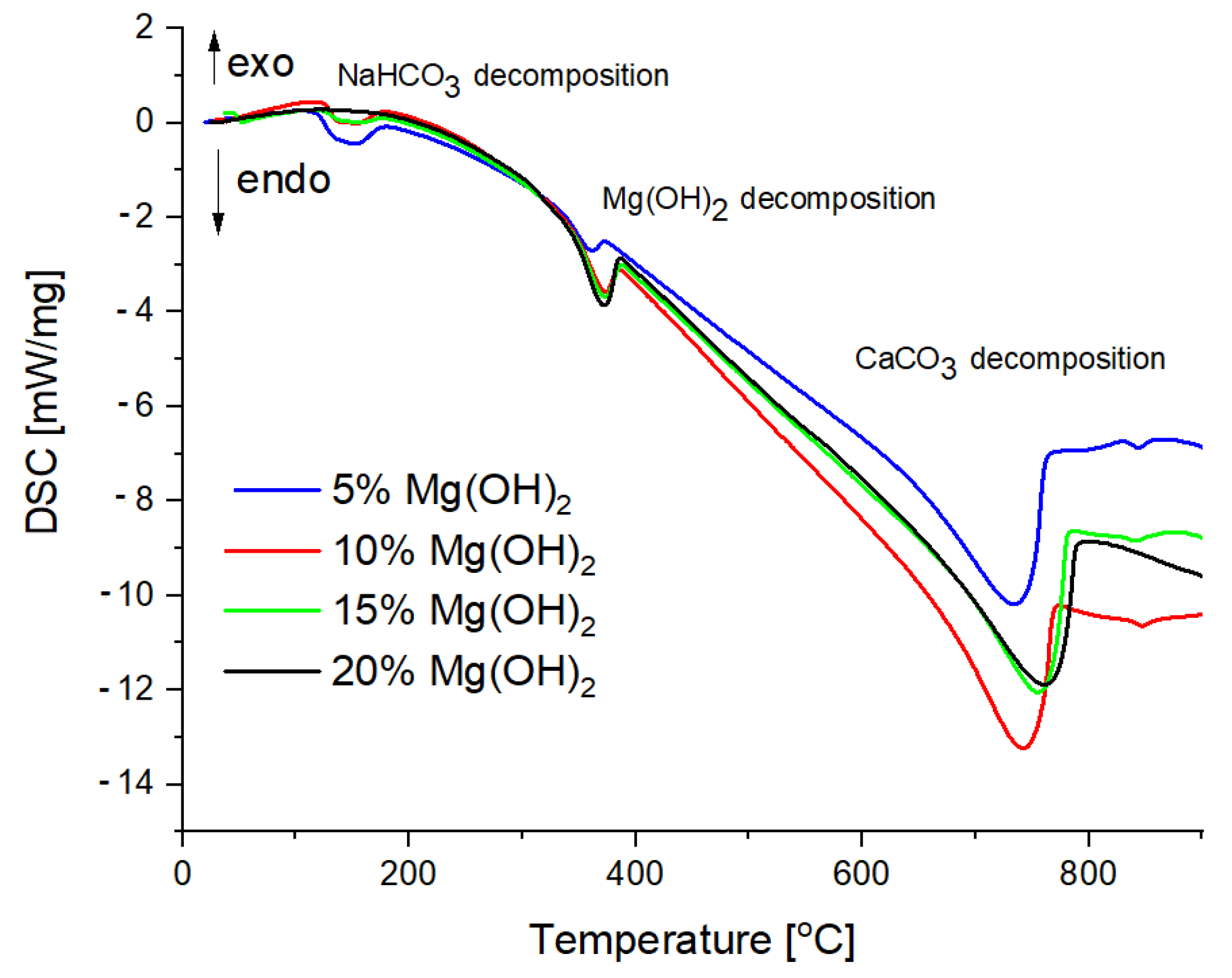
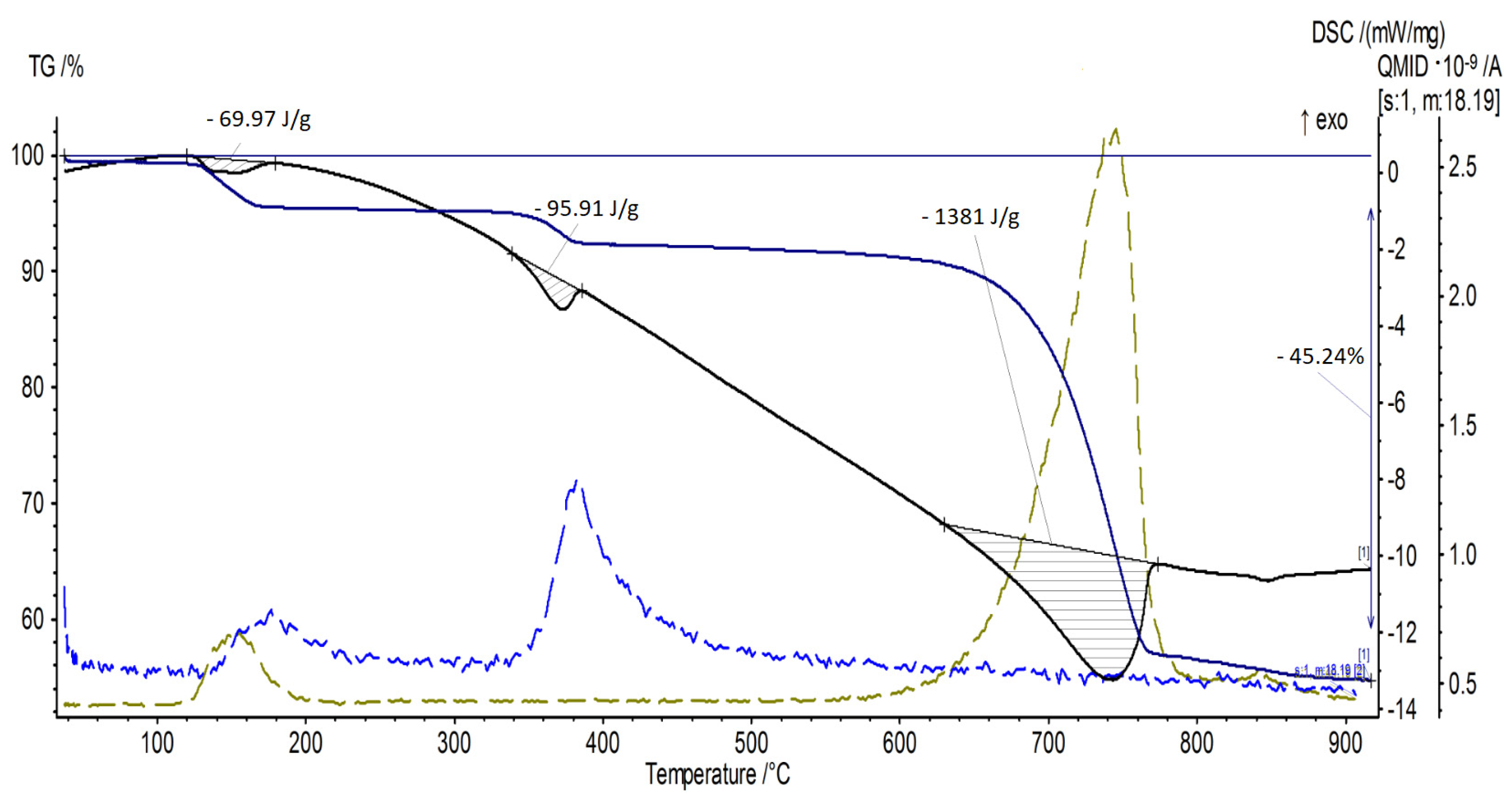
3.2. TG and DSC Analysis
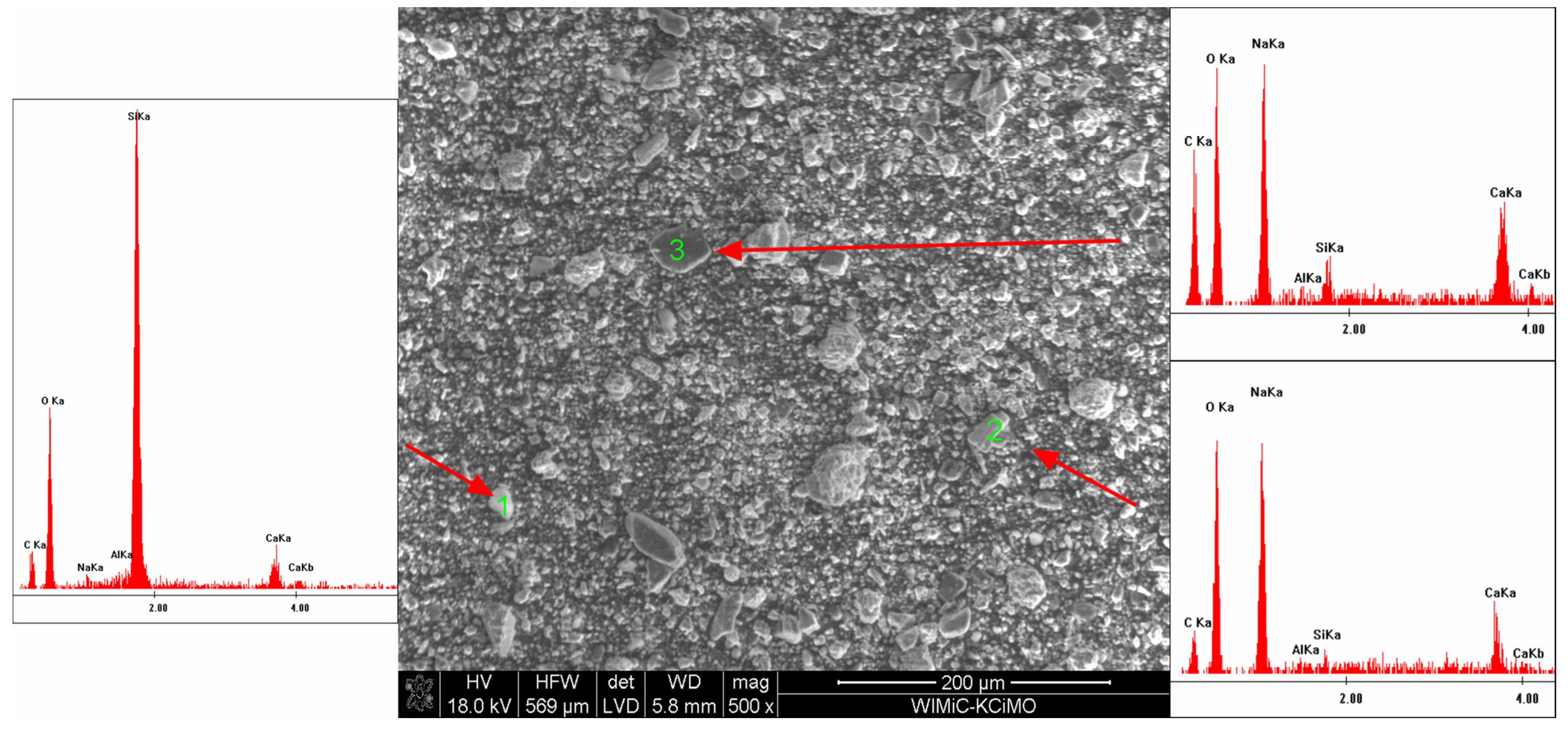
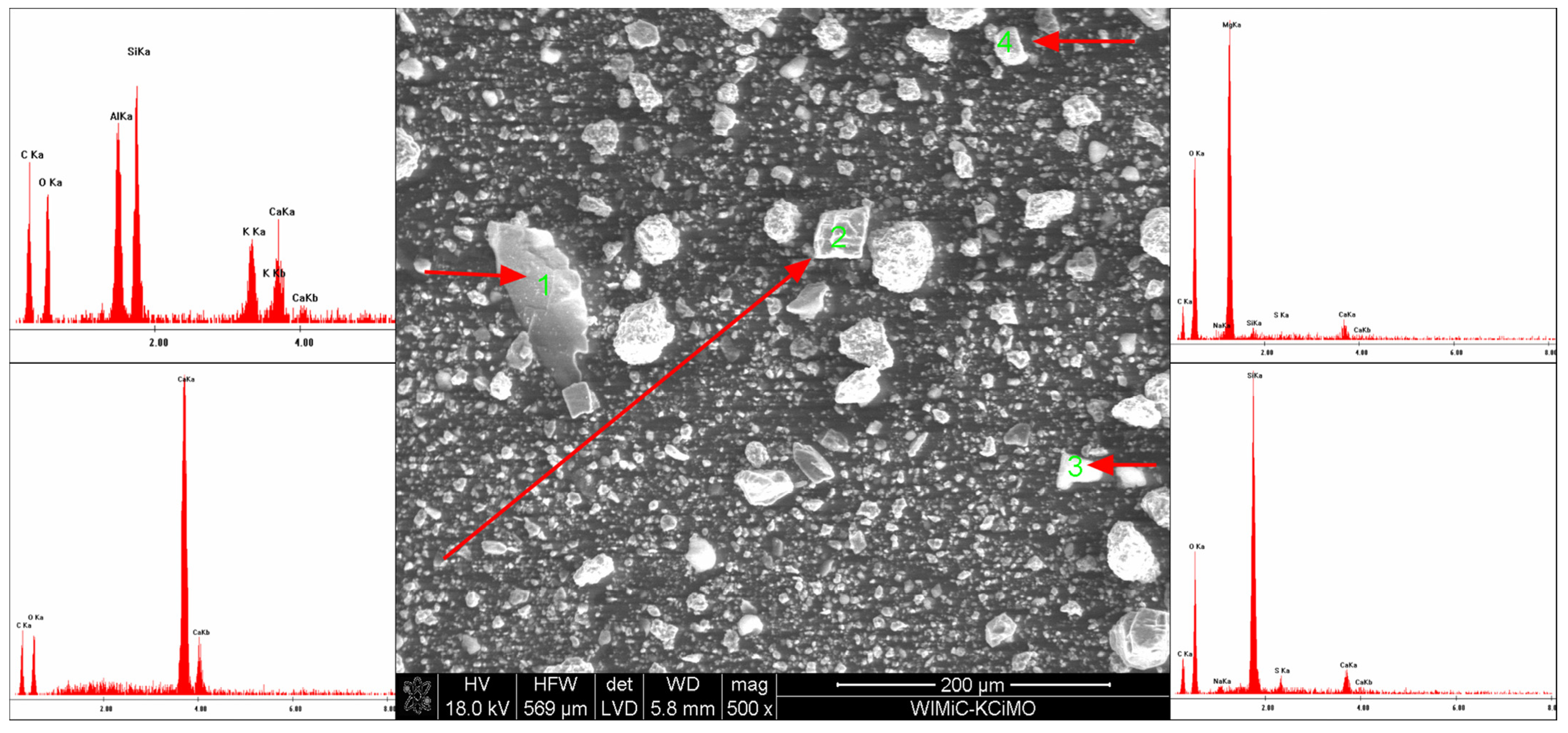
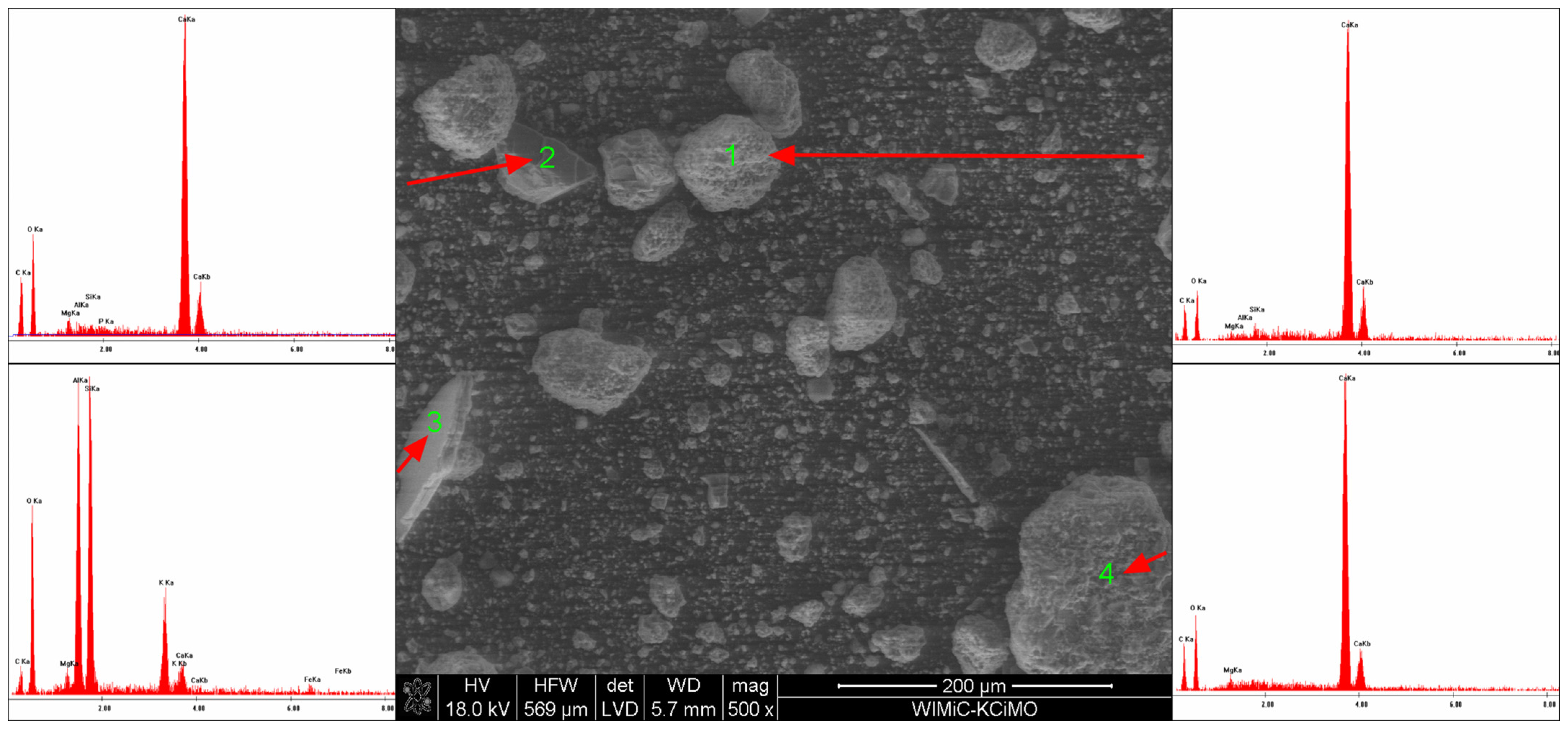
3.3. Scanning Electron Microscopy
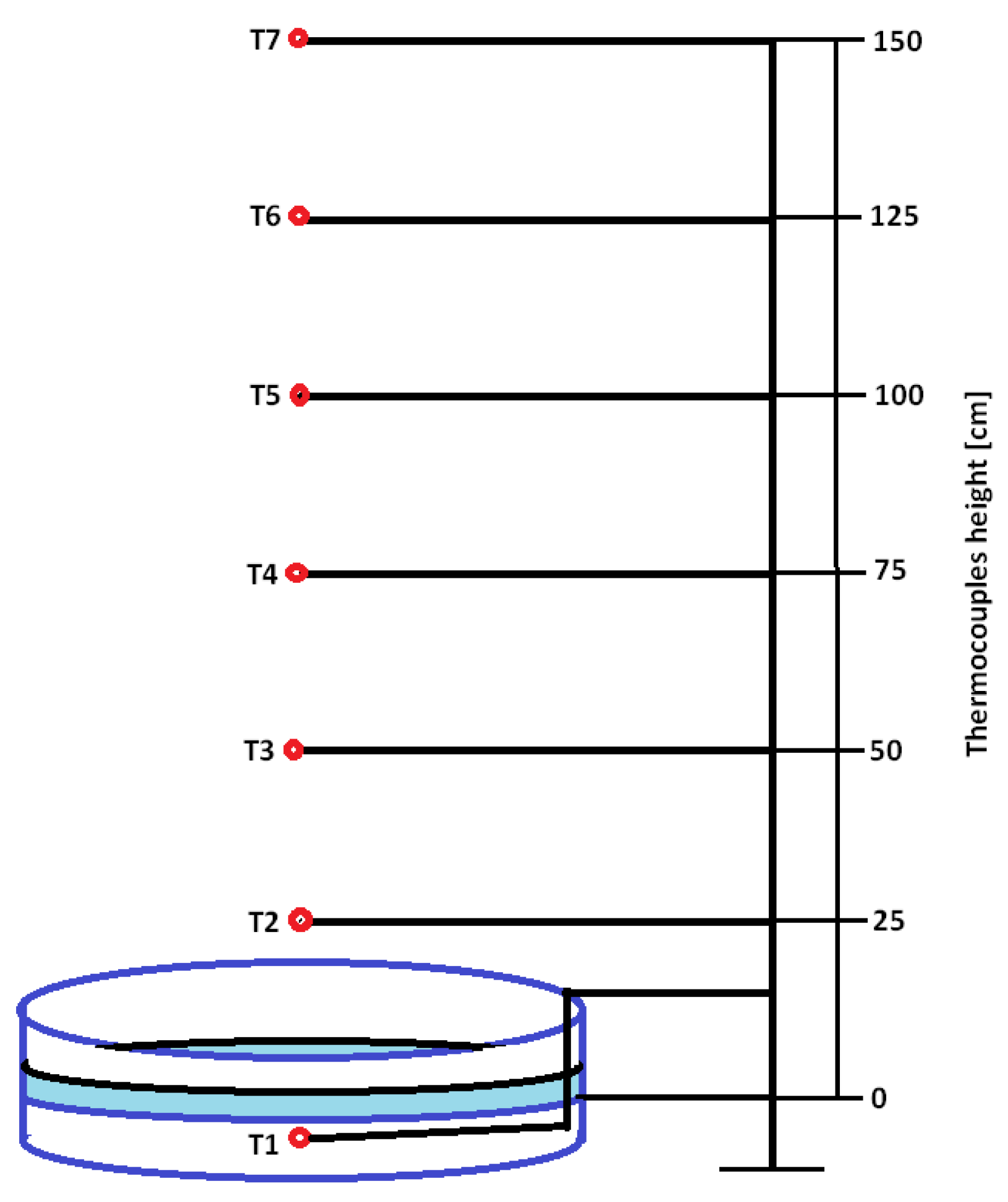
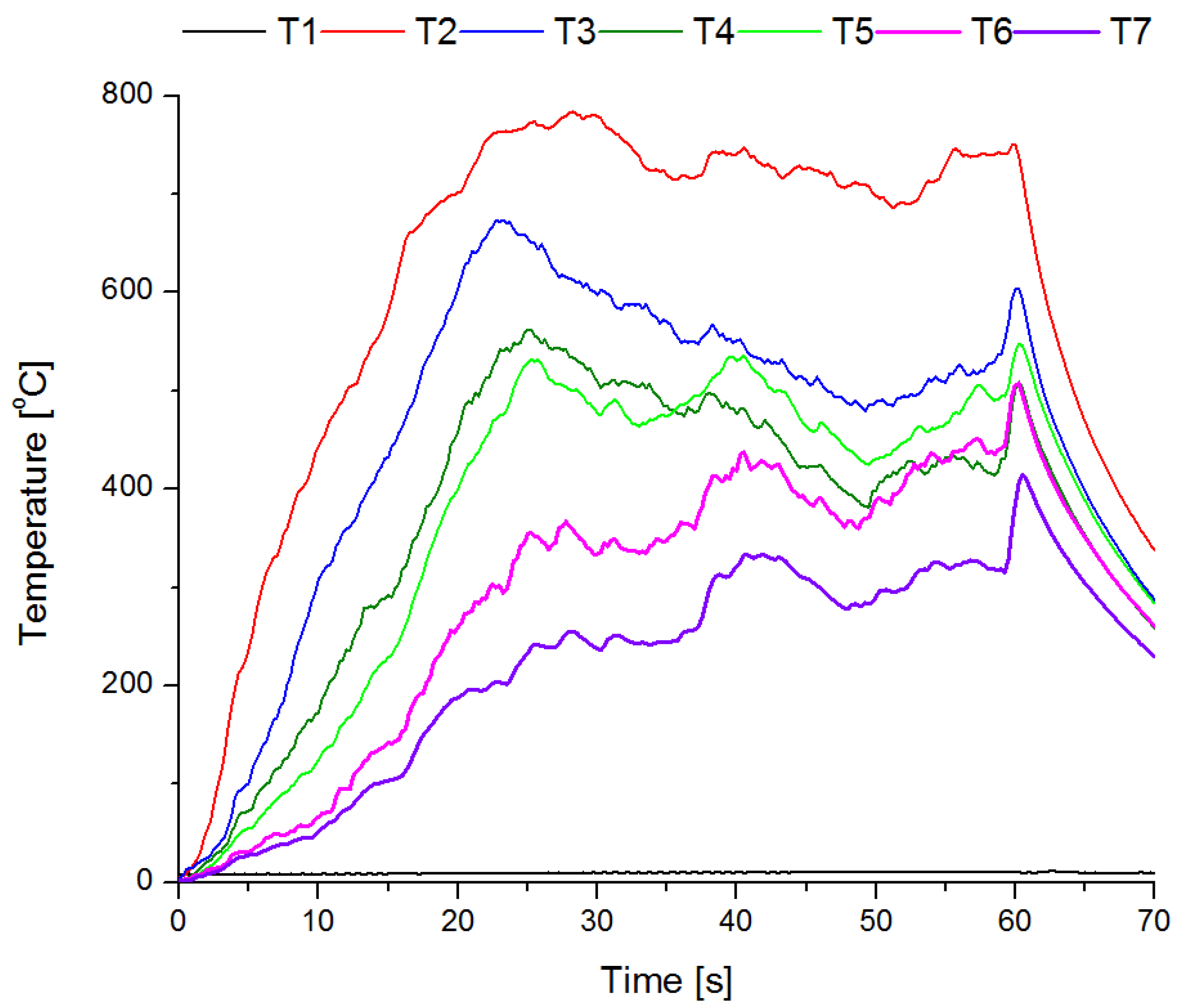
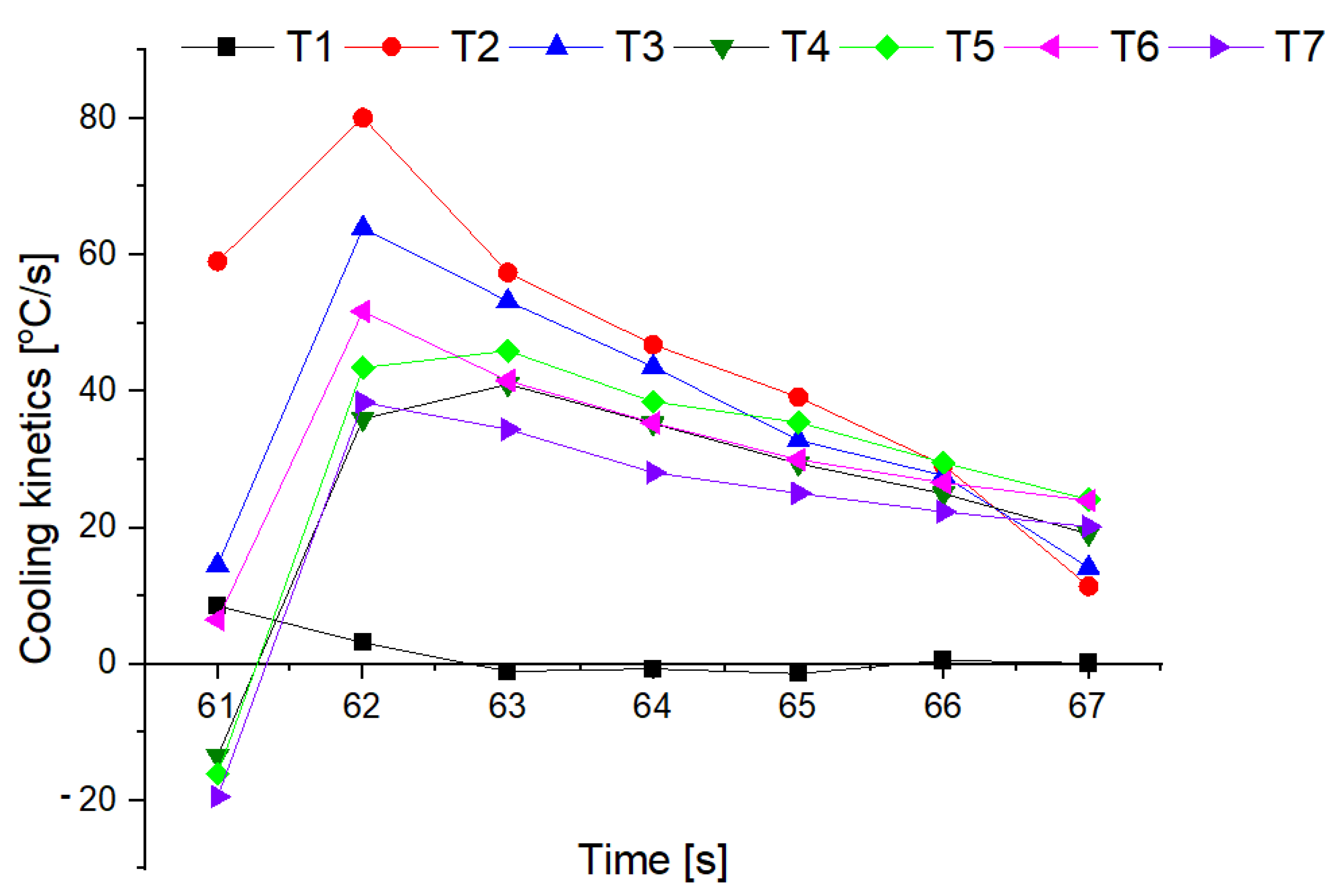
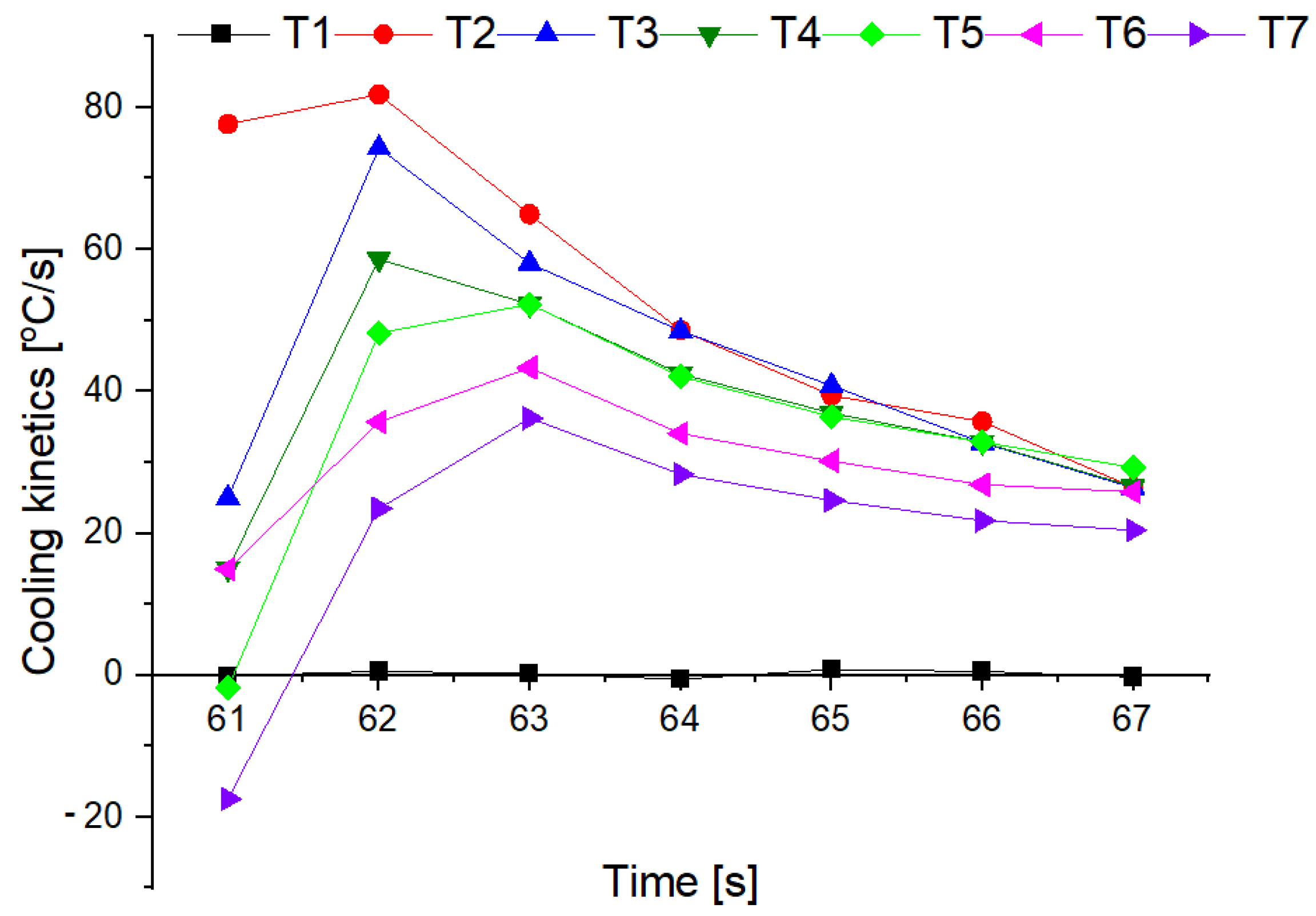
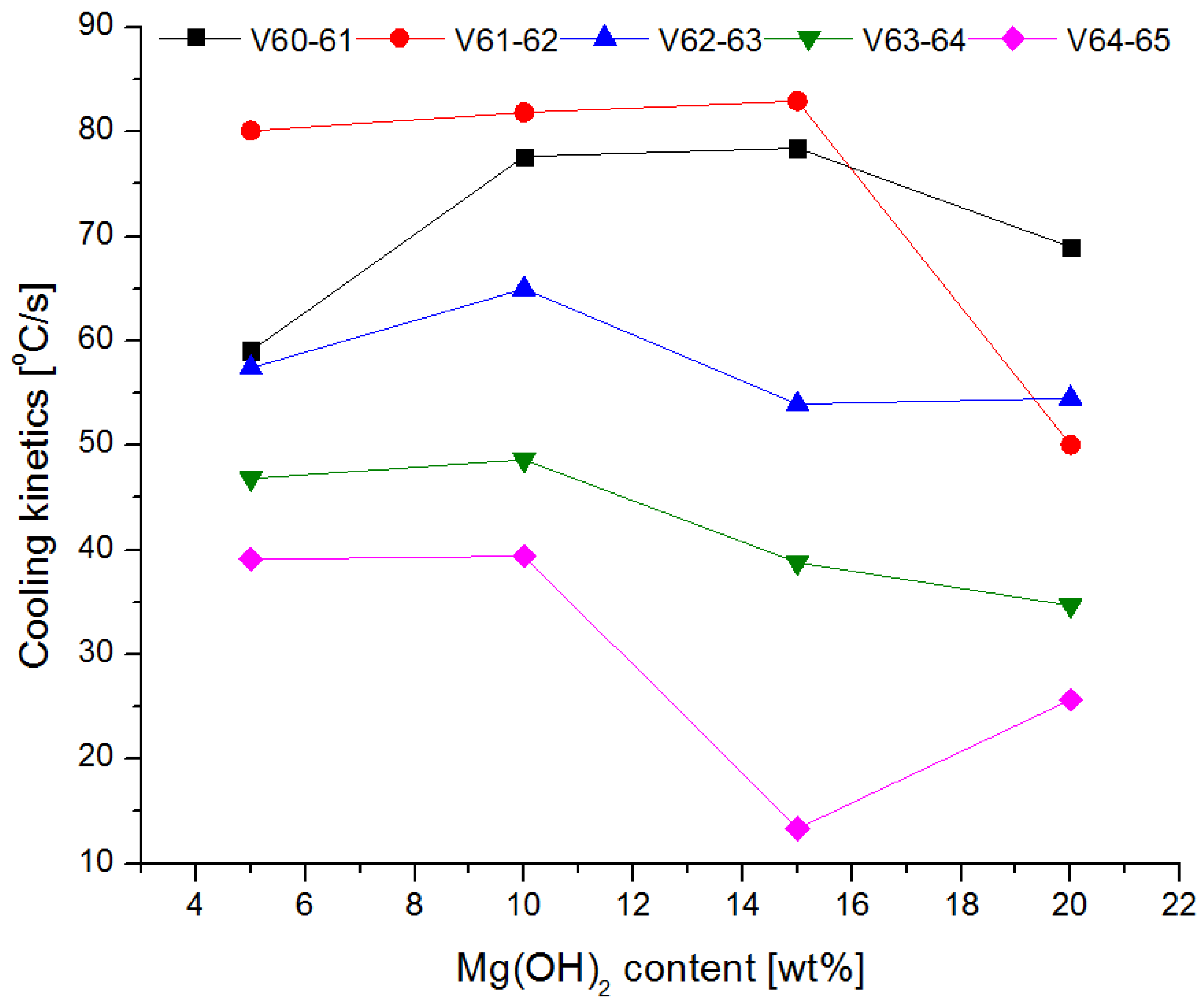
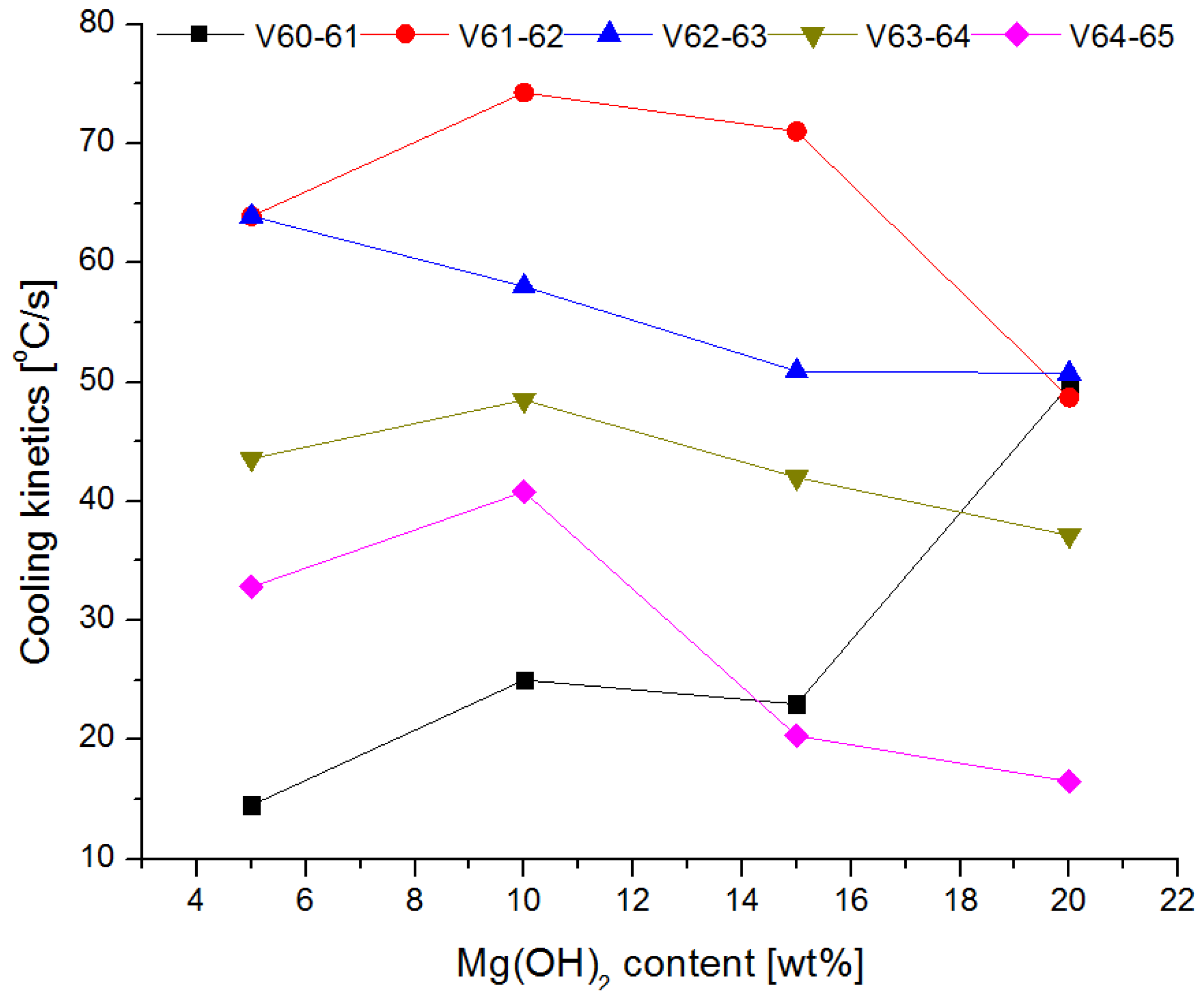
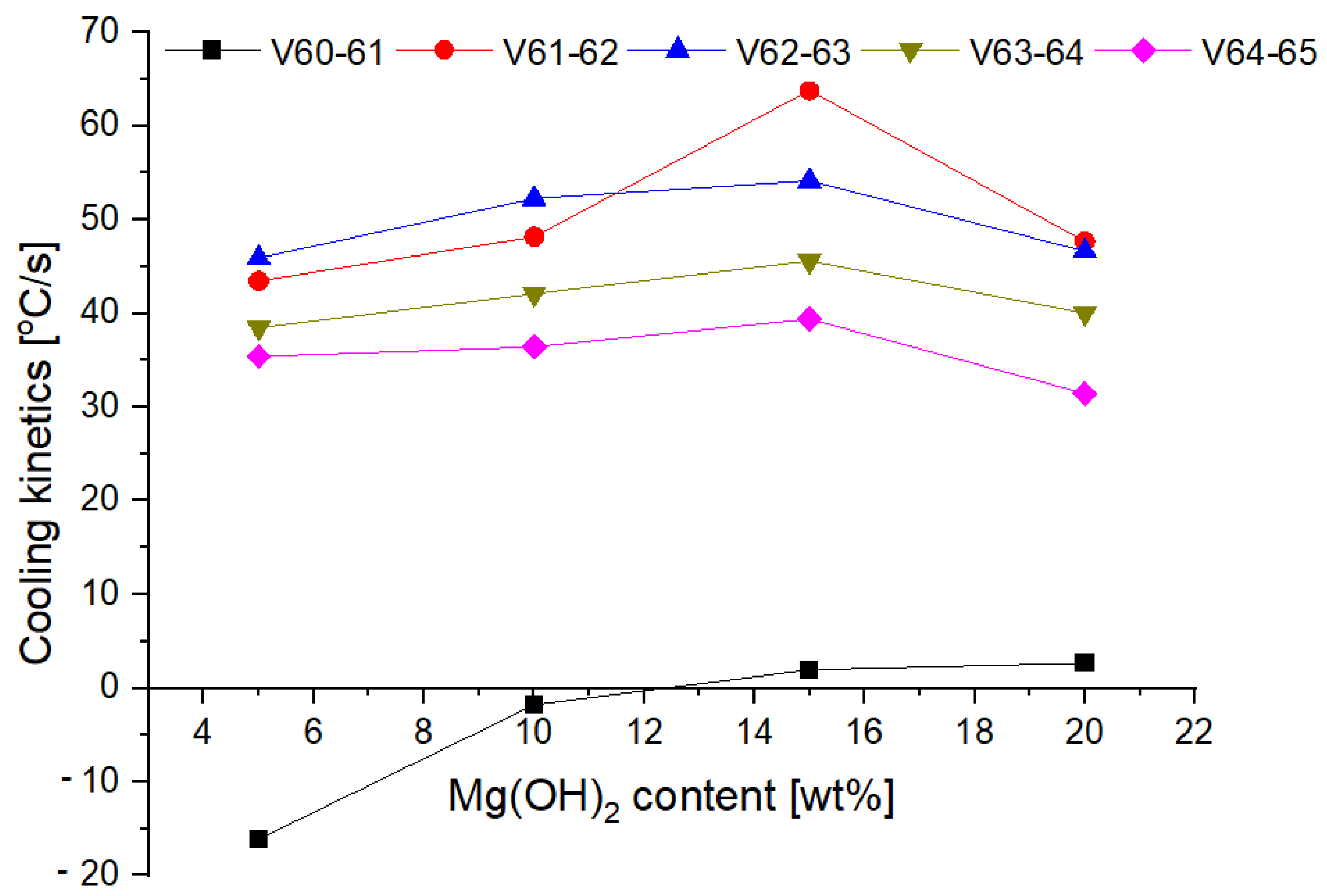
3.4. Fire Tests
4. Conclusions
- -
- The addition of magnesium hydroxide deteriorates the rheological properties and increases the ability to thicken and lump (the tangent of the flow curve slope varying from 0.258 for 5% of Mg(OH)2up to 0.330 for 20% of Mg(OH)2),which is disadvantageous from the point of view of using this compound as a fire extinguishing powder in popular portable fire extinguishing equipment;
- -
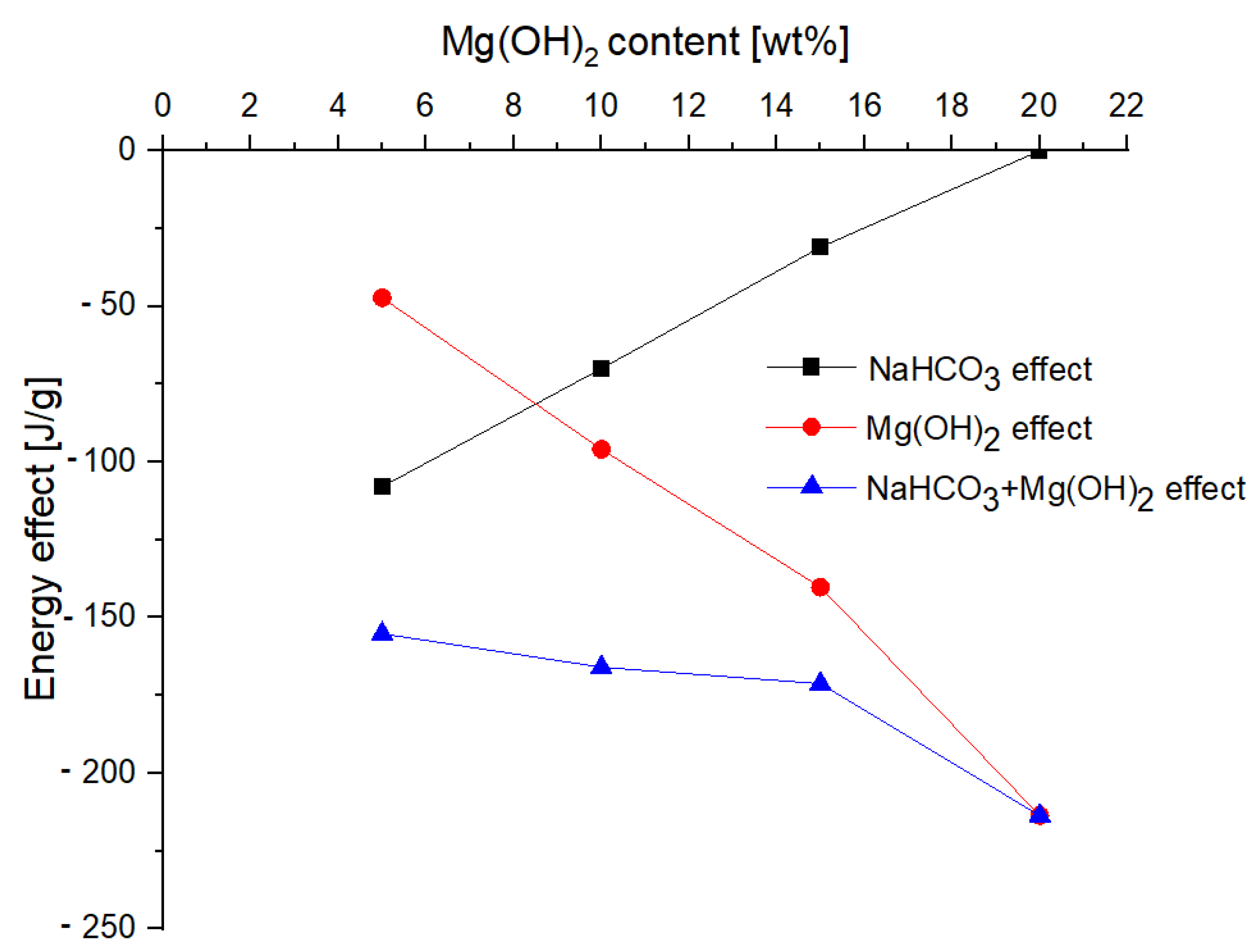
- The addition of magnesium hydroxide significantly improves the properties of the powder from the point of view of heat reception (Mg(OH)2increases the total energy of the chemical decomposition reaction (from −47.27 J/g for 5% of Mg(OH)2 up to −213.6 J/g for 20% of Mg(OH)2)), taking into account the entire temperature range, which means that the use of this compound would allow faster heat transferfrom the fire, and thus extinguish the fire using smaller amounts of the extinguishing powder;
- -
- The EDS analysis confirmed that the composition of the extinguishing powders, in addition to calcium carbonate, sodium bicarbonate and magnesium hydroxide, also includes sodium aluminosilicates and silica;
- -
- The conducted test fires showed that the complete resignation of sodium bicarbonate in the composition of the extinguishing powder makes it difficult to extinguish the test fire in group B. When the fire temperature drops below 350 °C (from t = 63 s), magnesium hydroxide does not decompose and makes it difficult to collect the heat from the fire;
- -
- The use of extinguishing powder containing 10–15% of magnesium hydroxide allows for better cooling and extinguishing properties, both at the beginning and at the end of the fire extinguishing process. In the first stage, the fire is extinguished by the decomposition of the magnesium hydroxide (when the temperature is above 350 °C), and finally by sodium bicarbonate (about 200 °C).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, W.; Yu, R.; Chang, Z.; Tan, Z.; Liu, X. The fire extinguishing mechanism of ultrafine composite dry powder agent containing Mg(OH)2. Int. J. Quantum Chem. 2021, 121, e26810. [Google Scholar] [CrossRef]
- Porshnov, D.; Ozols, V.; Ansone-Bertina, L.; Burlakovs, J.; Klavins, M. Thermal decomposition study of major refuse derived fuel components. Energy Procedia 2018, 147, 48–53. [Google Scholar] [CrossRef]
- Yuen, A.C.Y.; Chen, T.B.Y.; Yeoh, G.H.; Yang, W.; Cheung, S.C.-P.; Cook, M.; Yu, B.; Chan, Q.N.; Yip, H.L. Establishing pyrolysis kinetics for the modelling of the flammability and burning characteristics of solid combustible materials. J. Fire Sci. 2018, 36, 494–517. [Google Scholar] [CrossRef] [Green Version]
- Nyazika, T.; Jimenez, M.; Samyn, F.; Bourbigot, S. Pyrolysis modeling, sensitivity analysis, and optimization techniques for combustible materials: A review. J. Fire Sci. 2019, 37, 377–433. [Google Scholar] [CrossRef]
- Chen, T.B.Y.; Cordeiro, I.M.D.C.; Yuen, A.C.Y.; Yang, W.; Chan, Q.N.; Zhang, J.; Cheung, S.C.P.; Yeoh, G.H. An Investigation towards Coupling Molecular Dynamics with Computational Fluid Dynamics for Modelling Polymer Pyrolysis. Molecules 2022, 27, 292. [Google Scholar] [CrossRef]
- United Nations. Montreal Protocol on Substances That Deplete the Ozone Layer, 14th ed.; United Nations Treaty Series: New York, NY, USA, 2020. [Google Scholar]
- Liu, H.-Q.; Zong, R.-W.; Lo, S.; Hu, Y.; Zhi, Y.-R. Fire Extinguishing Efficiency of Magnesium Hydroxide Powders under Different Particle Size. Procedia Eng. 2018, 211, 447–455. [Google Scholar] [CrossRef]
- Du, D.; Shen, X.; Feng, L.; Hua, M.; Pan, X. Efficiency characterization of fire extinguishing compound superfine powder containing Mg(OH)2. J. Loss Prev. Process Ind. 2018, 57, 73–80. [Google Scholar] [CrossRef]
- Du, D.-X.; Pan, X.-H.; Hua, M. Experimental Study on Fire Extinguishing Properties of Compound Superfine Powder. Procedia Eng. 2018, 211, 142–148. [Google Scholar] [CrossRef]
- Ibrahim, H.; Patruni, J.R. Experimental investigation on extinguishing performance of a novel nanocomposite for gaseous fires. J. Loss Prev. Process Ind. 2020, 65, 104143. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, X.; Yan, K.; Ma, X.; Xiao, Q.; Wang, J.; Bai, J. Inhibition effects of Al(OH)3 and Mg(OH)2 on Al-Mg alloy dust explosion. J. Loss Prev. Process Ind. 2020, 66, 104206. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.; Yuan, B.; Zhang, H.; Shang, S.; Zhao, Q.; Dai, H.; He, S.; Zhang, Y.; Niu, Y. Insight into suppression performance and mechanisms of ultrafine powders on wood dust deflagration under equivalent concentration. J. Hazard. Mater. 2020, 394, 122584. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, X.; Yuan, B.; Zhang, H.; Dai, H.; He, S.; Zhang, Y.; Niu, Y.; Shen, S. Suppression of wood dust explosion by ultrafine magnesium hydroxide. J. Hazard. Mater. 2019, 378, 120723. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Jiang, Y.; Li, W.; Xiong, C.; Qiu, R. Investigation of the physical and chemical effects of fire suppression powder NaHCO3 addition on methane-air flames. Fuel 2019, 257, 116048. [Google Scholar] [CrossRef]
- Luo, Z.; Su, Y.; Chen, X.; Zheng, L. Effect of BC powder on hydrogen/methane/air premixed gas deflagration. Fuel 2019, 257, 116095. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Chen, X.; Liu, X.; Niu, Y.; Zhang, Y.; Yuan, B. Effect of dust explosion suppression by sodium bicarbonate with different granulometric distribution. J. Loss Prev. Process Ind. 2017, 49, 905–911. [Google Scholar] [CrossRef]
- Ibrahim, H.; Patruni, J.R. Experimental assessment on LPG fire extinguishing properties of three chemical powders before and after milling action. Fire Mater. 2020, 44, 747–756. [Google Scholar] [CrossRef]
- Huang, D.; Wang, X.; Yang, J. Influence of Particle Size and Heating Rate on Decomposition of BC Dry Chemical Fire Extinguishing Powders. Part. Sci. Technol. 2015, 33, 488–493. [Google Scholar] [CrossRef]
- Boroujerdnia, M.; Obeydavi, A.; Sabzi, M. Synthesis and characterisation of a novel Fe-based nanocomposite by mechanical alloying and spark plasma sintering. Powder Met. 2021, 64, 283–294. [Google Scholar] [CrossRef]
- Bahrami, A.; Mohammadnejad, A.; Sajadi, M. Microstructure and mechanical properties of spark plasma sintered AlCoFeMnNi high entropy alloy (HEA)-carbon nanotube (CNT) nanocomposite. J. Alloys Compd. 2021, 862, 158577. [Google Scholar] [CrossRef]
- Sabzi, M.; Anijdan, S.M.; Ghobeiti-Hasab, M.; Fatemi-Mehr, M. Sintering variables optimization, microstructural evolution and physical properties enhancement of nano-WC ceramics. J. Alloys Compd. 2018, 766, 672–677. [Google Scholar] [CrossRef]
- Yan, K.; Meng, X. An investigation on the aluminum dustexplosion suppression efficiency and mechanism of a NaHCO3/DE composite powder. Adv. Powder Technol. 2020, 31, 3246–3255. [Google Scholar] [CrossRef]
- Miao, N.; Zhong, S.; Yu, Q. Ignition characteristics of metal dusts generated during machining operations in the presence of calcium carbonate. J. Loss Prev. Process Ind. 2015, 40, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Xue, F.; Fu, Y.; Cheng, Y.; Yang, H.; Lu, S. A comparative study on the thermal runaway inhibition of 18650 lithium-ion batteries by different fire extinguishing agents. iScience 2021, 24, 102854. [Google Scholar] [CrossRef]
- Birchall, J. On the mechanism of flame inhibition by alkali metal salts. Combust. Flame 1970, 14, 85–95. [Google Scholar] [CrossRef]
- Jensen, D.E.; Jones, G.A. Kinetics of flame inhibition by sodium. J. Chem. Soc. Faraday Trans. 1982, 78, 2843–2850. [Google Scholar] [CrossRef]
- Jiang, H.; Bi, M.; Peng, Q.; Gao, W. Suppression of pulverized biomass dust explosion by NaHCO3 and NH4H2PO4. Renew. Energy 2019, 147, 2046–2055. [Google Scholar] [CrossRef]
- International Standard ISO 7165:2017; Fire Fighting-Portable Fire Extinguishers-Performance and Construction. ISO: Geneva, Switzerland, 2017.
- European Standard EN 3-7:2004+A1:2007; Portable Fire Extinguishers—Part 7: Characteristics, Performance Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2007.
- Yan, Q.-L.; He, G.-Q.; Liu, P.-J.; Gozin, M. Nanomaterials in Rocket Propulsion Systems, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Schroeder, S.L.M.; Gottfried, M. Temperature-Programmed Desorption (TPD) Thermal Desorption Spectroscopy (TDS). Available online: http://www.uhv.es/sites/marte/includes/doc/tds.pdf (accessed on 20 January 2022).
- Koshiba, Y.; Haga, T.; Ohtani, H. Flame inhibition by calcium compounds: Effects of calcium compounds on downward flame spread over solid cellulosic fuel. Fire Saf. J. 2019, 109, 102865. [Google Scholar] [CrossRef]




| Sample Number | Mg(OH)2 [wt%] | NaHCO3 [wt%] | CaCO3 [wt%] | Anti-Caking Agent [wt%] |
|---|---|---|---|---|
| 1 | 5 | 15 | 75 | 5 |
| 2 | 10 | 10 | 75 | 5 |
| 3 | 15 | 5 | 75 | 5 |
| 4 | 20 | 0 | 75 | 5 |
| Type of the Powder | |
|---|---|
| Free flowing | <0.10 |
| Easy flowing | <0.25 |
| Cohesive | <0.50 |
| Very cohesive | <1.00 |
| Sample Symbol | Type of the Powder | |
|---|---|---|
| 5% Mg(OH)2 | 0.258 | Cohesive |
| 10% Mg(OH)2 | 0.282 | Cohesive |
| 15% Mg(OH)2 | 0.307 | Cohesive |
| 20% Mg(OH)2 | 0.330 | Cohesive |
| Mg(OH)2 Content [wt%] | Δ |
|---|---|
| 5 | 30.324 |
| 10 | 33.922 |
| 15 | 37.351 |
| 20 | 36.470 |
| Sample Number | Mass Loss [wt%] | Decomposition of NaHCO3 [J/g] | Decomposition of Mg(OH)2 [J/g] | Decomposition of CaCO3 [J/g] | Total Energy Effect [J/g] |
|---|---|---|---|---|---|
| 1 | 43.37 | −107.9 | −47.27 | −1410 | −1565.17 |
| 2 | 45.24 | −69.97 | −95.91 | −1381 | −1546.88 |
| 3 | 44.31 | −30.99 | −140.2 | −1438 | −1609.19 |
| 4 | 41.56 | 0 | −213.6 | −1582 | −1795.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izak, P.; Biel, M.; Mastalska-Popławska, J.; Janik, P.; Mortka, P.; Lesiak, P. The Effect of Magnesium Hydroxide Addition on the Extinguishing Efficiency of Sodium Bicarbonate Powders. Materials 2022, 15, 3449. https://doi.org/10.3390/ma15103449
Izak P, Biel M, Mastalska-Popławska J, Janik P, Mortka P, Lesiak P. The Effect of Magnesium Hydroxide Addition on the Extinguishing Efficiency of Sodium Bicarbonate Powders. Materials. 2022; 15(10):3449. https://doi.org/10.3390/ma15103449
Chicago/Turabian StyleIzak, Piotr, Mateusz Biel, Joanna Mastalska-Popławska, Paweł Janik, Piotr Mortka, and Piotr Lesiak. 2022. "The Effect of Magnesium Hydroxide Addition on the Extinguishing Efficiency of Sodium Bicarbonate Powders" Materials 15, no. 10: 3449. https://doi.org/10.3390/ma15103449






