A Flexible and Wearable Nylon Fiber Sensor Modified by Reduced Graphene Oxide and ZnO Quantum Dots for Wide-Range NO2 Gas Detection at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
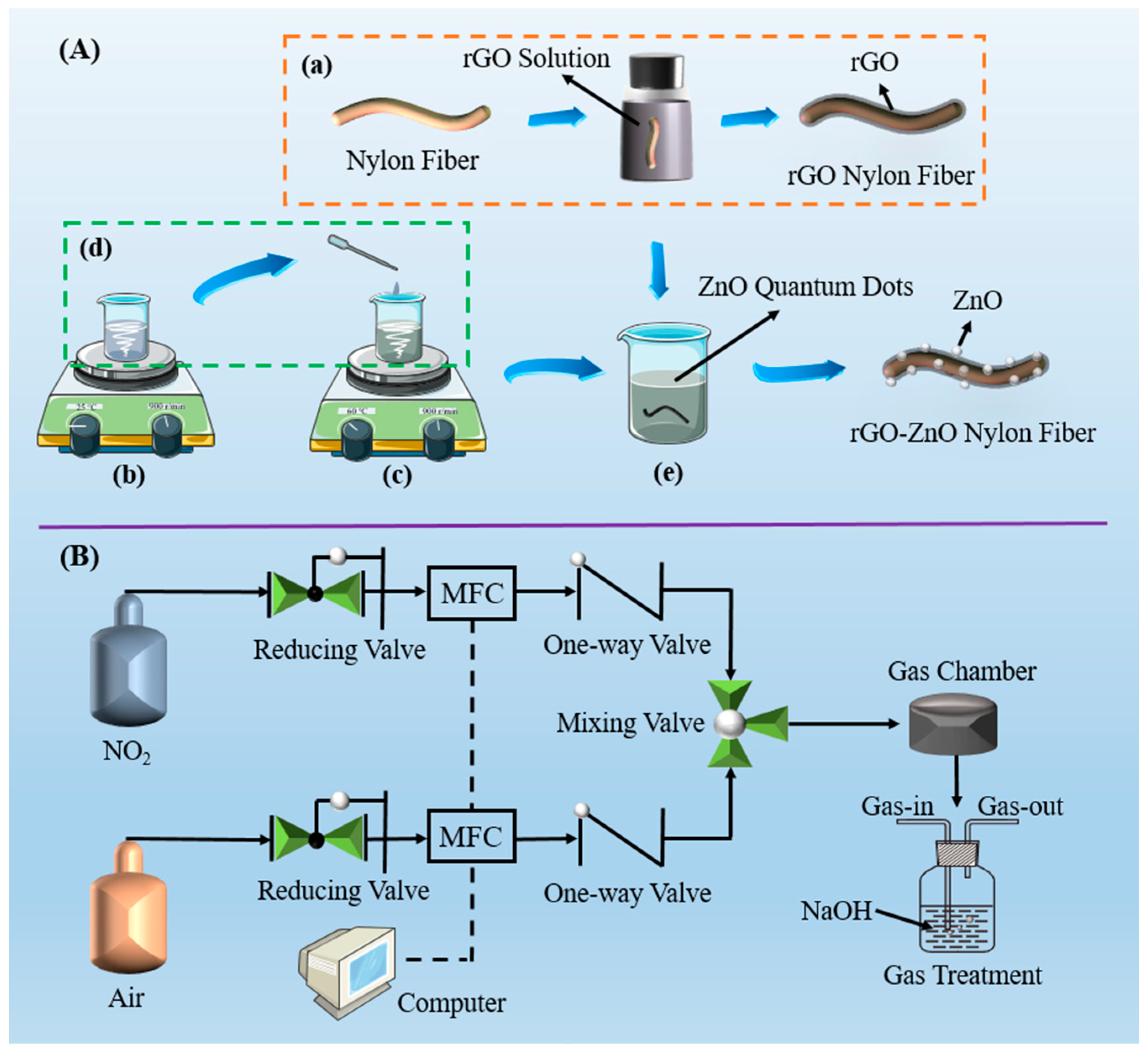
2.1. Materials and Gas Sensor Fabrication
2.2. Characterization
2.3. Gas Sensor Test and Evaluation Index
3. Results and Discussion
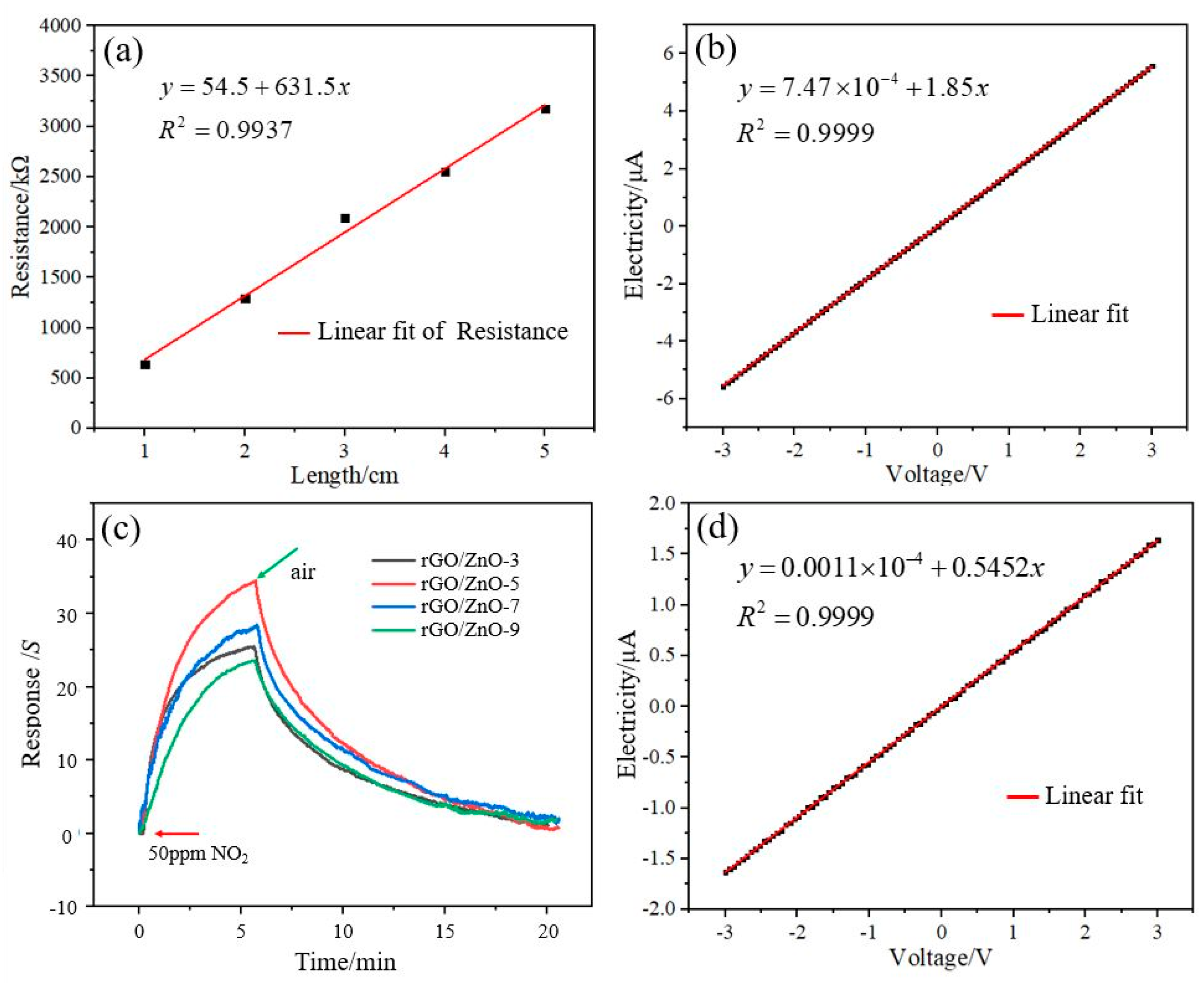
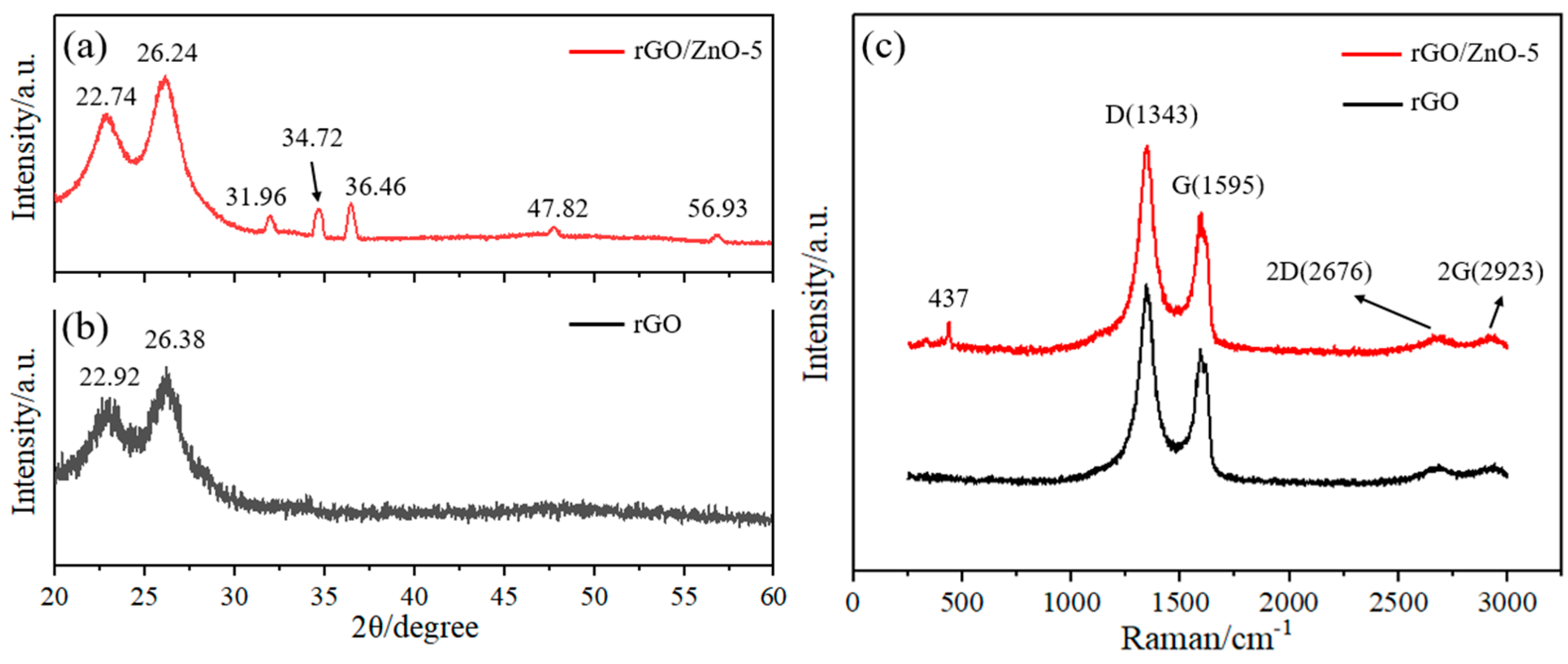
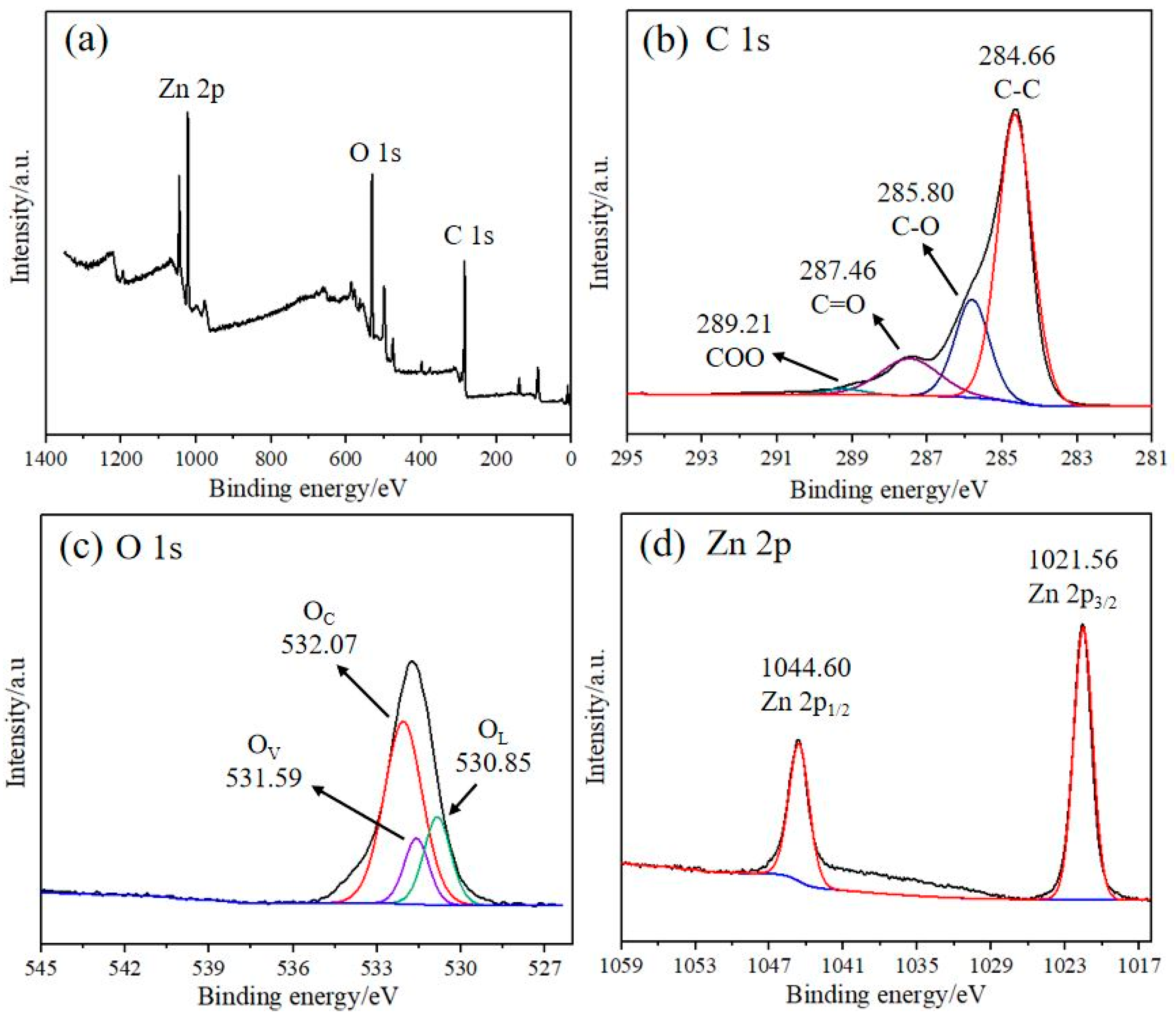
3.1. Characterization of the rGO/ZnO Nylon Fiber Sensor
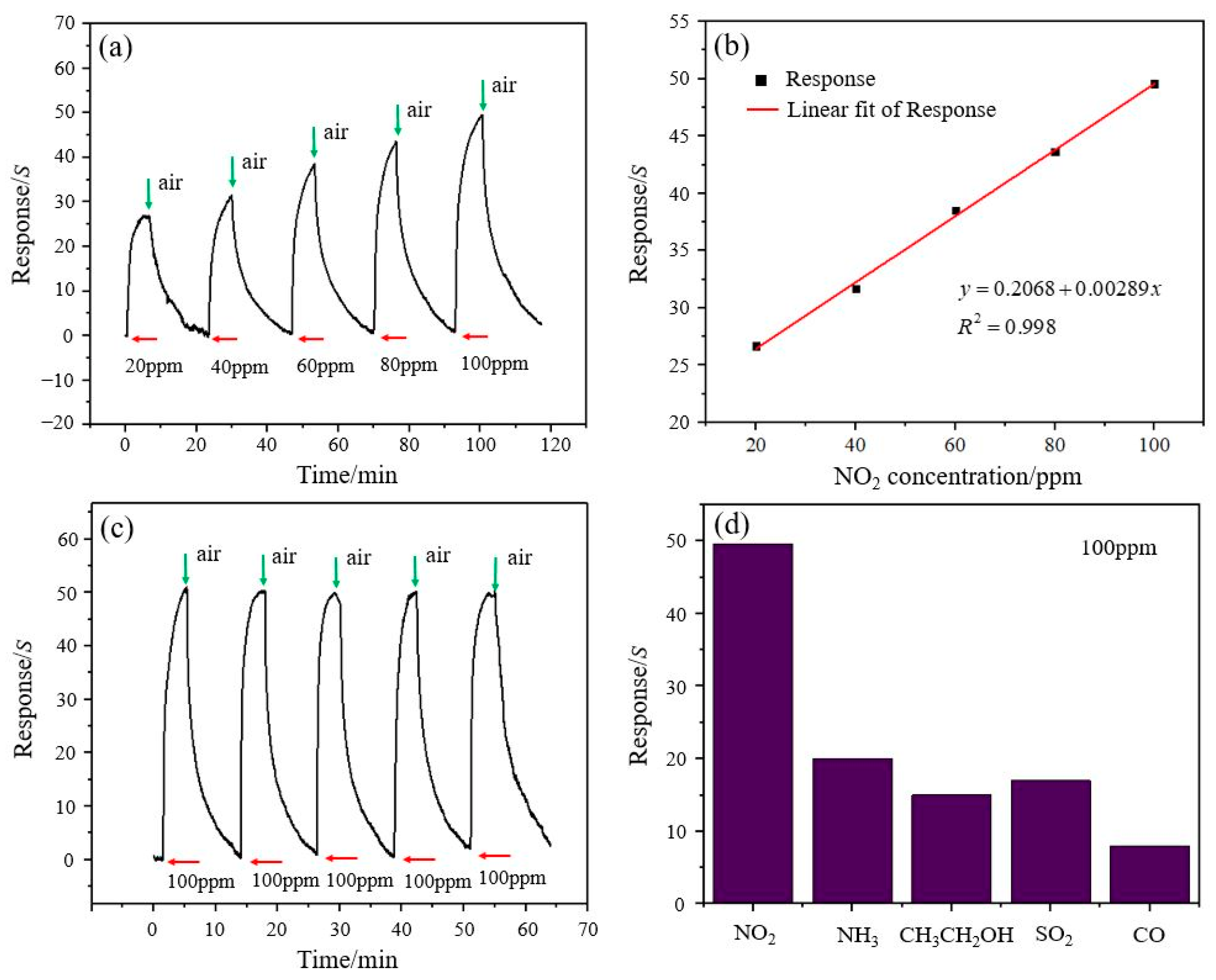
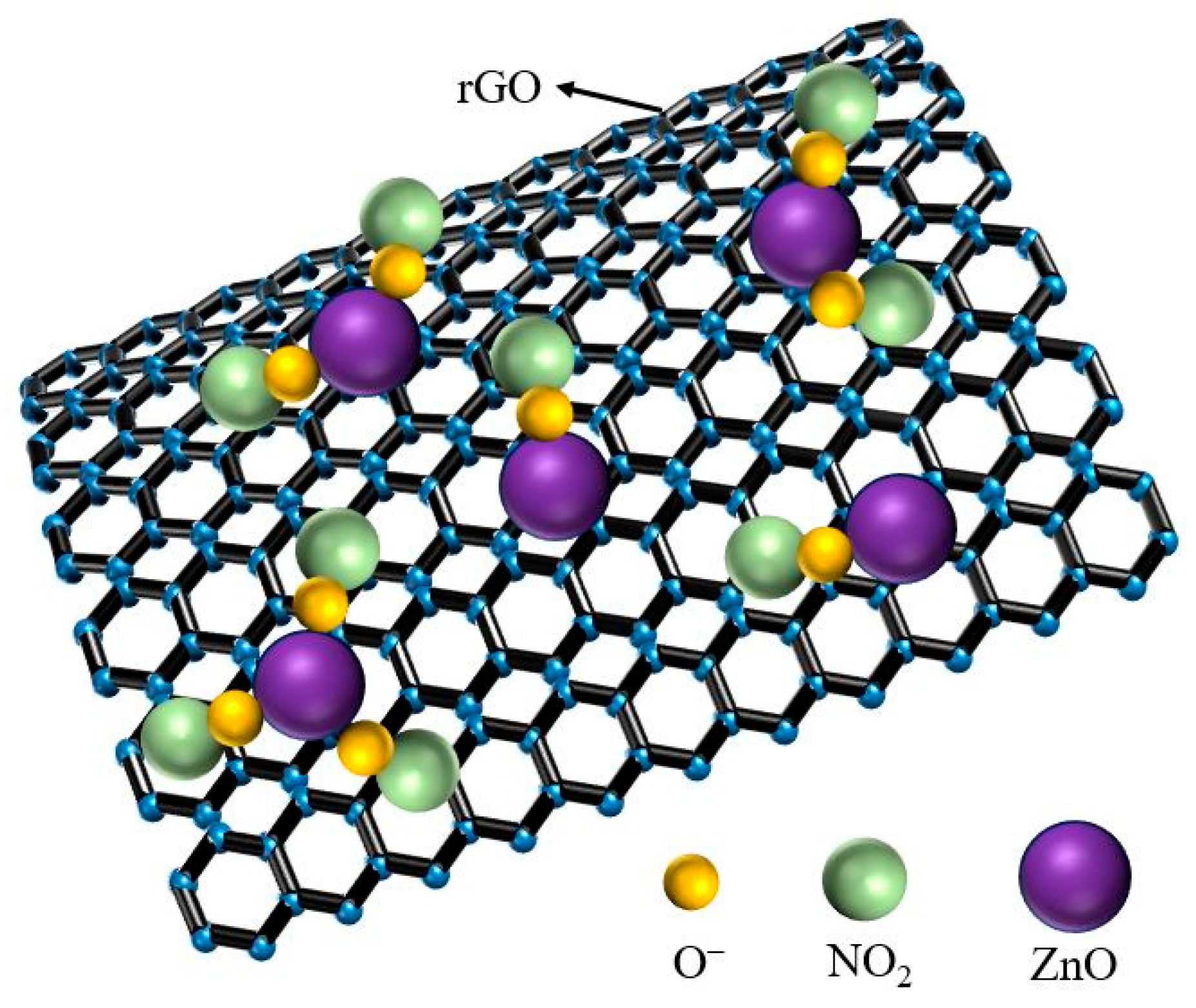
3.2. NO2 Gas Sensing Test and Analysis of the rGO/ZnO-5 Nylon Fiber Sensor
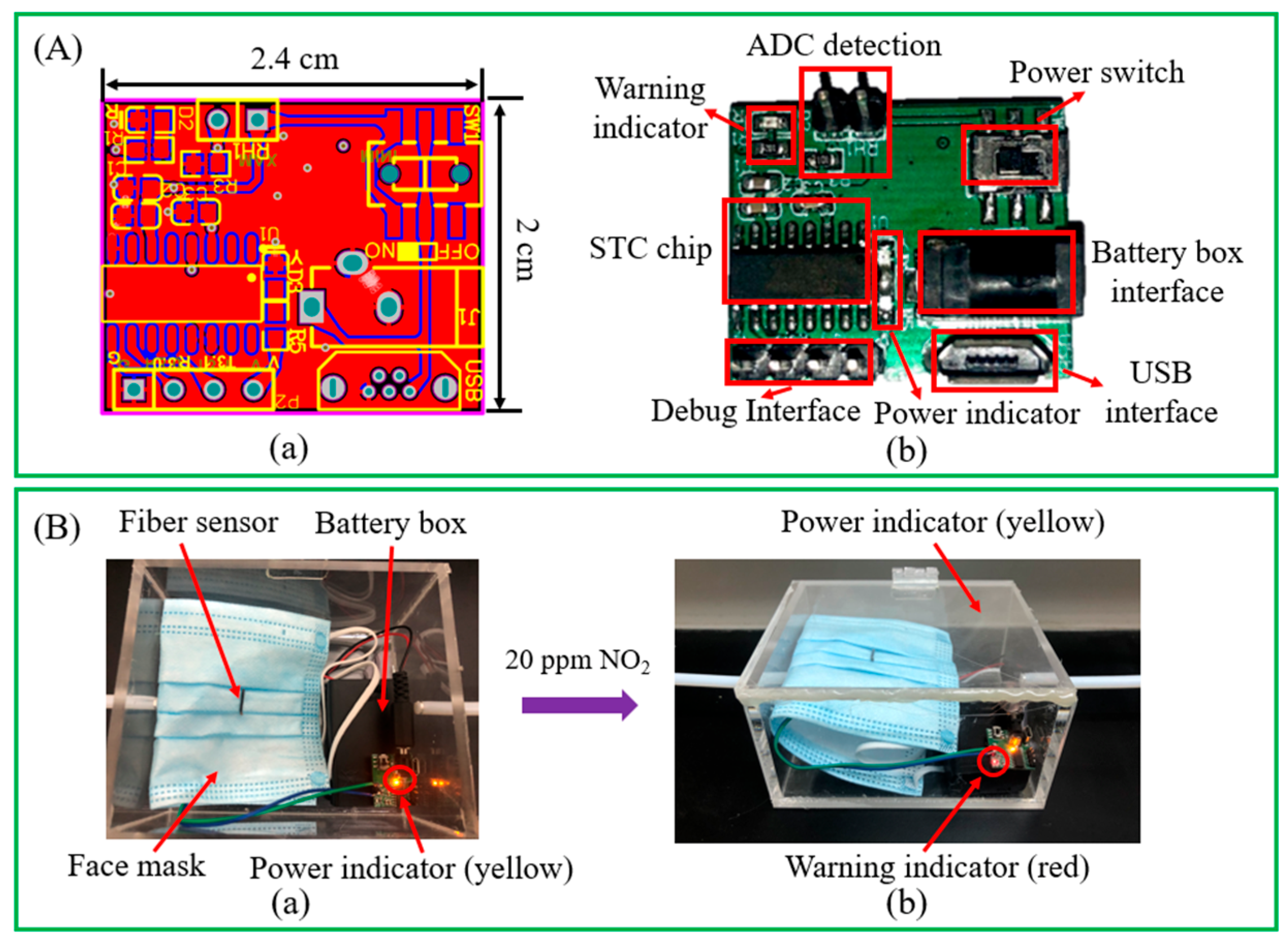
3.3. Over-Limit Warning Module and Wearable Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arani, M.H.; Jaafarzadeh, N.; Moslemzadeh, M.; Rezvani Ghalhari, M.; Bagheri Arani, S.; Mohammadzadeh, M. Dispersion of NO2 and SO2 pollutants in the rolling industry with AERMOD model: A case study to assess human health risk. J. Environ. Health Sci. 2021, 19, 1287–1298. [Google Scholar]
- Liao, J.; Li, Z.; Wang, G.; Chen, C.; Lv, S.; Li, M. ZnO nanorod/porous silicon nanowire hybrid structures as highly-sensitive NO2 gas sensors at room temperature. Phys. Chem. Chem. Phys. 2016, 18, 4835–4841. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, W.; Chu, Z.; Ding, G.; Yan, M.; Zhong, X.; Wang, R. Ultrasensitive ppb-level NO2 gas sensors at room temperature based on SnS2/rGO nanohybrids with P-N transition property and optoelectronic visible light enhancement performance. J. Mater. Chem. C 2019, 7, 8616–8625. [Google Scholar] [CrossRef]
- Leghrib, R.; Felten, A.; Pireaux, J.J.; Llobet, E. Gas sensors based on doped-CNT/SnO2 composites for NO2 detection at room temperature. Thin Solid Film. 2011, 520, 966–970. [Google Scholar] [CrossRef]
- Han, Y.; Ma, Y.; Liu, Y.; Xu, S.; Chen, X.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Yang, Z. Construction of MoS2/SnO2 heterostructures for sensitive NO2 detection at room temperature. Appl. Surf. Sci. 2019, 493, 613–619. [Google Scholar] [CrossRef]
- Jia, X.; Wang, X. Mosaic-Like Micropatterned Monolayer RGO/AgNPs Film Gas Sensor with Enhanced Room-Temperature NO2 Response/Recovery Properties. J. Microelectromech. Syst. 2019, 99, 833–840. [Google Scholar] [CrossRef]
- Xia, Y.; He, S.; Wang, J.; Zhou, L.; Wang, J.; Komarneni, S. MXene/WS2 hybrids for visible-light-activated NO2 sensing at room temperature. Chem. Commun. 2021, 57, 9136–9139. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, J.; Hao, J.; Zheng, S.; Wan, P.; Wang, T.; Fang, H.; Wang, Y. SnS2/SnS p–n heterojunctions with an accumulation layer for ultrasensitive room-temperature NO2 detection. Nanoscale 2019, 11, 13741–13749. [Google Scholar] [CrossRef]
- Wang, X.X.; Li, H.Y.; Guo, X. Flexible and transparent sensors for ultra-low NO2 detection at room temperature under visible light illumination. J. Mater. Chem. A 2020, 8, 14482–14490. [Google Scholar] [CrossRef]
- Pyeon, J.J.; Baek, I.H.; Song, Y.G.; Kim, G.S.; Cho, A.J.; Lee, G.Y.; Han, J.H.; Chung, T.M.; Hwang, C.S.; Kang, C.Y. Highly sensitive flexible NO2 sensor composed of vertically aligned 2D SnS2 operating at room temperature. J. Mater. Chem. C 2020, 8, 11874–11881. [Google Scholar] [CrossRef]
- Sya, B.; Ywa, B.; Zwa, B.; Mha, B.; Lei, D.; Jiang, Z.; Csi, P. Highly-sensitive NO2 gas sensors based on three-dimensional nanotube graphene and ZnO nanospheres nanocomposite at room temperature. Appl. Surf. Sci. 2021, 566, 150720. [Google Scholar]
- Sharma, N.; Sharma, V.; Sharma, S.K.; Sachdev, K. Gas sensing behaviour of green synthesized reduced graphene oxide (rGO) for H2 and NO. Mater. Lett. 2019, 236, 444–447. [Google Scholar] [CrossRef]
- Ghosal, S.; Bhattacharyya, P. A potential gas sensor device based on Pd/RGO/TiO2 nanotube ternary hybrid junction. Microelectron. Reliab. 2017, 78, 299–306. [Google Scholar] [CrossRef]
- Ngo, Y.L.T.; Hur, S.H. Low-temperature NO2 gas sensor fabricated with NiO and reduced graphene oxide hybrid structure. Mater. Res. Bull. 2016, 84, 168–176. [Google Scholar] [CrossRef]
- Chu, M.H.; Dat, D.Q.; Duy, N.V.; Quang, V.V.; Toan, N.V.; Hieu, N.V.; Hoa, N.D. Facile synthesis of ultrafine rGO/WO3 nanowire nanocomposites for highly sensitive toxic NH3 gas sensors. Mater. Res. Bull. 2020, 125, 110810.1–110810.10. [Google Scholar]
- Maddaka, R.; Chandrakalavathi, T.; Park, B.G.; Murali, G.; Kim, M.D. UV-light enhanced CO gas sensors based on InGaN nanorods decorated with p-Phenylenediamine-graphene oxide composite. Sens. Actuators B Chem. 2020, 307, 127649. [Google Scholar]
- Maddaka, R.; Park, B.G.; Kim, M.D.; Peta, K.R.; Chinh, N.D.; Kim, D.; Kim, S.G.; Murali, G. H2, H2S gas sensing properties of rGO/GaN nanorods at room temperature: Effect of UV illumination. Sens. Actuators B Chem. 2018, 264, 353–362. [Google Scholar]
- Nagornov, I.A.; Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Liquid-Phase Growth of Nanocrystalline ZnO Thin Films and Their Gas-Sensitive Properties. Russ. J. Inorg. Chem. 2022, 67, 539–546. [Google Scholar] [CrossRef]
- Zhang, Q.; Pang, Z.; Hu, W.; Li, J.; Xu, M. Performance degradation mechanism of the light-activated room temperature NO2 gas sensor based on Ag-ZnO nanoparticles. Appl. Surf. Sci. 2020, 541, 148418. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Cheng, X.; Zhang, X.; Huo, L. Fast detection of NO2 by porous SnO2 nanotoast sensor at low temperature. J. Hazard. Mater. 2021, 419, 126414. [Google Scholar] [CrossRef]
- Jhb, A.; Amb, C.; Sh, A.; Hyl, A.; Kys, A.; Sang, S.; Hyoun, W. Realization of low-temperature and selective NO2 sensing of SnO2 nanowires via synergistic effects of Pt decoration and Bi2O3 branching. Ceram. Int. 2020, 47, 5099–5111. [Google Scholar]
- Zhang, F.Z.; Lin, Q.J.; Han, F.; Wang, Z.W.; Tian, B.; Zhao, L.B.; Dong, T.; Jiang, Z.D. A Flexible and Wearable NO2 gas Detection and Early Warning Device based on Spraying Process and Interdigital Electrode at Room Temperature. Microsyst. Nanoeng. 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Navale, Y.H.; Navale, S.T.; Stadler, F.; Ramgirc, N.S.; Patila, V.B. Enhanced NO2 Sensing Aptness of ZnO nanowire/CuO nanoparticle Heterostructure-based Gas Sensors. Ceram. Int. 2018, 45, 1513–1522. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, L.; Zhu, Y.; Sun, Q.; Wang, L. Highly sensitive NO2 sensors based on organic field effect transistors with Al2O3/PMMA bilayer dielectrics by sol-spin coating. Org. Electron. 2019, 74, 69–76. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liu, Y.C. Design of Nanoscaled Surface Morphology of TiO2-Ag2O3 Composite Nanorods through Sputtering Decoration Process and Their Low-Concentration NO2 Gas-Sensing Behaviors. Nanomaterials 2019, 9, 1150. [Google Scholar] [CrossRef] [Green Version]
- Umar, A.; Ibrahim, A.A.; Algadi, H.; Albargi, H.; Akbar, S. Enhanced NO2 Gas Sensor Device based on supramolecularly assembled polyaniline/silver oxide/graphene oxide composites. Ceram. Int. 2021, 18, 25696–25707. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Nagornov, I.A.; Simonenko, T.L.; Simonenkoa, N.P.; Gorobtsov, P.; Arkhipushkin, I.A.; Simonenko, E.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Gas-sensitive nanostructured ZnO films praseodymium and europium doped: Electrical conductivity, selectivity, influence of UV irradiation and humidity. Appl. Surf. Sci. 2022, 589, 152974. [Google Scholar] [CrossRef]
- Liu, X.; Cui, J.; Sun, J.; Zhang, X. 3D graphene aerogel-supported SnO2 nanoparticles for efficient detection of NO2. RSC Adv. 2014, 4, 22601–22605. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhang, X. Novel 3D graphene aerogel-ZnO composites as efficient detection for NO2 at room temperature. Sens. Actuat. B Chem. 2015, 211, 220–226. [Google Scholar] [CrossRef]
- Ta, Q.T.H. Namgung, G.; Noh, J.S. Synthesis of Ag@rGO/g-C3N4 Layered Structures and Their Application to Toxic Gas Sensors: Effect of Ag Nanoparticles. Electron. Mater. Lett. 2019, 15, 750–759. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, D.; Tian, S.; Qin, Z.; Zeng, D.; Xie, C. CuInS2 QDs decorated ring-like NiO for significantly enhanced room-temperature NO2 sensing performances via effective interfacial charge transfer. Sens. Actuators 2018, 256, 1001–1010. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Wang, X.; Xie, C.; Zeng, D. Effect of Heterointerface on NO2 Sensing Properties of In-Situ Formed TiO2 QDs-Decorated NiO Nanosheets. Nanomaterials 2019, 9, 1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathi, K.; Pal, K. Ruthenium decorated tungsten disulfide quantum dots for CO2 gas sensor. Nanotechnology 2019, 31, 135502. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Hu, Z.X.; Zhan, Y.Z.; Li, H.Y.; Gao, N.; Tian, Z.; Zhou, L.; Zhang, B.; Tang, J.; Zhang, J.; et al. MoS2 Nanosheets Sensitized with Quantum Dots for Room Temperature Gas Sensors. Nano Micro Lett. 2020, 12, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purbia, R.; Kwon, Y.M.; Kim, H.D.; Yun, S.L.; Shin, H.; Baik, J.M. Zero-dimensional Heterostructures: N-Doped Graphene Dots/SnO2 for Ultrasensitive and Selective NO2 Gas Sensing at Low Temperatures. J. Mater. Chem. A 2020, 7, 44–56. [Google Scholar] [CrossRef]
- Feng, H.; Yang, S.; Jing, W.; Jiang, K.; Jiang, Z.; Liu, H.; Lei, L. A highly efficient synthetic process of graphene films with tunable optical properties. Appl. Surf. Sci. 2014, 314, 71–77. [Google Scholar]
- Virendra, S.; Daeha, J.; Lei, Z.; Soumen, D.; Saiful, K.; Sudipta, S. Graphene based materials: Past, present and future. Pro. Mater. Sci. 2011, 8, 1178–1271. [Google Scholar]
- Ramli, M.M.; Hanim, K.N.; Muda, M.R.; Isa, S.M.; Jamlos, M.F. The Effect of Different Reduction Methods on Conductivity of Reduced-Graphene Oxide (r-GO). Appl. Mech. Mater. 2015, 815, 216–220. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, G.; Liu, L.; Zhao, Y.; Gao, B.; Zhang, F.; Liu, X.; Yan, X.; Liang, P.; Sun, G.; et al. Highly sensitive sensors based on quasi-2D rGO/SnS2 hybrid for rapid detection of NO2 gas. Sens. Actuat. B Chem 2019, 291, 216–225. [Google Scholar] [CrossRef]
- Chithaiah, P.; Raju, M.M.; Kulkarni, G.U.; Rao, C. Simple synthesis of nanosheets of rGO and nitrogenated rGO. Beilstein J. Nanotechnol. 2020, 11, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Zhang, X.; Gu, W.; Feng, X.; Zhang, L.; Weng, Y. Synthesis of ZnO particles with multi-layer and biomorphic porous microstructures and ZnO/rGO composites and their applications for photocatalysis. Chem. Phys. Lett. 2018, 711, 100–106. [Google Scholar] [CrossRef]
- Qu, G.P.; Fan, G.J.; Zhou, M.J.; Rong, X.; Li, T.; Zhang, R. Graphene-modified ZnO nanostructures for low-temperature NO2 sensing. ACS Omega 2019, 4, 4221–4232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Ma, Y.; Song, P.; Leng, J.; Wang, Q. Gas sensor based on rGO/ZnO aerogel for efficient detection of NO2 at room temperature. J. Mater. Sci. Mater. Electron. 2021, 32, 10058–10069. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Hendi, A.H.; Hossain, M.K.; Yamani, Z.H.; Moqbel, R.A.; Hezam, A. UV-activated gold decorated rGO/ZnO heterostructured nanocomposite sensor for efficient room temperature H2 detection. Sens. Actuators B Chem. 2019, 290, 666–675. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, G.; Sun, J.; Cao, X.; Li, D. The effect of oxygen vacancies in ZnO at an Au/ZnO interface on its catalytic selective oxidation of glycerol. J. Catal. 2019, 377, 271–282. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Zhang, Z.; Yue, J.; Zakaria, Q.M.D.; Chen, C.; Jiang, X.; Yu, A. Crystal plane-dependent gas-sensing properties of zinc oxide nanostructures: Experimental and theoretical studies. Phys. Chem. Chem. Phys. 2014, 16, 11471–11480. [Google Scholar] [CrossRef]
- Hai, B.; Hui, G.; Jin, W.; Yan, D.; Bin, L.; Zi, X.; Fu, G.; Dun, C.; Rong, Z.; You, Z. A room-temperature NO2 gas sensor based on CuO nanoflakes modified with rGO nanosheets. Sens. Actuators B Chem. 2021, 337, 129783. [Google Scholar]
- Geng, X.; Lu, P.; Zhang, C.; Lahem, D.; Olivier, M.; Debliquy, M. Room-temperature NO2 gas sensors based on rGO@ZnO(1–x) composites: Experiments and molecular dynamics simulation. Sens. Actuators 2019, 282, 690–702. [Google Scholar] [CrossRef]
- Patil, M.A.; Vvg, E.; Kyr, B.; Hpd, C.; Shm, D. Fast response and highly selective nitrogen dioxide gas sensor based on Zinc Stannate thin films—ScienceDirect. Mater. Sci. Energy Technol. 2020, 3, 36–42. [Google Scholar]
- Guo, L.; Hao, Y.W.; Li, P.L.; Song, J.F.; Yang, R.Z.; Fu, X.Y.; Xie, S.Y.; Zhao, J.; Zhang, Y.L. Gas Sensing Properties of Graphene Oxide Reduced by Two-beam-laser Interference. Sci. Rep. 2018, 8, 4918. [Google Scholar] [CrossRef]
- Adu, A.; Ggu, B.; Shj, C.; Ngd, C.; Wl, B.; Hkc, C.; Byac, J. ZnO decorated flexible and strong graphene fibers for sensing NO2 and H2S at room temperature. Sens. Actuators B Chem. 2020, 308, 127690. [Google Scholar]
- Jing, L.; Xin, L.; Sun, J. One step solvothermal synthesis of urchin-like ZnO nanorods/graphene hollow spheres and their NO2 gas sensing properties. Ceram. Int. 2015, 42, 2085–2090. [Google Scholar]
- Bhati, V.S.; Ranwa, S.; Rajamani, S.; Kumari, K.; Kumar, M. Improved sensitivity with low limit of detection of hydrogen gas sensor based on rGO loaded Ni doped ZnO nanostructures. ACS Appl. Mater. Inter. 2018, 10, 11116–11124. [Google Scholar] [CrossRef]
- Morsy, M.; Yahia, I.S.; Zahran, H.Y.; Meng, F.; Ibrahim, M. Portable and Battery Operated Ammonia Gas Sensor Based on CNTs/rGO/ZnO Nanocomposite. J. Electron. Mater. 2019, 48, 7328–7335. [Google Scholar] [CrossRef]
- Qiu, J.W.; Hu, X.; Min, X.; Quan, W.; Wang, X. The observation of switchable dual conductive channels and related nitric oxide gas sensing properties in N-rGO/ZnO heterogeneous structure. ACS Appl. Mater. Inter. 2020, 12, 19755–19767. [Google Scholar] [CrossRef] [PubMed]


| Sensor Structure | NO2 (ppm) | Temp (°C) | Response (S) | Tres/Trec (s) | Baseline Drift | Ref. |
|---|---|---|---|---|---|---|
| Zn2SnO4 film | 40 | 200 | 29.3 Rg/R0 | 8/58 | No | Patil et al. [49] |
| RGO (two-beam laser interference) | 20 | RT | 27% △R/R0 | 10/7 | Yes | Guo et al. [51] |
| ZnO/graphene aerogel | 100 | RT | 8.9% △R/R0 | ~500/~500 | Yes | Liu et al. [29] |
| rGO/ZnO flowers and nanoparticles | 1.5 | RT | 1.4% △R/R0 | 405/760 | No | Adu et al. [51] |
| rGO/ZnO nanorods | 100 | RT | 17.4% (△R/R0) | 780/1980 | Yes | Jing et al. [52] |
| rGO/ZnO QDs | 100 | RT | 49.6% △R/R0 | 216/668 | No | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Q.; Zhang, F.; Zhao, N.; Zhao, L.; Wang, Z.; Yang, P.; Lu, D.; Dong, T.; Jiang, Z. A Flexible and Wearable Nylon Fiber Sensor Modified by Reduced Graphene Oxide and ZnO Quantum Dots for Wide-Range NO2 Gas Detection at Room Temperature. Materials 2022, 15, 3772. https://doi.org/10.3390/ma15113772
Lin Q, Zhang F, Zhao N, Zhao L, Wang Z, Yang P, Lu D, Dong T, Jiang Z. A Flexible and Wearable Nylon Fiber Sensor Modified by Reduced Graphene Oxide and ZnO Quantum Dots for Wide-Range NO2 Gas Detection at Room Temperature. Materials. 2022; 15(11):3772. https://doi.org/10.3390/ma15113772
Chicago/Turabian StyleLin, Qijing, Fuzheng Zhang, Na Zhao, Libo Zhao, Zuowei Wang, Ping Yang, Dejiang Lu, Tao Dong, and Zhuangde Jiang. 2022. "A Flexible and Wearable Nylon Fiber Sensor Modified by Reduced Graphene Oxide and ZnO Quantum Dots for Wide-Range NO2 Gas Detection at Room Temperature" Materials 15, no. 11: 3772. https://doi.org/10.3390/ma15113772







