The Microstructural Evolution and Grain Growth Kinetics of Fine-Grained Extruded Mg-Nd-Zn-Zr Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Alloy Preparation
2.2. Microstructure Analysis
2.3. Grain Growth Kinetics
3. Results and Discussion
3.1. The Initial Microstructure
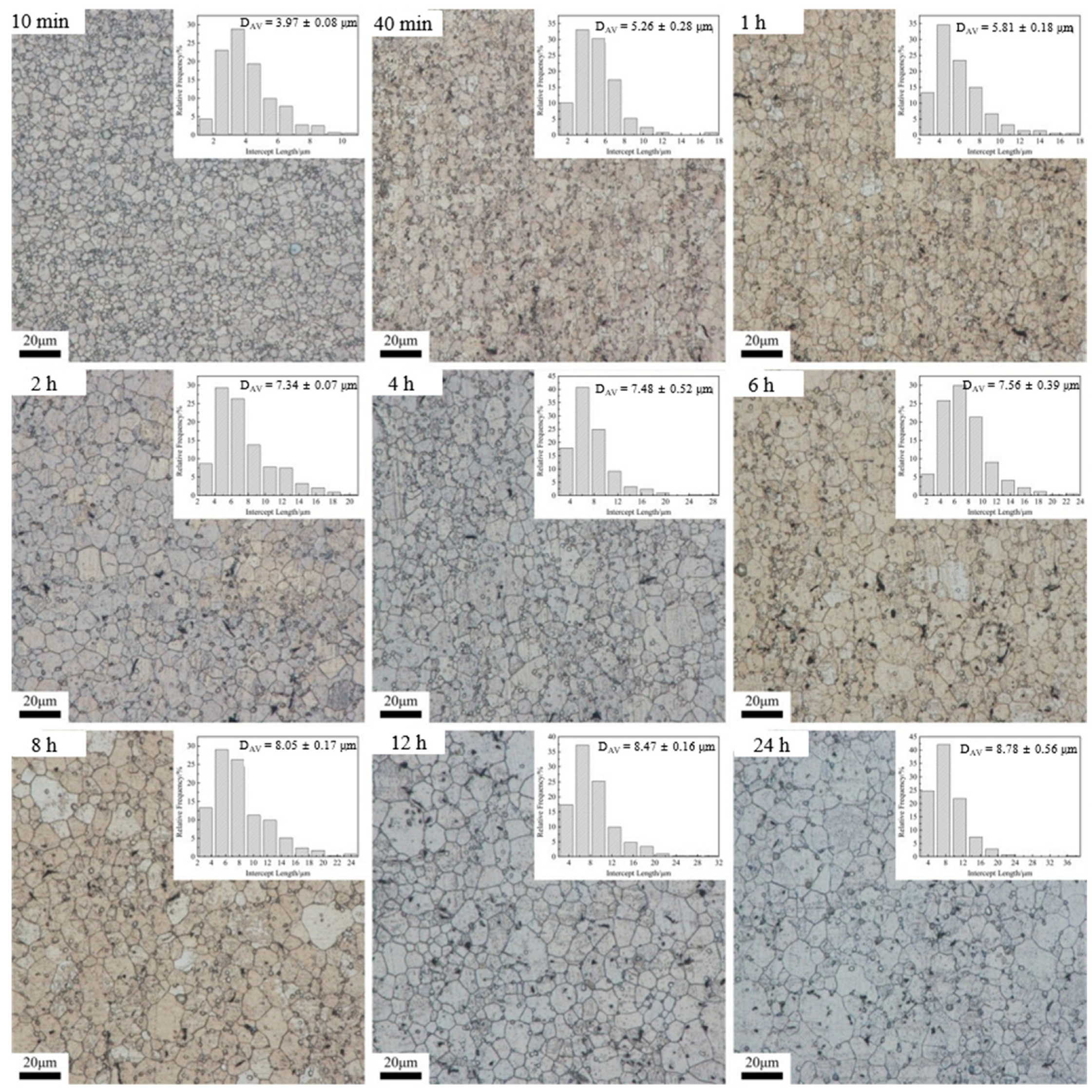
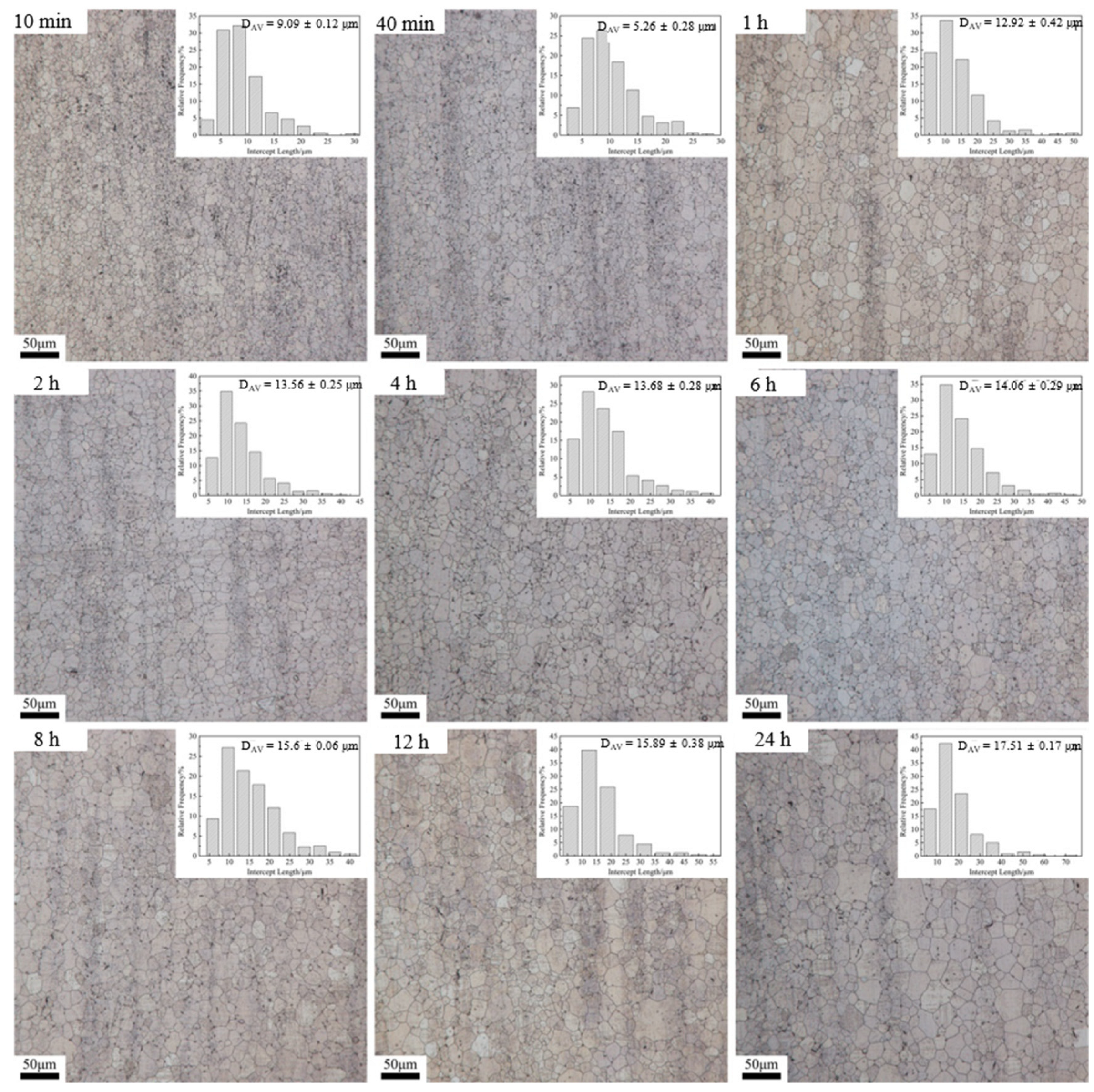
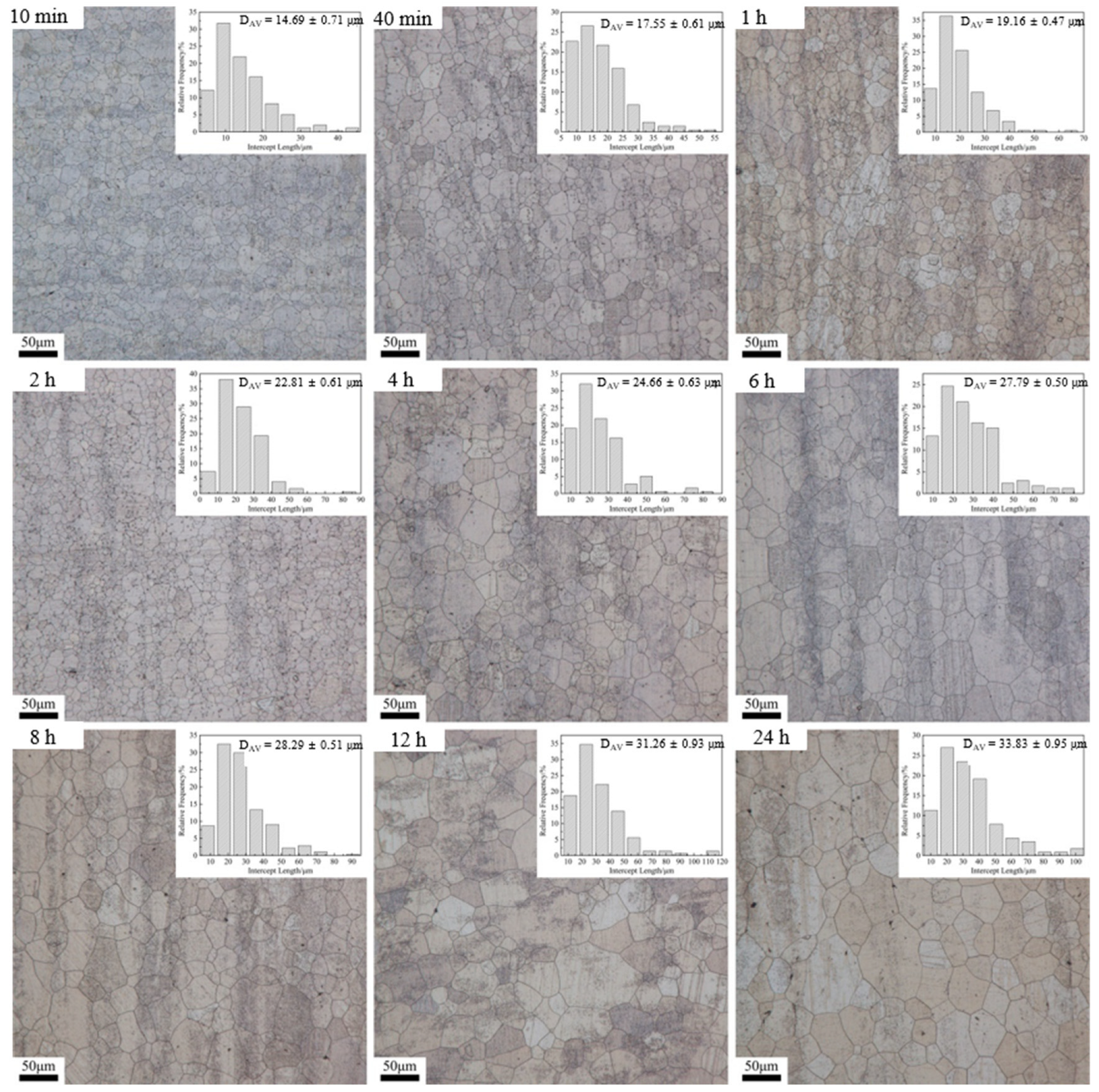
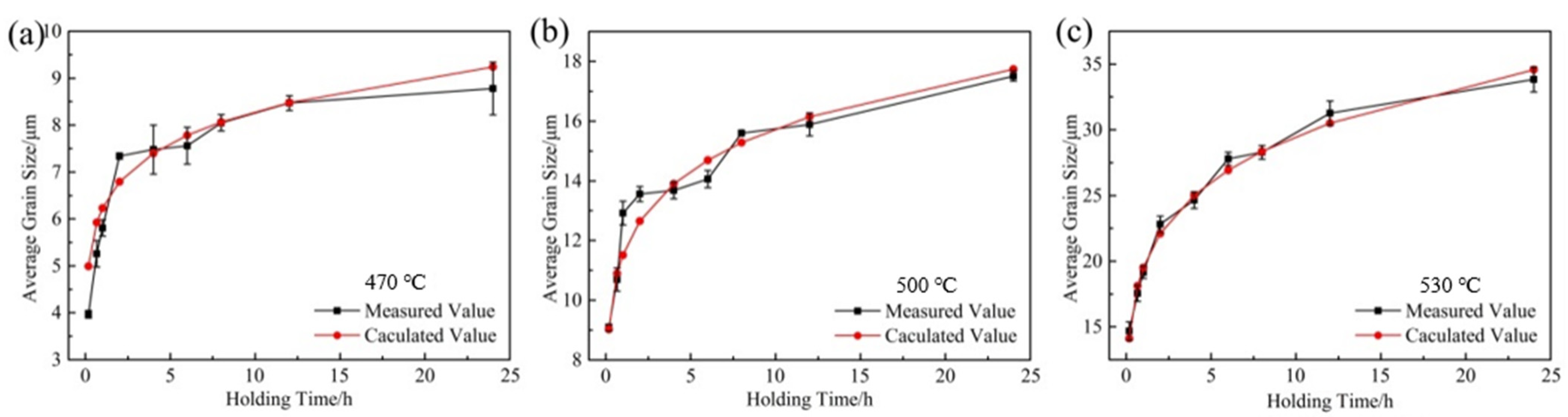
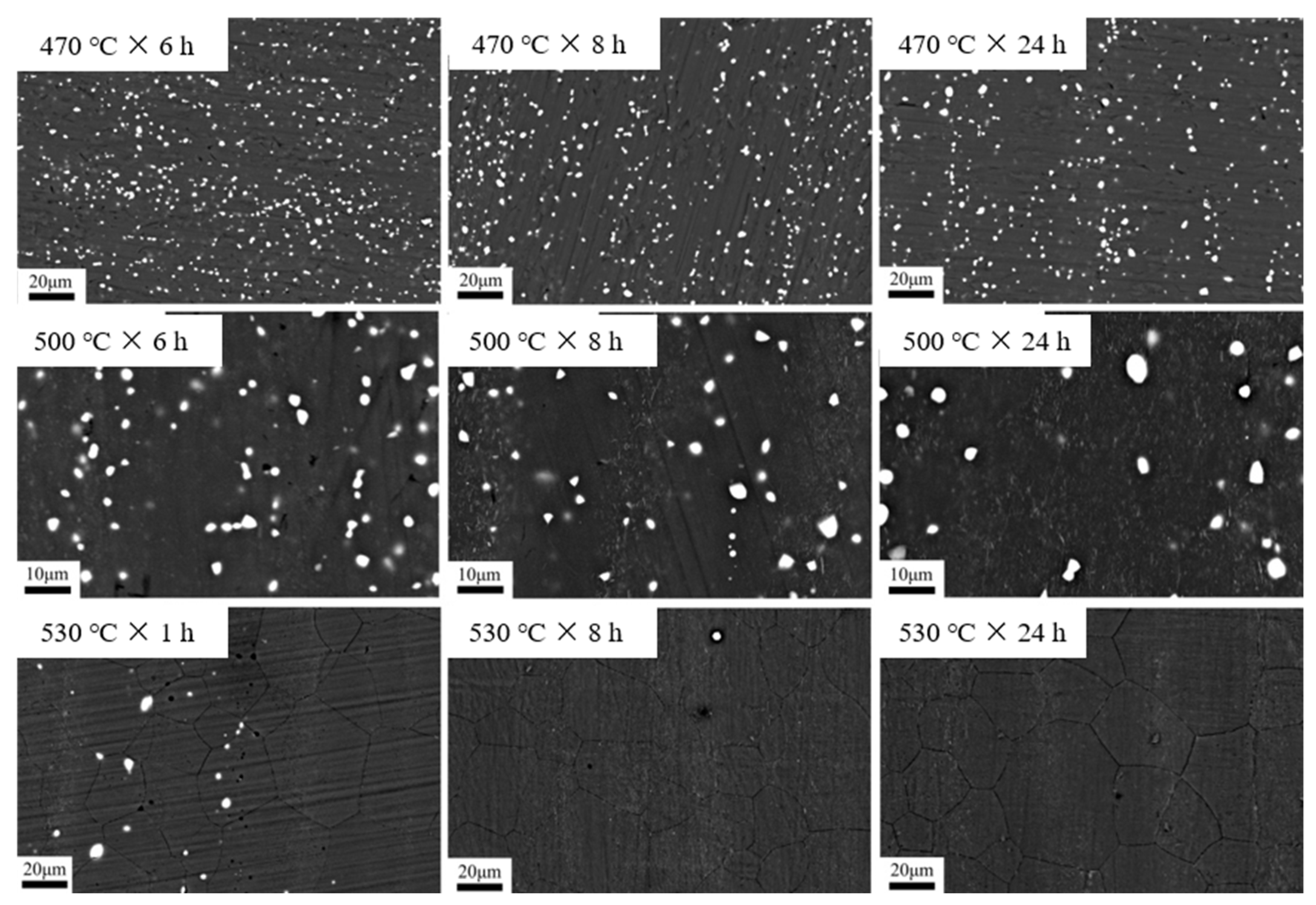
3.2. Dependence of Microstructure on Heat Treatment Condition
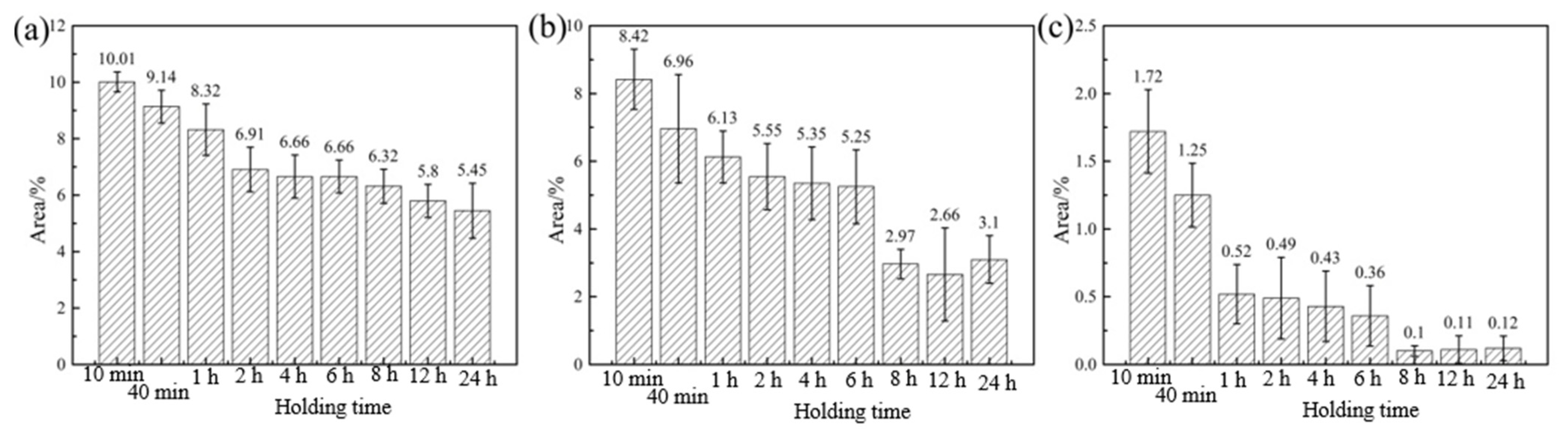
3.3. Dependence of Phase Content on Heat Treatment Regime
3.4. Phase Transition
4. Discussion
4.1. The Abnormal Grain Growth
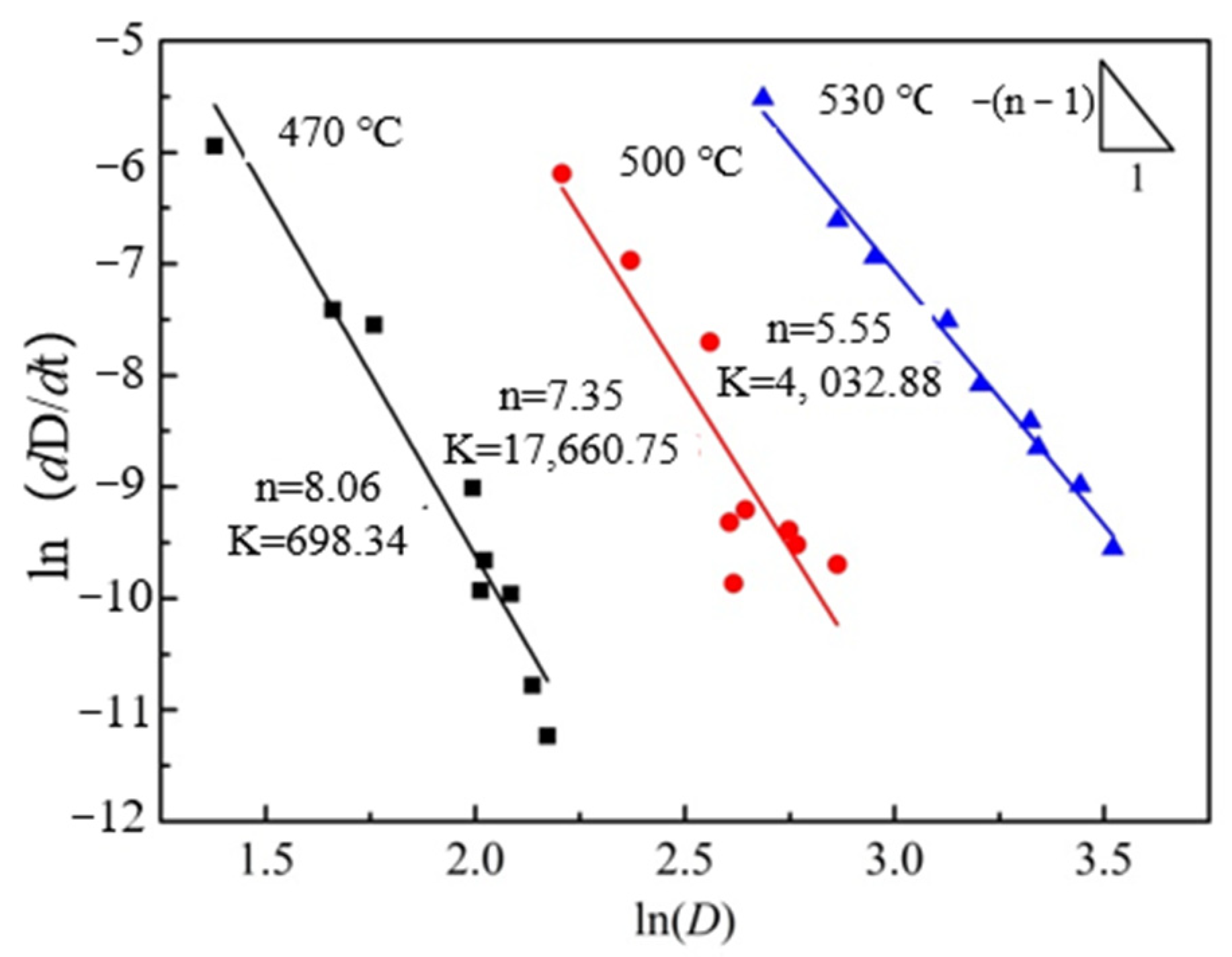
4.2. Analysis of Grain Growth Kinetics
4.3. Dissolution Sequence of the Second Phase
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurya, R.; Mittal, D.; Balani, K. Effect of heat-treatment on microstructure, mechanical and tribological properties of Mg-Li-Al based alloy. J. Mater. Res. Technol. 2020, 9, 4749–4762. [Google Scholar] [CrossRef]
- Chowdary, S.; Dumpala, R.; Kondaiah, V.V. Influence of heat treatment on the machinability and corrosion behavior of AZ91 Mg alloy. J. Magnes. Alloys 2018, 6, 52–58. [Google Scholar] [CrossRef]
- Ma, L.; Mishra, R.K.; Peng, L.; Luo, A.A.; Ding, W.; Sachdev, A.K. Texture and mechanical behavior evolution of age-hardenable Mg-Nd-Zn extrusions during aging treatment. Mater. Sci. Eng. A 2011, 529, 151–155. [Google Scholar] [CrossRef]
- Ashrafizadeh, S.M.; Mahmudi, R. Effects of Gd, Y, and La rare-earth elements on the microstructural stability and elevated-temperature mechanical properties of AZ81 magnesium alloy. Metall. Mater. Trans. 2019, 50, 5957–5968. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Yan, H.; Chen, R.S.; Han, E.H. The preferential growth and related textural evolution during static recrystallization in a cold-rolled Mg-Zn-Gd alloy. J. Magnes. Alloys 2020, 9, 818–828. [Google Scholar] [CrossRef]
- Hoseini-Athar, M.M.; Mahmudi, R.; Babu, R.P.; Hedström, P. Microstructure, texture, and strain-hardening behavior of extruded Mg-Gd-Zn alloys. Mater. Sci. Eng. A 2019, 772, 138833. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, E.; Cao, G.; Wang, L.; Lun, Y.; Feng, Y. Microstructure and mechanical properties of Mg-Nd-Zn-Zr alloy processed by integrated extrusion and equal channel angular pressing. J. Alloys Compd. 2017, 705, 118–125. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, X.; Liu, J.; Zhang, R.; Wang, X.; Yuan, Y.; Li, Z.; Han, Z. The microstructure, fracture mechanism and their correlation with the mechanical properties of as-cast Mg-Nd-Zn-Zr alloy under the effect of cooling rate. Mater. Sci. Eng. A 2021, 801, 140382. [Google Scholar] [CrossRef]
- Caceres, C.H.; Davidson, C.J.; Griffiths, J.R.; Newton, C.L. Effects of solidification rate and ageing on the microstructure and mechanical properties of AZ91 alloy. Mater. Sci. Eng. A 2002, 325, 344–355. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, G.; Niu, J.; Fu, P.; Ding, W. Microstructure, mechanical properties, biocorrosion behavior, and cytotoxicity of as-extruded Mg-Nd-Zn-Zr alloy with different extrusion ratios. J. Mech. Behav. Biomed. Mater. 2012, 9, 153–162. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, W.; Wang, H.; Yang, J.; Chen, W.; Cui, G.; Wang, G. Microstructures evolution, texture characteristics and mechanical properties of Mg-2.5Nd-0.5Zn-0.5Zr alloy during the high strain rate hot-rolling. Mater. Sci. Eng. A 2020, 803, 140488. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Yang, J.; Zhang, W.; Sun, Y.; Pan, J.; Chen, W. Improving the ductility of Mg-2.5Nd-0.5Zn-0.5Zr alloy by multi-pass hot rolling. J. Mater. Res. Technol. 2021, 14, 2124–2130. [Google Scholar] [CrossRef]
- Doiphode, R.L.; Murty, S.N.; Prabhu, N.; Kashyap, B.P. Grain growth in calibre rolled Mg-3Al-1Zn alloy and its effect on hardness. J. Magnes. Alloys 2015, 3, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Luo, Q.; Qin, E.W. Influencing factors of abnormal grain growth in Mg alloy by phase field method. Mater. Today Commun. 2019, 22, 100790. [Google Scholar] [CrossRef]
- Jin, Z.; Yu, D.; Wu, X.; Yin, K.; Yan, K. Drag effects of solute and second phase distributions on the grain growth kinetics of pre-extruded Mg-6Zn alloy. J. Mater. Sci. Technol. 2016, 12, 52–58. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Guo, Z. Static recrystallization and grain growth during annealing of an extruded Mg-Zn-Zr-Er magnesium alloy. J. Magnes. Alloys 2013, 1, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Sellars, C.M.; Whiteman, J.A. Recrystallization and grain growth in hot rolling. Met. Sci. J. 1978, 13, 187–194. [Google Scholar] [CrossRef]
- Wang, X.; Hu, L.; Liu, K.; Zhang, Y. Grain growth kinetics of bulk AZ31 magnesium alloy by hot pressing. J. Alloys Compd. 2012, 527, 193–196. [Google Scholar] [CrossRef]
- Chang, J.W.; Guo, X.W.; Fu, P.H.; Peng, L.M.; Ding, W.J. Effect of heat treatment on corrosion and electrochemical behavior of Mg-3Nd-0.2Zn-0.4Zr (wt.%) alloy. Electrochim. Acta 2007, 52, 3160–3167. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, E.; Wang, L.; Wu, T.; Feng, Y. Effect of pre-compressive strain on microstructure and mechanical properties of Mg-2.7Nd-0.4Zn-0.5Zr alloy. Mater. Sci. Eng. A 2015, 647, 28–33. [Google Scholar] [CrossRef]
- Rios, P.R. Abnormal grain growth development from uniform grain size distributions. Acta Mater. 1997, 45, 1785–1789. [Google Scholar] [CrossRef]
- Novikov, V.Y. Microstructure evolution during grain growth in materials with disperse particles. Mater. Lett. 2012, 68, 413–415. [Google Scholar] [CrossRef]
- Roostaei, M.; Shirdel, M.; Parsa, M.H.; Mahmudi, R.; Mirzadeh, H. Microstructural evolution and grain growth kinetics of GZ31 magnesium alloy. Mater. Charact. 2016, 118, 584–592. [Google Scholar] [CrossRef]
- Shirdel, M.; Mirzadehx, M.; Parsa, M.H. Microstructural evolution during normal/abnormal grain growth in austenitic stainless steel. Metall. Mater. Trans. A 2014, 45, 5185–5193. [Google Scholar] [CrossRef]
- Na, S.M.; Atwater, K.M.; Flatau, A.B. Particle pinning force thresholds for promoting abnormal grain growth in magnetostrictive Fe-Ga alloy sheets. Scr. Mater. 2015, 100, 1–4. [Google Scholar] [CrossRef]
- Bednarczyk, W.; Kawałko, J.; Rutkowski, B.; Wątroba, M.; Gao, N.; Starink, M.J.; Bała, P.; Langdon, T.G. Abnormal grain growth in a Zn-0.8Ag alloy after processing by high-pressure torsion. Acta Mater. 2021, 207, 116667. [Google Scholar] [CrossRef]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena || Grain Growth Following Recrystallization; Elsevier: Amsterdam, The Netherlands, 2004; pp. 333–378. [Google Scholar]
- Burke, J.E.; Turnbull, D. Recrystallization and grain growth. Prog. Met. Phys. 1952, 3, 220–292. [Google Scholar] [CrossRef]
- Bhattacharyya, J.J.; Agnew, S.R.; Muralidharan, G. Texture enhancement during grain growth of magnesium alloy AZ31B. Acta Mater. 2015, 86, 80–94. [Google Scholar] [CrossRef] [Green Version]
- Hoseini-Athar, M.M.; Mahmudi, R.; Babu, R.P.; Hedström, P. Effect of Zn content on the microstructural stability and grain growth kinetics of fine-grained extruded Mg-Gd-Zn alloys. J. Alloys Compd. 2020, 831, 154766. [Google Scholar] [CrossRef]
- Azzeddine, H.; Bradai, D. On the texture and grain growth in hot-deformed and annealed WE54 alloy. Int. J. Mater. Res. 2012, 1103, 1351–1360. [Google Scholar] [CrossRef]
- Miao, Q.; Hu, L.; Wang, X.; Wang, E. Grain growth kinetics of a fine-grained AZ31 magnesium alloy produced by hot rolling. J. Alloys Compd. 2010, 493, 87–90. [Google Scholar] [CrossRef]
- Thein, M.A.; Lu, L.; Lai, M.O. Kinetics of grain growth in nanocrystalline magnesium-based metal-metal composite synthesized by mechanical alloying. Compos. Sci. Technol. 2006, 66, 531–537. [Google Scholar] [CrossRef]
- Natarajan, A.R.; Solomon, E.L.; Puchala, B.; Marquis, E.A.; Van der Ven, A. On the early stages of precipitation in dilute Mg-Nd alloys. Acta Mater. 2016, 108, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Kaichi, S.; Kenji, H. The structures of precipitates in an Mg-0.5at%Nd age-hardened alloy studied by HAADF-STEM technique. Mater. Trans. 2011, 52, 1860–1867. [Google Scholar] [CrossRef] [Green Version]
- Nie, J. Precipitation and hardening in magnesium alloys. Metall. Mater. Trans. A 2012, 46, 3891–3939. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Mishra, R.K.; Balogh, M.P.; Peng, L.; Luo, A.A.; Sachdev, A.K.; Ding, W. Effect of Zn on the microstructure evolution of extruded Mg-3Nd (-Zn)-Zr (wt.%) alloys. Mater. Sci. Eng. A 2012, 543, 12–21. [Google Scholar] [CrossRef]
- Sanaty-Zadeh, A.; Luob, A.A.; Stone, D.S. Comprehensive study of phase transformation in age-hardening of Mg-3Nd-0.2Zn by means of scanning transmission electron microscopy. Acta Mater. 2015, 94, 294–306. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.J.; Liao, H.H.; Ding, W.J.; Peng, L.M.; Fu, P.H. Chemical composition optimization of gravity cast Mg-yNd-xZn-Zr alloy. Mater. Sci. Eng. A 2008, 496, 177–188. [Google Scholar] [CrossRef]
- Liang, M.J.; Liao, H.H.; Ding, W.J.; Peng, L.M.; Fu, P.H. Microstructure characterization on Mg-2Nd-4Zn-1Zr alloy duringheat treatment. Trans. Nonferrous Met. Soc. China 2012, 22, 2327–2333. [Google Scholar] [CrossRef]
- Nuttall, P.A.; Pike, T.J.; Noble, B. Metallography of dilute Mg-Nd-Zn alloys. Metallography 1980, 13, 3–20. [Google Scholar] [CrossRef]
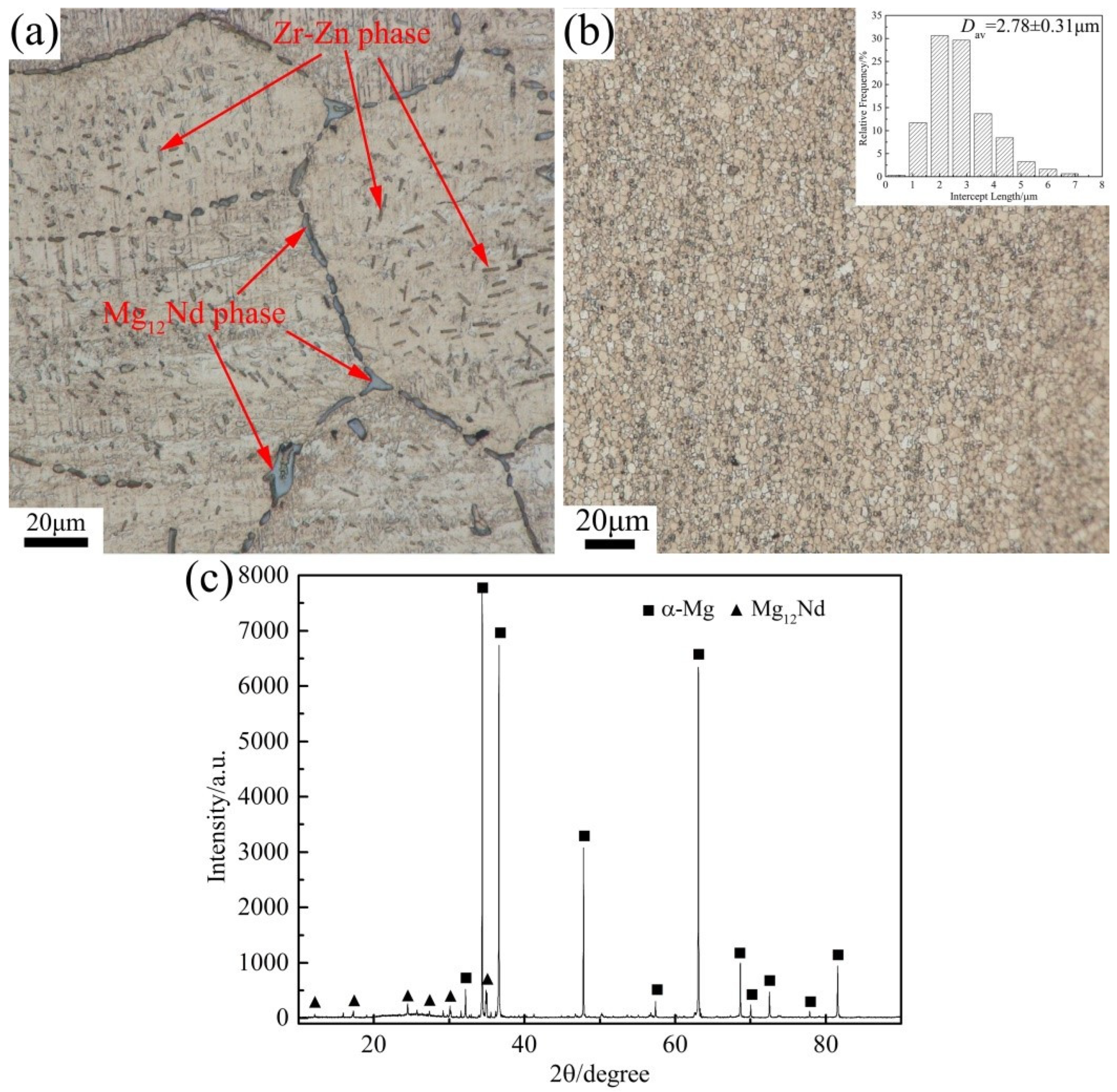


| Element | Nd | Zn | Zr | Mg |
|---|---|---|---|---|
| Composition | 2.58 | 0.54 | 0.54 | Balance |
| Area | Element Content (at.%) | Area | Element Content (at.%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mg | Nd | Zn | Zr | Mg | Nd | Zn | Zr | ||
| A | 99.7 | 0.3 | 0 | 0 | M | 99.36 | 0.28 | 0 | 0.37 |
| B | 97.93 | 1.43 | 0.64 | 0 | N | 98.42 | 1.58 | 0 | 0 |
| C | 99.46 | 0.54 | 0 | 0 | O | 96.12 | 3.88 | 0 | 0 |
| D | 97.51 | 1.96 | 0.53 | 0 | P | 98.78 | 0.95 | 0 | 0.27 |
| E | 99.62 | 0.38 | 0 | 0 | Q | 97.02 | 2.98 | 0 | 0 |
| F | 94.28 | 4.98 | 0.75 | 0 | R | 100 | 0 | 0 | 0 |
| G | 96.67 | 2.66 | 0.67 | 0 | S | 99.73 | 0.27 | 0 | 0 |
| H | 98.62 | 0.4 | 0.38 | 0.6 | U | 99.1 | 0.65 | 0.25 | 0 |
| I | 95.94 | 3.33 | 0.73 | 0 | V | 99.78 | 0.22 | 0 | 0 |
| J | 91.7 | 7.07 | 1.23 | 0 | W | 99.76 | 0.24 | 0 | 0 |
| K | 99.58 | 0.42 | 0 | 0 | X | 99.62 | 0.38 | 0 | 0 |
| L | 96.43 | 2.81 | 0.76 | 0 | Y | 97.56 | 1.86 | 0.59 | 0 |
| Time | 470 °C | 500 °C | 530 °C | |||
|---|---|---|---|---|---|---|
| Dmax/μm | Dmax/Dav | Dmax/μm | Dmax/Dav | Dmax/μm | Dmax/Dav | |
| 10 min | 10.75 | 2.71 | 30.64 | 3.37 | 45.22 | 3.08 |
| 40 min | 13.34 | 2.54 | 36.67 | 3.43 | 55.18 | 3.14 |
| 1 h | 15.89 | 2.73 | 47.02 | 3.64 | 67.95 | 3.55 |
| 2 h | 20.49 | 2.79 | 48.83 | 3.60 | 83.56 | 3.66 |
| 4 h | 24.31 | 3.25 | 49.04 | 3.58 | 97.22 | 3.94 |
| 6 h | 23.39 | 3.09 | 48.3 | 3.44 | 95.11 | 3.42 |
| 8 h | 26.07 | 3.24 | 49.72 | 3.19 | 85.65 | 3.03 |
| 12 h | 28.97 | 3.42 | 65.02 | 4.09 | 118.78 | 3.80 |
| 24 h | 39.15 | 4.46 | 87.85 | 5.02 | 132.98 | 3.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, X.; Li, X.; Zhan, L.; Wang, G.; Ding, J.; Yang, J. The Microstructural Evolution and Grain Growth Kinetics of Fine-Grained Extruded Mg-Nd-Zn-Zr Alloy. Materials 2022, 15, 3556. https://doi.org/10.3390/ma15103556
Jiao X, Li X, Zhan L, Wang G, Ding J, Yang J. The Microstructural Evolution and Grain Growth Kinetics of Fine-Grained Extruded Mg-Nd-Zn-Zr Alloy. Materials. 2022; 15(10):3556. https://doi.org/10.3390/ma15103556
Chicago/Turabian StyleJiao, Xueyan, Xinjie Li, Liqiang Zhan, Gang Wang, Jin Ding, and Jianlei Yang. 2022. "The Microstructural Evolution and Grain Growth Kinetics of Fine-Grained Extruded Mg-Nd-Zn-Zr Alloy" Materials 15, no. 10: 3556. https://doi.org/10.3390/ma15103556





