Inhibition of Alkali-Carbonate Reaction by Fly Ash and Metakaolin on Dolomitic Limestones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cementitious Materials
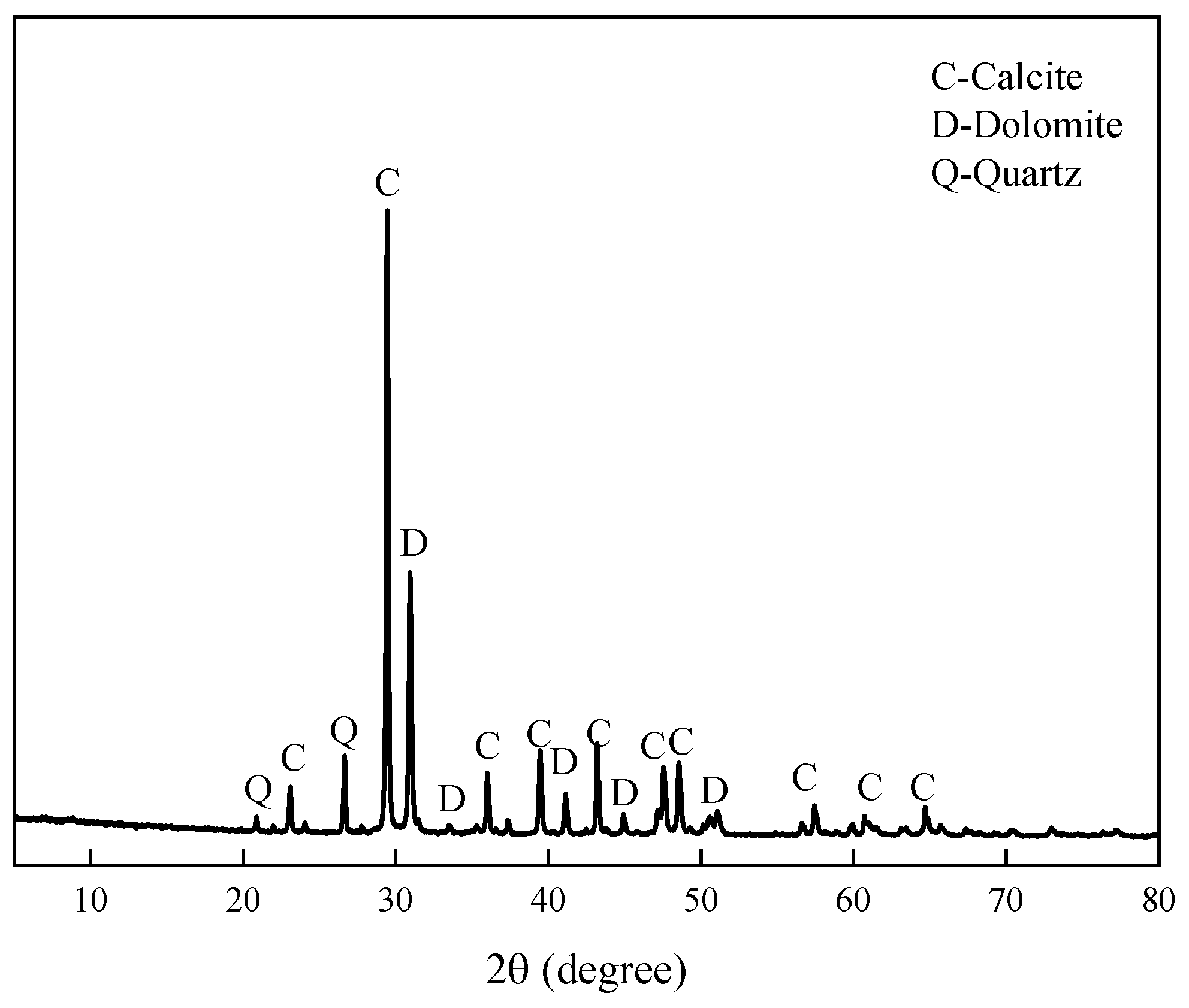
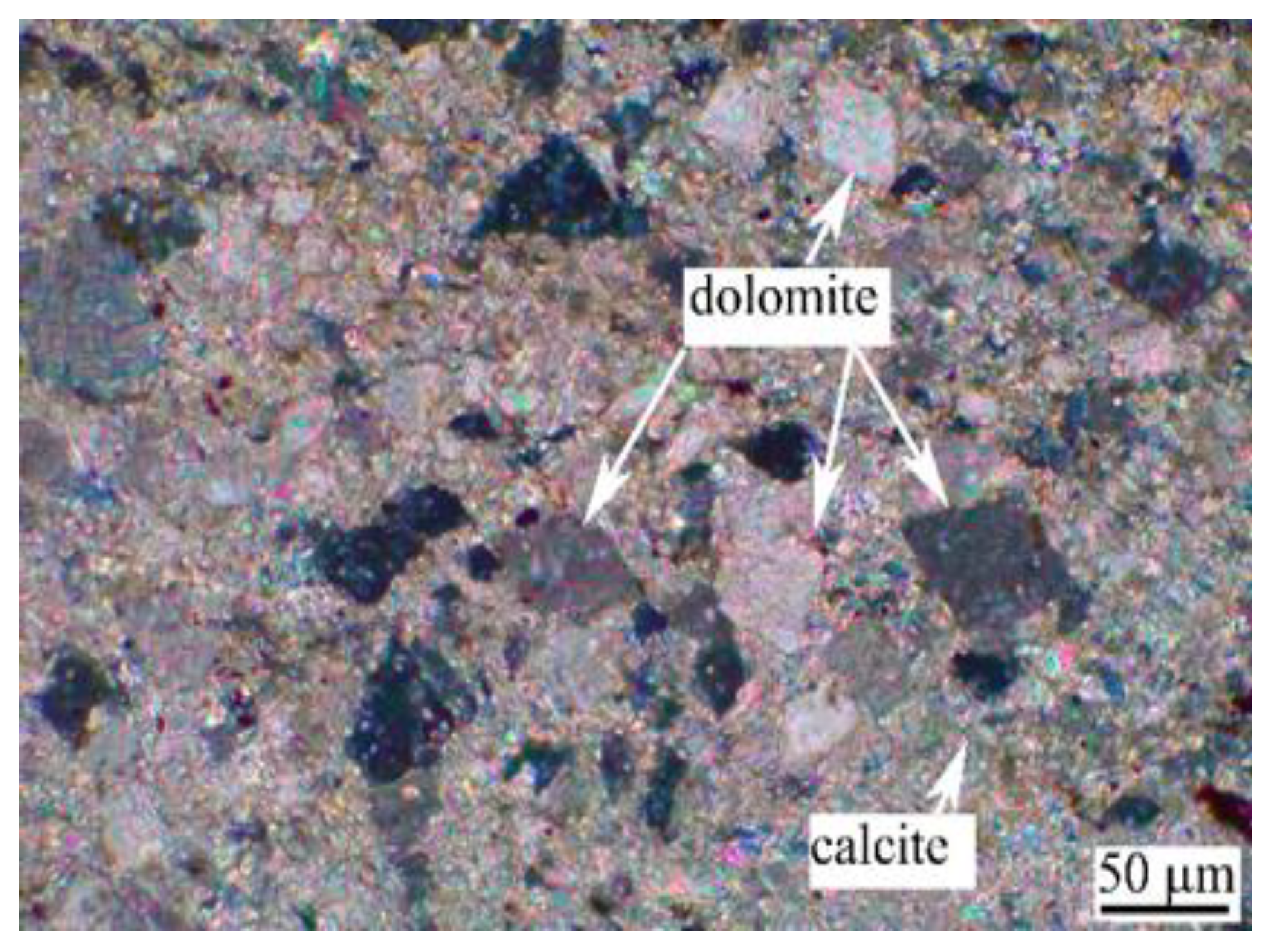
2.1.2. Aggregates
2.2. Methods
2.2.1. Determination of Aggregate Alkali Activity
2.2.2. Concrete Microbars Test
2.2.3. Determination of Hydration Products
2.2.4. Mercury Porosimetry Analyses
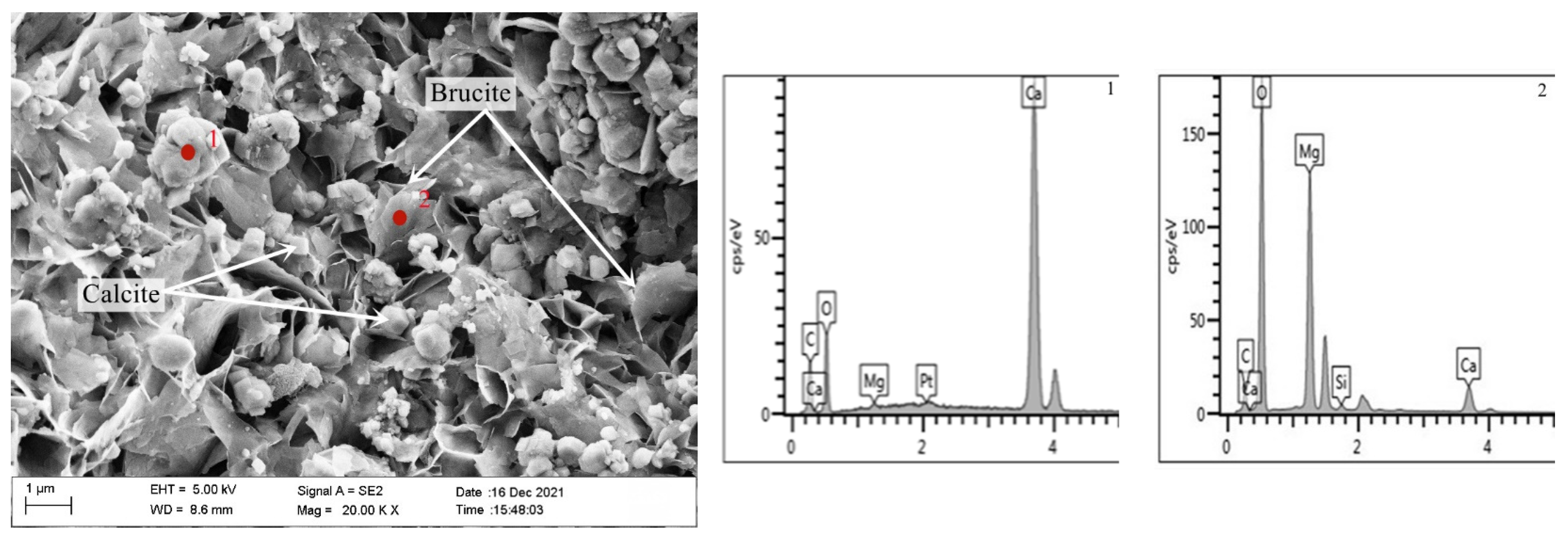
2.2.5. Microscopic Analysis of ACR Products
3. Results and Discussion
3.1. Alkali Activity of Aggregate
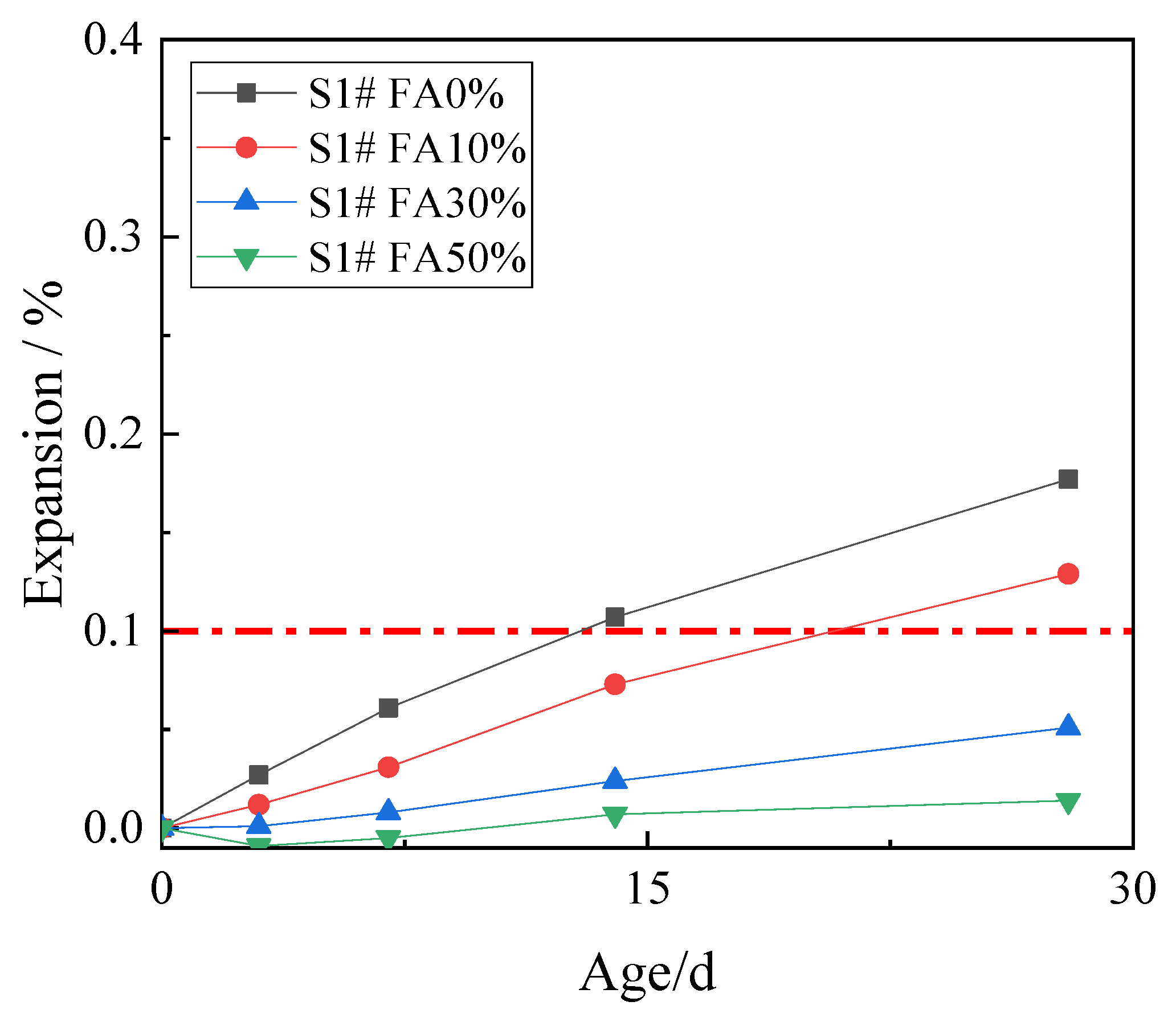
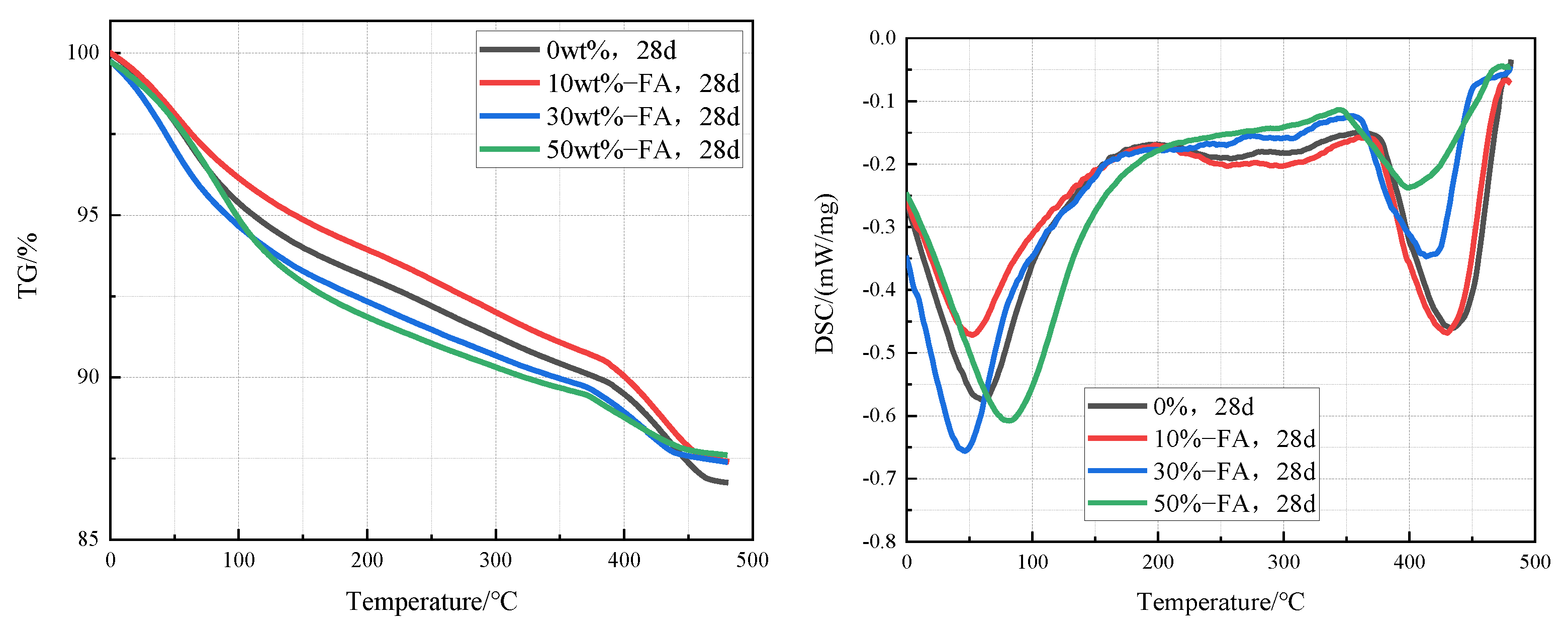
3.2. Inhibition of ACR with Fly Ash
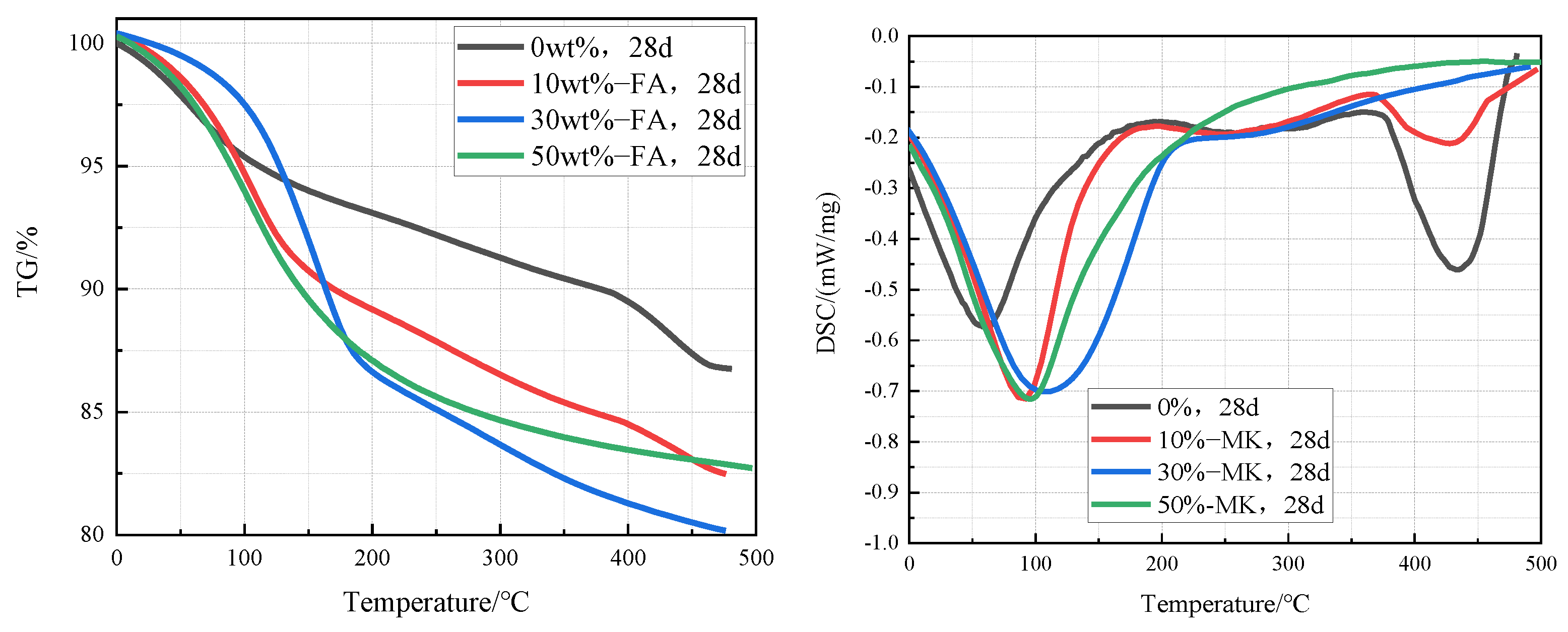
3.3. Inhibition of ACR with Metakaolin
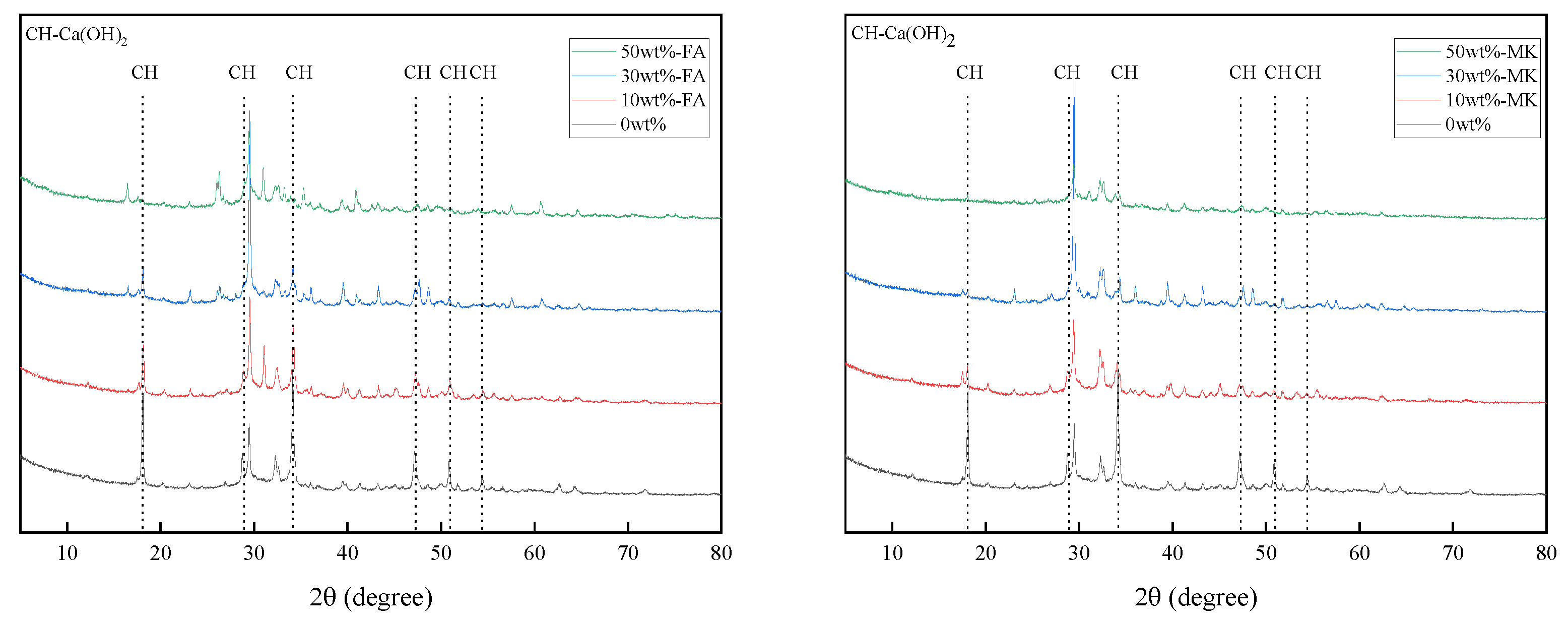
3.4. Effects of Fly Ash and Metakaolin on Ca(OH)2 in Cement Paste
3.4.1. XRD Analyses
3.4.2. TG-DSC Analyses
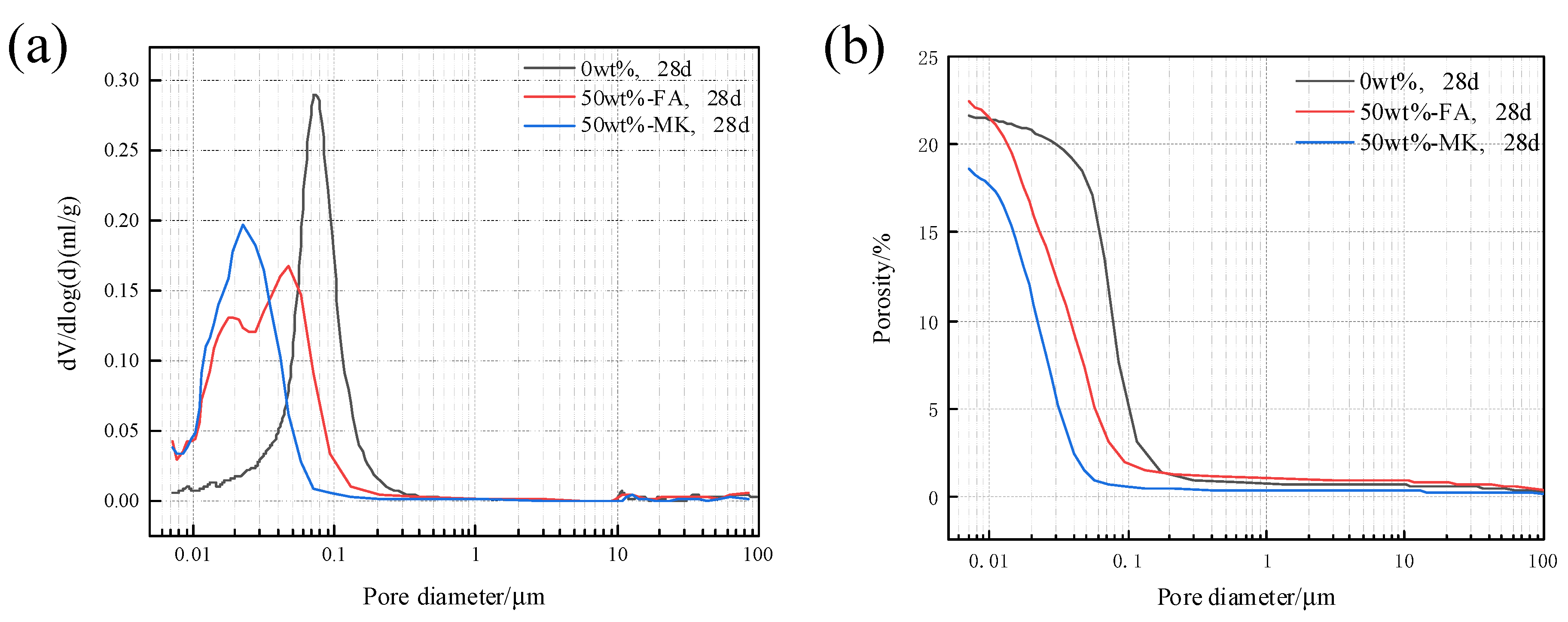
3.5. Effects of Fly Ash and Metakaolin on the Pore Structure of Concrete Microbars
3.6. Effect of Fly Ash and Metakaolin on Aggregate Reaction Degree
3.7. Effects of Fly Ash and Metakaolin on Expansion Cracks
4. Conclusions
- (1)
- Four methods were used to determine that the dolomitic limestone S1# is alkali-carbonate reactive aggregate.
- (2)
- In the concrete microbars test, metakaolin and fly ash can effectively inhibit the ACR of reactive aggregate, and the more the content of fly ash and metakaolin, the more efficient the effect of inhibition. Less fly ash cannot effectively inhibit the alkali–aggregate reaction of active aggregate. When the content of fly ash and metakaolin is the same, metakaolin is more effective than fly ash.
- (3)
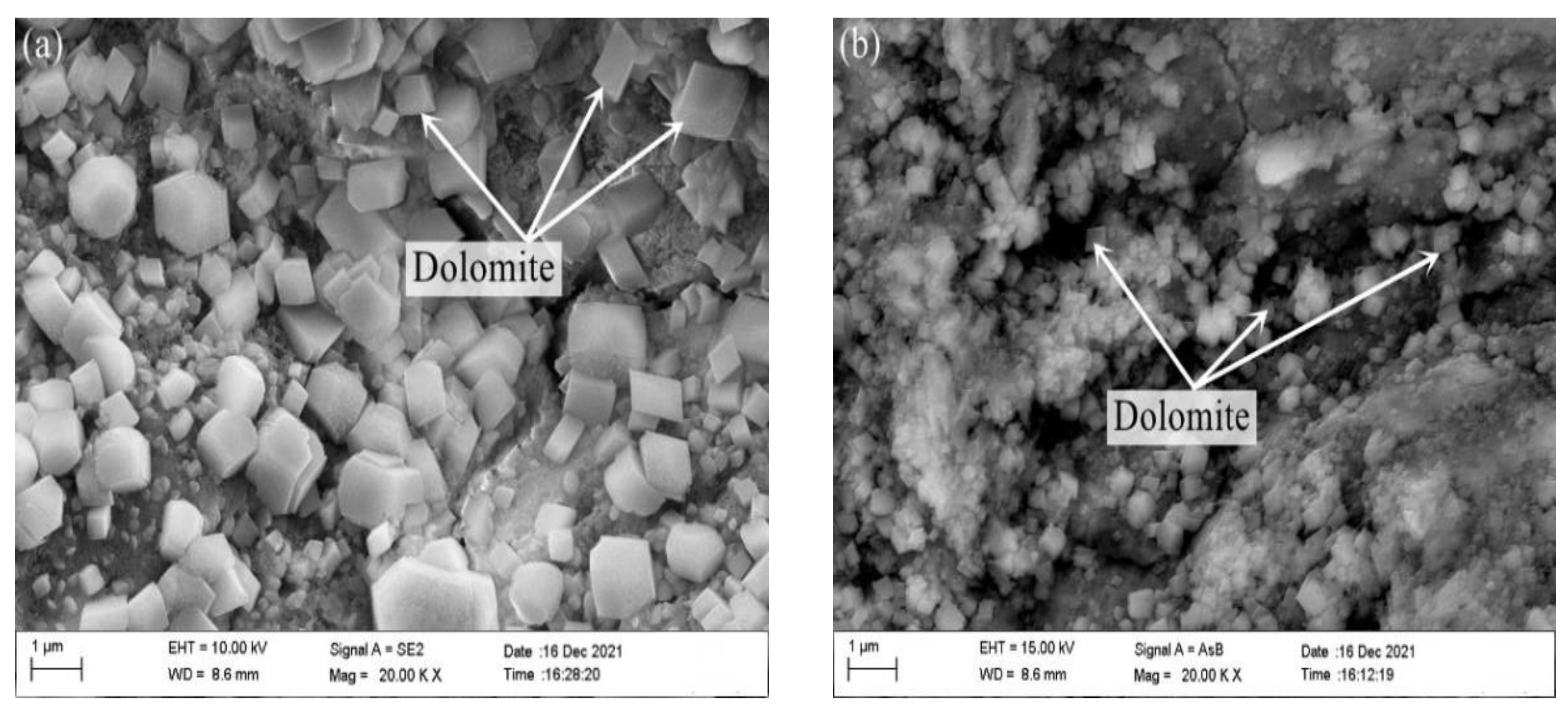
- The products of ACR calcite and brucite can be clearly observed in the SEM image, but no ASR gel was found, which indicates that the expansion of dolomitic limestone S1# was caused by ACR.
- (4)
- Metakaolin and fly ash participate in the secondary hydration of cement, which consumes a large amount of the hydration product Ca(OH)2 of Portland cement and reduces the alkalinity of the cement hydration product, so the ACR be inhibited. The pore structure of concrete containing fly ash and metakaolin becomes denser, which prevents the diffusion of K+ and Na+ to the active aggregate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swenson, E.G. A reactive aggregate undetected by ASTM test. ASTM Bull. 1957, 226, 48–51. [Google Scholar]
- Swenson, E.G.; Gillott, J.E. Alkali-carbonate rock reaction. Highw. Res. Rec. 1964, 45, 21–40. [Google Scholar]
- Gillott, J.E. Petrology of Dolomitic Limestones, Kingston, Ontario, Canada. Geol. Soc. Am. Bull. 1963, 74, 759–778. [Google Scholar] [CrossRef] [Green Version]
- McCoy, W.J.; Caldwell, A.G. New approach to inhibiting alkali-aggregate expansion. J. Proc. 1951, 47, 693–706. [Google Scholar]
- Vredenburgh, L.D.; Lemish, J. Evaluation of autoclave induced expansion of some Iowa carbonate rocks. Proc. Iowa Acad. Sci. 1964, 71, 335–341. [Google Scholar]
- Diamond, S. A review of alkali-silica reaction and expansion mechanisms 1. Alkalies in cements and in concrete pore solutions. Cem. Concr. Res. 1975, 5, 329–345. [Google Scholar] [CrossRef]
- Abd-Elssamd, A.; Ma, Z.J.; Hou, H.; Le Pape, Y. Influence of mineralogical and chemical compositions on alkali-silica-reaction of Tennessee limestones. Constr. Build. Mater. 2020, 261, 119916. [Google Scholar] [CrossRef]
- Galı, S.; Ayora, C.; Alfonso, P.; Tauler, E.; Labrador, M. Kinetics of dolomite–portlandite reaction: Application to Portland cement concrete. Cem. Concr. Res. 2001, 31, 933–939. [Google Scholar] [CrossRef]
- French, W.J.; Poole, A.B. Deleterious reactions between dolomites from Bahrain and cement paste. Cem. Concr. Res. 1974, 4, 925–937. [Google Scholar] [CrossRef]
- Hansen, W.C. Basic chemistry of reactions of aggregates in Portland cement concrete. J. Mater. 1967, 2, 408. [Google Scholar]
- Hansen, W.C. Solid-liquid reactions in portland cement paste. Mater. Res. Stand. 1962, 2, 490–493. [Google Scholar]
- Rogers, C.A. Evaluation of the potential for expansion and cracking of concrete caused by the alkali-carbonate reaction. Cem. Concr. Aggre. 1986, 8, 13–23. [Google Scholar]
- Penkala, B. Alkali-aggregate reactivity investigations in Poland—A review. In Proceedings of the International Conference on Alkali-Aggregate Reaction, Ottawa, ON, Canada, 18–22 August 1986; pp. 221–225. [Google Scholar]
- Whitmore, D. Use of an applied electric field to drive lithium ions into alkali-silica reactive structures. In Proceedings of the International Conference on Alkali-Aggregate Reaction, Quebec, QC, Canada, 11–16 June 2000; pp. 1089–1098. [Google Scholar]
- Tang, M.S.; Liu, Z.; Han, S.F. Mechanism of alkali-carbonate reaction. In Proceedings of the International Conference on Alkali-Aggregate Reaction, Ottawa, ON, Canada, 18–22 August 1986; pp. 275–279. [Google Scholar]
- Sommer, H.; Nixon, P.J.; Sims, I. AAR-5: Rapid preliminary screening test for carbonate aggregates. Mater. Struct. 2005, 38, 787–792. [Google Scholar] [CrossRef]
- ASTM C1260-94; Standard Test Method for Potential Alkali Reactivity of Aggregates. American Society for Testing and Materials: West Conshohocken, PA, USA, 2014.
- ASTM C586–11; Standard test method for potential alkali reactivity of carbonate rocks as concrete aggregates. American Society for Testing and Materials: West Conshohocken, PA, USA, 2011.
- ASTM C1293-08b; Standard test method for determination of length change of concrete due to alkali-silica reaction. American Society for Testing and Materials: West Conshohocken, PA, USA, 2008.



| Materials | CaO | MgO | SiO2 | Al2O3 | Fe2O3 | K2O | Na2O | SO3 | LO I | Totals |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 64.00 | 2.35 | 19.43 | 4.73 | 2.96 | 0.47 | 0.26 | 2.58 | 2.81 | 99.59 |
| Fly ash | 4.40 | 1.11 | 50.10 | 29.77 | 8.95 | 0.89 | 0.39 | 1.15 | 1.81 | 98.57 |
| Metakaolin | 0.37 | 0.43 | 52.00 | 43.00 | 1.10 | 0.40 | 0.30 | 0.14 | 0.50 | 98.24 |
| Sample | Chemical Composition/% | |||||
|---|---|---|---|---|---|---|
| Loss | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | |
| S1# | 41.58 | 3.16 | 0.26 | 0.93 | 44.61 | 4.39 |
| Sample Name | Cement/g | Fly Ash/g | Metakaolin/g | Aggregates/g | w/c |
|---|---|---|---|---|---|
| S1#0% | 900 | - | - | 900 | 0.32 |
| S1#FA10% | 810 | 90 | - | 900 | |
| S1#FA30% | 630 | 270 | - | 900 | |
| S1#FA50% | 450 | 450 | 900 | ||
| S1#MK10% | 810 | - | 90 | 900 | |
| S1#MK30% | 630 | - | 270 | 900 | |
| S1#MK50% | 450 | - | 450 | 900 |
| Sample | Expansion of Mortar Bars at Age of 14 Days | Expansion of Concrete Microbars at Age of 28 Days | Expansion of Concrete Prisms at Age of 1 Year | Expansion of Rock Prisms at Age of 84 Days | Reactivity |
|---|---|---|---|---|---|
| S1# | 0.087% | 0.177% | 0.0427% | 0.114% | ACR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Mao, Z.; Huang, X.; Deng, M. Inhibition of Alkali-Carbonate Reaction by Fly Ash and Metakaolin on Dolomitic Limestones. Materials 2022, 15, 3538. https://doi.org/10.3390/ma15103538
Cao H, Mao Z, Huang X, Deng M. Inhibition of Alkali-Carbonate Reaction by Fly Ash and Metakaolin on Dolomitic Limestones. Materials. 2022; 15(10):3538. https://doi.org/10.3390/ma15103538
Chicago/Turabian StyleCao, Huan, Zhongyang Mao, Xiaojun Huang, and Min Deng. 2022. "Inhibition of Alkali-Carbonate Reaction by Fly Ash and Metakaolin on Dolomitic Limestones" Materials 15, no. 10: 3538. https://doi.org/10.3390/ma15103538
APA StyleCao, H., Mao, Z., Huang, X., & Deng, M. (2022). Inhibition of Alkali-Carbonate Reaction by Fly Ash and Metakaolin on Dolomitic Limestones. Materials, 15(10), 3538. https://doi.org/10.3390/ma15103538





