Hydration Mechanisms of Alkali-Activated Cementitious Materials with Ternary Solid Waste Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
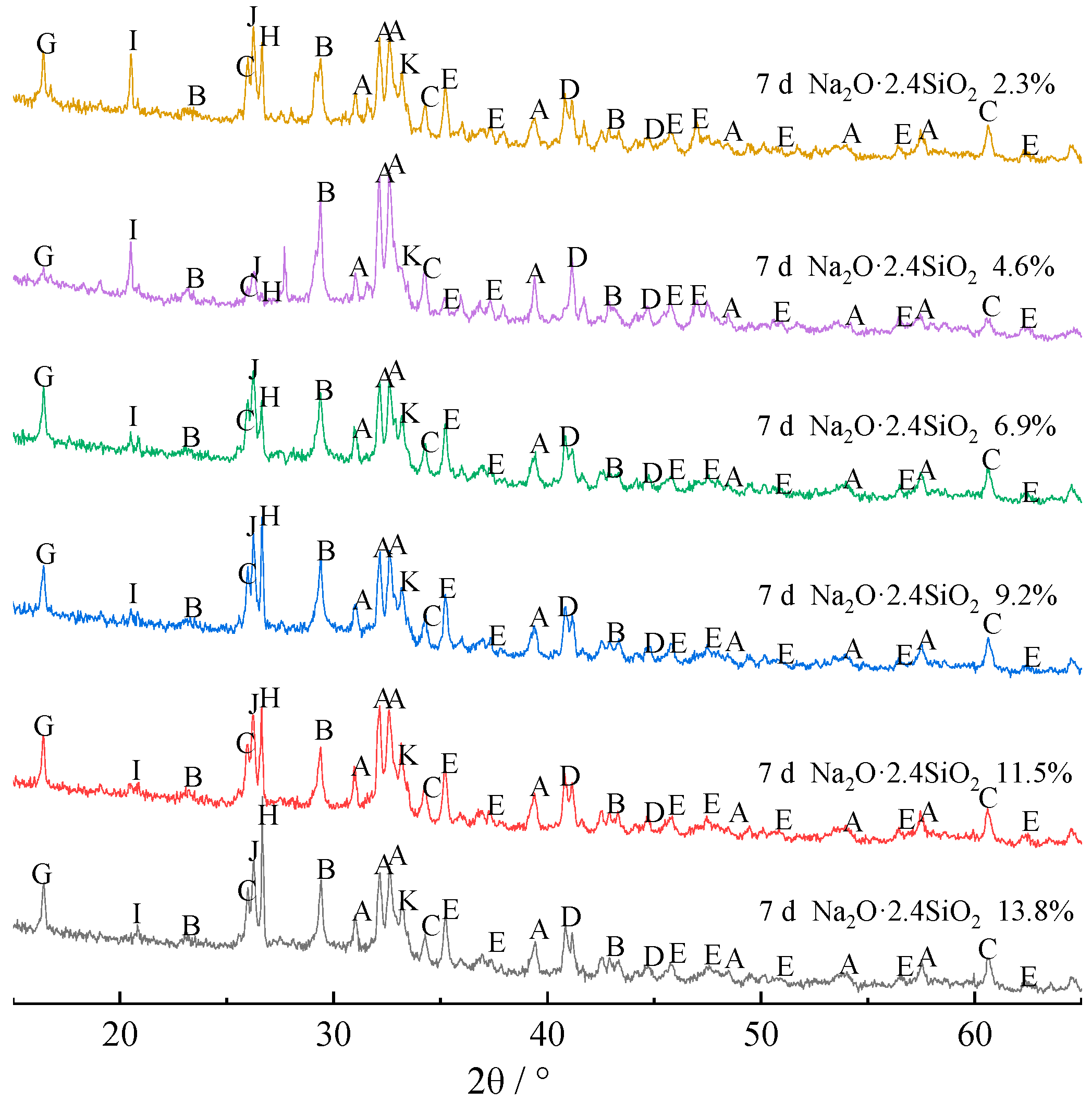
3.1. Phase Analysis
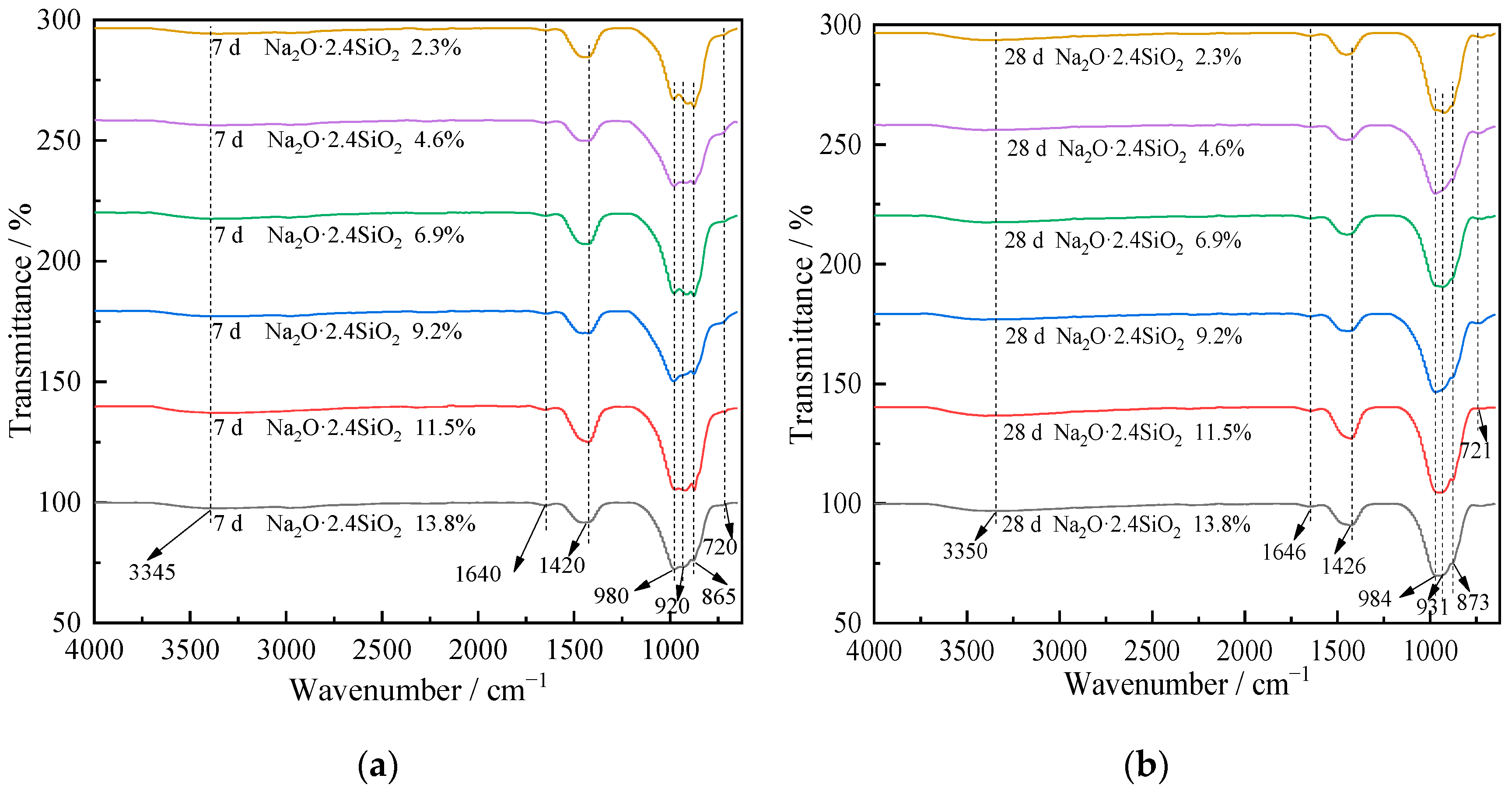
3.2. Chemical Structure Analysis
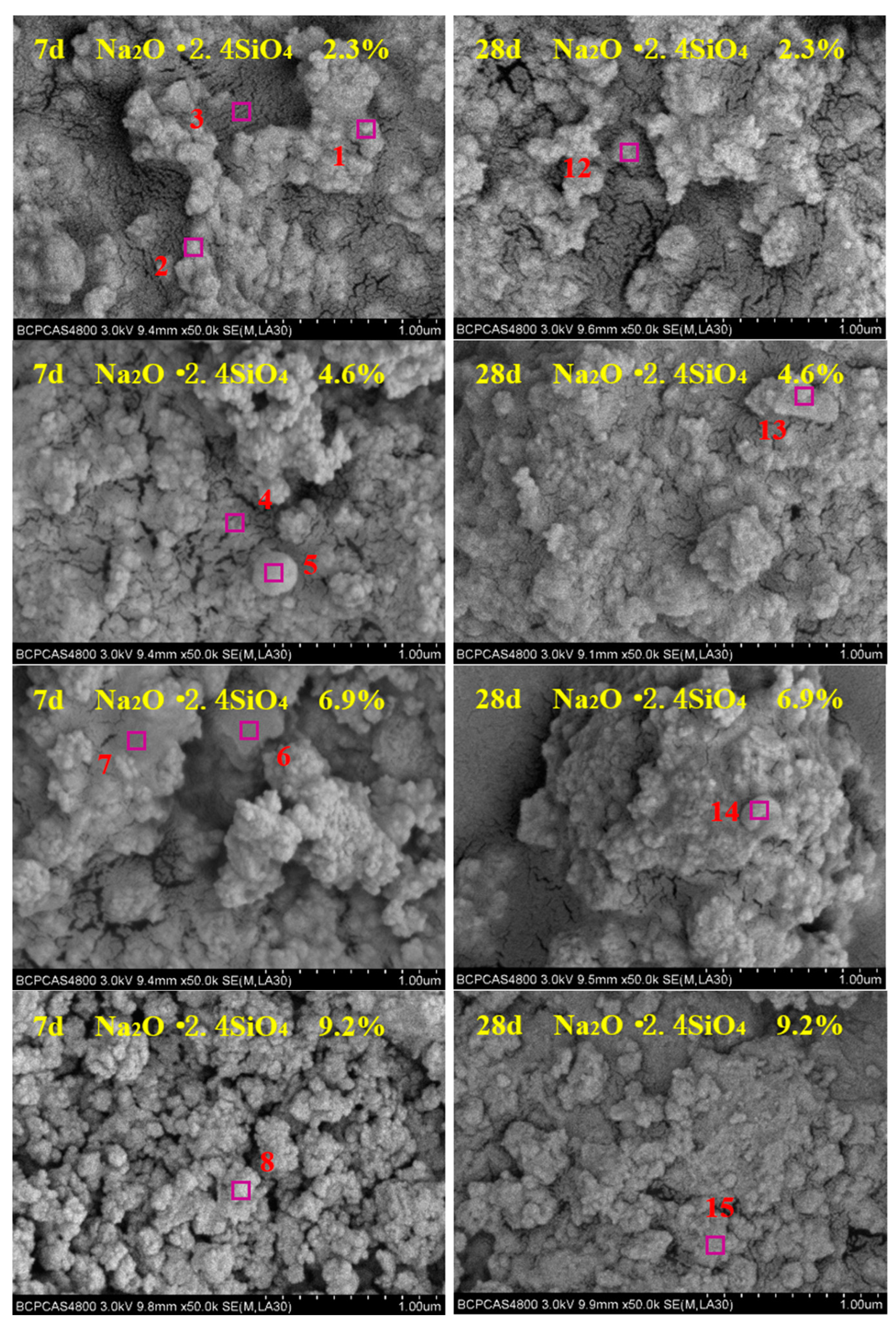
3.3. Micromorphology Analysis
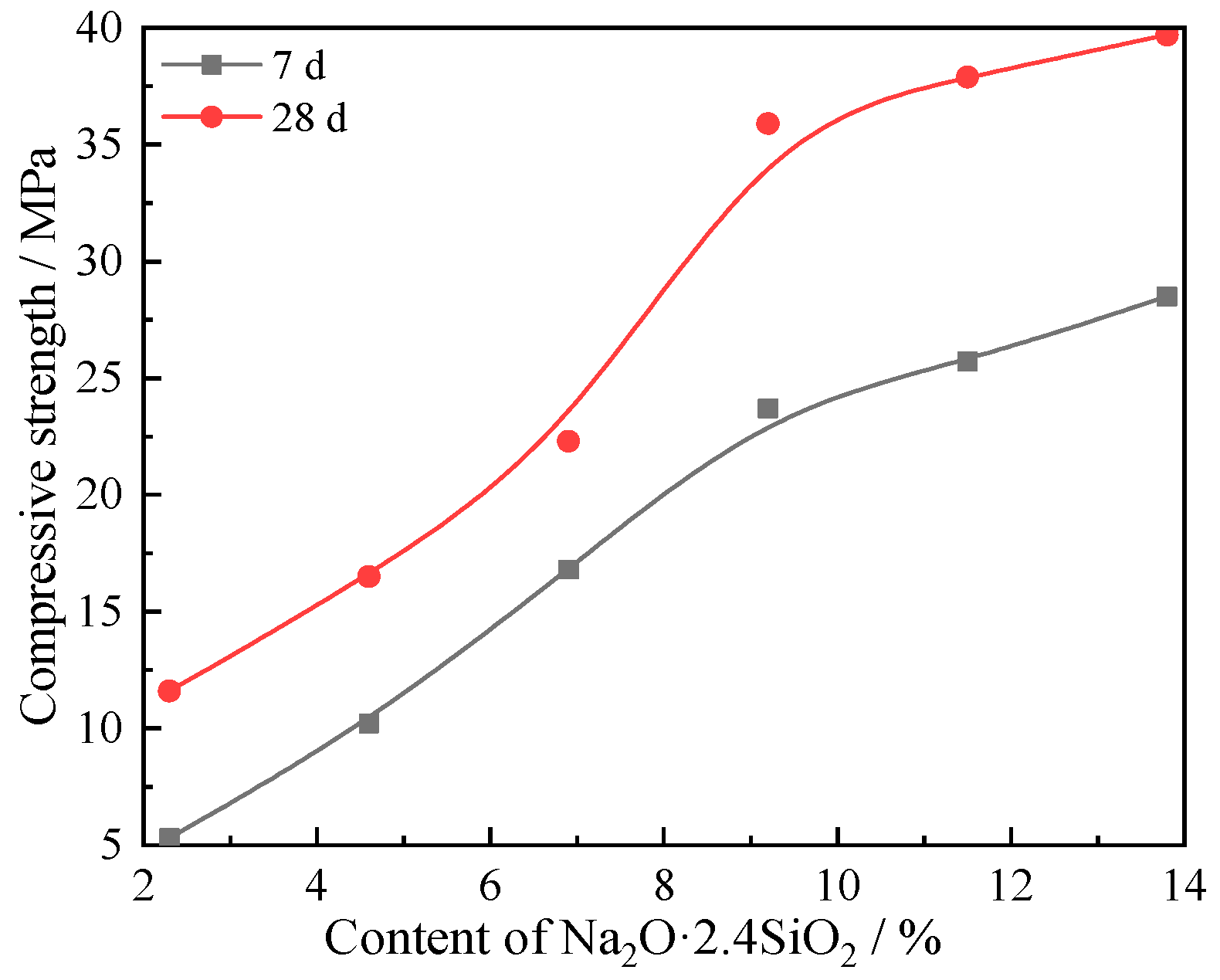
3.4. Macro Properties Analysis
4. Conclusions
- (1)
- From the hydration process, the alkali-activated cementitious composites contain Q0 and Q3 structural SiO4, in which Q3 structural SiO4 comes from the sodium silicate alkali-activator with a modulus of 2.4, while Q0 structural SiO4 comes from Si-O and Al-O bonds of FA and BFS, which are depolymerized under the polarization of OH− in a strong alkaline environment to form Q0 structural AlO4 and SiO4.
- (2)
- The alkali-activated cementitious material is a binary composite system composed of C(N)-A-S-H and C-S-H. The β-dicalcium silicate in CSS hydrates to form C-S-H and Ca(OH)2. There are three main reactions of Ca (OH)2. Part of Ca(OH)2 reacts with Q0 structural AlO4 and SiO4 to produce lawsonite and wairakite with a low polymerization degree of the Si-O and Al-O bonds, and part of Ca(OH)2 reacts with Q0 structural AlO4 and Q3 structural SiO4 with the participation of Na+ from the sodium silicate solution. A small part of Ca(OH)2 carbonizes with CO2 in the air to form calcite.
- (3)
- Due to the metastable state of glass phases, the glass phase is more likely than the crystalline phase to be modified during the hydration reaction. A large amount of mullite remained in hydrates with limited alkali-activated hydration reaction. However, with prolonged curing time, and when the sodium silicate content is more than 9.2%, the mullite and beidellite disappear.
- (4)
- With increasing sodium silicate content, the hydrate micromorphology at 7 and 28 days both become more uniform and dense, and the compressive strength increases, while the setting time decreases. When the content of sodium silicate is 9.2%, the macro properties of the composites effectively reach saturation. The compressive strength for composites with 9.2% sodium silicate was 23.7 and 35.9 MPa at 7 and 28 days, respectively, and the initial setting time and final setting time were 60 and 92 min, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prud, H.E.; Michaud, P.; Joussein, E. Silica fume as porogent agent in geo-materials at low temperature. Eur. Ceram. Soc. 2010, 30, 1641–1648. [Google Scholar]
- Wang, Y.; Wang, Y.; Zhang, M. Effect of sand content on engineering properties of fly ash-slag based strain hardening geopolymer composites. Build. Eng. 2021, 34, 21–25. [Google Scholar] [CrossRef]
- Khan, A.; Raut, A.; Chandrudu, C.R. Design and development of sustainable geopolymer using industrial copper byproduct. Clean. Prod. 2021, 278, 45–49. [Google Scholar] [CrossRef]
- Chalangaran, N.M.M. Jabbari. Experimental Investigation into Sound Transmission Loss through Concrete Containing Recycled Rubber. Va. Technol. 2019, 5, 20–35. [Google Scholar]
- Li, H.; Zhang, J.; Wang, C. Construct and research advance in clean and cyclic utilizations of associated resources in high-alumina coal fly ash. Clean Coal Technol. 2018, 24, 1–8. [Google Scholar]
- He, S.; Li, H.; Li, S. Kinetics of desilication process of fly ash with high aluminum from Kinetics of desilication process of fly ash with high aluminum from. Nonferrous Met. 2014, 24, 1888–1894. [Google Scholar]
- Yan, C.; Zhao, H.; Zhang, J. The cementitious composites using calcium silicate slag as partial cement. Clean. Prod. 2020, 256, 35–39. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, B.; Dang, H. Study on properties and application feasibility of alkali-activated red mud cementitious materials. Highw. Eng. 2021, 4, 1–9. [Google Scholar]
- Du, T.; Liu, Y.; Yu, Y. Influence of sodium silicate on fly ash slag geopolymer strength and stimulating mechanism. Highw. Transp. Res. Dev. 2021, 38, 41–49. [Google Scholar]
- Huang, L.; Ma, Q.; Guo, R. Experimental study on hydration products of alkali-activated slag. Bull. Chin. Ceram. Soc. 2020, 39, 1194–1200. [Google Scholar]
- Shi, D.; Ye, J.; Zhang, W. Effects of activator content on properties, mineralogy, hydration and microstructure of alkali-activated materials synthesized from calcium silicate slag and ground granulated blast furnace slag. Build. Eng. 2020, 32, 101791. [Google Scholar] [CrossRef]
- Lee, B.; Kim, G.; Kim, R. Strength development properties of geopolymer paste and mortar with respect to amorphous Si/Al ratio of fly ash. Constr. Build. Mater. 2017, 151, 512–519. [Google Scholar] [CrossRef]
- Farhan, N.A.; Sheikh, M.N.; Hadi, M.N.S. Investigation of engineering properties of normal and high strength fly ash based geopolymer and alkali-activated slag concrete compared to ordinary Portland cement concrete. Constr. Build. Mater. 2019, 196, 26–42. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Li, Z. Feasibility study of red mud-blast furnace slag based geopolymer grouting material: Effect of superplasticizers. Constr. Build. Mater. 2021, 267, 120910. [Google Scholar] [CrossRef]
- Bensted, J.; Barnes, P. Structure and Performance of Cements, 2nd ed.; Applied Science Publishers: London, UK, 1983; pp. 225–237. [Google Scholar]
- Shi, C.; Krivenko, P.V.; Della, R. Alkali-Activated Cements and Concretes; Taylor & Francis Group: London, UK, 2006; pp. 278–284. [Google Scholar]
- Cong, P.; Mei, L. Using silica fume for improvement of fly ash/slag based geopolymer activated with calcium carbide residue and gypsum. Constr. Build. Mater. 2021, 275, 56–58. [Google Scholar] [CrossRef]
- Nocun-Wczelik, W. Effect of Na and Al on the phase composition and morphology of autoclaved calcium silicate hydrates. Cem. Concr. Res. 1999, 29, 1759–1767. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Jiménez, A.F. Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Kumar, R.; Bhattacharjee, B. Porosity, pore size distribution and in situ strength of concrete. Cem. Concr. Res. 2003, 33, 155–164. [Google Scholar] [CrossRef]




| Constituents | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | Na2O | K2O | SO3 | P2O5 | F | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSS | 31.08 | 2.25 | 5.97 | 50.35 | 3.61 | 2.31 | 0.36 | 3.21 | 0.42 | 0.15 | 0.29 |
| BFS | 34.57 | 0.51 | 10.50 | 42.75 | 4.13 | 0.77 | 0.46 | 2.78 | 3.34 | 0.12 | 0.07 |
| FA | 42.67 | 2.57 | 42.36 | 4.30 | 3.20 | 0.58 | 0.39 | 1.27 | 1.46 | 0.47 | 0.73 |
| Serial Number | Proportion of Na2O2·4SiO2 (wt%) | Na2O2·4SiO2 (mL) | Distilled Water (mL) |
|---|---|---|---|
| 1 | 13.8 | 186 | 58 |
| 2 | 11.5 | 155 | 86 |
| 3 | 9.2 | 124 | 114 |
| 4 | 6.9 | 93 | 142 |
| 5 | 4.6 | 62 | 170 |
| 6 | 2.3 | 31 | 197 |
| Wavenumber/cm−1 | Chemical Group Vibration |
|---|---|
| 3450–3000 | Stretching vibration of the water molecule |
| 1650–1600 | Bending vibration of the water molecule |
| 1450–1400 | Asymmetric stretching vibration of CO32− |
| 975–965 | Asymmetric stretching vibration of C-S-H |
| 950–900 | Asymmetric stretching vibration of -Si-O-Si(Al)- |
| 890–850 | Bending vibration of -Si(Al)-OH |
| 730–710 | Bending vibration of -Si-O-Si(Al)- |
| Region | Micromorphology | Atomic Molar Ratio (at%) | Corresponding Phase | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | Al | Si | Ca | Na | Mg | Fe | C | |||
| 1 | granular-like | 55.31 | 2.21 | 14.22 | 26.31 | 0.50 | 1.22 | 0.23 | - | β-Dicalcium silicate |
| 2 | granular-like | 54.56 | 1.75 | 15.42 | 26.22 | 0.52 | 1.13 | 0.40 | - | β-Dicalcium silicate |
| 3 | worm-like | 68.97 | 14.61 | 14.36 | 0.22 | 1.63 | 0.10 | 0.11 | - | Beidellite |
| 4 | worm-like | 69.38 | 14.82 | 14.29 | 0.11 | 1.19 | 0.12 | 0.09 | - | Beidellite |
| 5 | spherical-like | 60.46 | 27.71 | 10.52 | - | 0.27 | - | 1.04 | - | Mullite |
| 6 | flake-like | 63.15 | 0.42 | 17.78 | 16.52 | - | 0.81 | 1.32 | - | C-S-H |
| 7 | flocculent-like | 70.21 | 5.02 | 20.64 | 2.03 | 2.10 | - | - | - | Boggsite |
| 8 | block-like | 59.03 | 0.22 | 0.10 | 20.22 | - | 0.10 | 0.10 | 20.23 | Calcite |
| 9 | flocculent-like | 70.15 | 5.41 | 20.21 | 2.13 | 2.10 | - | - | - | Boggsite |
| 10 | block-like | 58.56 | 0.33 | 0.21 | 20.32 | - | 0.11 | 0.33 | 20.14 | Calcite |
| 11 | strip-like | 63.23 | 5.01 | 21.01 | 5.23 | 5.21 | 0.11 | 0.20 | - | Tobermorite |
| 12 | flocculent-like | 70.41 | 5.21 | 20.15 | 2.13 | 2.10 | - | - | - | Boggsite |
| 13 | flake-like | 65.95 | 0.23 | 16.72 | 16.33 | 0.12 | 0.44 | 0.21 | - | C-S-H |
| 14 | spherical-like | 62.48 | 25.85 | 9.96 | - | 0.59 | 0.34 | 0.78 | - | Mullite |
| 15 | columnar-like | 66.05 | 0.05 | 33.63 | - | 0.27 | - | - | - | Quartz |
| 16 | layer-like | 56.08 | 12.50 | 25.05 | 6.20 | 0.05 | 0.11 | 0.01 | - | Wairakite |
| 17 | flake-like | 66.12 | 0.23 | 16.55 | 16.33 | 0.12 | 0.44 | 0.21 | - | C-S-H |
| 18 | strip-like | 62.68 | 5.97 | 19.52 | 5.86 | 5.44 | 0.32 | 0.21 | - | Tobermorite |
| 19 | strip-like | 62.57 | 5.68 | 19.84 | 5.96 | 5.42 | 0.32 | 0.21 | - | Tobermorite |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhang, D.; Fang, C.; Jiao, Y.; Kang, D.; Yan, C.; Zhang, J. Hydration Mechanisms of Alkali-Activated Cementitious Materials with Ternary Solid Waste Composition. Materials 2022, 15, 3616. https://doi.org/10.3390/ma15103616
Yang Z, Zhang D, Fang C, Jiao Y, Kang D, Yan C, Zhang J. Hydration Mechanisms of Alkali-Activated Cementitious Materials with Ternary Solid Waste Composition. Materials. 2022; 15(10):3616. https://doi.org/10.3390/ma15103616
Chicago/Turabian StyleYang, Zhijie, De Zhang, Chengyang Fang, Yang Jiao, Dong Kang, Changwang Yan, and Ju Zhang. 2022. "Hydration Mechanisms of Alkali-Activated Cementitious Materials with Ternary Solid Waste Composition" Materials 15, no. 10: 3616. https://doi.org/10.3390/ma15103616






