Liquid Superlubricity Enabled by the Synergy Effect of Graphene Oxide and Lithium Salts
Abstract
:1. Introduction
2. Materials and Methods
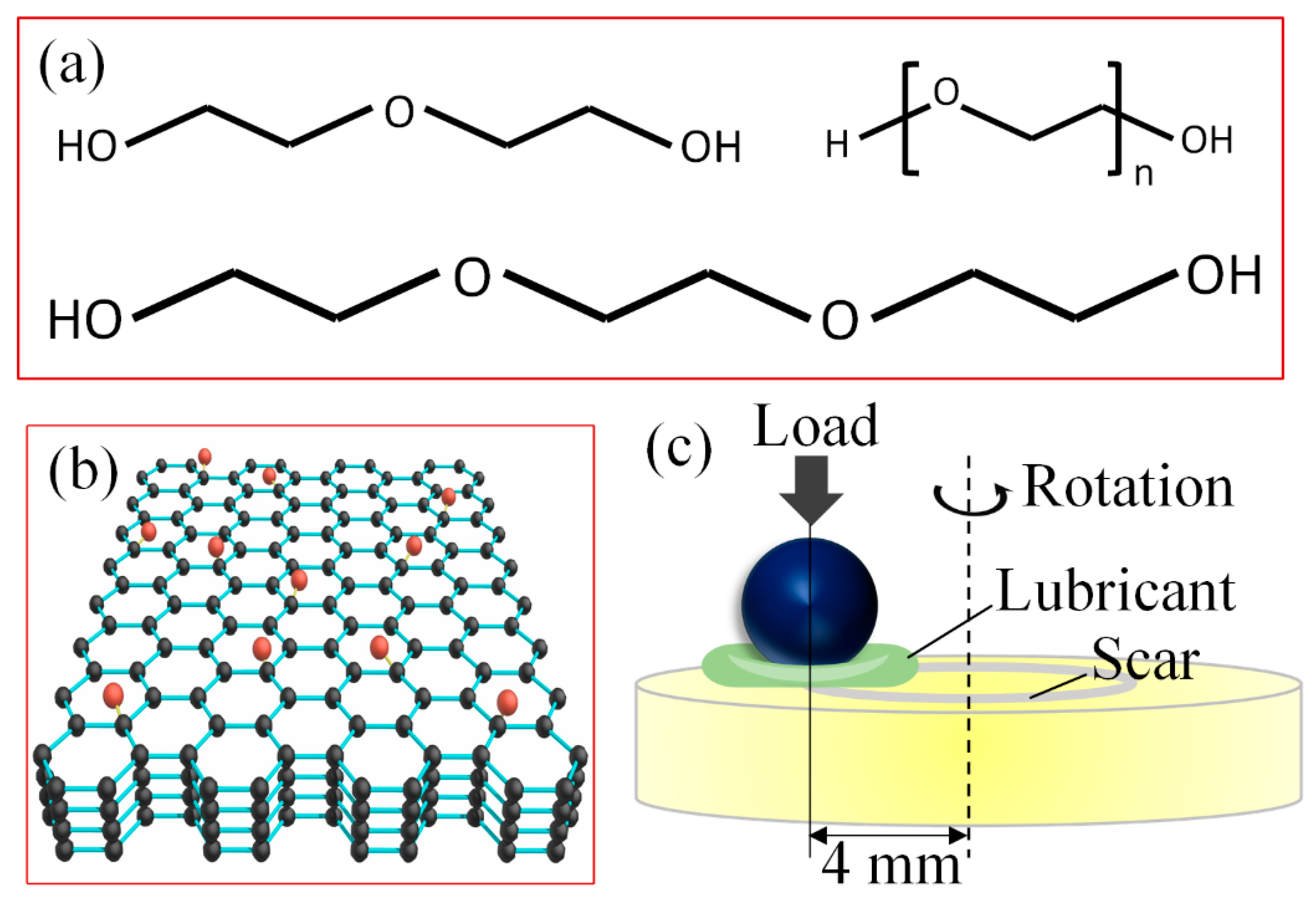
2.1. Materials
2.2. Tribological Experiments
2.3. Surface Characterization
3. Results
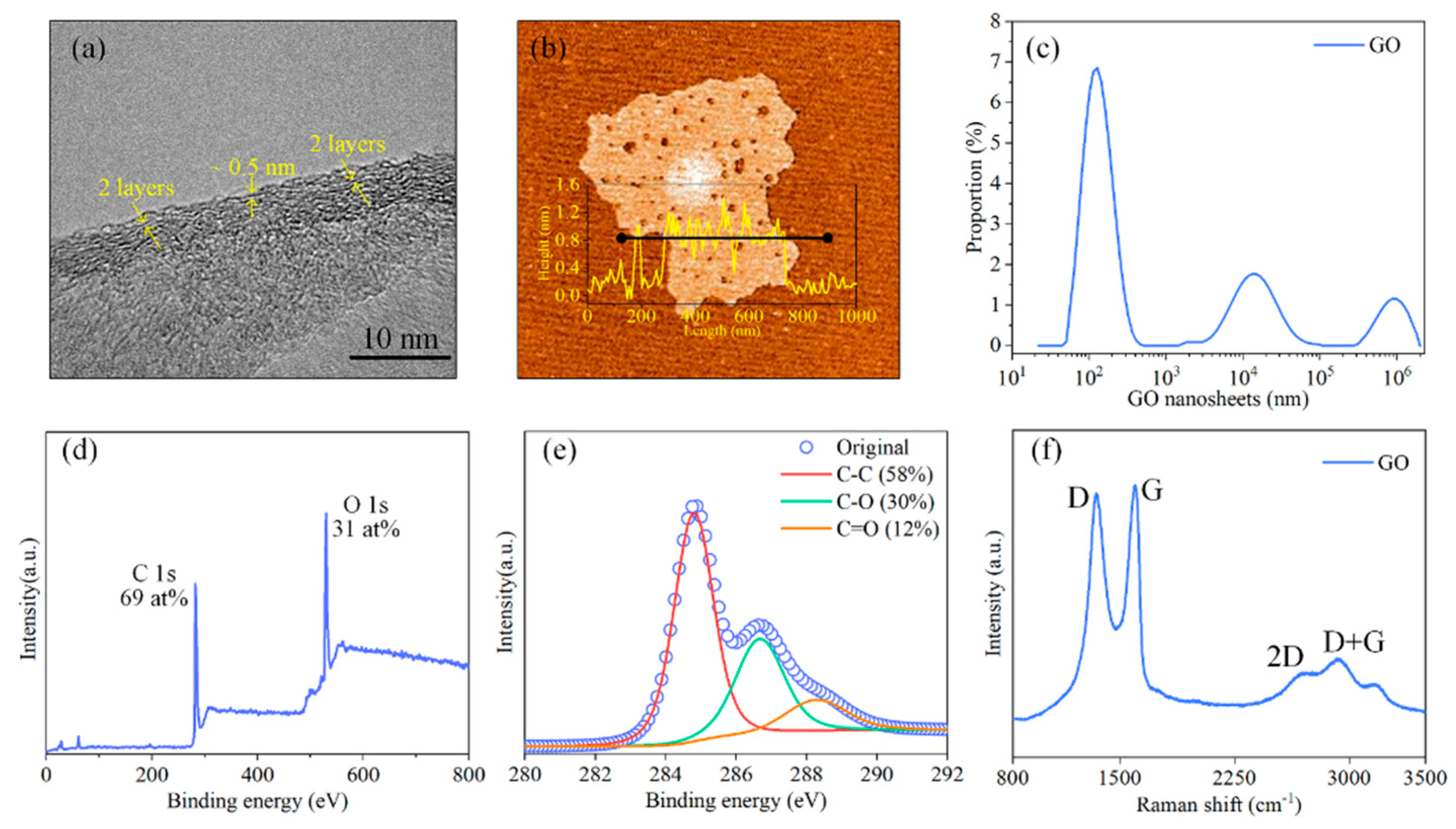
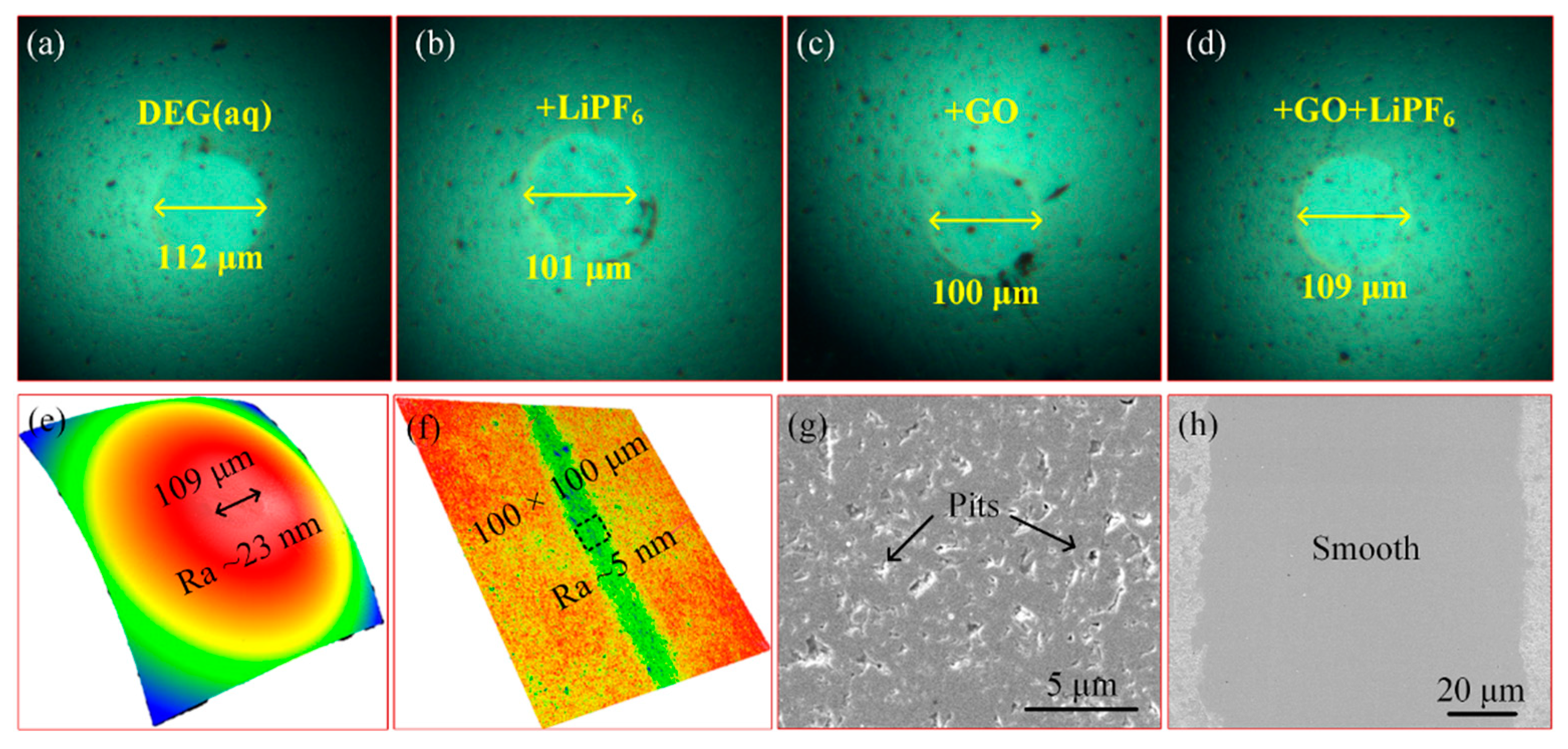
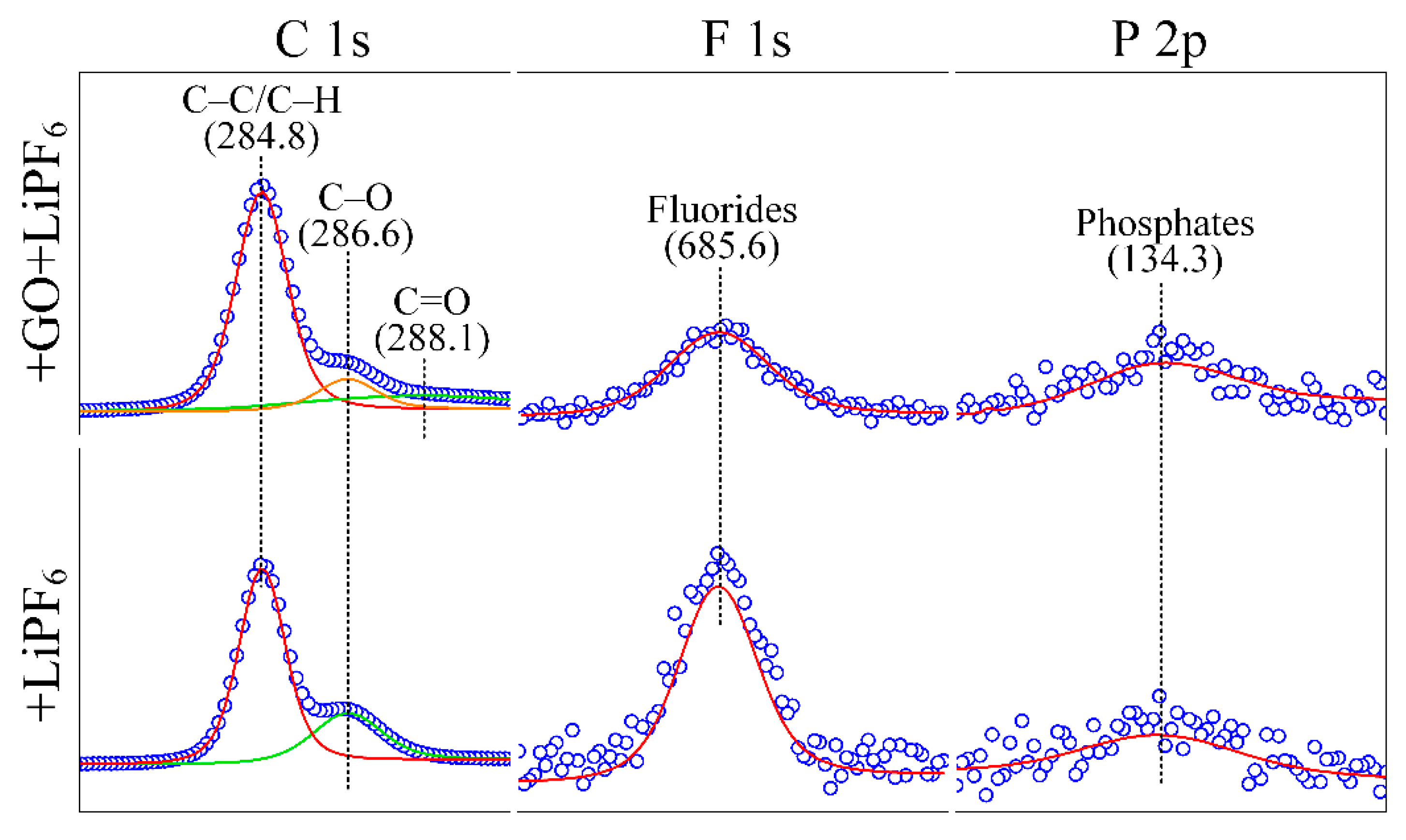
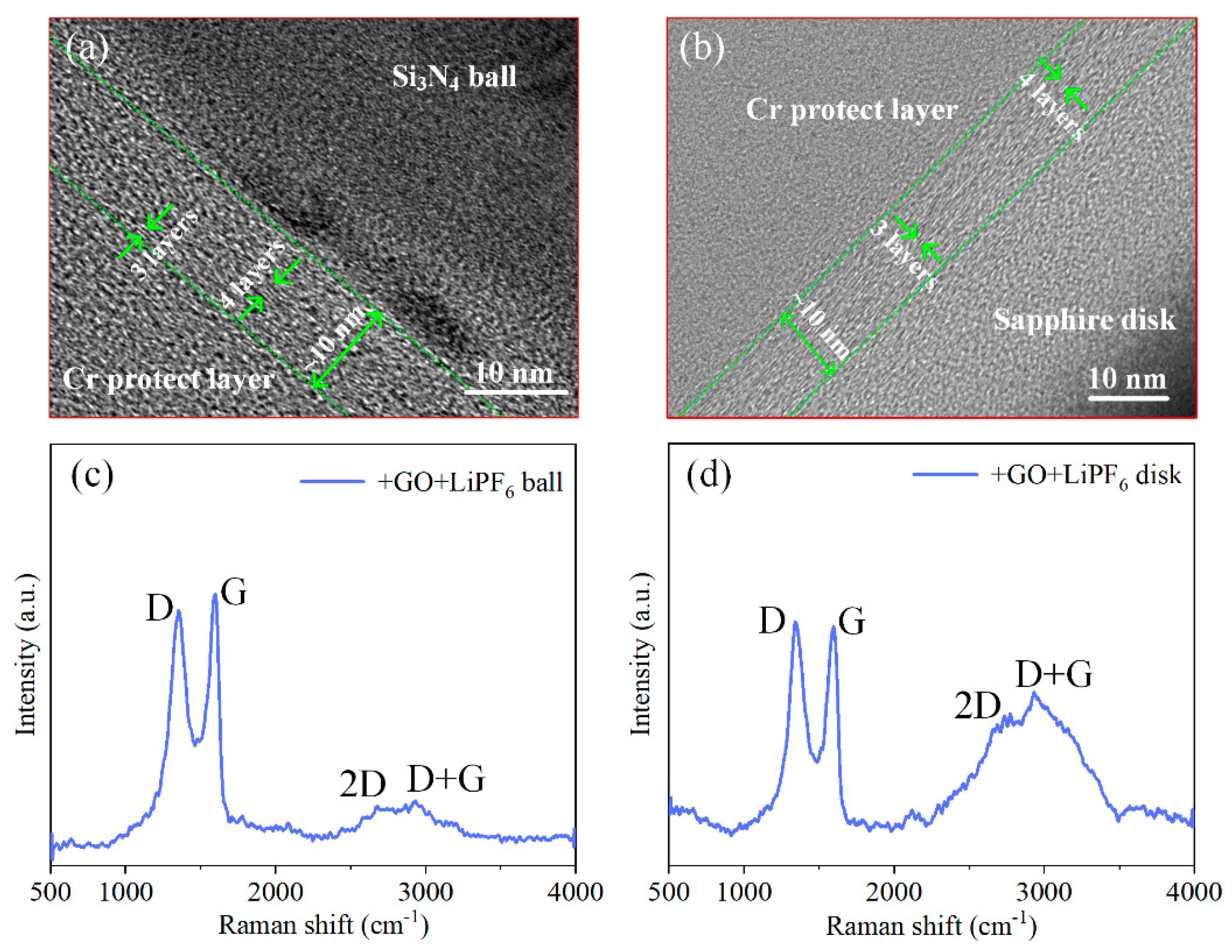
3.1. Material Characterization
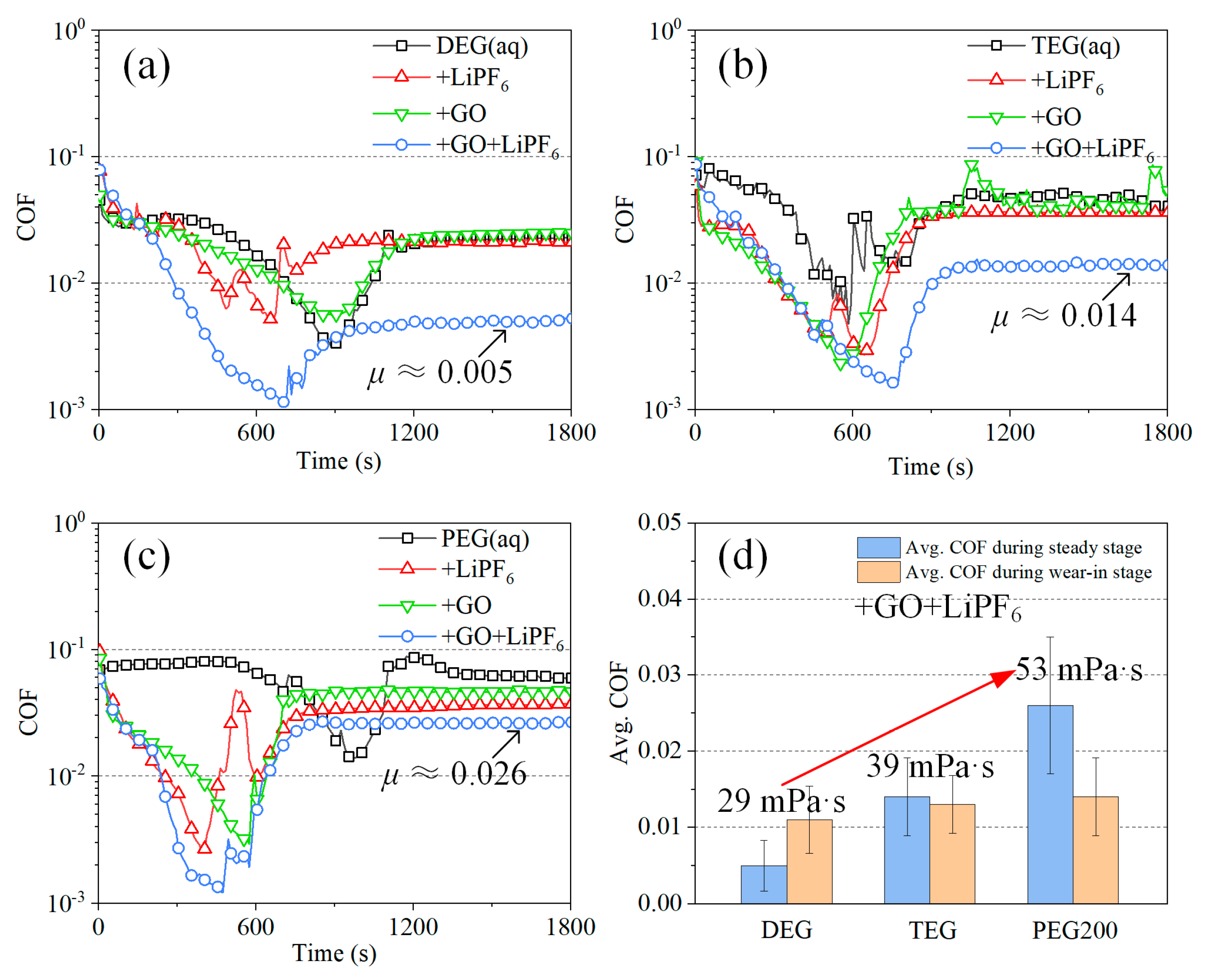
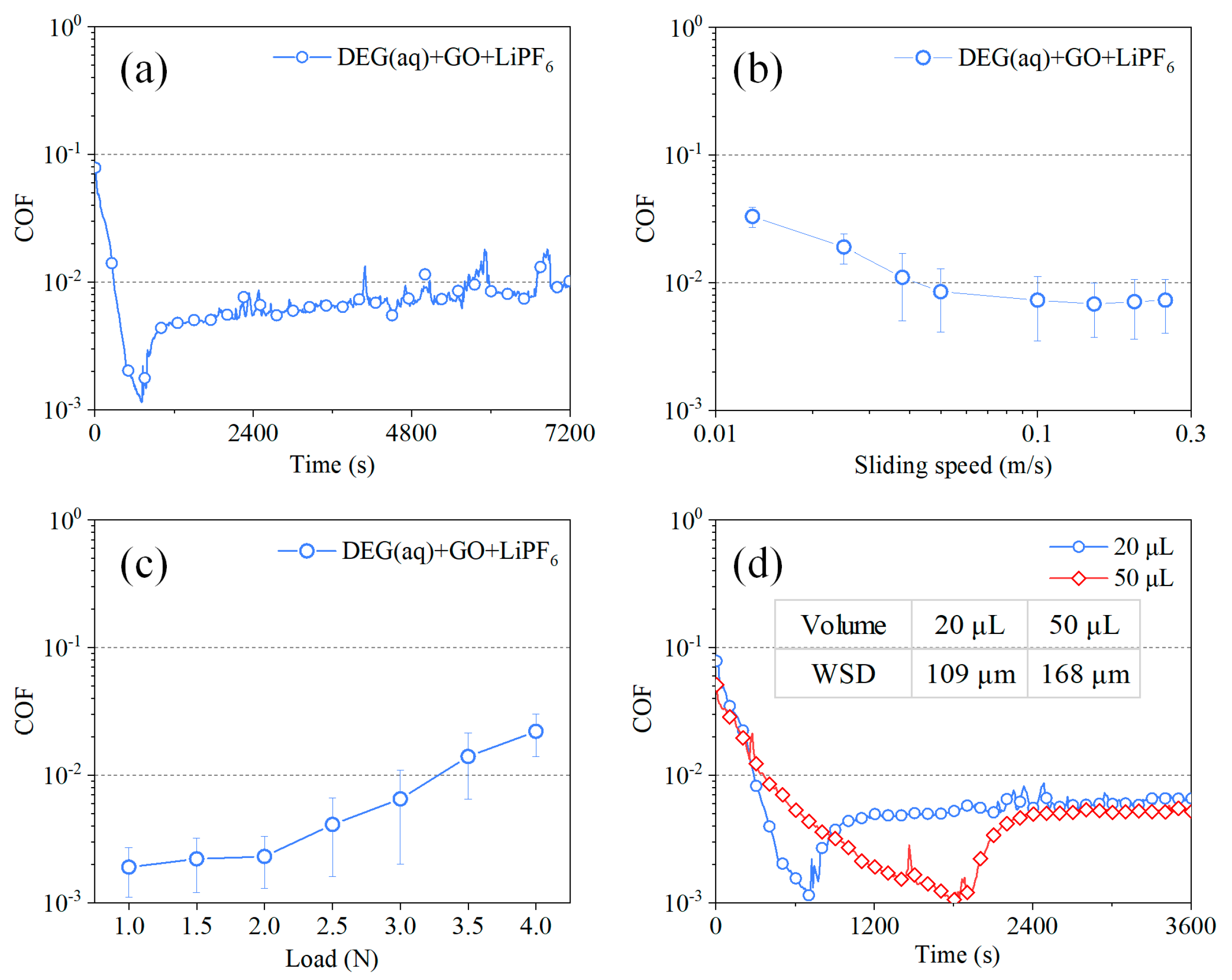
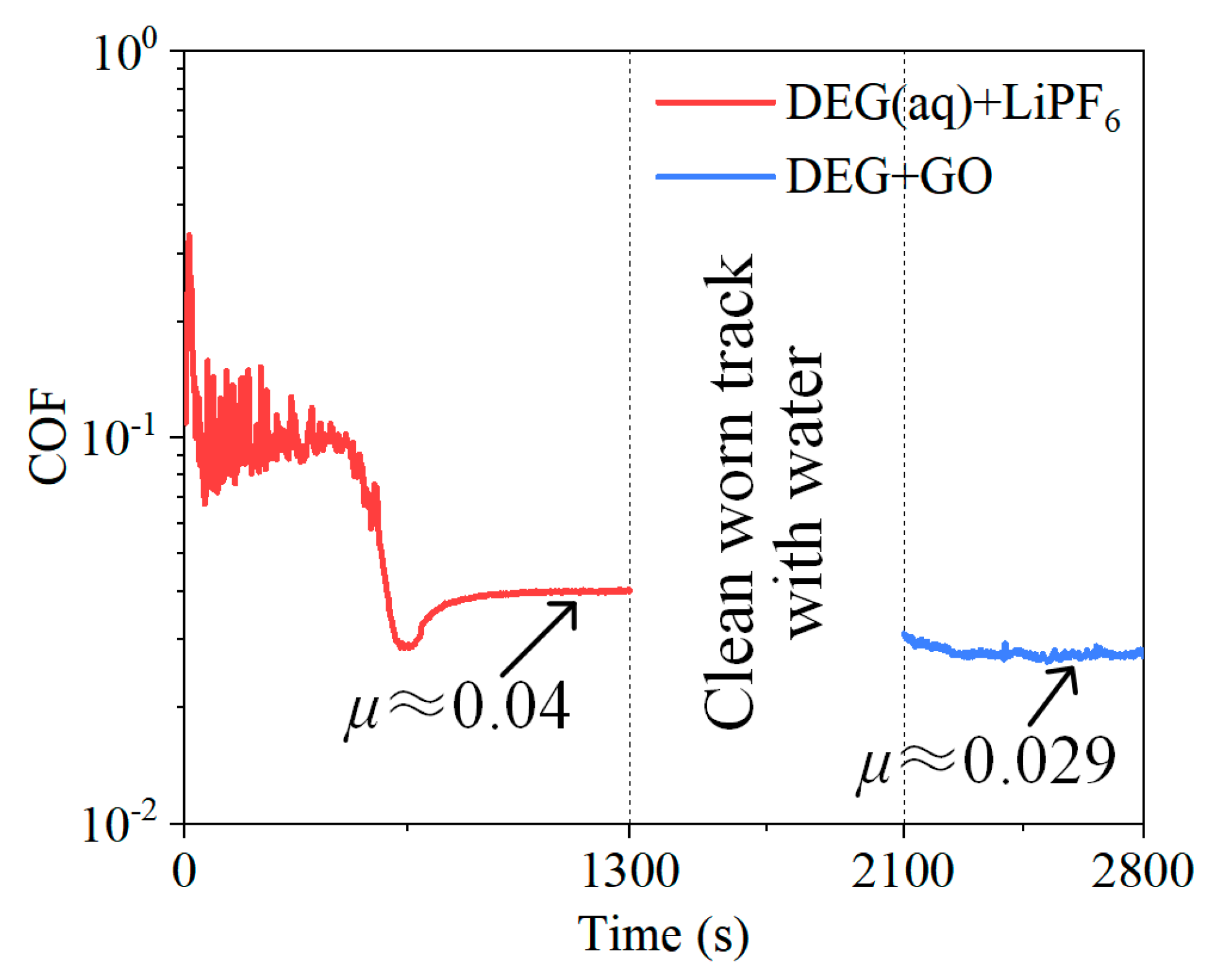
3.2. Tribological Experiments
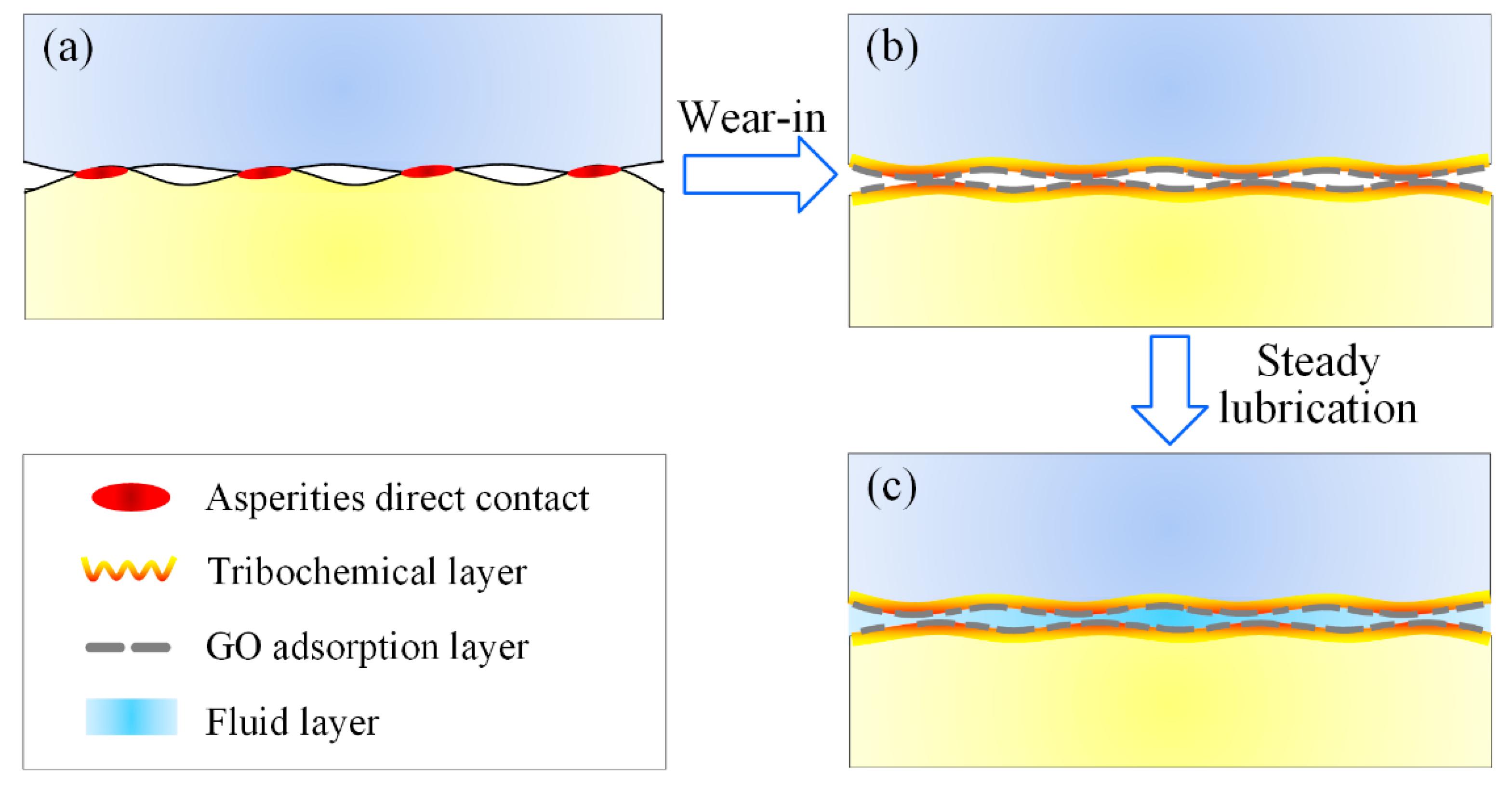
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.B.; Liu, M.; Ma, L.R. Origin of friction and the new frictionless technology—Superlubricity: Advancements and future outlook. Nano Energy 2021, 86, 106092. [Google Scholar] [CrossRef]
- Fan, M.J.; Liang, Y.M.; Zhou, F.; Liu, W. Dramatically improved friction reduction and wear resistance by in situ formed ionic liquids. RSC Adv. 2012, 2, 6824–6830. [Google Scholar] [CrossRef]
- Hirano, M.; Shinjo, K. Atomic locking and friction. Phys. Rev. B 1990, 41, 11837–11851. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, A.; Martin, J.M.; Luo, J.B. Superlubricity, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Yu, G.M.; Gong, Z.B.; Jiang, B.; Wang, D.; Bai, C.; Zhang, J. Superlubricity for hydrogenated diamond like carbon induced by thin MoS2 and DLC layer in moist air. Diam. Relat. Mater. 2020, 102, 107668. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Xu, Q.; Zhang, J.; Hu, Y.; Ma, T.; Zheng, Q.; Luo, J. Superlubricity between graphite layers in ultrahigh vacuum. ACS Appl. Mater. Interfaces 2020, 12, 43167–43172. [Google Scholar] [CrossRef]
- Gao, K.X.; Lai, Z.G.; Jia, Q.; Zhang, B.; Wei, X.; Zhang, J. Bilayer a−C:H/MoS2 film to realize superlubricity in open atmosphere. Diam. Relat. Mater. 2020, 108, 107973. [Google Scholar] [CrossRef]
- Li, P.; Ju, P.; Ji, L.; Li, H.; Liu, X.; Chen, L.; Zhou, H.; Chen, J. Toward robust macroscale superlubricity on engineering steel substrate. Adv. Mater. 2020, 32, 2002039. [Google Scholar] [CrossRef]
- Tomizawa, H.; Fischer, T.E. Friction and wear of silicon nitride and silicon carbide in water: Hydrodynamic lubrication at low sliding speed obtained by tribochemical wear. ASLE Trans. 1986, 30, 41–46. [Google Scholar] [CrossRef]
- Li, J.J.; Zhang, C.H.; Luo, J.B. Superlubricity behavior with phosphoric acid−water network induced by rubbing. Langmuir 2011, 27, 9413–9417. [Google Scholar] [CrossRef]
- Han, T.Y.; Zhang, C.H.; Luo, J.B. Macroscale superlubricity enabled by hydrated alkali metal ions. Langmuir 2018, 34, 11281–11291. [Google Scholar] [CrossRef]
- Ge, X.Y.; Li, J.J.; Zhang, C.; Liu, Y.; Luo, J. Superlubricity and antiwear properties of in situ formed ionic liquids at ceramic interfaces induced by tribochemical reactions. ACS Appl. Mater. Interfaces 2019, 11, 6568–6574. [Google Scholar] [CrossRef] [PubMed]
- Arad, S.; Rapoport, L.; Moshkovich, A.; van Moppes, D.; Karpasas, M.; Golan, R.; Golan, Y. Superior biolubricant from a species of red microalga. Langmuir 2006, 22, 7313–7317. [Google Scholar] [CrossRef] [PubMed]
- Røn, T.; Javakhishvili, I.; Hvilsted, S.; Jankova, K.; Lee, S. Ultralow friction with hydrophilic polymer brushes in water as segregated from silicone matrix. Adv. Mater. Interfaces 2016, 3, 1500472. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.Y.; Halmans, T.; Li, J.; Luo, J. Molecular behaviors in thin film lubrication—Part three: Superlubricity attained by polar and nonpolar molecules. Friction 2019, 7, 625–636. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, Y.; Li, J.; Luo, J. Investigation of superlubricity achieved by polyalkylene glycol aqueous solutions. Adv. Mater. Interfaces 2016, 3, 1600531. [Google Scholar] [CrossRef]
- Zeng, Q.F.; Yu, F.; Dong, G.N. Superlubricity behaviors of Si3N4/DLC films under PAO oil with nano boron nitride additive lubrication. Surf. Interface Anal. 2013, 45, 1283–1290. [Google Scholar] [CrossRef]
- Liu, Y.F.; Ge, X.Y.; Li, J.J. Graphene lubrication. Appl. Mater. Today 2020, 20, 100662. [Google Scholar] [CrossRef]
- Ge, X.Y.; Li, J.J.; Luo, R.; Zhang, C.; Luo, J. Macroscale superlubricity enabled by synergy effect of graphene-oxide nanoflakes and ethanediol. ACS Appl. Mater. Interfaces 2018, 10, 40863–40870. [Google Scholar] [CrossRef]
- Ge, X.Y.; Li, J.J.; Wang, H.; Zhang, C.; Liu, Y.; Luo, J. Macroscale superlubricity under extreme pressure enabled by the combination of graphene-oxide nanosheets with ionic liquid. Carbon 2019, 151, 76–83. [Google Scholar] [CrossRef]
- Yi, S.; Chen, X.C.; Li, J.J.; Liu, Y.; Ding, S.; Luo, J. Macroscale superlubricity of Si-doped diamond-like carbon film enabled by graphene oxide as additives. Carbon 2021, 176, 358–366. [Google Scholar] [CrossRef]
- Ederer, J.; Janoš, P.; Ecorchard, P.; Tolasz, J.; Štengl, V.; Beneš, H.; Perchacz, M.; Pop-Georgievski, O. Determination of amino groups on functionalized graphene oxide for polyurethane nanomaterials: XPS quantitation vs. functional speciation. RSC Adv. 2017, 7, 12464–12473. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.C.; Bai, H.W.; Wang, Y.J.; Liu, Z.; Zhang, X.; Sun, D.D. Self-assembling TiO2 nanorods on large graphene oxide sheets at a two-phase interface and their anti-recombination in photocatalytic applications. Adv. Funct. Mater. 2010, 20, 4175–4181. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Zhang, L.G.; Li, G.T.; Guo, Y.; Qi, H.; Zhang, G. Significantly enhancing tribological performance of epoxy by filling with ionic liquid functionalized graphene oxide. Carbon 2018, 136, 309–319. [Google Scholar] [CrossRef]
- Gao, X.; Chen, L.; Ji, L.; Liu, X.; Li, H.; Zhou, H.; Chen, J. Humidity-sensitive macroscopic lubrication behavior of an as-sprayed graphene oxide coating. Carbon 2018, 140, 124–130. [Google Scholar] [CrossRef]
- Xu, H.; Han, X.Y.; Dai, X.; Liu, W.; Wu, J.; Zhu, J.; Kim, D.; Zou, G.F.; Sablon, K.A.; Sergeev, A.; et al. High detectivity and transparent few-layer MoS2/glassy-graphene heterostructure photodetectors. Adv. Mater. 2018, 30, 1706561. [Google Scholar] [CrossRef]
- Arul, R.; Oosterbeek, R.N.; Robertson, J.; Xu, G.; Jin, J.; Simpson, M.C. The mechanism of direct laser writing of graphene features into graphene oxide films involves photoreduction and thermally assisted structural rearrangement. Carbon 2016, 99, 423–431. [Google Scholar] [CrossRef]
- Nasybulin, E.; Xu, W.; Engelhard, M.H.; Nie, Z.; Burton, S.D.; Cosimbescu, L.; Gross, M.E.; Zhang, J.-G. Effects of electrolyte salts on the performance of Li–O2 batteries. J. Phys. Chem. C 2013, 117, 2635–2645. [Google Scholar] [CrossRef]
- Dedryvère, R.; Laruelle, S.; Grugeon, S.; Gireaud, L.; Tarascon, J.M.; Gonbeau, D. XPS identification of the organic and inorganic components of the electrode/electrolyte interface formed on a metallic cathode. J. Electrochem. Soc. 2005, 152, 689–696. [Google Scholar] [CrossRef]
- Khan, T.; Koide, S.; Tamura, Y.; Yamamoto, H.; Morina, A.; Neville, A. Effects of using alternative extreme pressure (EP) and anti-wear (AW) additives with oxy−nitrided samples. Tribol. Lett. 2018, 66, 43. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Kato, K.; Hirayama, T. The transition of wear mode during the running-in process of silicon nitride sliding in water. Wear 1997, 205, 55–63. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, X.; Chai, Z.; Shi, Q.; Liu, Y.; Tang, J.; Wang, W. Liquid Superlubricity Enabled by the Synergy Effect of Graphene Oxide and Lithium Salts. Materials 2022, 15, 3546. https://doi.org/10.3390/ma15103546
Ge X, Chai Z, Shi Q, Liu Y, Tang J, Wang W. Liquid Superlubricity Enabled by the Synergy Effect of Graphene Oxide and Lithium Salts. Materials. 2022; 15(10):3546. https://doi.org/10.3390/ma15103546
Chicago/Turabian StyleGe, Xiangyu, Zhiyuan Chai, Qiuyu Shi, Yanfei Liu, Jiawei Tang, and Wenzhong Wang. 2022. "Liquid Superlubricity Enabled by the Synergy Effect of Graphene Oxide and Lithium Salts" Materials 15, no. 10: 3546. https://doi.org/10.3390/ma15103546
APA StyleGe, X., Chai, Z., Shi, Q., Liu, Y., Tang, J., & Wang, W. (2022). Liquid Superlubricity Enabled by the Synergy Effect of Graphene Oxide and Lithium Salts. Materials, 15(10), 3546. https://doi.org/10.3390/ma15103546







