Nano-Hybrid Au@LCCs Systems Displaying Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Materials and Methods
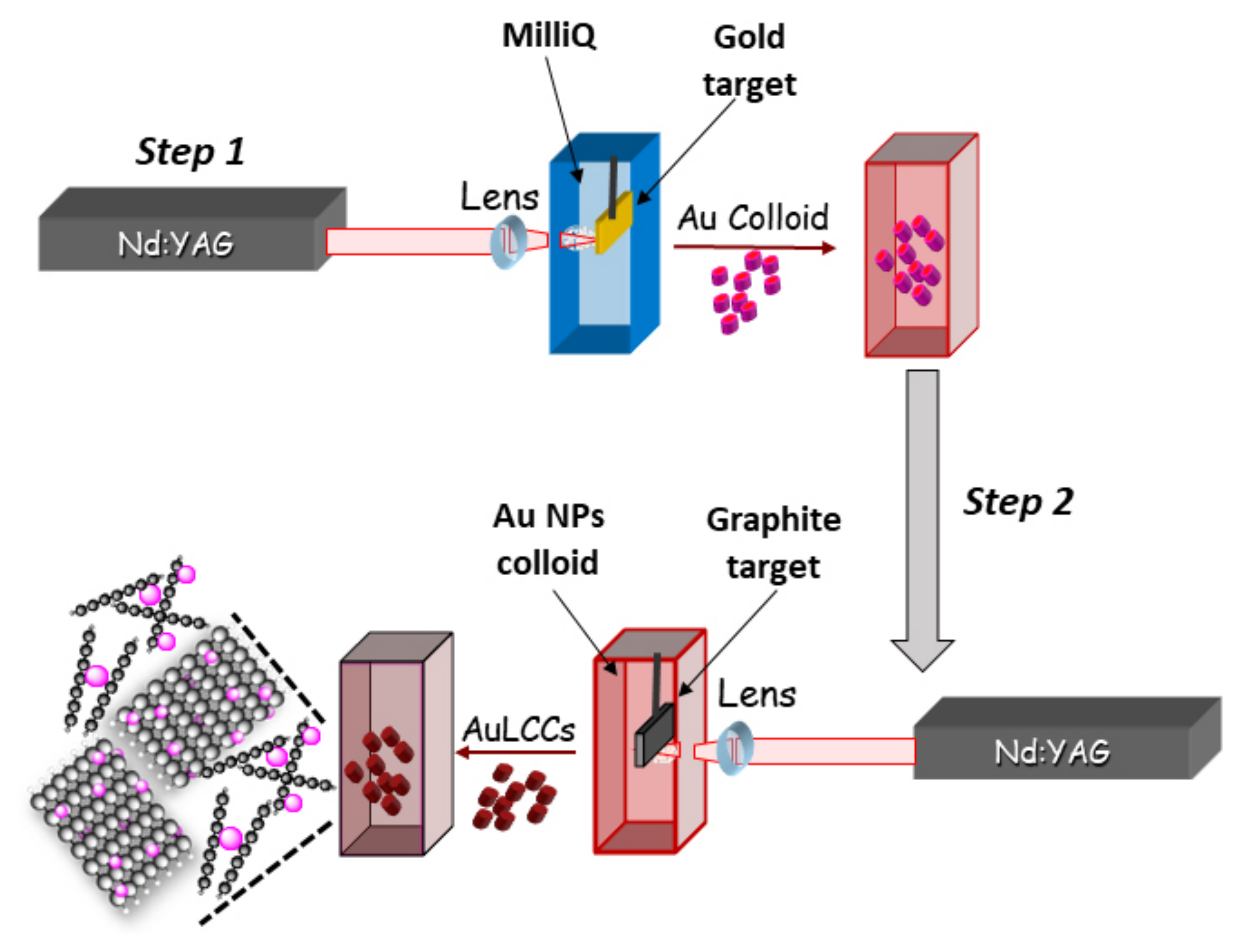
2.1. Au@LCC Synthesis and Characterization
2.2. Cell Cultures
2.3. Citotoxicity Assay
2.4. Intestinal Epithelial Cells Inflammation Model
2.5. Real-Time Pcr
2.6. Statistical Analysis
3. Results
3.1. Physico-Chemical Characterization of Au@LCC Nanocolloids
3.2. Biocompatibility on NIH/3T3 Fibroblasts
3.3. Anti-Inflammatory and Antioxidant Effects on Intestinal Epithelial Cells Treated with Tnf-
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Au | Gold |
| LCC | Linear Carbon Chain |
| NPs | Nanoparticles |
| PLAL | Pulsed Laser Ablation in Liquid |
| STEM | Scanning Transmission Electron Microscopy |
| XPS | X-ray photoelectron spectroscopy |
| TEER | Trans-Epithelial Electrical Resistance |
| SPR | Surface Plasmon Resonance |
References
- Echavarren, J.; Gall, M.A.Y.; Haertsch, A.; Leigh, D.A.; Spence, J.T.J.; Tetlow, D.J.; Tian, C. Sequence-Selective Decapeptide Synthesis by the Parallel Operation of Two Artificial Molecular Machines. J. Am. Chem. Soc. 2021, 143, 5158–5165. [Google Scholar] [CrossRef]
- Santoro, A.; Holub, J.; Fik-Jaskółka, M.A.; Vantomme, G.; Lehn, J.M. Dynamic Helicates Self-Assembly from Homo- and Heterotopic Dynamic Covalent Ligand Strands. Chem. Eur. J. 2020, 26, 15664–15671. [Google Scholar] [CrossRef] [PubMed]
- Foti, C.; Mineo, P.G.; Nicosia, A.; Scala, A.; Neri, G.; Piperno, A. Recent Advances of Graphene-Based Strategies for Arsenic Remediation. Front. Chem. 2020, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nastasi, F.; Santoro, A.; Serroni, S.; Campagna, S.; Kaveevivitchai, N.; Thummel, R.P. Early photophysical events of a ruthenium(ii) molecular dyad capable of performing photochemical water oxidation and of its model compounds. Photochem. Photobiol. Sci. 2019, 18, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dong, C.; Yu, G.; Cheng, W.; Liang, Y.; Pan, Y.; Li, H.; Ji, H. Smart and dual-targeted BSA nanomedicine with controllable release by high autolysosome levels. Colloids Surf. Biointerf. 2019, 182, 110325. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Barattucci, A.; Bonaccorsi, P.; Giannetto, A.; La Ganga, G.; Musarra-Pizzo, M.; Salerno, T.M.G.; Santoro, A.; Sciortino, M.T.; Puntoriero, F.; et al. Carbohydrates and Charges on Oligo(phenylenethynylenes): Towards the Design of Cancer Bullets. Chem. Eur. J. 2018, 24, 16972–16976. [Google Scholar] [CrossRef]
- Seok, J.M.; Jeong, J.E.; Lee, S.J.; Im, S.H.; Lee, J.H.; Kim, W.D.; Lee, K.; Park, S.A. Bio-plotted hydrogel scaffold with core and sheath strand-enhancing mechanical and biological properties for tissue regeneration. Colloids Surf. Biointerf. 2021, 205, 111919. [Google Scholar] [CrossRef]
- Neri, G.; Corsaro, C.; Fazio, E. Plasmon-Enhanced Controlled Drug Release from Ag-PMA Capsules. Molecules 2020, 25, 2267. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, N.; Mishra, P.; Malhotra, B.D. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci. Total Environ. 2021, 754, 142363. [Google Scholar] [CrossRef]
- Halim, A.; Luo, Q.; Ju, Y.; Song, G. A Mini Review Focused on the Recent Applications of Graphene Oxide in Stem Cell Growth and Differentiation. Nanomaterials 2018, 8, 736. [Google Scholar] [CrossRef]
- Sajjadi, M.; Nasrollahzadeh, M.; Jaleh, B.; Soufi, G.J.; Iravani, S. Carbon-based nanomaterials for targeted cancer nanotherapy: Recent trends and future prospects. J. Drug Target. 2021, 29, 716–741. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, D.; Currò, M.; Ientile, R.; Verderio, E.A.; Scala, A.; Mazzaglia, A.; Pennisi, R.; Musarra-Pizzo, M.; Zagami, R.; Neri, G.; et al. Intracellular Fate and Impact on Gene Expression of Doxorubicin/Cyclodextrin-Graphene Nanomaterials at Sub-Toxic Concentration. Int. J. Mol. Sci. 2020, 21, 4891. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, K.; Wang, H.; Liu, W.; Zhang, Z.; Liu, J.; Shi, J. A fullerene based hybrid nanoparticle facilitates enhanced photodynamic therapy via changing light source and oxygen consumption. Colloids Surf. Biointerf. 2020, 186, 110700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, T.; Jiang, H.; Xie, Y.; Zhang, X.; Nie, H.; Yang, Y.; Qian, M.; Li, W.; Hao, T.; et al. Sugar-originated carbon nanodots selectively damage the tumor and enhance the sensitivity of chemotherapy. Nano Today 2021, 38, 101200. [Google Scholar] [CrossRef]
- Shevtsova, T.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Donchak, V.; Harhay, K.; Korolko, S.; Budkowski, A.; Stetsyshyn, Y. Temperature-responsive hybrid nanomaterials based on modified halloysite nanotubes uploaded with silver nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2022, 641, 128525. [Google Scholar] [CrossRef]
- Abed, A.; Derakhshan, M.; Karimi, M.; Shirazinia, M.; Mahjoubin-Tehran, M.; Homayonfal, M.; Hamblin, M.R.; Mirzaei, S.A.; Soleimanpour, H.; Dehghani, S.; et al. Platinum Nanoparticles in Biomedicine: Preparation, Anti-Cancer Activity, and Drug Delivery Vehicles. Front. Pharmacol. 2022, 13, 797804. [Google Scholar] [CrossRef]
- Lee, J.; Shi, Y.M.; Grün, P.; Gube, M.; Feldbrügge, M.; Bode, H.; Hennicke, F. Identification of Feldin, an Antifungal Polyyne from the Beefsteak Fungus Fistulina hepatica. Biomolecules 2020, 10, 1502. [Google Scholar] [CrossRef]
- Murata, K.; Suenaga, M.; Kai, K. Genome Mining Discovery of Protegenins A–D, Bacterial Polyynes Involved in the Antioomycete and Biocontrol Activities of Pseudomonas protegens. ACS Chem. Biol. 2021. [Google Scholar] [CrossRef]
- Shi Shun, A.L.K.; Tykwinski, R.R. Synthesis of Naturally Occurring Polyynes. Angew. Chem. Int. Ed. 2006, 45, 1034–1057. [Google Scholar] [CrossRef]
- Meng, L.Z.; Huang, W.H.; Wang, C.Z.; Yuan, C.S.; Li, S.P. Anticancer Activities of Polyynes from the Root Bark of Oplopanax horridus and Their Acetylated Derivatives. Molecules 2014, 19, 6142–6162. [Google Scholar] [CrossRef]
- Basso, A.; Guanti, G.; Riva, R.; Banfi, L. From Natural to Rationally Designed Artificial Enediynes. In Polyynes; CRC Press: Boca Raton, FL, USA, 2005; Chapter 19; pp. 453–492. [Google Scholar] [CrossRef]
- Del Rosso, T.; Louro, S.; Deepak, F.; Romani, E.; Zaman, Q.; Tahir; Pandoli, O.; Cremona, M.; Freire Junior, F.; De Beule, P.; et al. Biocompatible Au@Carbynoid/Pluronic-F127 nanocomposites synthesized by pulsed laser ablation assisted CO2 recycling. Appl. Surf. Sci. 2018, 441, 347–355. [Google Scholar] [CrossRef]
- Fazio, E.; Gökce, B.; Giacomo, A.D.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- Fazio, E.; Neri, F.; Patane, S.; D’urso, L.; Compagnini, G. Optical limiting effects in linear carbon chains. Carbon 2011, 49, 306–310. [Google Scholar] [CrossRef]
- Fazio, E.; D’Urso, L.; Consiglio, G.; Giuffrida, A.; Compagnini, G.; Puglisi, O.; Patanè, S.; Neri, F.; Forte, G. Nonlinear Scattering and Absorption Effects in Size-Selected Diphenylpolyynes. J. Phys. Chem. 2014, 118, 28812–28819. [Google Scholar] [CrossRef]
- Fazio, E.; Saija, R.; Santoro, M.; Abir, S.; Neri, F.; Tommasini, M.; Ossi, P.M. On the Optical Properties of Ag–Au Colloidal Alloys Pulsed Laser Ablated in Liquid: Experiments and Theory. J. Phys. Chem. 2020, 124, 24930–24939. [Google Scholar] [CrossRef]
- Peggiani, S.; Marabotti, P.; Lotti, R.A.; Facibeni, A.; Serafini, P.; Milani, A.; Russo, V.; Li Bassi, A.; Casari, C.S. Solvent-dependent termination, size and stability in polyynes synthesized via laser ablation in liquids. Phys. Chem. Chem. Phys. 2020, 22, 26312–26321. [Google Scholar] [CrossRef]
- Peggiani, S.; Facibeni, A.; Milani, A.; Castiglioni, C.; Russo, V.; Li Bassi, A.; Casari, C.S. In situ synthesis of polyynes in a polymer matrix via pulsed laser ablation in a liquid. Mater. Adv. 2020, 1, 2729–2736. [Google Scholar] [CrossRef]
- D’Urso, L.; Grasso, G.; Messina, E.; Bongiorno, C.; Scuderi, V.; Scalese, S.; Puglisi, O.; Spoto, G.; Compagnini, G. Role of Linear Carbon Chains in the Aggregation of Copper, Silver, and Gold Nanoparticles. J. Phys. Chem. 2010, 114, 907–915. [Google Scholar] [CrossRef]
- Grasso, G.; D’Urso, L.; Messina, E.; Cataldo, F.; Puglisi, O.; Spoto, G.; Compagnini, G. A mass spectrometry and surface enhanced Raman spectroscopy study of the interaction between linear carbon chains and noble metals. Carbon 2009, 47, 2611–2619. [Google Scholar] [CrossRef]
- Boukhvalov, D.; Zhidkov, I.; Kurmaev, E.; Fazio, E.; Cholakh, S.; D’Urso, L. Atomic and electronic structures of stable linear carbon chains on Ag-nanoparticles. Carbon 2018, 128, 296–301. [Google Scholar] [CrossRef]
- Fazio, E.; Patane, S.; D’urso, L.; Compagnini, G.; Neri, F. Enhanced nonlinear optical response of linear carbon chain colloid mixed with silver nanoparticles. Opt. Commun. 2012, 285, 2942–2946. [Google Scholar] [CrossRef]
- Zhidkov, I.S.; Kurmaev, E.Z.; Cholakh, S.O.; Fazio, E.; D’Urso, L. XPS study of interactions between linear carbon chains and colloidal Au nanoparticles. Mendeleev Commun. 2020, 30, 285–287. [Google Scholar] [CrossRef]
- Pan, B.; Xiao, J.; Li, J.; Liu, P.; Wang, C.; Yang, G. Carbyne with finite length: The one-dimensional sp carbon. Sci. Adv. 2015, 1, e1500857. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Takano, S.; Yamazoe, S.; Tsukuda, T. Synthesis of active, robust and cationic Au25 cluster catalysts on double metal hydroxide by long-term oxidative aging of Au25(SR)18. Nanoscale 2022, 14, 3031–3039. [Google Scholar] [CrossRef] [PubMed]
- Alba-Molina, D.; Santiago, A.R.P.; Giner-Casares, J.J.; Rodríguez-Castellón, E.; Martín-Romero, M.T.; Camacho, L.; Luque, R.; Cano, M. Tailoring the ORR and HER electrocatalytic performances of gold nanoparticles through metal–ligand interfaces. J. Mater. Chem. 2019, 7, 20425–20434. [Google Scholar] [CrossRef]
- Zuliani, A.; Ranjan, P.; Luque, R.; der Eycken, E.V.V. Heterogeneously Catalyzed Synthesis of Imidazolones via Cycloisomerizations of Propargylic Ureas Using Ag and Au/Al SBA-15 Systems. ACS Sustain. Chem. Eng. 2019, 7, 5568–5575. [Google Scholar] [CrossRef]
- Osugi, S.; Takano, S.; Masuda, S.; Harano, K.; Tsukuda, T. Few-nm-sized, phase-pure Au5Sn intermetallic nanoparticles: Synthesis and characterization. Dalton Trans. 2021, 50, 5177–5183. [Google Scholar] [CrossRef]
- Famta, P.; Famta, M.; Kaur, J.; Khursheed, R.; Kaur, A.; Khatik, G.; Pawde, D.; Rahman, S.; Tamilvanan, S. Protecting the Normal Physiological Functions of Articular and Periarticular Structures by Aurum Nanoparticle-Based Formulations: An Up-to-Date Insight. AAPS PharmSciTech 2020, 21, 95. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, M.J. Nano-gold displayed anti-inflammatory property via NF-kB pathways by suppressing COX-2 activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1149–1158. [Google Scholar] [CrossRef]
- Domsa, E.; Filip, A.; Olteanu, E.; Baldea, I.; Clichici, S.; Muresan, A.; David, L.; Moldovan, B.; Para, I.; Suciu, M.; et al. Gold nanoparticles phytoreduced with Cornus mas extract mitigate some of gliadin effects on Caco-2 cells. J. Physiol. Pharmacol. 2020, 71, 201–212. [Google Scholar] [CrossRef]
- De Araújo, R.F.; de Araújo, A.A.; Pessoa, J.B.; Freire Neto, F.P.; da Silva, G.R.; Leitão Oliveira, A.L.C.; de Carvalho, T.G.; Silva, H.F.; Eugênio, M.; Sant’Anna, C.; et al. Anti-inflammatory, analgesic and anti-tumor properties of gold nanoparticles. Pharmacol. Rep. 2017, 69, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Carr, K.; Smyth, S.; Mccullough, M.; Morris, J.; Moyes, S. Morphological aspects of interactions between microparticles and mammalian cells: Intestinal uptake and onward movement. Prog. Histochem. Cytochem. 2012, 46, 185–252. [Google Scholar] [CrossRef] [PubMed]
- Enea, M.; Pereira, E.; Silva, D.D.; Costa, J.; Soares, M.E.; de Lourdes Bastos, M.; Carmo, H. Study of the intestinal uptake and permeability of gold nanoparticles using bothin vitroandin vivoapproaches. Nanotechnology 2020, 31, 195102. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; He, L.; McClements, D.J.; Xiao, H. Uptake of Gold Nanoparticles by Intestinal Epithelial Cells: Impact of Particle Size on Their Absorption, Accumulation, and Toxicity. J. Agric. Food Chem. 2015, 63, 8044–8049. [Google Scholar] [CrossRef] [PubMed]
- Chelly, S.; Chelly, M.; Occhiuto, C.; Cimino, F.; Cristani, M.; Saija, A.; Molonia, M.S.; Ruberto, G.; D’Angelo, V.; Germanò, M.P.; et al. Evaluation of Antioxidant, Anti-Inflammatory and Antityrosinase Potential of Extracts from Different Aerial Parts of Rhanterium suaveolens from Tunisia. Chem. Biodivers. 2021, 18, e2100316. [Google Scholar] [CrossRef]
- Bashllari, R.; Molonia, M.S.; Muscarà, C.; Speciale, A.; Wilde, P.J.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside protects intestinal epithelial cells from palmitate-induced lipotoxicity. Arch. Physiol. Biochem. 2020, 1–8. [Google Scholar] [CrossRef]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef]
- Ferrari, D.; Francesco, C.; Fratantonio, D.; Molonia, M.; Romina, B.; Rossana, B.; Antonina, S.; Speciale, A. Cyanidin-3-O-Glucoside Modulates the In Vitro Inflammatory Crosstalk between Intestinal Epithelial and Endothelial Cells. Mediat. Inflamm. 2017, 2017, 3454023. [Google Scholar] [CrossRef]
- Speciale, A.; Anwar, S.; Ricciardi, E.; Chirafisi, J.; Saija, A.; Cimino, F. Cellular adaptive response to glutathione depletion modulates endothelial dysfunction triggered by TNF-α. Toxicol. Lett. 2011, 207, 291–297. [Google Scholar] [CrossRef]
- Schmittgen, T.; Livak, K. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Rorden, C. ezANOVA: Simple Analysis of Variance. Available online: https://people.cas.sc.edu/rorden/ezanova/index.html (accessed on 8 May 2022).
- Milani, A.; Tommasini, M.; Barbieri, V.; Lucotti, A.; Russo, V.; Cataldo, F.; Casari, C.S. Semiconductor-to-Metal Transition in Carbon-Atom Wires Driven by sp2 Conjugated End Groups. J. Phys. Chem. C 2017, 121, 10562–10570. [Google Scholar] [CrossRef]
- Bryce, M.R. A review of functional linear carbon chains (oligoynes, polyynes, cumulenes) and their applications as molecular wires in molecular electronics and optoelectronics. J. Mater. Chem. C 2021, 9, 10524–10546. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, M.; Wu, M.; Zhu, J.; Zhang, X. Multifunctional Carbon-Based Nanomaterials: Applications in Biomolecular Imaging and Therapy. Acs Omega 2018, 3, 9126–9145. [Google Scholar] [CrossRef] [PubMed]
- Fazio, E.; Neri, F. Nonlinear optical effects from Au nanoparticles prepared by laser plasmas in water. Appl. Surf. Sci. 2013, 272, 88–93. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Lucotti, A.; Tommasini, M.; Zoppo, M.D.; Castiglioni, C.; Zerbi, G.; Cataldo, F.; Casari, C.; Bassi, A.L.; Russo, V.; Bogana, M.; et al. Raman and SERS investigation of isolated sp carbon chains. Chem. Phys. Lett. 2006, 417, 78–82. [Google Scholar] [CrossRef]
- Compagnini, G.; Mita, V.; D’urso, L.; Cataliotti, R.; Puglisi, O. Spectroscopic study of polyynes obtained by laser ablation in liquids. J. Raman Spectrosc. 2008, 39, 177–181. [Google Scholar] [CrossRef]
- Arutyunyan, N.R.; Kononenko, V.V.; Gololobov, V.M.; Obraztsova, E.D. Resonant Effects in SERS Spectra of Linear Carbon Chains. Phys. Status Solidi B 2018, 255, 1700254. [Google Scholar] [CrossRef]
- Kubackova, J.; Izquierdo-Lorenzo, I.; Jancura, D.; Miskovsky, P.; Sanchez-Cortes, S. Adsorption of linear aliphatic α,ω-dithiols on plasmonic metal nanoparticles: A structural study based on surface-enhanced Raman spectra. Phys. Chem. Chem. Phys. 2014, 16, 11461–11470. [Google Scholar] [CrossRef]
- Tarakeshwar, P.; Buseck, P.R.; Kroto, H.W. Pseudocarbynes: Charge-Stabilized Carbon Chains. J. Phys. Chem. Lett. 2016, 7, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Tarakeshwar, P.; Fujikado, N.M.; Evraets, K.; Jones, A.K.; Meneghetti, M.; Buseck, P.R.; Sayres, S.G. Pseudocarbynes: Linear Carbon Chains Stabilized by Metal Clusters. J. Phys. Chem. 2020, 124, 19355–19361. [Google Scholar] [CrossRef]
- Kutrovskaya, S.; Osipov, A.; Baryshev, S.; Zasedatelev, A.; Samyshkin, V.; Demirchyan, S.; Pulci, O.; Grassano, D.; Gontrani, L.; Hartmann, R.R.; et al. Excitonic Fine Structure in Emission of Linear Carbon Chains. Nano Lett. 2020, 20, 6502–6509. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corp: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Bayazit, M.K.; Hodge, S.A.; Clancy, A.J.; Menzel, R.; Chen, S.; Shaffer, M.S.P. Carbon nanotube anions for the preparation of gold nanoparticle–nanocarbon hybrids. Chem. Commun. 2016, 52, 1934–1937. [Google Scholar] [CrossRef]
- Paula, M.M.S.; Petronilho, F.; Vuolo, F.; Ferreira, G.K.; De Costa, L.; Santos, G.P.; Effting, P.S.; Dal-Pizzol, F.; Dal-Bó, A.G.; Frizon, T.E.; et al. Gold nanoparticles and/or N-acetylcysteine mediate carrageenan-induced inflammation and oxidative stress in a concentration-dependent manner. J. Biomed. Mater. Res. Part A 2015, 103, 3323–3330. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, S.; Kim, Y.J.; Perumalsamy, H.; Lee, S.; Hwang, E.; Yi, T.H. Green synthesis of gold nanoparticles using Euphrasia officinalis leaf extract to inhibit lipopolysaccharide-induced inflammation through NF-κB and JAK/STAT pathways in RAW 264.7 macrophages. Int. J. Nanomed. 2019, 14, 2945–2959. [Google Scholar] [CrossRef]
- Rizwan, H.; Mohanta, J.; Si, S.; Pal, A. Gold nanoparticles reduce high glucose-induced oxidative-nitrosative stress regulated inflammation and apoptosis via tuberin-mTOR/NF-κB pathways in macrophages. Int. J. Nanomed. 2017, 12, 5841–5862. [Google Scholar] [CrossRef]
- Sumbayev, V.V.; Yasinska, I.M.; Garcia, C.P.; Gilliland, D.; Lall, G.S.; Gibbs, B.F.; Bonsall, D.R.; Varani, L.; Rossi, F.; Calzolai, L. Gold Nanoparticles Downregulate Interleukin-1β-Induced Pro-Inflammatory Responses. Small 2013, 9, 472–477. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, Y.; Zhang, K.; Guo, Z.; Liu, Y.; Zhou, P.; Liu, Z.; Lu, X. Gold nanoparticles alleviates the lipopolysaccharide-induced intestinal epithelial barrier dysfunction. Bioengineered 2021, 12, 6472–6483. [Google Scholar] [CrossRef]
- Abdelmegid, A.; Abdo, F.; Ahmed, F.; Kattaia, A. Therapeutic effect of gold nanoparticles on DSS-induced ulcerative colitis in mice with reference to interleukin-17 expression. Sci. Rep. 2019, 9, 10176. [Google Scholar] [CrossRef]
- Ko, W.C.; Wang, S.J.; Hsiao, C.Y.; Hung, C.T.; Hsu, Y.J.; Chang, D.C.; Hung, C.F. Pharmacological Role of Functionalized Gold Nanoparticles in Disease Applications. Molecules 2022, 27, 1551. [Google Scholar] [CrossRef] [PubMed]
- Pigulski, B.; Gulia, N.; Szafert, S. Reactivity of Polyynes: Complex Molecules from Simple Carbon Rods. Eur. J. Org. Chem. 2019, 2019, 1420–1445. [Google Scholar] [CrossRef]
- Waza, A.A.; Hamid, Z.; Ali, S.; Bhat, S.A.; Bhat, M.A. A review on heme oxygenase-1 induction: Is it a necessary evil. Inflamm. Res. 2018, 67, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of Cell Protection by Heme Oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Puentes-Pardo, J.D.; Moreno-SanJuan, S.; Carazo, Á.; León, J. Heme Oxygenase-1 in Gastrointestinal Tract Health and Disease. Antioxidants 2020, 9, 1214. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; He, J.; Yin, X.; Shi, Y.; Wan, J.; Tian, Z. The protective effect of lithocholic acid on the intestinal epithelial barrier is mediated by the vitamin D receptor via a SIRT1/Nrf2 and NF-κB dependent mechanism in Caco-2 cells. Toxicol. Lett. 2019, 316, 109–118. [Google Scholar] [CrossRef]
- Chi, J.H.; Kim, Y.H.; Sohn, D.H.; Seo, G.S.; Lee, S.H. Ameliorative effect of Alnus japonica ethanol extract on colitis through the inhibition of inflammatory responses and attenuation of intestinal barrier disruption in vivo and in vitro. Biomed. Pharmacother. 2018, 108, 1767–1774. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Li, F.; Xin, Y.; Duan, Z. Contributions of HO-1-Dependent MAPK to Regulating Intestinal Barrier Disruption. Biomol. Ther. 2021, 29, 175–183. [Google Scholar] [CrossRef]
- Wu, W.B.; Lai, T.H.; Shieh, J.M.; Tsou, C.J. Gold nanoparticles induce heme oxygenase-1 expression through Nrf2 activation and Bach1 export in human vascular endothelial cells. Int. J. Nanomed. 2015, 10, 5925. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.; Lin, C.H.; Chen, S.J.; Yen, C.; Huang, R.N. Nanogold induces anti-inflammation against oxidative stress induced in human neural stem cells exposed to amyloid-beta peptide. Neurochem. Int. 2021, 145, 104992. [Google Scholar] [CrossRef]
- Bredeck, G.; Halamoda-Kenzaoui, B.; Bogni, A.; Lipsa, D.; Bremer-Hoffmann, S. Tiered testing of micro- and nanoplastics using intestinal in vitro models to support hazard assessments. Environ. Int. 2022, 158, 106921. [Google Scholar] [CrossRef] [PubMed]
- Ude, V.; Brown, D.; Viale, L.; Kanase, N.; Stone, V.; Johnston, H. Impact of copper oxide nanomaterials on differentiated and undifferentiated Caco-2 intestinal epithelial cells; assessment of cytotoxicity, barrier integrity, cytokine production and nanomaterial penetration. Part. Fibre Toxicol. 2017, 14, 31. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condorelli, M.; Speciale, A.; Cimino, F.; Muscarà, C.; Fazio, E.; D’Urso, L.; Corsaro, C.; Neri, G.; Mezzasalma, A.M.; Compagnini, G.; et al. Nano-Hybrid Au@LCCs Systems Displaying Anti-Inflammatory Activity. Materials 2022, 15, 3701. https://doi.org/10.3390/ma15103701
Condorelli M, Speciale A, Cimino F, Muscarà C, Fazio E, D’Urso L, Corsaro C, Neri G, Mezzasalma AM, Compagnini G, et al. Nano-Hybrid Au@LCCs Systems Displaying Anti-Inflammatory Activity. Materials. 2022; 15(10):3701. https://doi.org/10.3390/ma15103701
Chicago/Turabian StyleCondorelli, Marcello, Antonio Speciale, Francesco Cimino, Claudia Muscarà, Enza Fazio, Luisa D’Urso, Carmelo Corsaro, Giulia Neri, Angela Maria Mezzasalma, Giuseppe Compagnini, and et al. 2022. "Nano-Hybrid Au@LCCs Systems Displaying Anti-Inflammatory Activity" Materials 15, no. 10: 3701. https://doi.org/10.3390/ma15103701
APA StyleCondorelli, M., Speciale, A., Cimino, F., Muscarà, C., Fazio, E., D’Urso, L., Corsaro, C., Neri, G., Mezzasalma, A. M., Compagnini, G., Neri, F., & Saija, A. (2022). Nano-Hybrid Au@LCCs Systems Displaying Anti-Inflammatory Activity. Materials, 15(10), 3701. https://doi.org/10.3390/ma15103701













