A First-Principles Study on the Hydration Behavior of (MgO)n Clusters and the Effect Mechanism of Anti-Hydration Agents
Abstract
:1. Introduction
2. Calculation Methods
3. Results
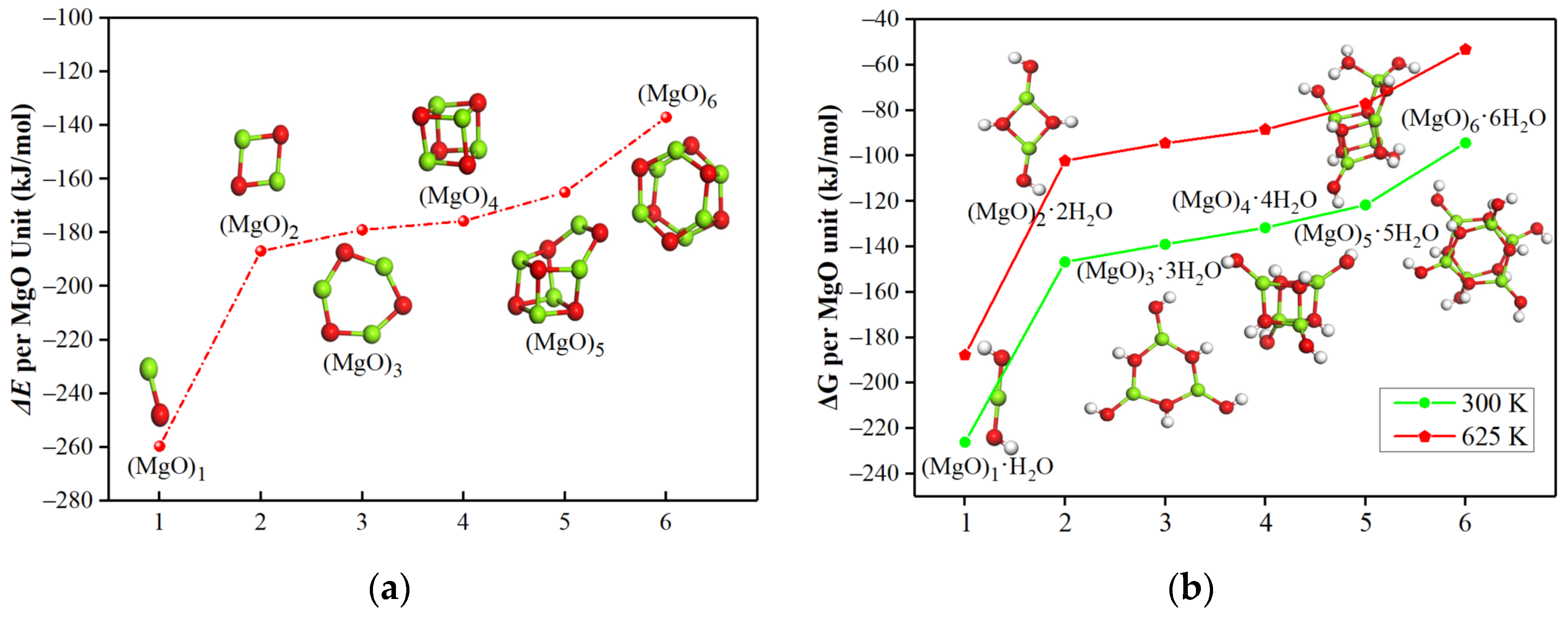
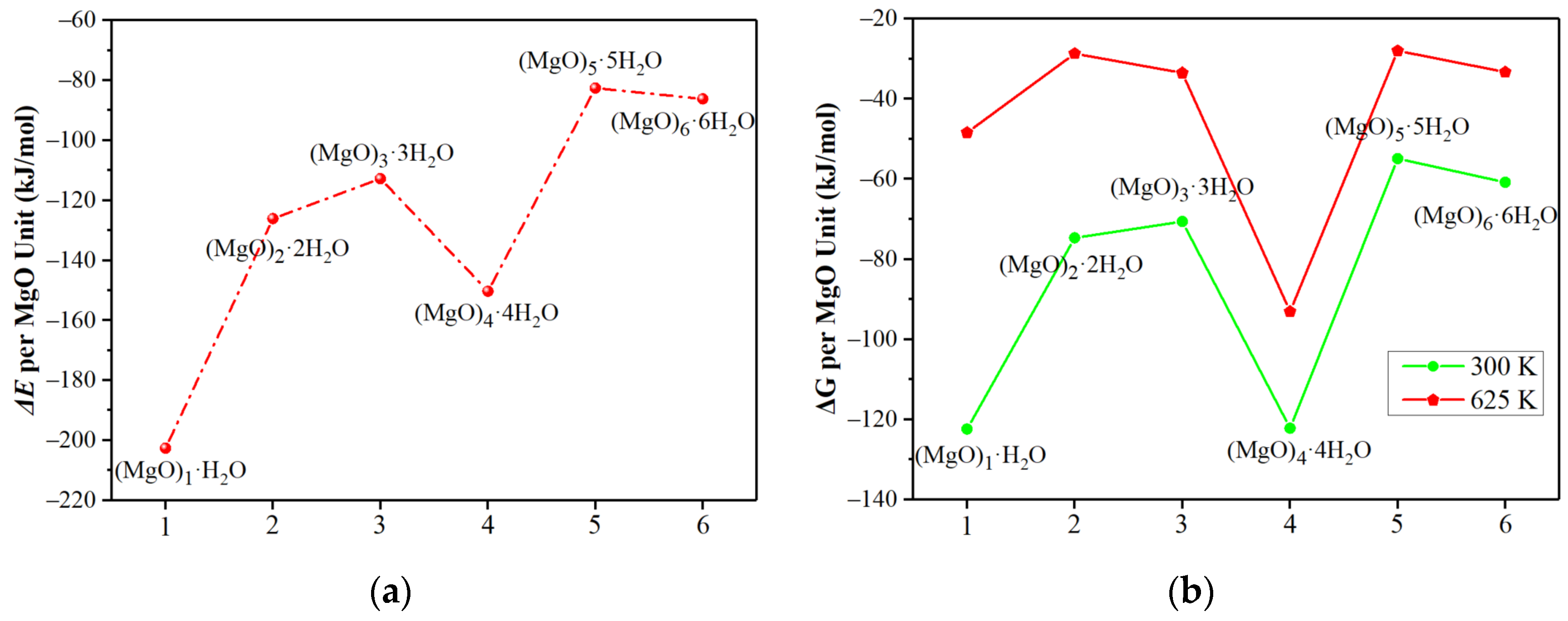
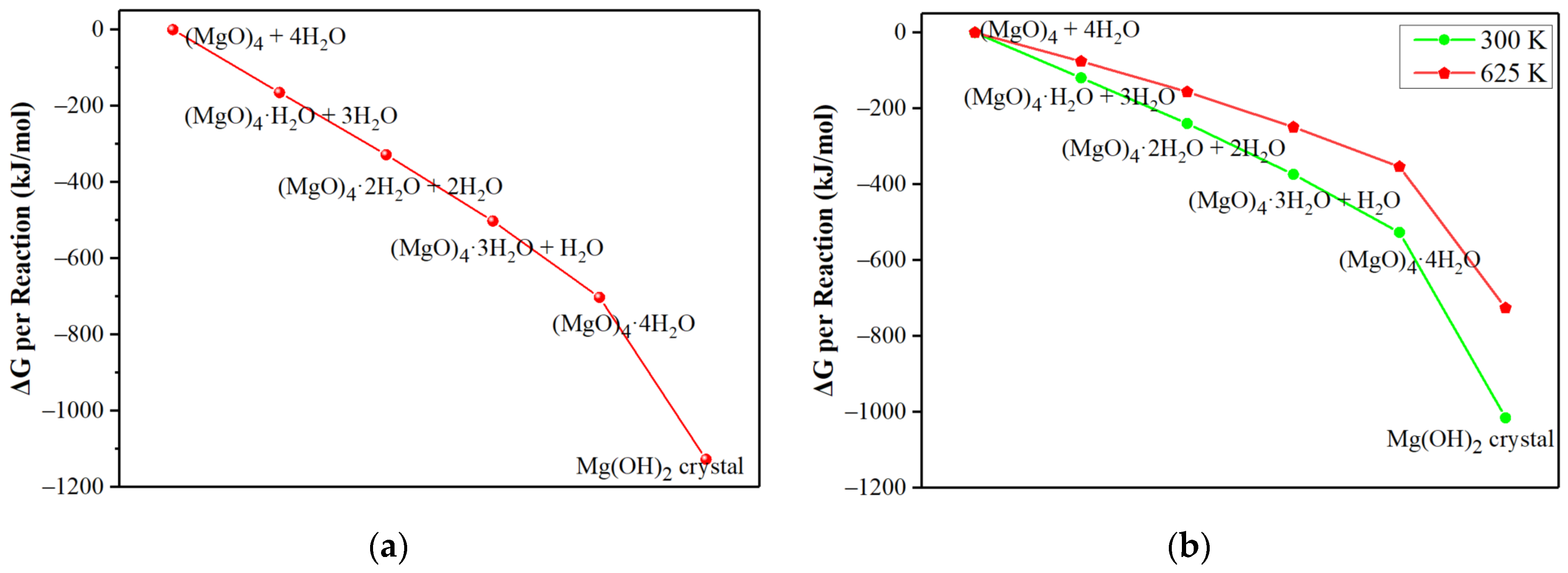
3.1. Hydration Mechanism of (MgO)n·nH2O Clusters
3.2. Effect Mechanism of Anti-Hydration Agents on Hydration of (MgO)4 Cluster
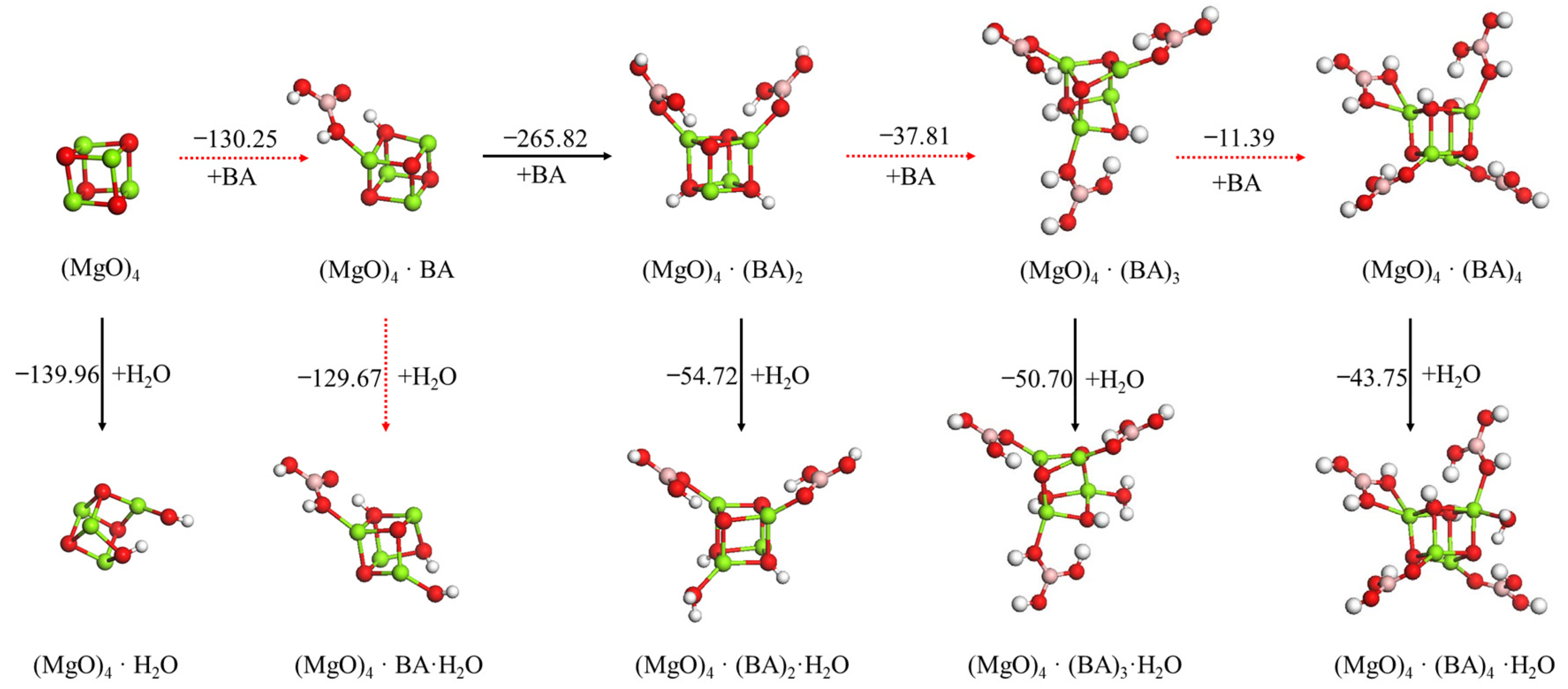
3.2.1. Effect Mechanism of Boric Acid on Hydration of (MgO)4 Cluster
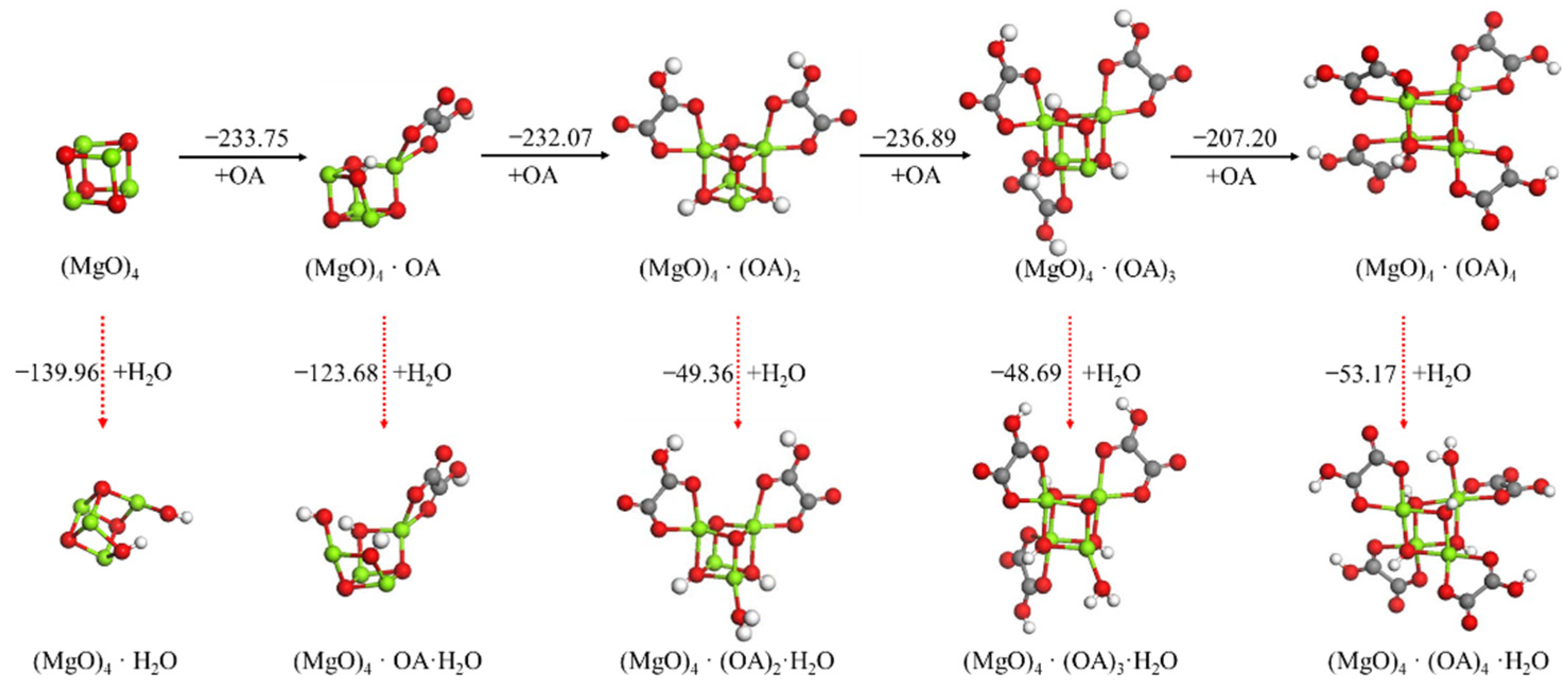
3.2.2. Effect Mechanism of Oxalic Acid on Hydration of (MgO)4 Cluster
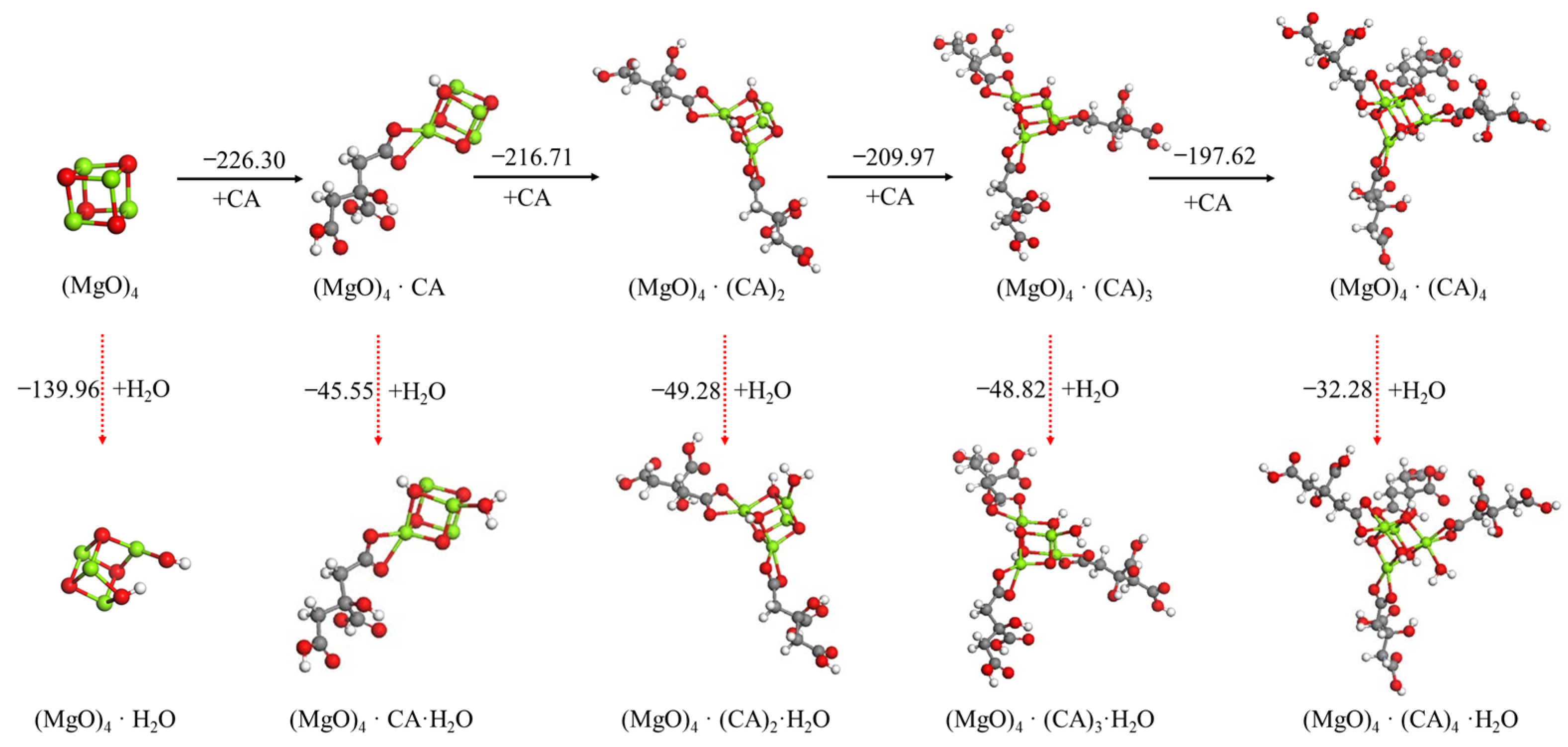
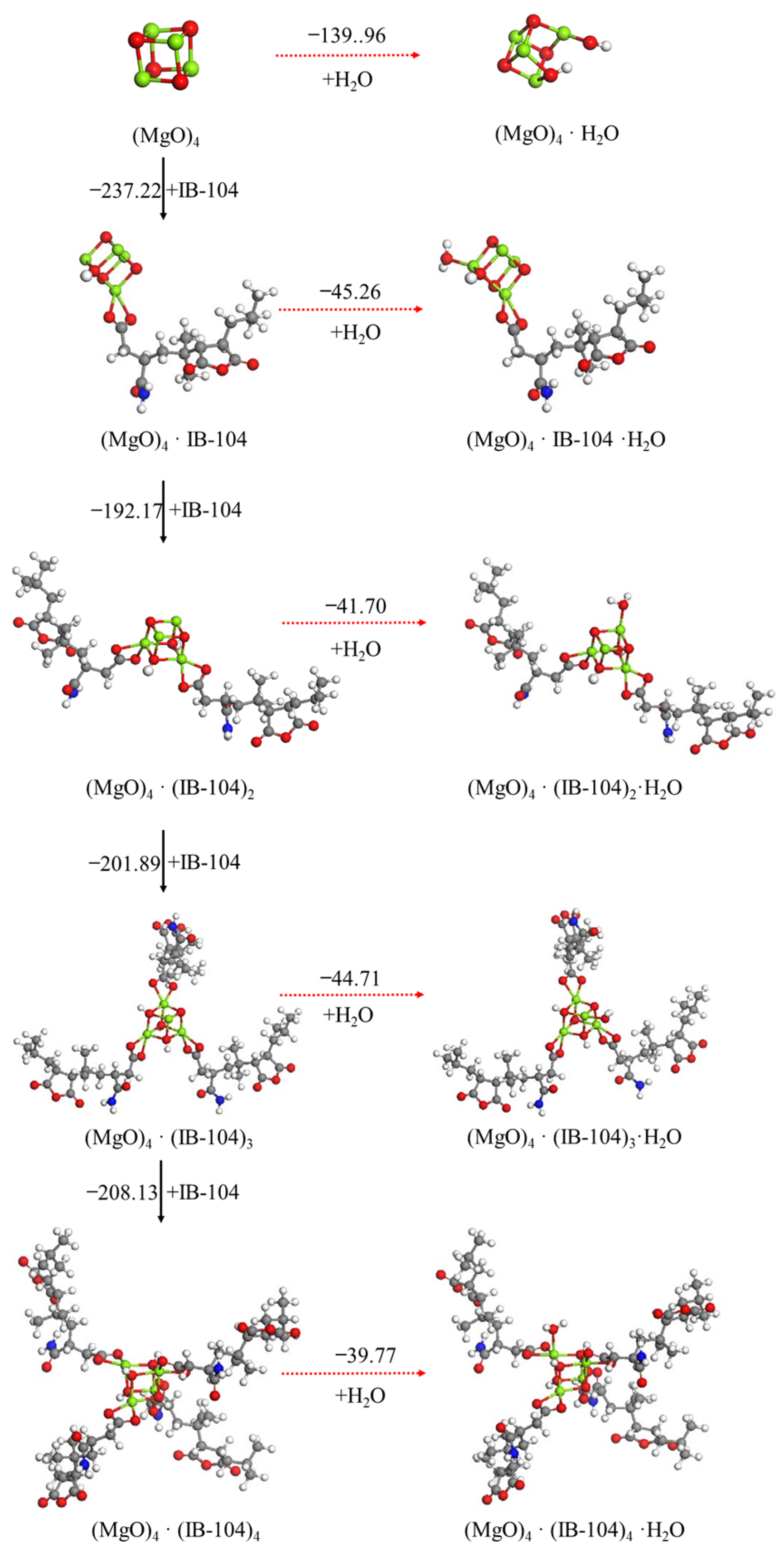
3.2.3. Effect Mechanism of Tartaric Acid, Citric Acid and ISOBAM-104 on Hydration of (MgO)4 Cluster
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahraki, A.; Ghasemi-Kahrizsangi, S.; Nemati, A. Performance improvement of MgO–CaO refractories by the addition of nano-sized Al2O3. Mater. Chem. Phys. 2017, 198, 354–359. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Boroujerdnia, M. Effect of micro and nano-Al2O3 addition on the microstructure and properties of MgO–C refractory ceramic composite. Mater. Chem. Phys. 2017, 189, 230–236. [Google Scholar] [CrossRef]
- Dehsheikh, H.G.; Ghasemi-Kahrizsangi, S. Performance improvement of MgO–C refractory bricks by the addition of Nano-ZrSiO4. Mater. Chem. Phys. 2017, 202, 369–376. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Karamian, E.; Boroujerdnia, M.; Payandeh, K. Effect of MgAl2O4 nanoparticles addition on the densification and properties of MgO–CaO refractories. Ceram. Int. 2017, 43, 5014–5019. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Sedeh, M.B.; Dehsheikh, H.G.; Shahraki, A.; Farooghi, M. Densification and properties of ZrO2 nanoparticles added magnesia–doloma refractories. Ceram. Int. 2016, 42, 15658–15663. [Google Scholar] [CrossRef]
- Cai, M.; Liang, Y.; Yin, Y.; Nie, J. Effect of citric acid on the hydration process of colloidal silica-bonded magnesia gunning materials. Ceram. Int. 2019, 45, 15514–15519. [Google Scholar] [CrossRef]
- Kurosawa, R.; Takeuchi, M.; Ryu, J. Fourier-transform infrared and X-ray diffraction analyses of the hydration reaction of pure magnesium oxide and chemically modified magnesium oxide. RSC Adv. 2021, 11, 24292–24311. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Z.; Wang, S. Influence of calcination temperature on the structure and hydration of MgO. Constr. Build. Mater. 2020, 262, 120776. [Google Scholar] [CrossRef]
- Shah, V.; Scott, A. Hydration and microstructural characteristics of MgO in the presence of metakaolin and silica fume. Cem. Concr. Compos. 2021, 121, 104068. [Google Scholar] [CrossRef]
- He, J.; Zheng, W.; Bai, W.; Hu, T.; He, J.; Song, X. Effect of reactive MgO on hydration and properties of alkali-activated slag pastes with different activators. Constr. Build. Mater. 2021, 271, 121608. [Google Scholar] [CrossRef]
- Warmuz, K.; Madej, D. Effect of the particle size on the reactivity of MgO–Al2O3 hydrating mixtures: A long-term kinetic investigation of hydrotalcite synthesis. Appl. Clay Sci. 2021, 211, 106196. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Wang, J.; Huang, H.; Wang, S. Atomic-level insights into nano-salt droplets wetting on the MgO surface using molecular dynamics simulations. Corros. Sci. 2020, 167, 108549. [Google Scholar] [CrossRef]
- Da Silva Alvim, R.; Borges, I., Jr.; Leitão, A.A. Proton migration on perfect, vacant, and doped MgO (001) surfaces: Role of dissociation residual groups. J. Phys. Chem. C 2018, 122, 21841–21853. [Google Scholar] [CrossRef]
- Oncak, M.; Włodarczyk, R.; Sauer, J. Water on the MgO (001) surface: Surface reconstruction and ion solvation. J. Phys. Chem. Lett. 2015, 6, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Alessio, M.; Usvyat, D.; Sauer, J. Chemically accurate adsorption energies: CO and H2O on the MgO (001) surface. J. Chem. Theory Comput. 2018, 15, 1329–1344. [Google Scholar] [CrossRef]
- Souza, T.M.; Braulio, M.A.L.; Luz, A.P.; Bonadia, P.; Pandolfellli, V.C. Systemic analysis of MgO hydration effects on alumina–magnesia refractory castables. Ceram. Int. 2012, 38, 3969–3976. [Google Scholar] [CrossRef]
- Chen, S.J.; Lu, P.G.; Chen, G.R.; Cheng, J.J. Improved hydration resistance of synthesized magnesia–calcia clinker by surface modification. J. Am. Ceram. Soc. 2004, 87, 2164–2167. [Google Scholar] [CrossRef]
- Amaral, L.F.; Oliveira, I.R.; Bonadia, P.; Salomao, R.; Pandolfelli, V.C. Chelants to inhibit magnesia (MgO) hydration. Ceram. Int. 2011, 37, 1537–1542. [Google Scholar] [CrossRef]
- Nouri-Khezrabad, M.; Luz, A.P.; Golestani-Fard, F.; Rezaie, H.R.; Pandolfelli, V.C. Citric acid role and its migration effects in nano-bonded refractory castables. Ceram. Int. 2014, 40, 14523–14527. [Google Scholar] [CrossRef]
- Salomão, R.; Pandolfelli, V.C. Citric acid as anti-hydration additive for magnesia containing refractory castables. Ceram. Int. 2011, 37, 1839–1842. [Google Scholar] [CrossRef]
- Dos Santos, T., Jr.; dos Santos, J.; Luz, A.P.; Pagliosa, C.; Pandolfelli, V.C. Kinetic control of MgO hydration in refractory castables by using carboxylic acids. J. Eur. Ceram. Soc. 2018, 38, 2152–2163. [Google Scholar] [CrossRef]
- Xing, X.D.; Hermann, A.; Kuang, X.Y.; Ju, M.; Lu, C.; Jin, Y.Y.; Xia, X.X.; Maroulis, G. Insights into the geometries, electronic and magnetic properties of neutral and charged palladium clusters. Sci. Rep. 2016, 6, 19656. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Lv, J.; Kuang, X.Y.; Ding, L.P.; Lu, C.; Wng, J.J.; Jin, Y.Y.; Maroulis, G. Systematic theoretical investigation of geometries, stabilities and magnetic properties of iron oxide clusters (FeO)nμ (n = 1–8, μ= 0,±1): Insights and perspectives. RSC Adv. 2015, 5, 6560–6570. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Shi, T.T.; Qi, J.L.; Wang, Q. Calculation on the CO2 influences on the structure, stability of (MgO)m (m = 1–6) clusters. Phase Transit. 2018, 91, 1223–1231. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Malliavin, M.J.; Coudray, C. Ab initio calculations on (MgO)n, (CaO)n, and (NaCl)n clusters (n = 1–6). J. Chem. Phys. 1997, 106, 2323–2330. [Google Scholar] [CrossRef]
- Bawa, F.; Panas, I. Competing pathways for MgO, CaO, SrO, and BaO nanocluster growth. Phys. Chem. Chem. Phys. 2002, 4, 103–108. [Google Scholar] [CrossRef]
- De la Puente, E.; Aguado, A.; Ayuela, A.; Lopez, J.M. Structural and electronic properties of small neutral (MgO)n clusters. Phys. Rev. B 1997, 56, 7607. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Wang, H.L.; Cheng, J.G.; Tang, L.L.; Zhao, J.J. Lowest-energy structures of (MgO)n (n= 2–7) clusters from a topological method and first-principles calculations. Comput. Theor. Chem. 2012, 980, 62–67. [Google Scholar] [CrossRef]
- Dong, R.B.; Chen, X.S.; Wang, X.F.; Lu, W. Structural transition of hexagonal tube to rocksalt for (MgO)3n, 2 ≤ n ≤ 10. J. Chem. Phys. 2008, 129, 044705. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kumar, V.; Sluiter, M.; Kawazoe, Y. First principles studies of magnesium oxide clusters by parallelized Tohoku University Mixed-Basis program TOMBO. Comput. Mater. Sci. 2006, 36, 171–175. [Google Scholar] [CrossRef]
- Nguyen, N.Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial activities and mechanisms of magnesium oxide nanoparticles (nMgO) against pathogenic bacteria, yeasts, and biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruhwirth, O.; Herzog, G.W.; Hollerer, I.; Rachetti, A. Dissolution and hydration kinetics of MgO. Surf. Technol. 1985, 24, 301–317. [Google Scholar] [CrossRef]
- Maity, S.; Ghosh, S.; Ghosh, A. Elucidating the secondary effect in the Lewis acid mediated anodic shift of electrochemical oxidation of a Cu (II) complex with a N2O2 donor unsymmetrical ligand. Dalton Trans. 2019, 48, 14898–14913. [Google Scholar] [CrossRef]
- Li, Z.G.; Ji, Z.S.; Jiang, L.L.; Yu, S.W. Effect of additives on the properties of magnesium oxysulfate cement. J. Intell. Fuzzy Syst. 2017, 33, 3021–3025. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Dong, L.; Huang, L.; Huang, Z.; Li, F.; Zhang, H.; Zhang, S. A First-Principles Study on the Hydration Behavior of (MgO)n Clusters and the Effect Mechanism of Anti-Hydration Agents. Materials 2022, 15, 3521. https://doi.org/10.3390/ma15103521
Gao Y, Dong L, Huang L, Huang Z, Li F, Zhang H, Zhang S. A First-Principles Study on the Hydration Behavior of (MgO)n Clusters and the Effect Mechanism of Anti-Hydration Agents. Materials. 2022; 15(10):3521. https://doi.org/10.3390/ma15103521
Chicago/Turabian StyleGao, Yu, Long Dong, Liang Huang, Zhong Huang, Faliang Li, Haijun Zhang, and Shaowei Zhang. 2022. "A First-Principles Study on the Hydration Behavior of (MgO)n Clusters and the Effect Mechanism of Anti-Hydration Agents" Materials 15, no. 10: 3521. https://doi.org/10.3390/ma15103521





