Green Deal and Circular Economy of Bottom Ash Waste Management in Building Industry—Alkali (NaOH) Pre-Treatment
Abstract
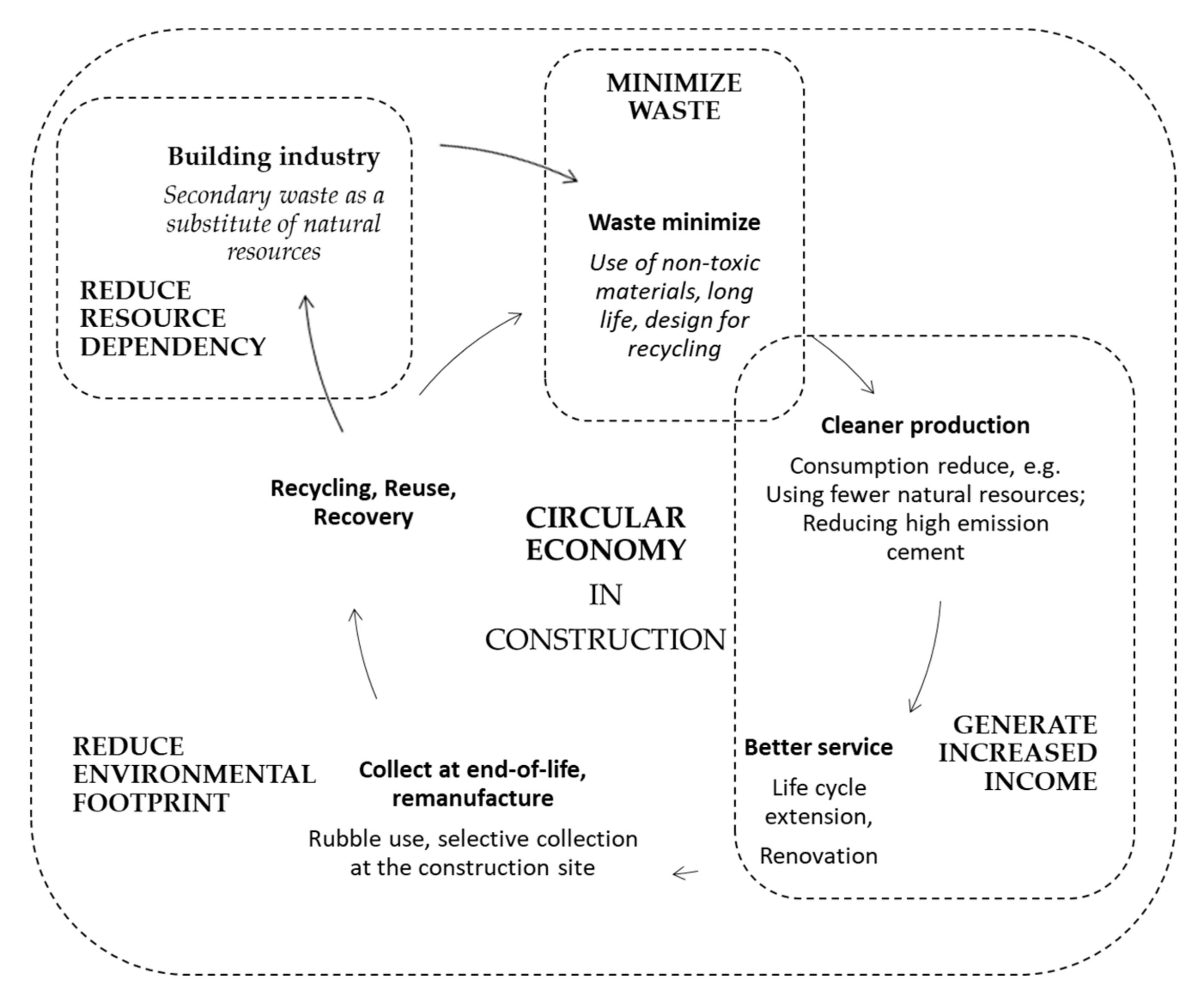
:1. Introduction
1.1. Current Market Situation with Residual Waste and Secondary Waste
1.2. Life Cycle Assessment (LCA)
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Bottom Ash Characterization
2.2.2. Construction Properties
2.2.3. Leachability
2.2.4. Life Cycle Assessment
- ○
- Eighteen detailed waypoints that are relatively accurate but difficult to interpret;
- ○
- Three simple to understand, but more imprecise endpoints:
- for human health;
- about the ecosystem;
- for natural resources.
- Human health, expressed as the number of years life lost and the number of years lived disabled. These are combined as Disability Adjusted Life Years (DALYs), an index that is also used by the World Bank and WHO. The unit is years;
- Ecosystems, expressed as the loss of species over a certain area, during a certain time. The unit is years;
- Resources surplus costs, expressed as the surplus costs of future resource production over an infinitive timeframe (assuming constant annual production), considering a 3% discount rate. The unit is 2000US $.
3. Results
3.1. Botton Ash Characterization
- Carbon does not change in all samples;
- Sulphur decreases from 0.48% in MSWIBA before valorization to 0.20% in MSWIBA after valorization and 0.07% after NaOH pre-treatment;
- Chloride decreases from 0.41% in MSWIBA before valorization to 0.12% in MSWIBA after valorization and <0.01% after NaOH pre-treatment;
- Manganese decreases strongly after valorization (from 1178.35 ppm to 463.46 ppm) and less strongly after NaOH treatment (403.04 ppm);
- Nickel decreases after valorization, but does not decrease after NaOH treatment;
- Lead decreases after valorization, but less strongly after NaOH treatment;
- Cobalt decreases after valorization and after NaOH treatment;
- Chrome decreases after valorization and after NaOH treatment;
- Copper decreases after valorization and decreases more after NaOH treatment.
3.2. Building Research
3.3. Polution Leachability
3.4. LCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Regulation of the European Parliament and of the Council. Establishing the Framework for Achieving Climate Neutrality and Amending Regulation (EU) 2018/1999 (European Climate Law). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1588581905912&uri=CELEX%3A52020PC0080&fbclid=IwAR1QjYzmS7WNyVgr1IcRxrJhglSH3_K0moNbSm1OQR5X7PxoyP6k9z-q-c8 (accessed on 20 August 2021).
- Nøst, E.; Borgan, Ø.; Øistein, D.; Rui, H. Variation in chemical composition of MSWI fly ash and dry scrubber residues. Waste Manag. 2021, 126, 623–631. [Google Scholar] [CrossRef]
- Wajda, A. Management of wastes from energy industry in the frame of circular economy on the example of microspheres. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, Vienna, Austria, 3–6 December 2018; Volume 18, pp. 71–78. [Google Scholar] [CrossRef]
- European Commision. European Green Deal: Commission Aims for Zero Pollution in Air, Water and Soil. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_2345?fbclid=IwAR1oZpeCcqFDjCkMb80j23Iza22LmeKZQUCLvvEzfULlwjttKixTgeQnKww (accessed on 20 August 2021).
- The Circular Economy: Vision, Problems and Smart City Solutions. Available online: https://hub.beesmart.city/en/strategy/the-circular-economy-and-smart-city-solutions?fbclid=IwAR00Ud9F9fB8-fqi9fXQ5B08qZRQaQXRTeYN5fX8R_fr-nHu79_ytCN2g18 (accessed on 20 August 2021).
- Lausselet, C.; Lund, K.M.; Bratteb, H. LCA and scenario analysis of a Norwegian net-zero GHG emission neighbourhood: The importance of mobility and surplus energy from PV technologies. Build. Environ. 2021, 189, 107528. [Google Scholar] [CrossRef]
- Climate Change—United Nations Sustainable Development. Goal 13: Take Urgent Action to Combat Climate Change and Its Impacts. Available online: https://www.un.org/sustainabledevelopment/climate-change/?fbclid=IwAR1nC2m2YioXkEvzdXQRa_AL5hPkL-chZe2QIVRNQz4TOHwPEsRGLHwCMkg (accessed on 20 August 2021).
- The EU Green Deal–A Roadmap to Sustainable Economies. Available online: https://www.switchtogreen.eu/the-eu-green-deal-promoting-a-green-notable-circular-economy/?fbclid=IwAR3Qjwk5f6D-OQGlSniG0iZSE8uACoVsI4fkEeTs6RsH38GCG-FcN6nAZdE (accessed on 20 August 2021).
- Martínez-García, R.; Guerra-Romero, I.M.; Morán-del Pozo, J.M.; de Brito, J.; Juan-Valdés, A. Recycling Aggregates for Self-Compacting Concrete Production: A Feasible Option. Materials 2020, 13, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pikoń, K.; Poranek, N.; Czajkowski, A.; Łaźniewska-Piekarczyk, B. Poland’s Proposal for a Safe Solution of Waste Treatment during the COVID-19 Pandemic and Circular Economy Connection. Appl. Sci. 2021, 11, 3939. [Google Scholar] [CrossRef]
- European Commission. Zero Pollution Action Plan. Available online: https://ec.europa.eu/environment/strategy/zero-pollution-action-plan_pl (accessed on 20 August 2021).
- Bogacka, M.; Poranek, N.; Łaźniewska-Piekarczyk, B.; Pikoń, K. Removal of Pollutants from Secondary Waste from an Incineration Plant: The Review of Methods. Energies 2020, 13, 6322. [Google Scholar] [CrossRef]
- Purchase, C.K.; Al Zulayq, D.M.; O’Brien, B.T.; Kowalewski, M.J.; Berenjian, A.; Tarighaleslami, A.H.; Seifan, M. Circular Economy of Construction and Demolition Waste: A Literature Review on Lessons, Challenges, and Benefits. Materials 2021, 15, 76. [Google Scholar] [CrossRef]
- Kajda-Szcześniak, M.; Jaworski, T.; Wajda, A. Theoretical and experimental model of the combustion process in a layer on the grate of the waste thermal treatment installation. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, Sofia, Bulgaria, 30 June–6 July 2019; Volume 19, pp. 759–766. [Google Scholar] [CrossRef]
- Patel, A.K.; Wajda, A.; Brociek, R.; Pleszczy, M. Optimization of Energy Recovery from Hazardous Waste in a Waste Incineration Plant with the Use of an Application. Processes 2022, 10, 462. [Google Scholar] [CrossRef]
- Woo, B.-H.; Jeon, I.-K.; Yoo, D.-H.; Kim, S.-S.; Lee, J.-B.; Kim, H.-G. Utilization of Municipal Solid Waste Incineration Bottom Ash as Fine Aggregate of Cement Mortars. Sustainability 2021, 13, 8832. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, L.; Zhu, Y.; Zhang, B.; Hu, G.; Xu, B.; He, C.; Maio, F.D. The fate of heavy metals and salts during the wet treatment of municipal solid waste incineration bottom ash. Waste Manag. 2021, 121, 33–41. [Google Scholar] [CrossRef]
- Xuan, D.; Poon, C.S. Removal of metallic Al and Al/Zn alloys in MSWI bottom ash by alkaline treatment. J. Hazard. Mater. 2018, 344, 73–80. [Google Scholar] [CrossRef]
- Poranek, N.; Łaźniewska-Piekarczyk, B.; Czajkowski, A.; Pikoń, K. Circular Economy for Municipal Solid Waste Incineration Bottom Ash (MSWIBA) Management in Mortars with CSA and CEM I, MSWIBA Glassy Phase, and DTG. Energies 2022, 15, 135. [Google Scholar] [CrossRef]
- Schnabel, K.; Brück, F.; Mansfeldt, T.; Weigand, H. Full-scale accelerated carbonation of waste incinerator bottom ash under continuous-feed conditions. Waste Manag. 2021, 125, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.C. Life cycle based risk assessment of recycled materials in roadway construction. Waste Manag. 2007, 27, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Birgisdóttir, H.; Bhander, G.; Hauschild, M.Z.; Christensen, T.H. Life cycle assessment of disposal of residues from municipal solid waste incineration: Recycling of bottom ash in road construction or landfilling in Denmark evaluated in the ROAD-RES model. Waste Manag. 2007, 27, S75–S84. [Google Scholar] [CrossRef] [PubMed]
- Pikoń, K.; Krawczyk, P.; Badyda, K.; Bogacka, M. Predictive analysis of waste co-combustion with fossil fuels using the life cycle assessment (LCA) methodology. Energies 2019, 12, 3691. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, L.; Carnevale, E.A. Evaluation of the environmental sustainability of different waste-to-energy plant configurations. Waste Manag. 2018, 73, 232–246. [Google Scholar] [CrossRef]
- Abdulkareem, M.; Havukainen, J.; Nuortila-Jokinen, J.; Horttanainen, M. Environmental and economic perspective of waste-derived activators on alkali-activated mortars. J. Clean. Prod. 2021, 280, 124651. [Google Scholar] [CrossRef]
- Morselli, L.; Bartoli, M.; Bertacchini, M.; Brighetti, A.; Luzi, J.; Passarini, F.; Masoni, P. Tools for evaluation of impact associated with MSW incineration: LCA and integrated environmental monitoring system. Ecol. Chem. Eng. 2005, 25, 191–196. [Google Scholar] [CrossRef]
- Marieta, C.; Guerrero, A.; Leon, I. Municipal solid waste incineration fly ash to produce eco-friendly binders for sustainable building construction. Waste Manag. 2021, 120, 114–124. [Google Scholar] [CrossRef]
- EWC Codes|List of Waste|European Waste Codes. Available online: https://www.pureplanetrecycling.co.uk/list-of-waste/ (accessed on 28 October 2021).
- Division of Soils due to the Division of pH. Available online: hps://creavecommons.org/licenses/by-sa/3.0 (accessed on 28 October 2021).
- Turk, J.; Cotič, Z.; Mladenovič, A.; Šajna, A. Environmental evaluation of green concretes versus conventional concrete by means of LCA. Waste Manag. 2015, 45, 194–205. [Google Scholar] [CrossRef]
- Tyszkiewicz, Z.E.; Czubaszek, R.; Roj-Rojewski, S.; Wydawnicza, O.; Białostockiej, P. Podstawowe Metody Laboratoryjnej Analizy Gleby; Basic Methods Labolatory Soil Analysis; Oficyna Wydawnicza Politechniki Białostockiej: Białystok, Poland, 2019. [Google Scholar] [CrossRef]
- Al-Mallahi, J.; Furuichi, T.; Ishii, K. Appropriate conditions for applying NaOH-pretreated two-phase olive milling waste for codigestion with food waste to enhance biogas production. Waste Manag. 2016, 48, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, T.; Yan, Y.; Ma, K. Influence of sodium hydroxide addition on characteristics and environmental risk of heavy metals in biochars derived from swine manure. Waste Manag. 2020, 105, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi-Eshkaftaki, M.; Rahmanian-Koushkaki, H. An optimum strategy for substrate mixture and pretreatment in biogas plants: Potential application for high-pH waste management. Waste Manag. 2020, 113, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Paluszak, Z.; Skowron, K.; Gryń, G.; Wiśniewska, E.; Szkudlarek-Kowalczyk, M.; Ziętara, A. The effect of disinfection of municipal sewage with sodium hydroxide solution on survival of E.coli and faecal streptococci group D. Ekol. I Tech. 2011, 19, 243–246. [Google Scholar]
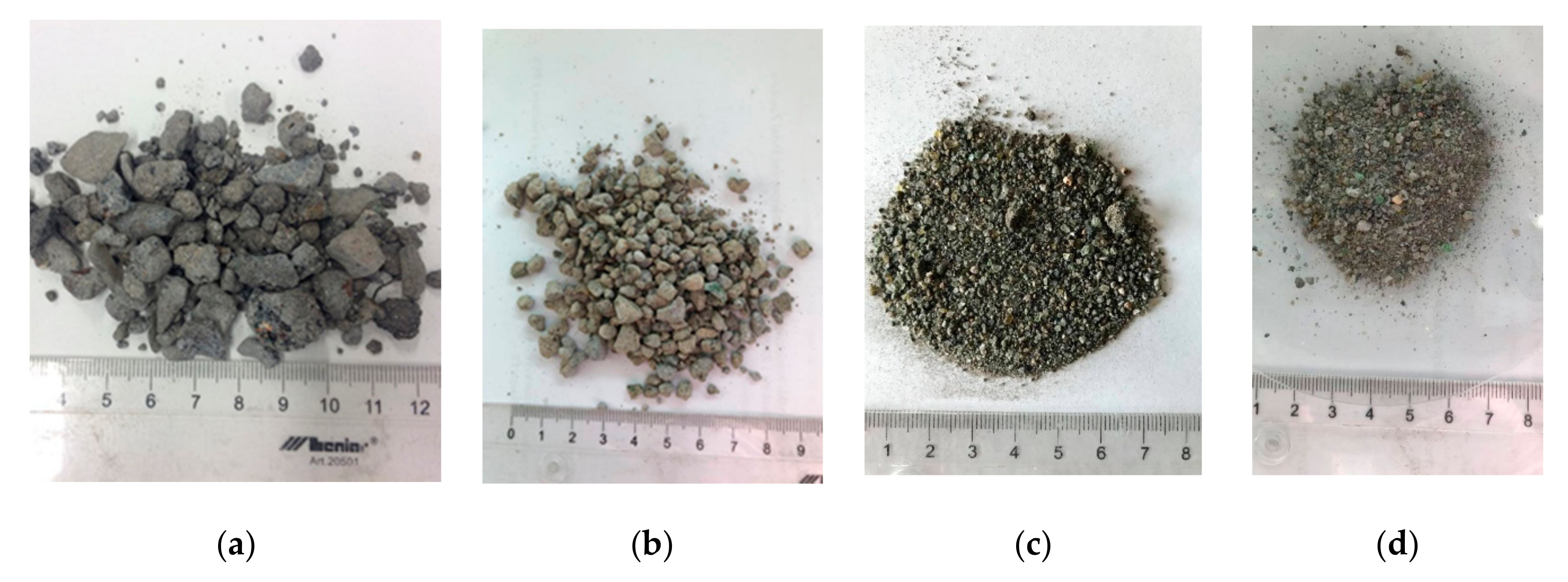

| Parameter | Standard |
|---|---|
| Moisture (M) | PN-Z-15008-02: 1993 |
| Total Carbon (TC) | PN-EN 15407: 2011 |
| Total Sulfur (S) | PN-ISO 351: 1999 |
| Chlorine (Cl) | PN-ISO 587: 2000 |
| Heavy Metals | PN-EN 16174: 2012, PN-EN ISO 11885: 2009 |
| Water Extract (Leeachability) | PN-EN 12457-2: 2006 |
| Sodium, Potassium, Lithium, Calcium, Bar (Na, K, Li, Ca, Ba) | PN-ISO 9964-2/Ak: 1997 |
| Parameter | Symbol | Unit | MSWIBA before Valorization | MSWIBA after Valorization | After NaOH Pre-Treatment | |||
|---|---|---|---|---|---|---|---|---|
| Average Result | Result Standard Deviation | Average Result | Result Standard Deviation | Average Result | Result Standard Deviation | |||
| Moisture | M | % | 4.18 | 0.14 | 8.65 | 0.15 | n.d. ** | n.d. ** |
| Total Carbon | TC | % | 0.85 | 0.46 | 0.85 | 0.43 | 0.84 | 0.38 |
| Sulphur | S | % | 0.48 | 0.16 | 0.20 | 0.09 | 0.07 | 0.01 |
| Chloride | Cl− | % | 0.41 | 0.12 | 0.12 | 0.04 | <0.01 | n.d. ** |
| Manganese | Mn | ppm | 1178.35 | 43.83 | 463.46 | 21.64 | 403.04 | 17.36 |
| Cadmium | Cd | ppm | LOQ * | n.d. ** | LOQ * | n.d. ** | LOQ * | n.d. ** |
| Nickel | Ni | ppm | 45.99 | 5.78 | 10.41 | 2.96 | 11.68 | 2.85 |
| Lead | Pb | ppm | 379.50 | 12.69 | 176.47 | 10.36 | 154.61 | 10.97 |
| Cobalt | Co | ppm | 13.58 | 2.65 | 6.18 | 1.85 | 2.92 | 0.34 |
| Chrome | Cr | ppm | 1618.31 | 39.26 | 49.32 | 4.36 | 20.72 | 2.39 |
| Copper | Cu | ppm | 2954.00 | 50.36 | 2484.10 | 42.36 | 1192.10 | 42.44 |
| Type of Cement in Mortar | 28-Day Bending Strength of Mortar (MPa) | 28-Day Bending Strength Standard Deviation | 28-Day Compressive Strength of Mortar (MPa) | 28-Day Compressive Strength Standard Deviation |
|---|---|---|---|---|
| 30% MSWIBA after valorization and CEM I 42.5R | 4.60 | 0.23 | 25.90 | 1.90 |
| 30% MSWIBA NaOH pretreatment and CEM I 42.5R | 4.65 | 0.70 | 29.92 | 2.90 |
| Na | K | Li | Ca | Ba | ||
|---|---|---|---|---|---|---|
| Mortar with MSWIBA before valorization/acid environment | Result | 21.97 | 24.67 | 0.24 | 41.45 | 10.34 |
| Standard Deviation | 1.44 | 1.52 | 0.02 | 0.58 | 0.98 | |
| Mortar with MSWIBA after valorization/acid environment | Result | 23.45 | 24.39 | 0.22 | 36.76 | 10.53 |
| Standard Deviation | 0.74 | 0.60 | 0.01 | 0.97 | 0.79 | |
| Mortar with NaOH pre-treatment MSWIBA/acid environment | Result | 32.63 | 18.74 | 0.22 | 39.45 | 9.71 |
| Standard Deviation | 1.02 | 0.63 | 0.01 | 0.55 | 0.34 | |
| Mortar with MSWIBA before valorization/aggressive environment | Result | 15.52 | 29.56 | 0.33 | 74.19 | 13.66 |
| Standard Deviation | 0.39 | 0.55 | 0.01 | 0.82 | 0.68 | |
| Mortar with MSWIBA after valorization/aggressive environment | Result | 15.53 | 28.47 | 0.31 | 65.51 | 13.01 |
| Standard Deviation | 0.22 | 0.41 | 0.00 | 0.40 | 0.60 | |
| Mortar with NaOH pre-treatment MSWIBA/aggressive environment | Result | 31.51 | 21.82 | 0.31 | 73.45 | 10.68 |
| Standard Deviation | 1.30 | 0.94 | 0.01 | 0.86 | 0.56 | |
| Mortar with MSWIBA before valorization/neutral environment | Result | 11.83 | 13.67 | 0.15 | 16.57 | 8.62 |
| Standard Deviation | 0.13 | 0.71 | 0.00 | 0.31 | 0.73 | |
| Mortar with MSWIBA after valorization/neutral environment | Result | 12.60 | 15.08 | 0.15 | 16.30 | 8.59 |
| Standard Deviation | 0.75 | 0.62 | 0.00 | 0.33 | 0.46 | |
| Mortar with NaOH pre-treatment MSWIBA/neutral environment | Result | 17.80 | 14.94 | 0.16 | 17.90 | 8.66 |
| Standard Deviation | 0.38 | 0.30 | 0.00 | 0.41 | 0.26 | |
| Mortar with MSWIBA before valorization/alkaline environment | Result | 30.50 | n.d. * | 2.36 | 49.97 | n.d. * |
| Standard Deviation | 1.33 | n.d. * | 0.02 | 0.35 | n.d. * | |
| Mortar with MSWIBA after valorization/alkaline environment | Result | 34.07 | n.d. * | 2.37 | 50.10 | n.d. * |
| Standard Deviation | 0.40 | n.d. * | 0.22 | 0.73 | n.d. * | |
| Mortar with NaOH pre-treatment MSWIBA/alkaline environment | Result | 44.41 | n.d. * | 2.32 | 54.54 | n.d. * |
| Standard Deviation | 0.56 | n.d. * | 0.30 | 0.58 | n.d. * |
| Impact Category | Unit | Scenario 1 | Scenario 2 | Scenario 3 |
|---|---|---|---|---|
| Human health | DALY | 6.99 × 10−07 | 3.41 × 10−07 | 4.99 × 10−07 |
| Ecosystems | species.yr | 3.40 × 10−09 | 1.61 × 10−09 | 2.24 × 10−09 |
| Resources | $ | 7.02 × 10−03 | 3.89 × 10−03 | 6.92 × 10−03 |
| Impact Category | Unit | Process 1 Sodium Hydroxide (50% NaOH) | Process 2 Sodium Hydroxide, from Amalgam Technology (50% NaOH) | Process 3 Sodium Hydroxide, from Concentrating Membrane (50% NaOH) |
|---|---|---|---|---|
| Human health | DALY | 4.99 × 10−07 | 5.20 × 10−07 | 4.83 × 10−07 |
| Ecosystems | species.yr | 2.24 × 10−09 | 2.29 × 10−09 | 2.19 × 10−09 |
| Resources | $ | 6.92 × 10−03 | 7.12 × 10−03 | 6.70 × 10−03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poranek, N.; Łaźniewska-Piekarczyk, B.; Lombardi, L.; Czajkowski, A.; Bogacka, M.; Pikoń, K. Green Deal and Circular Economy of Bottom Ash Waste Management in Building Industry—Alkali (NaOH) Pre-Treatment. Materials 2022, 15, 3487. https://doi.org/10.3390/ma15103487
Poranek N, Łaźniewska-Piekarczyk B, Lombardi L, Czajkowski A, Bogacka M, Pikoń K. Green Deal and Circular Economy of Bottom Ash Waste Management in Building Industry—Alkali (NaOH) Pre-Treatment. Materials. 2022; 15(10):3487. https://doi.org/10.3390/ma15103487
Chicago/Turabian StylePoranek, Nikolina, Beata Łaźniewska-Piekarczyk, Lidia Lombardi, Adrian Czajkowski, Magdalena Bogacka, and Krzysztof Pikoń. 2022. "Green Deal and Circular Economy of Bottom Ash Waste Management in Building Industry—Alkali (NaOH) Pre-Treatment" Materials 15, no. 10: 3487. https://doi.org/10.3390/ma15103487
APA StylePoranek, N., Łaźniewska-Piekarczyk, B., Lombardi, L., Czajkowski, A., Bogacka, M., & Pikoń, K. (2022). Green Deal and Circular Economy of Bottom Ash Waste Management in Building Industry—Alkali (NaOH) Pre-Treatment. Materials, 15(10), 3487. https://doi.org/10.3390/ma15103487










