Role of Liquid-Phase Amount in Ceramization of Silicone Rubber Composites and Its Controlling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Sample Pyrolysis
2.4. Characterization
3. Results and Discussion
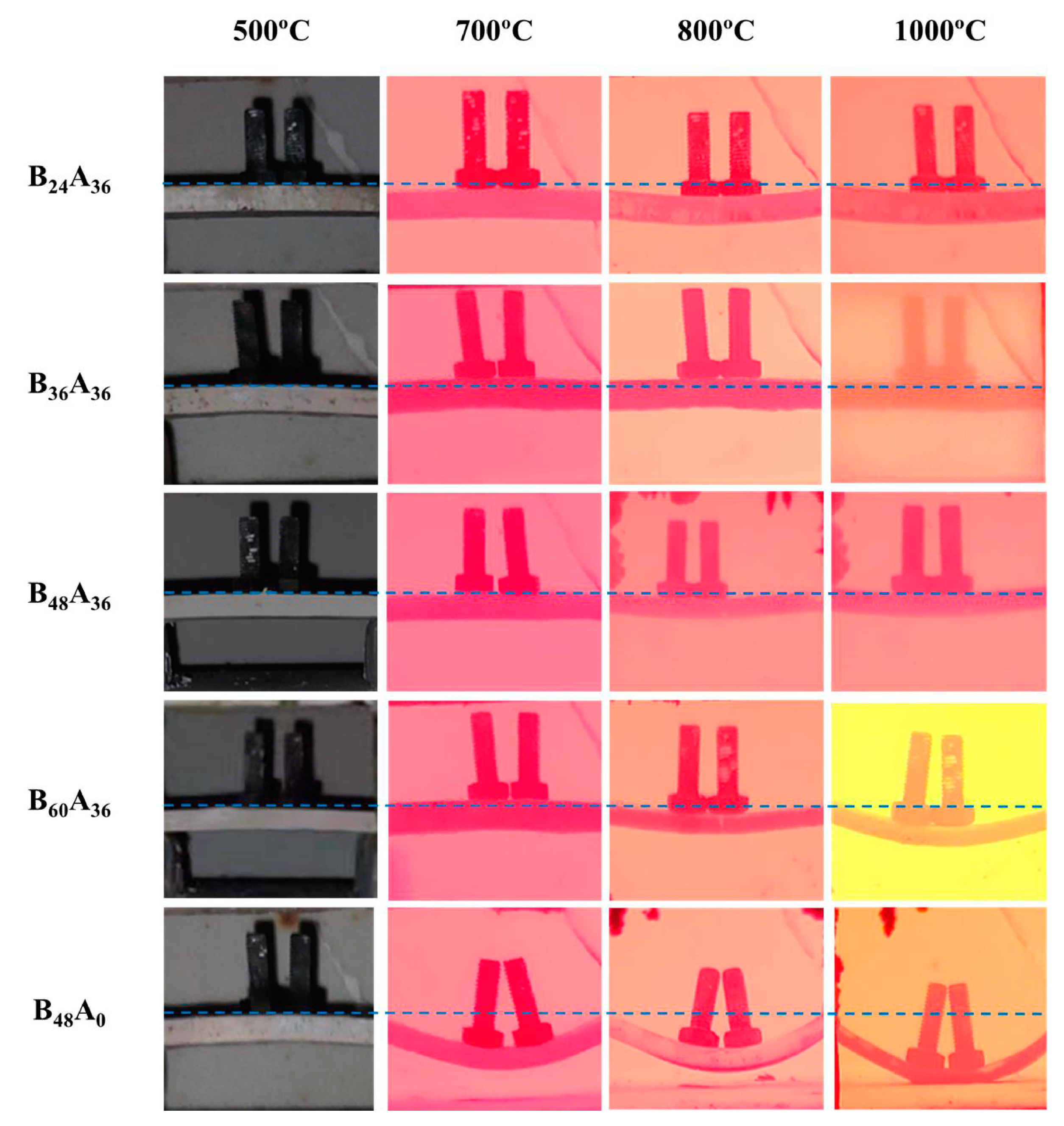
3.1. Microstructure of Residual Products
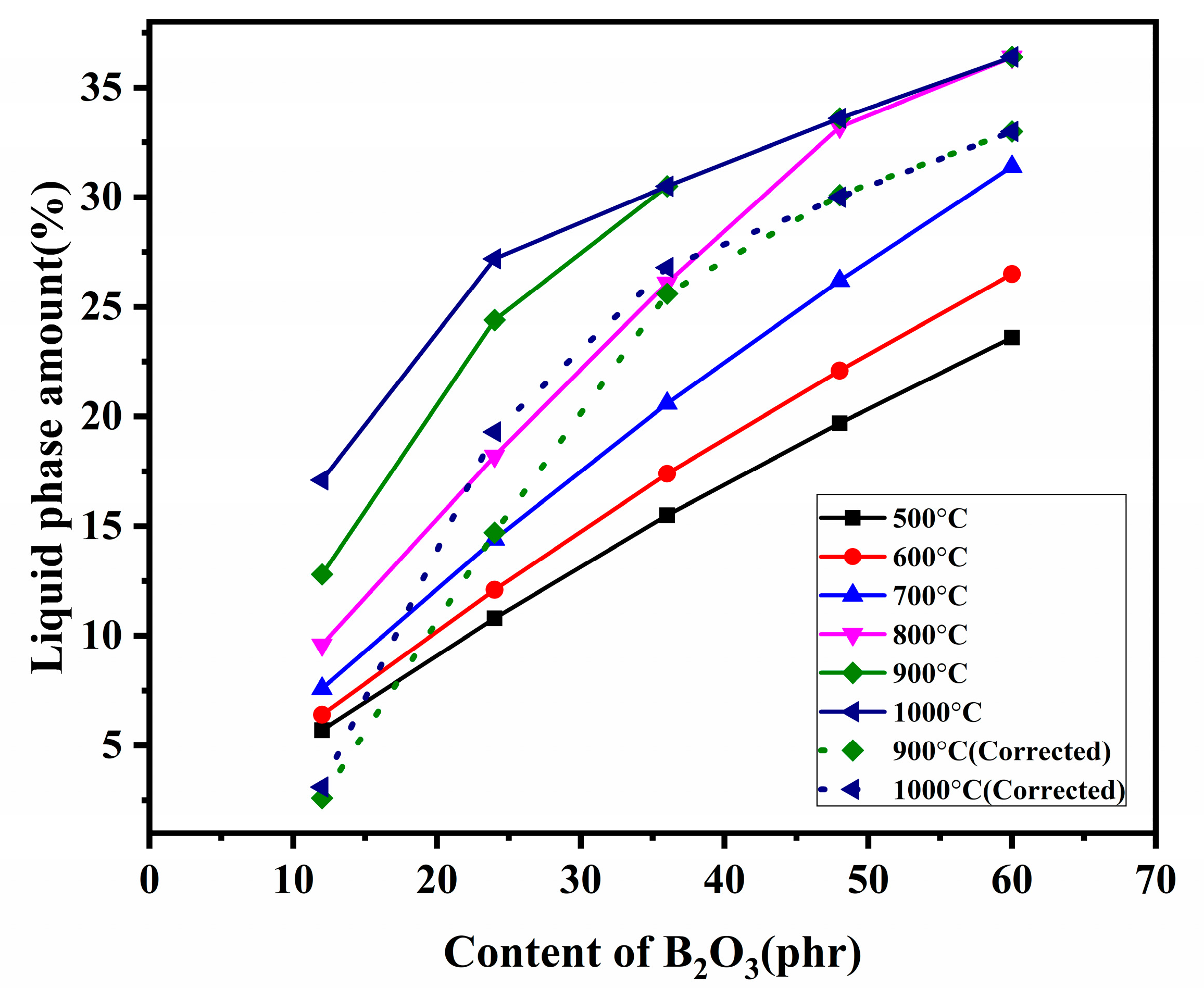
3.2. Phase analysis
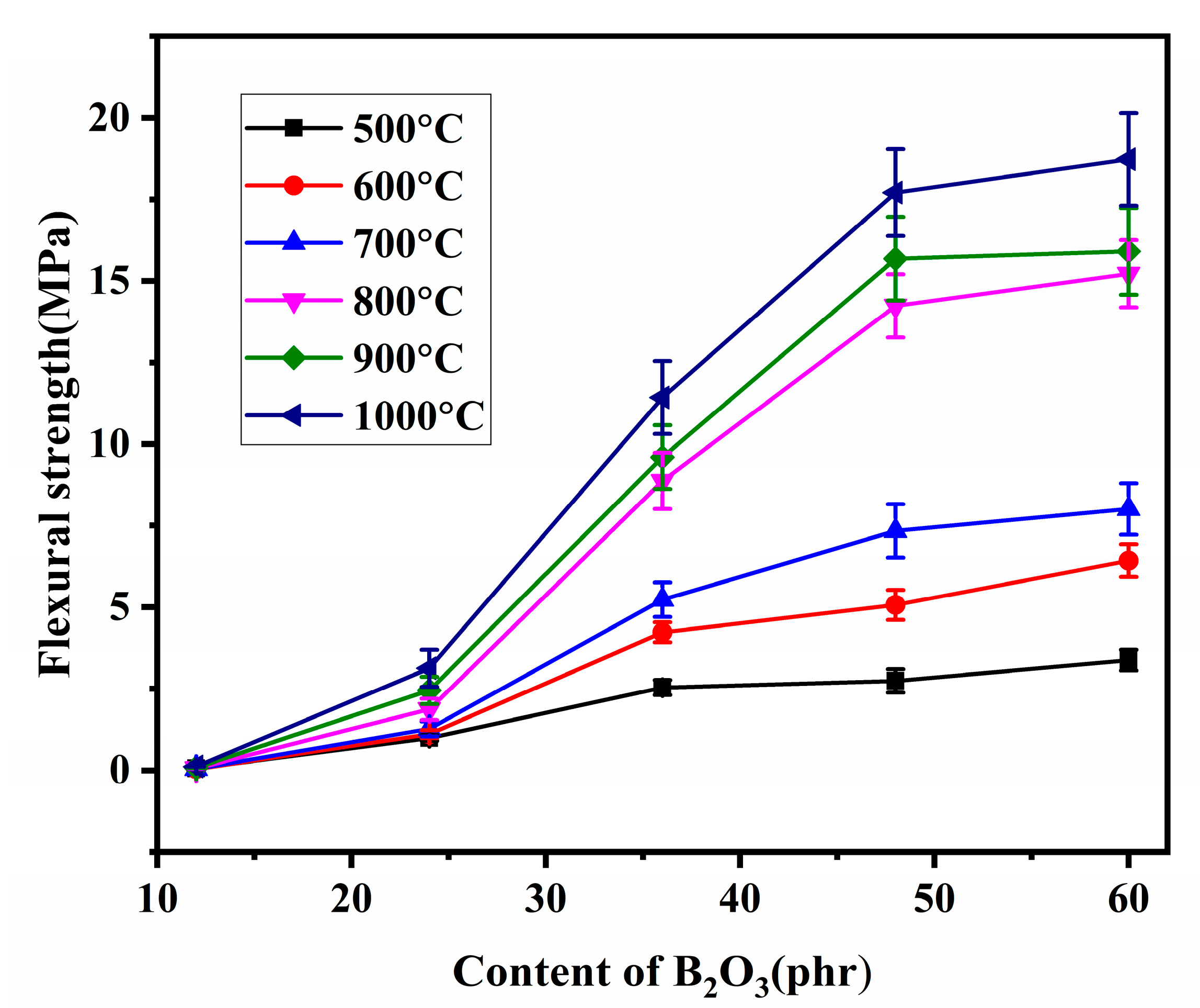
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camino, C.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane Thermal Degradation Part 1. Kinetic Aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.M.; Ganachaud, F. Flame Retardancy of Silicone-Based Materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Su, L.M.; Cui, C.H.; Shang, Y.J.; Zheng, F. Research Progress of Ceramifiable Polymer Fireproof Composite. Mater. Sci. Eng. Powder Metall. 2009, 14, 290–294. [Google Scholar]
- Hshieh, F.-Y. Shielding Effects of Silica-Ash Layer on the Combustion of Silicones and Their Possible Applications on the Fire Retardancy of Organic Polymers. Fire Mater. 1998, 22, 69–76. [Google Scholar] [CrossRef]
- Mansouri, J.; Burford, R.P.; Cheng, Y.B. Pyrolysis Behaviour of Silicone-Based Ceramifying Composites. Mater. Sci. Eng. A 2006, 425, 7–14. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, F.; Huang, Z.; Dai, J.; Shi, M. Improved Ablation Resistance of Silicone Rubber Composites by Introducing Montmorillonite and Silicon Carbide Whisker. Materials 2016, 9, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, Y.; Zhang, L.X.; Zhang, L.Q.; Lu, Y.L. Study on Ablative Properties and Mechanisms of Hydrogenated Nitrile Butadiene Rubber (HNBR) Composites Containing Different Fillers. Polym. Degrad. Stab. 2011, 96, 808–817. [Google Scholar] [CrossRef]
- Anyszka, D.M.; Bieliński, Z.; Pędzich, M.Z.-N. Influence of Boron Oxide on Ceramization of Silicone-Basing Composites. In Proceedings of the 12th Conference of the European Ceramic Society, Stockholm, Sweden, 19–23 June 2011. [Google Scholar]
- Mansouri, J.; Wood, C.A.; Roberts, K.; Cheng, Y.-B.; Burford, R.P. Investigation of the Ceramifying Process of Modified Silicone–Silicate Compositions. J. Mater. Sci. 2007, 42, 6046–6055. [Google Scholar] [CrossRef]
- Hu, S.; Chen, F.; Li, J.G.; Shen, Q.; Huang, Z.X.; Zhang, L.M. The Ceramifying Process and Mechanical Properties of Silicone Rubber/Ammonium Polyphosphate/ Aluminium Hydroxide/Mica Composites. Polym. Degrad. Stab. 2016, 126, 196–203. [Google Scholar] [CrossRef]
- Guo, J.; Gao, W.; Wang, Y.; Liang, D.; Li, H.; Zhang, X. Effect of Glass Frit with Low Softening Temperature on the Properties, Microstructure and Formation Mechanism of Polysiloxane Elastomer-Based Ceramizable Composites. Polym. Degrad. Stab. 2017, 136, 71–79. [Google Scholar] [CrossRef]
- Gong, X.H.; Wu, T.Y.; Ma, J.; Zhao, D.; Shen, Y.C.; Wang, T.W. Improved Self-Supporting Property of Ceramifying Silicone Rubber Composites by Forming Crystalline Phase at High Temperatures. J. Alloys Compd. 2017, 706, 322–329. [Google Scholar] [CrossRef]
- Hernández, M.F.; Suárez, G.; Cipollone, M.; Conconi, M.S.; Aglietti, E.F.; Rendtorff, N.M. Formation, Microstructure and Properties of Aluminum Borate Ceramics Obtained from Alumina and Boric Acid. Ceram. Int. 2017, 43, 2188–2195. [Google Scholar] [CrossRef]
- Whittaker, M.L.; Sohn, H.Y.; Cutler, R.A. Oxidation Kinetics of Aluminum Diboride. J. Solid State Chem. 2013, 207, 163–169. [Google Scholar] [CrossRef]
- Yoshida, M.; Takeuchi, S.; Pan, J.; Sasaki, G.; Fuyama, N.; Fuj, T.; Fukunaga, H. Preparation and Characterization of Aluminum Borate Whisker Reinforced Magnesium Alloy Composites by Semi-Solid Process. Adv. Compos. Mater. 1999, 8, 259–268. [Google Scholar] [CrossRef]
- Suganuma, K.; Fujita, T.; Sasaki, G.; Suzuki, N. Evaluation of Strength and Heat-Resistance for Aluminum-Borate Whisker Reinforced AC8A Aluminum Alloy Composite. J. Japan Inst. Light Met. 1991, 41, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.K.; Zerbetto, S.; Colombo, P.; Pantano, C.G. Glass–Ceramics and Composites Containing Aluminum Borate Whiskers. Ceram. Int. 2010, 36, 1589–1596. [Google Scholar] [CrossRef]
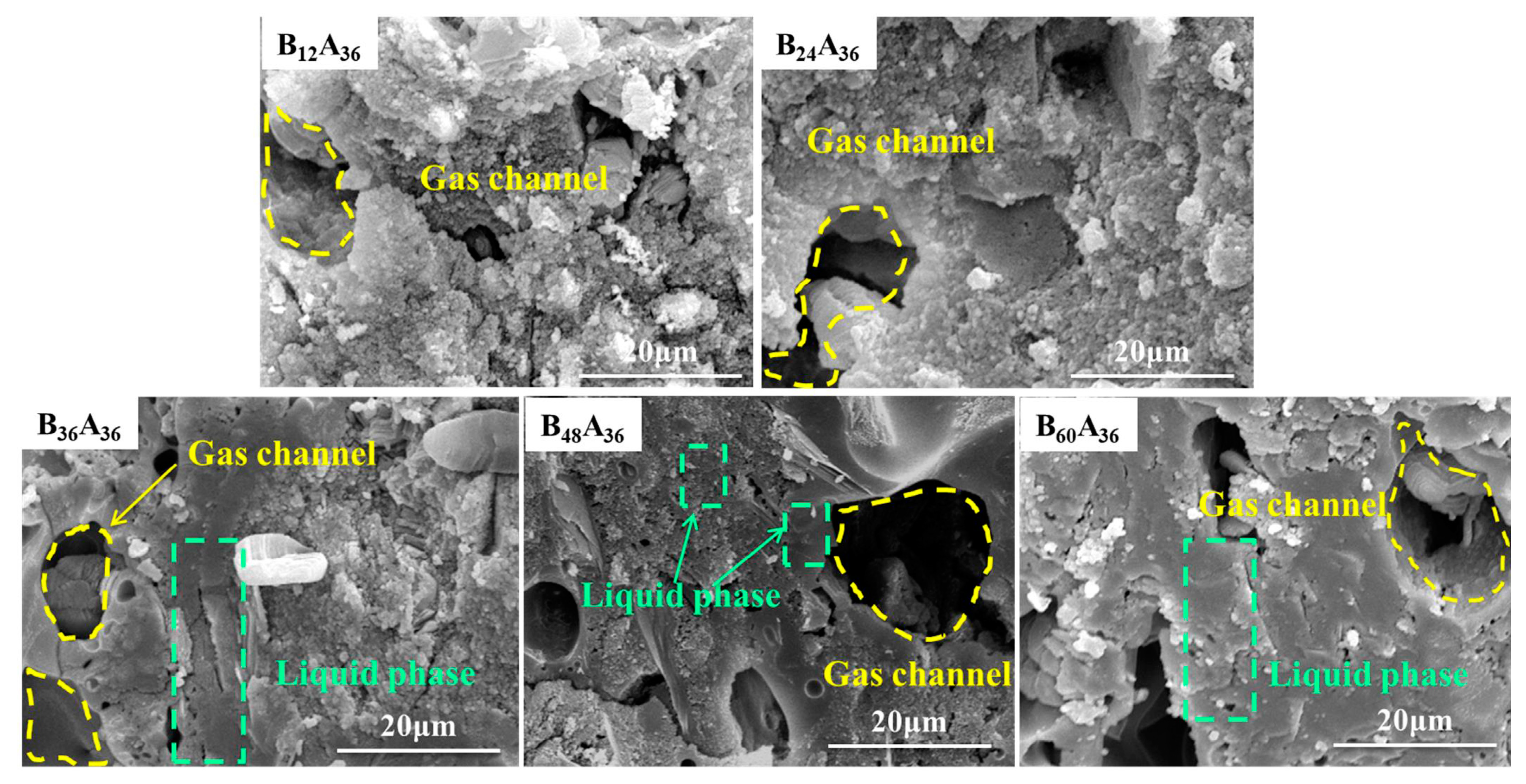
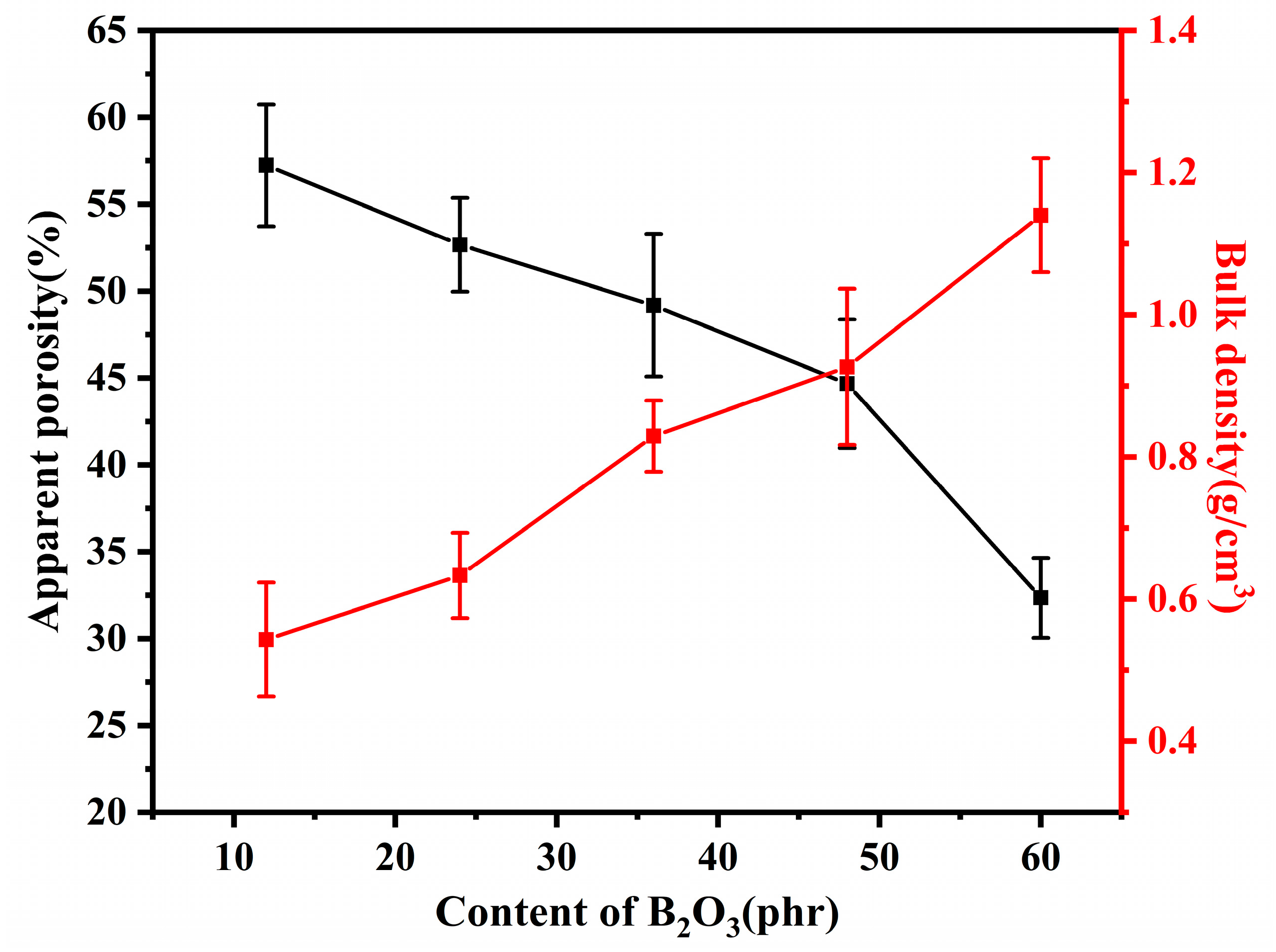

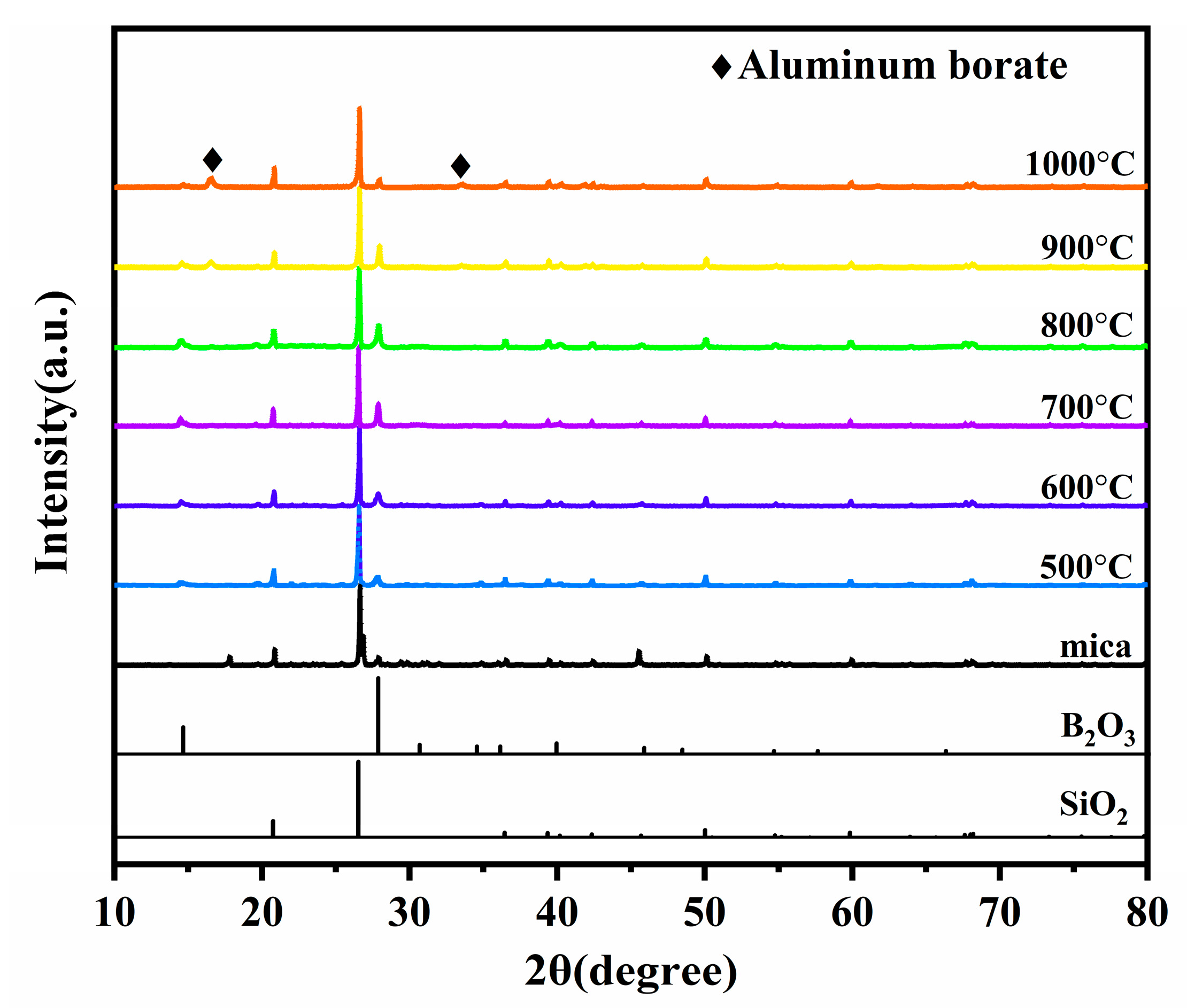
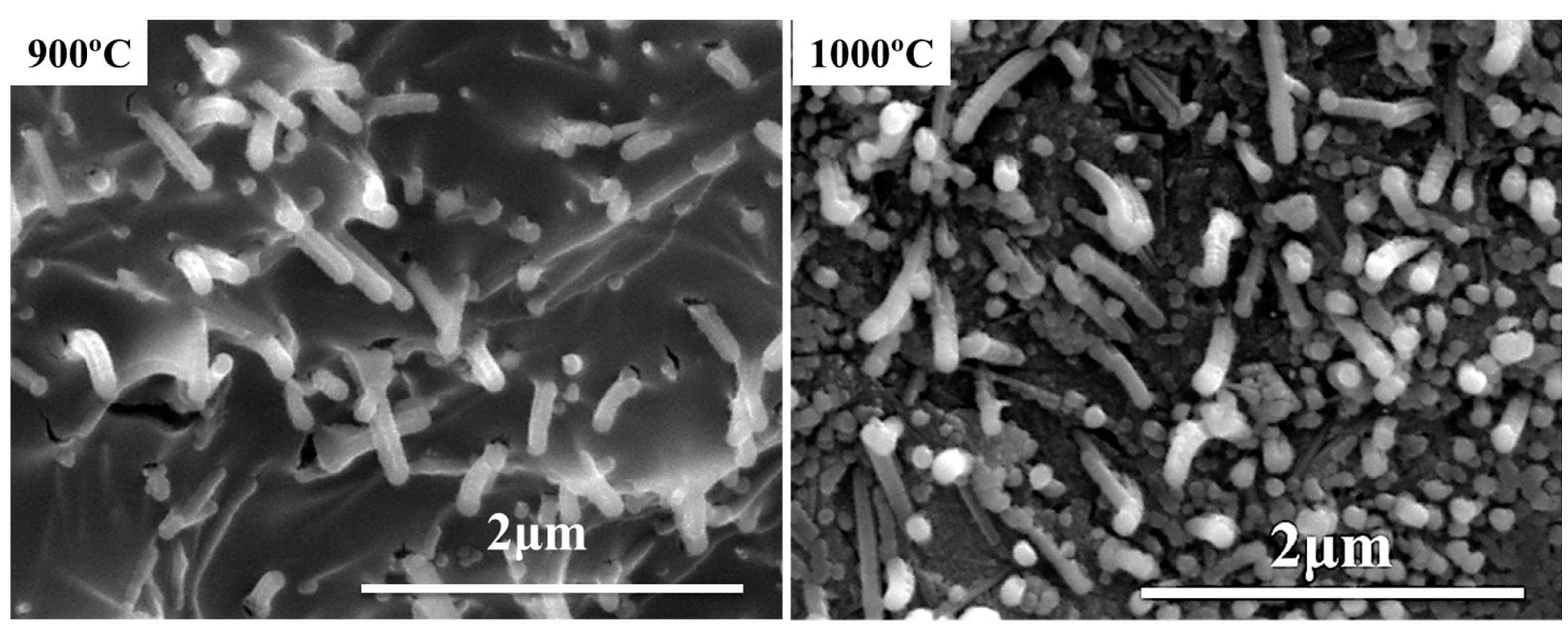
| Compositions (phr) | Silicone Rubber | SiO2 | Mica | B2O3 | Nano-γ-Al2O3 | Fumed Silica | DCBP | |
|---|---|---|---|---|---|---|---|---|
| Samples | ||||||||
| B12A36 | 100 | 24 | 36 | 12 | 36 | 30 | 2 | |
| B24A36 | 100 | 24 | 36 | 24 | 36 | 30 | 2 | |
| B36A36 | 100 | 24 | 36 | 36 | 36 | 30 | 2 | |
| B48A36 | 100 | 24 | 36 | 48 | 36 | 30 | 2 | |
| B60A36 | 100 | 24 | 36 | 60 | 36 | 30 | 2 | |
| B48A0 | 100 | 24 | 36 | 48 | 0 | 30 | 2 | |
| Temperature (°C) | Consumption of Nano-γ-Al2O3 (%) | Consumption of B2O3 (%) |
|---|---|---|
| 900 | 89 | 20 |
| 1000 | 89 | 21 |
| Temperature (°C) | Bending Angle (°) | ||||
|---|---|---|---|---|---|
| B24A36 | B36A36 | B48A36 | B60A36 | B48A0 | |
| 500 | - | - | - | - | - |
| 700 | - | - | - | - | 25 |
| 800 | 10 | - | 12 | 14 | 31 |
| 1000 | 10 | - | 12 | 20 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, H.; Zhang, S.; Pan, L.; Yang, S.; Zhang, J.; Shi, M.; Huang, Z.; Li, J.; Shen, Q. Role of Liquid-Phase Amount in Ceramization of Silicone Rubber Composites and Its Controlling. Materials 2022, 15, 3675. https://doi.org/10.3390/ma15103675
Pang H, Zhang S, Pan L, Yang S, Zhang J, Shi M, Huang Z, Li J, Shen Q. Role of Liquid-Phase Amount in Ceramization of Silicone Rubber Composites and Its Controlling. Materials. 2022; 15(10):3675. https://doi.org/10.3390/ma15103675
Chicago/Turabian StylePang, Haibo, Shiquan Zhang, Lei Pan, Suohui Yang, Jian Zhang, Minxian Shi, Zhixiong Huang, Junguo Li, and Qiang Shen. 2022. "Role of Liquid-Phase Amount in Ceramization of Silicone Rubber Composites and Its Controlling" Materials 15, no. 10: 3675. https://doi.org/10.3390/ma15103675






