Processing and Mechanical Properties of Highly Filled PP/GTR Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
2.3. Rheological Properties Measurements
2.4. FTIR Analysis of the Compounds Chemical Composition
2.5. Measurements of Thermal Properties
2.6. Measurements of Mechanical Properties
2.7. Measurements of Morphology
3. Results
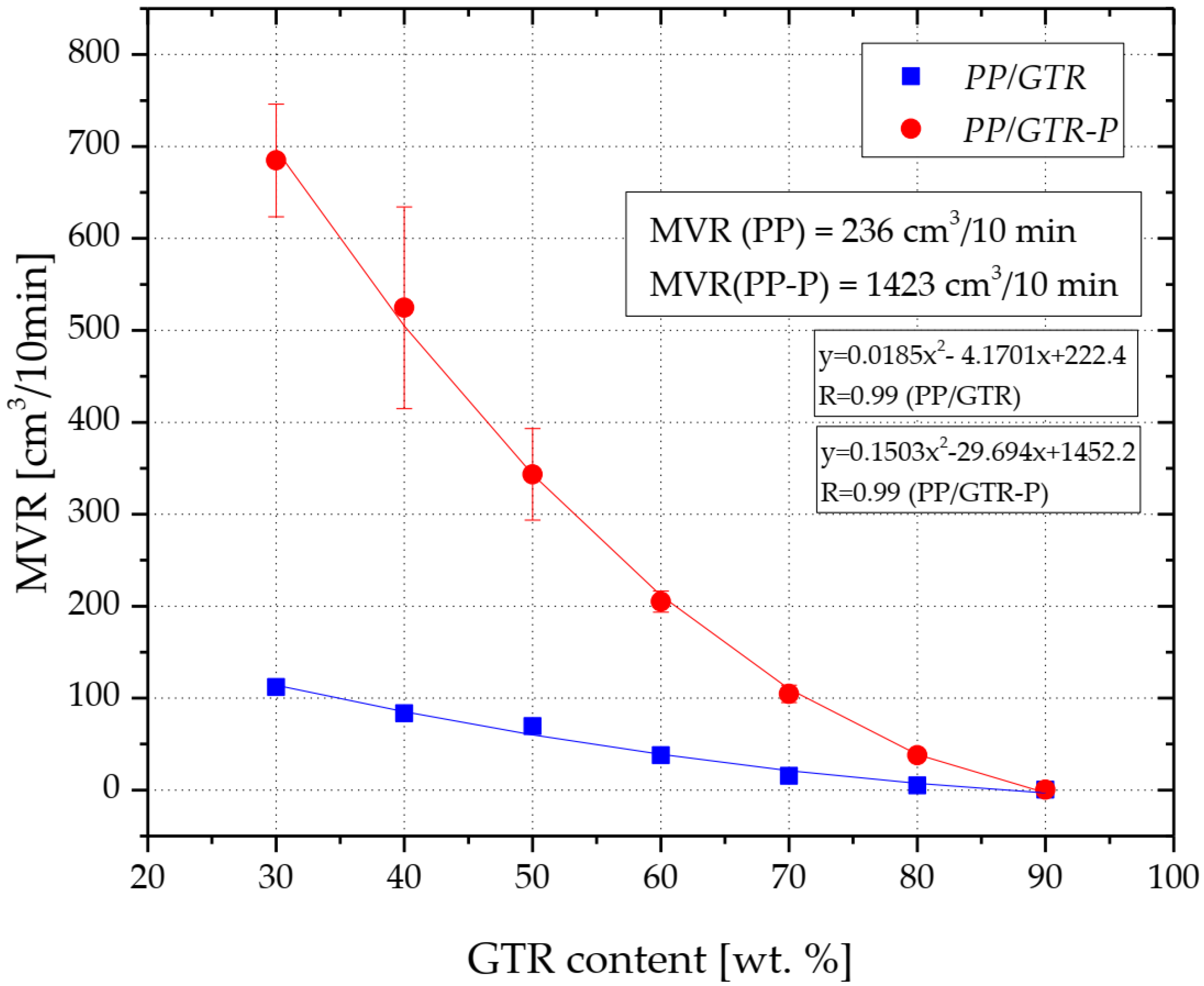
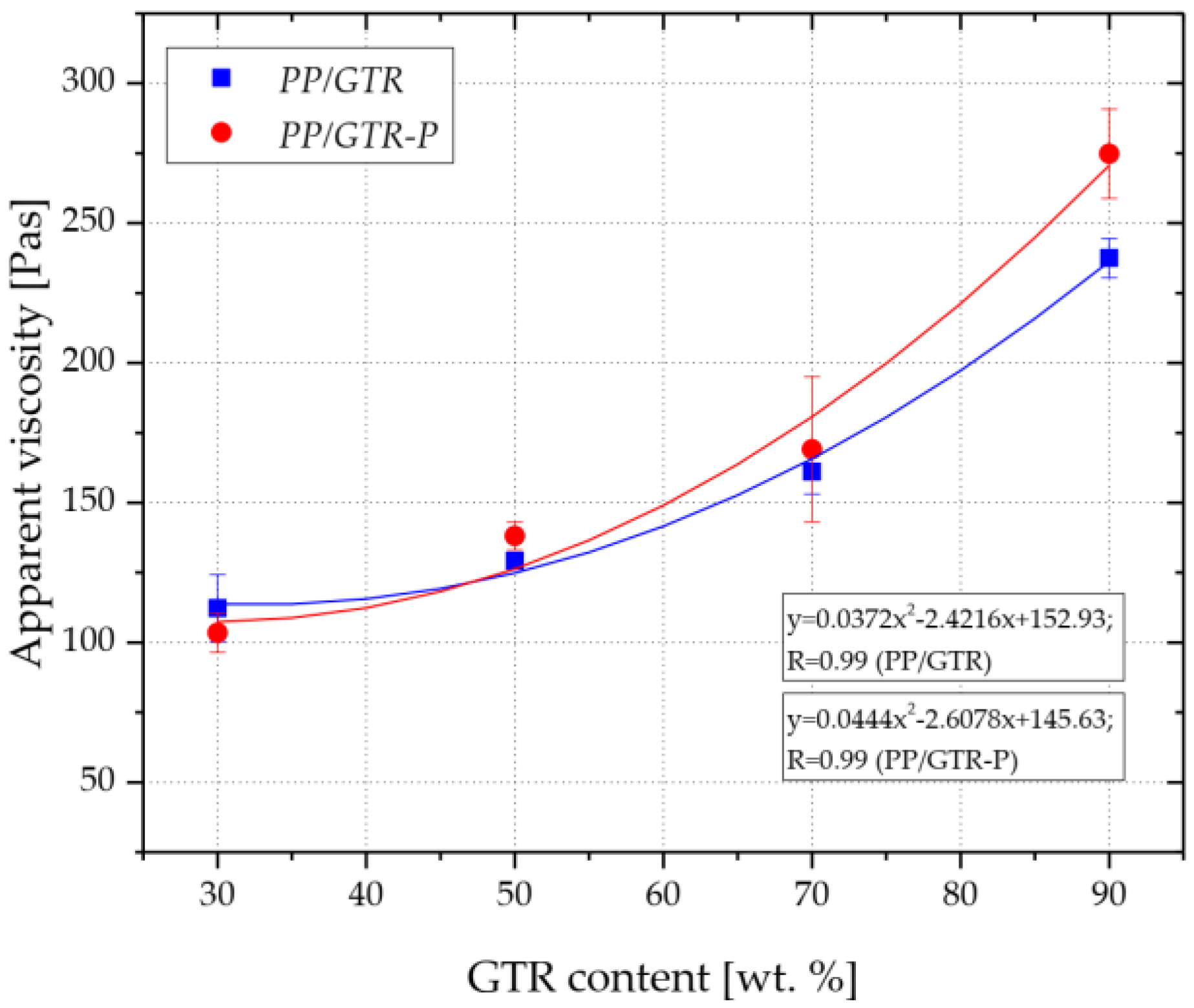
3.1. Rheological Properties
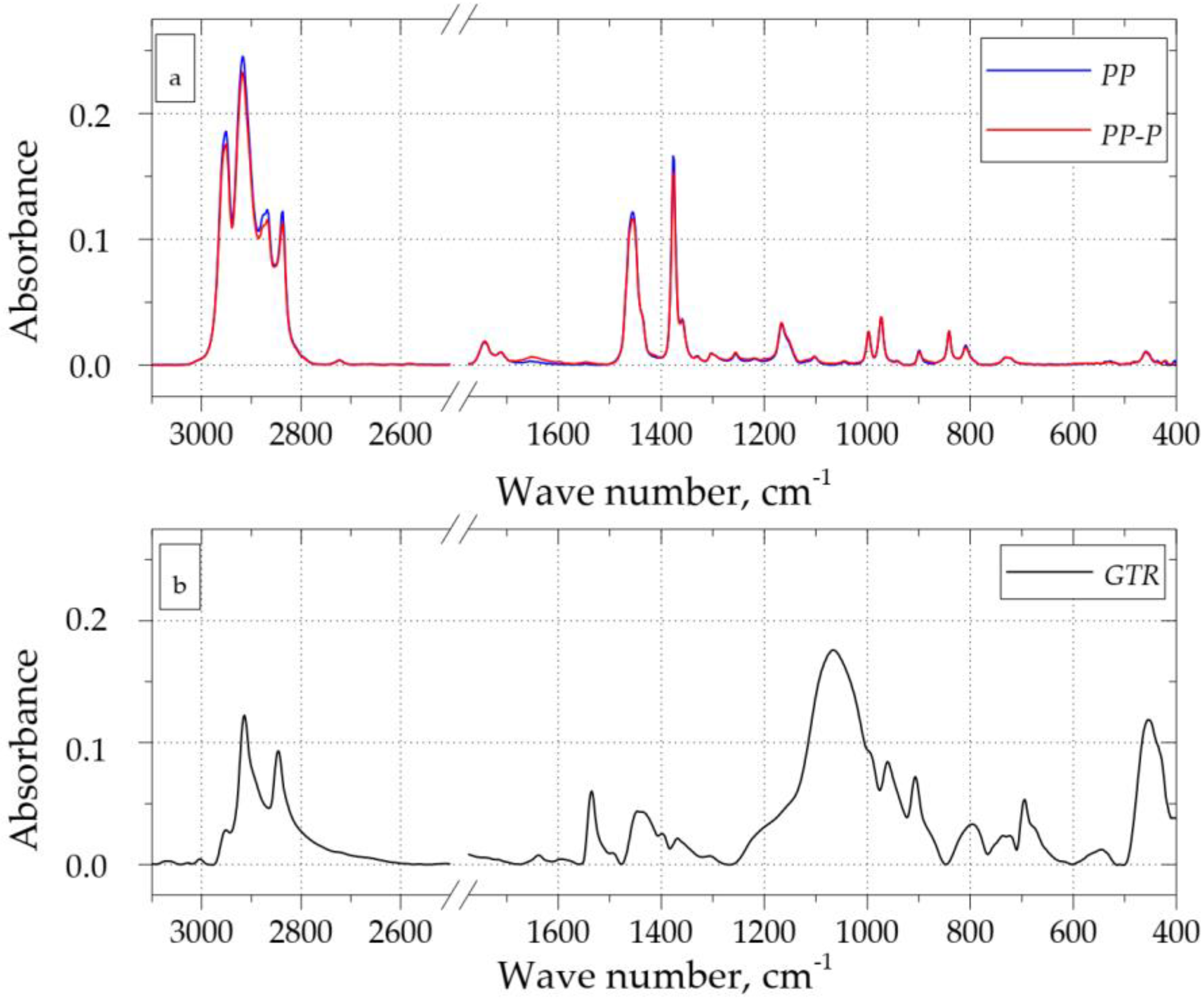
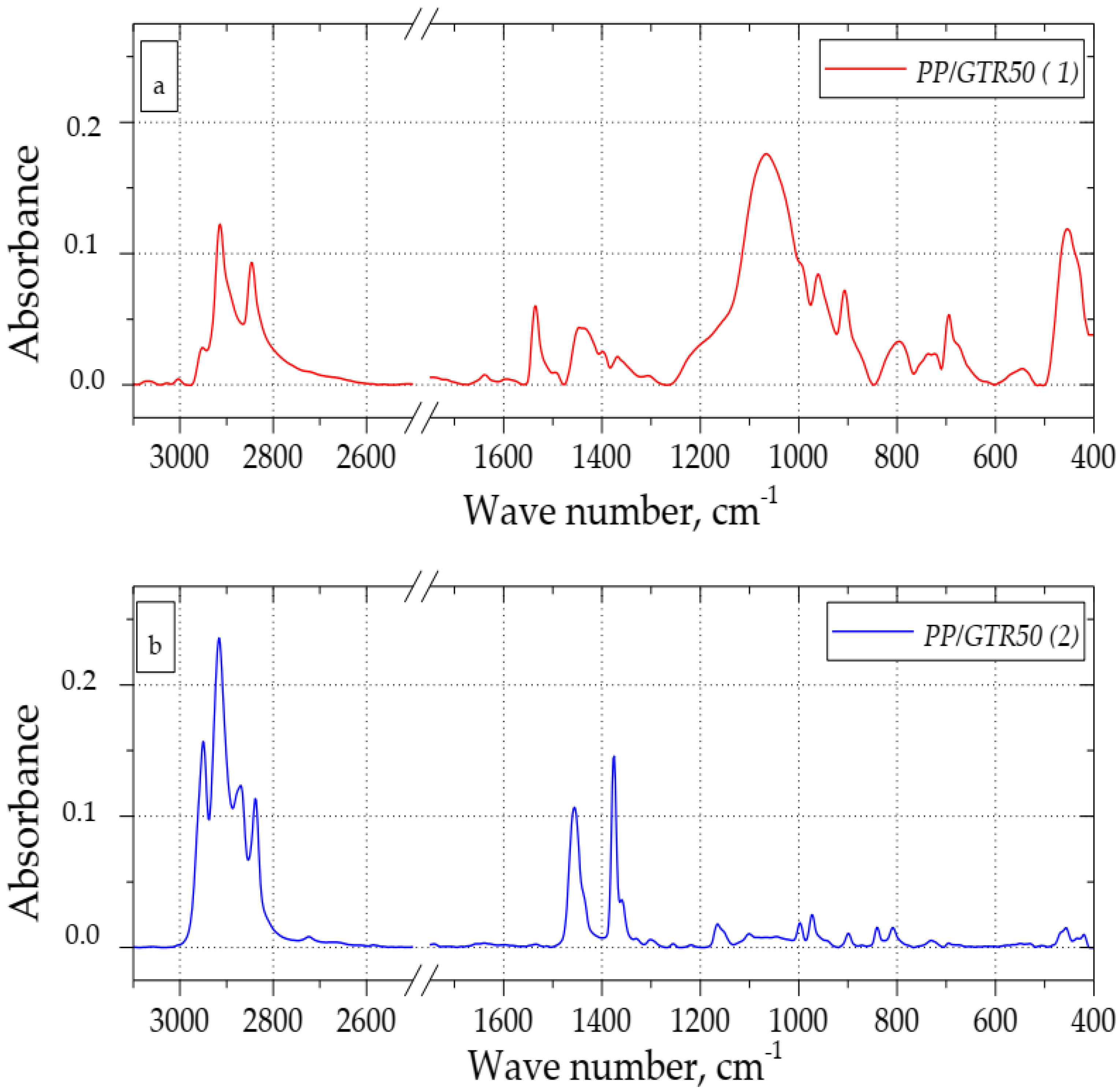
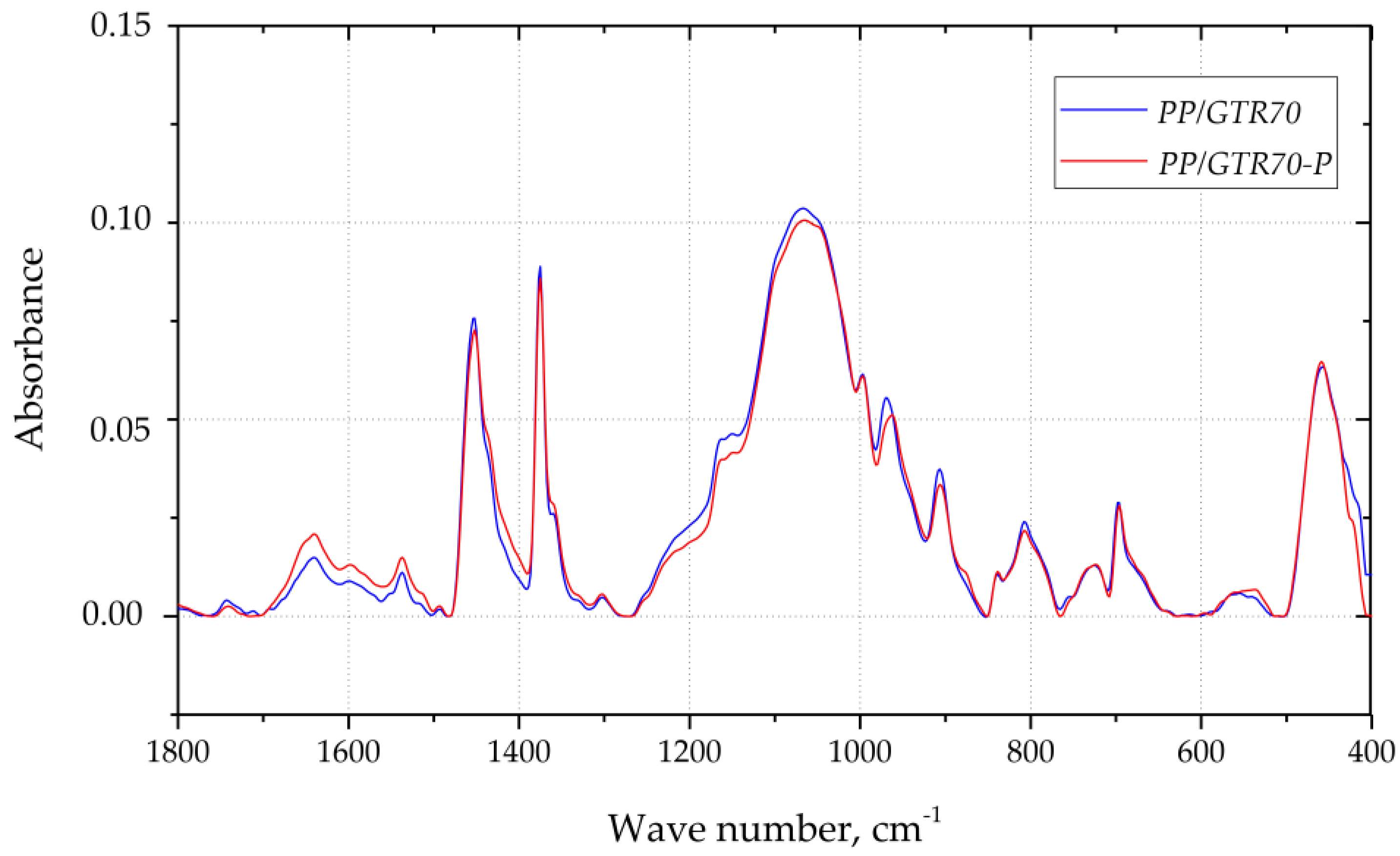
3.2. FT-IR Analysis
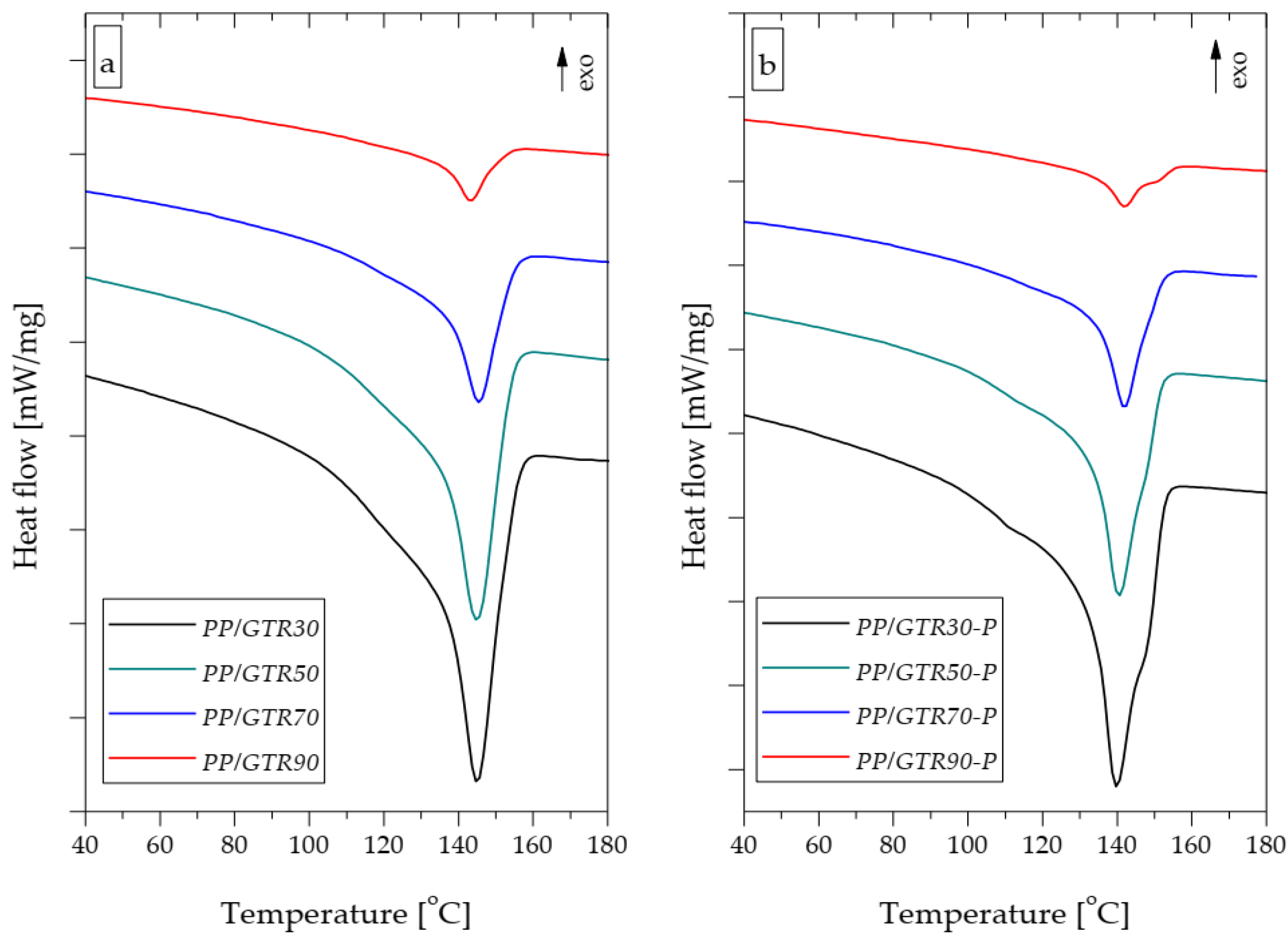
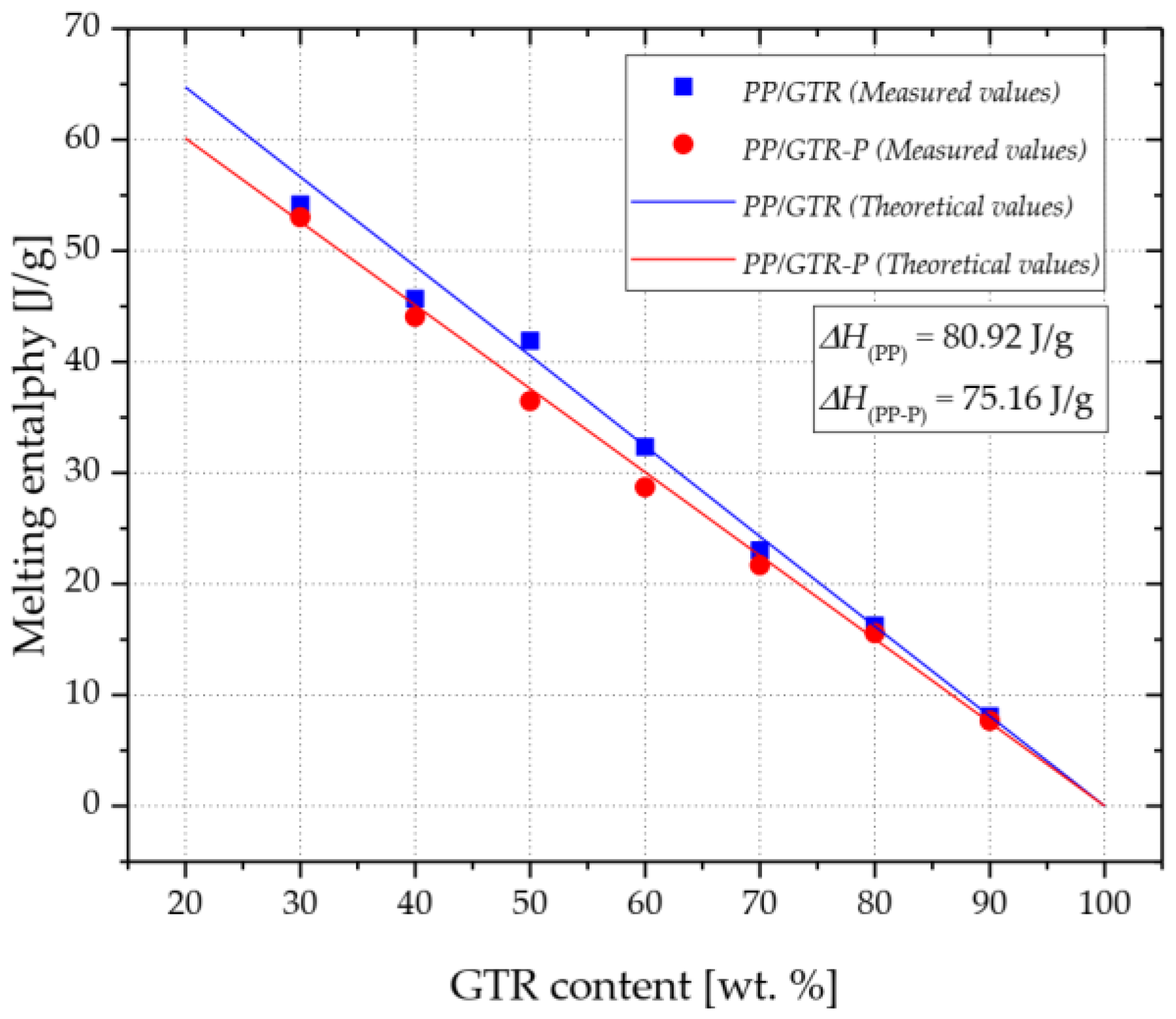
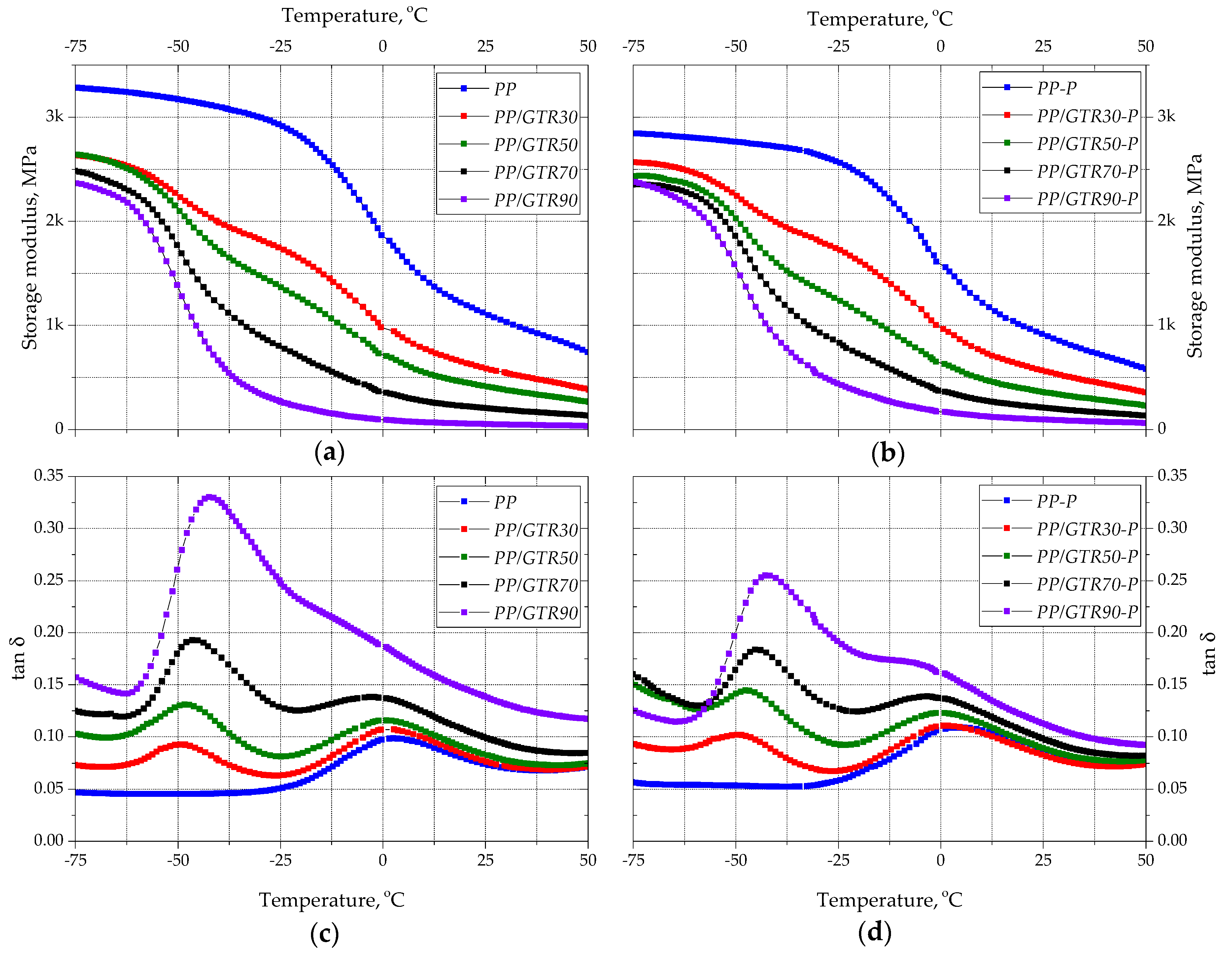
3.3. Thermal Properties
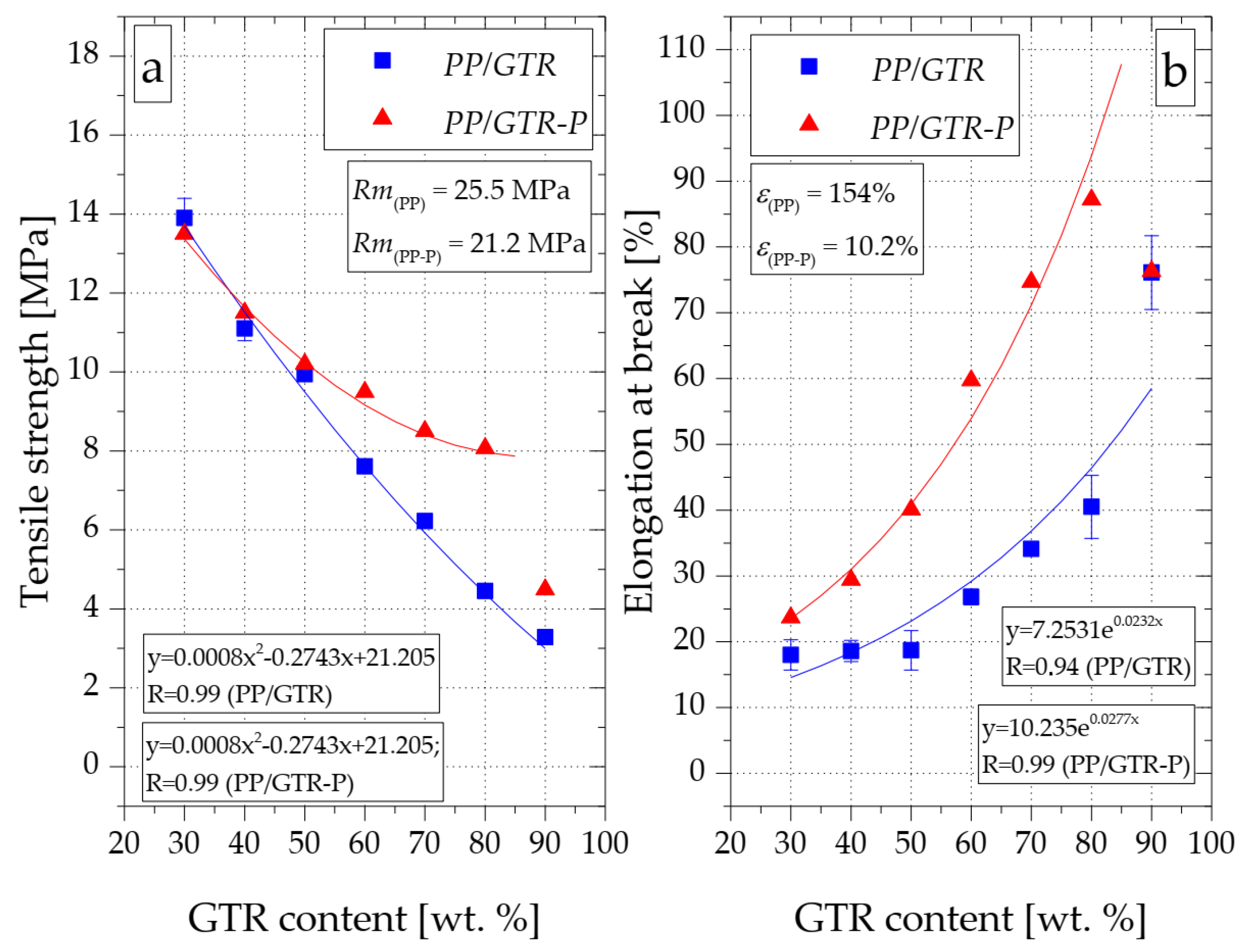
3.4. Mechanical Properties
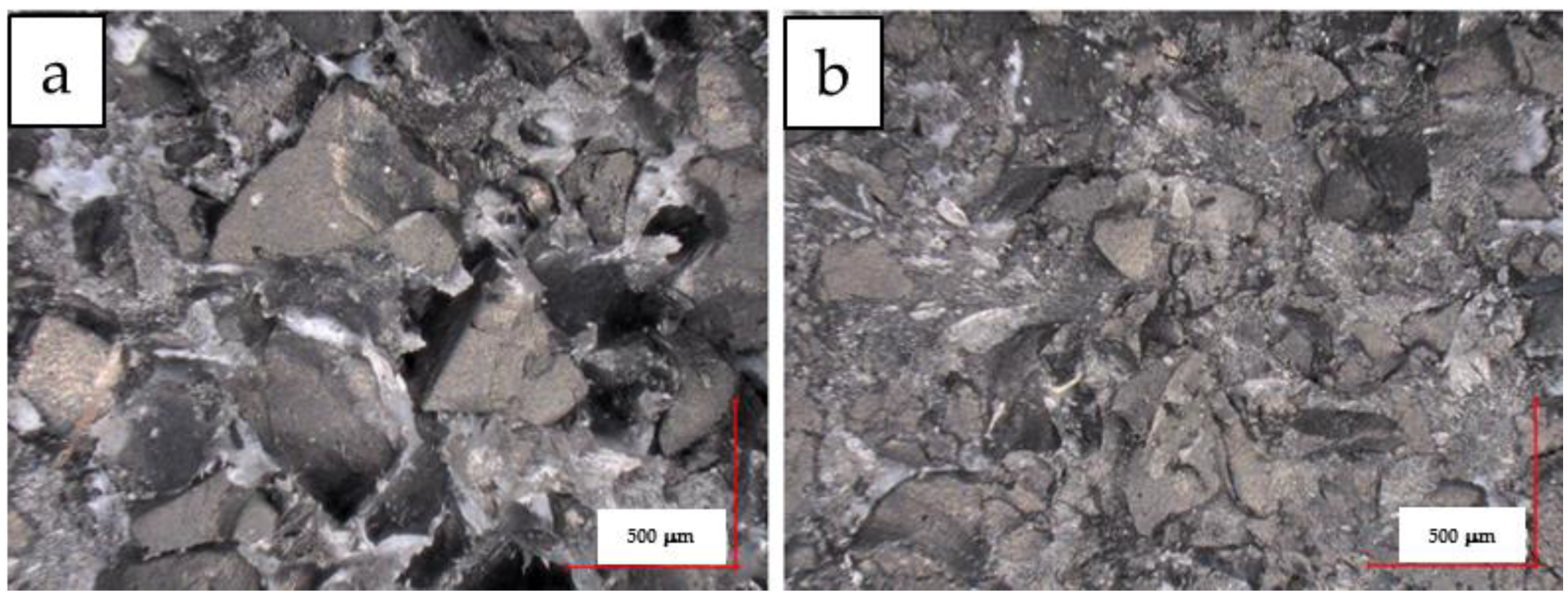
3.5. Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kościuszko, A.; Sterzyński, T.; Piszczek, K. Multilayer hybrid polypropylene composite with single and wood-polymer composites layers. Polimery 2018, 63, 755–761. [Google Scholar] [CrossRef]
- Kościuszko, A.; Czyżewski, P.; Wajer, Ł.; Ościak, A.; Bieliński, M. Properties of polypropylene composites filled with microsilica waste. Polimery 2020, 65, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Barczewski, M.; Andrzejewski, J.; Majchrowski, R.; Dobrzycki, K.; Formela, K. Mechanical Properties, Microstructure and Surface Quality of Polypropylene Green Composites as a Function of Sunflower Husk Waste Filler Particle Size and Content. J. Renew. Mater. 2021, 9, 841–853. [Google Scholar] [CrossRef]
- Gozdecki, C.; Wilczyński, A.; Kociszewski, M.; Zajchowski, S. Properties of wood–plastic composites made of milled particleboard and polypropylene. Eur. J. Wood Wood Prod. 2015, 73, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, K.; Piszczek, K.; Zajchowski, S.; Mirowski, J. Rheological properties of wood polymer composites at high shear rates. Polym. Test. 2016, 51, 58–62. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Lewandowski, K.; Daniel Nowak, D.; Matykiewicz, D.; Andrzejewski, J.; Skórczewska, K.; Piasecki, A. Effect of Basalt Powder Surface Treatments on Mechanical and Processing Properties of Polylactide-Based Composites. Materials 2020, 13, 5436. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.; Sohn, J.S.; Kweon, B.C.; Cha, S.W. Shrinkage Optimization in Talc- and Glass-Fiber-Reinforced Polypropylene Composites. Materials 2019, 12, 764. [Google Scholar] [CrossRef] [Green Version]
- Kościuszko, A.; Marciniak, D.; Sykutera, D. Post-Processing time dependence of shrinkage and mechanical properties of injection-molded polypropylene. Materials 2021, 14, 22. [Google Scholar] [CrossRef]
- Bazan, P.; Nosal, P.; Kozub, B.; Kuciel, S. Biobased Polyethylene Hybrid Composites with Natural Fiber: Mechanical, Thermal Properties, and Micromechanics. Materials 2020, 13, 2967. [Google Scholar] [CrossRef]
- Pokorný, J.; Šál, J.; Ševčík, R. The Role of Processing Procedures on Properties of Waste Tires Recycled Products. AIP Conf. Proc. 2021, 2429, 020029. [Google Scholar]
- Formela, K. Sustainable development of waste tires recycling technologies—Recent advances, challenges and future trends. Adv. Ind. Eng. Polym. Res. 2021, 4, 209–222. [Google Scholar] [CrossRef]
- Hoyer, S.; Kroll, L.; Sykutera, D. Technology comparison for the production of fine rubber powder from end of life tyres. Procedia Manuf. 2020, 43, 193–200. [Google Scholar] [CrossRef]
- Tamayo, A.; Rubio, F.; Pérez-Aparicio, R.; Saiz-Rodríguez, L.; Rubio, J. Preparation and properties of sustainable brake pads with recycled end-of-life tire rubber particles. Polymers 2021, 13, 3371. [Google Scholar] [CrossRef] [PubMed]
- Kosmela, P.; Olszewski, A.; Zedler, Ł.; Burger, P.; Formela, K.; Hejna, A. Ground tire rubber filled flexible polyurethane foam—effect of waste rubber treatment on composite performance. Materials 2021, 14, 3807. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Morera, J.; Verdugo-Manzanares, R.; González, S.; Verdejo, R.; Lopez-Manchado, M.A.; Santana, M.H. On the use of mechano-chemically modified ground tire rubber (GTR) as recycled and sustainable filler in styrene-butadiene rubber (SBR) composites. J. Compos. Sci. 2021, 5, 68. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mészáros, L.; Bárány, T. Ground tyre rubber (GTR) in thermoplastics, thermosets, and rubbers. J. Mater. Sci. 2013, 48, 1–38. [Google Scholar] [CrossRef]
- Serdar, M.; Baričević, A.; Bjegović, D.; Lakušić, S. Possibilities of use of products from waste tyre recycling in concrete industry. J. Appl. Eng. Sci. 2014, 12, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Serdar, M.; Baričević, A.; Rukavina, M.J.; Pezer, M.; Bjegović, D.; Štirmer, N. Shrinkage behaviour of fibre reinforced concrete with recycled tyre polymer fibres. Int. J. Polym. Sci. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Zhou, S.; Wang, S. Structural evolution of recycled tire rubber in asphalt. J. Appl. Polym. Sci. 2016, 133, 42954. [Google Scholar] [CrossRef]
- Fernández-Ruiz, R.; Redrejo, M.J.; Pérez-Aparicio, R.; Saiz-Rodríguez, L. Quantification of recycled rubber content of end-of-life tyres in asphalt bitumen by total-reflection X-ray fluorescence spectrometry, Spectrochim. Acta Part B At. Spectrosc. 2020, 166, 105803. [Google Scholar] [CrossRef]
- Alkadi, F.; Lee, J.; Yeo, J.S.; Hwang, S.H.; Choi, J.W. 3D Printing of Ground Tire Rubber Composites. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 211–222. [Google Scholar] [CrossRef]
- Dou, Y.; Rodrigue, D. Rotomolding of Foamed and Unfoamed GTR-LLDPE Blends: Mechanical, Morphological and Physical Properties. Cell. Polym. 2018, 37, 55–68. [Google Scholar] [CrossRef]
- Kakroodi, A.R.; Rodrigue, D. Highly filled thermoplastic elastomers from ground tire rubber, maleated polyethylene and high density polyethylene. Plast. Rubber Compos. 2013, 42, 115–122. [Google Scholar] [CrossRef]
- Mujal-Rosas, R.; Marin-Genesca, M.; Ballart-Prunell, J. Dielectric properties of various polymers (PVC, EVA, HDPE, and PP) reinforced with ground tire rubber (GTR). Sci. Eng. Compos. Mater. 2015, 22, 231–243. [Google Scholar] [CrossRef]
- Hrdlička, Z.; Trnka, T.; Čadek, D.; Kadeřábková, A.; Kuta, A. Thermoplastic blends based on waste tyre rubber and polyamide 12. KGK Kautsch. Gummi Kunstst. 2018, 71, 18–21. [Google Scholar]
- Lu, Y.; Yang, Y.; Xiao, P.; Feng, Y.; Liu, L.; Tian, M.; Li, X.; Zhang, L. Effect of interfacial enhancing on morphology, and rheological properties of polypropylene-ground tire rubber powder blend. J. Appl. Polym. Sci. 2017, 134, 45354. [Google Scholar] [CrossRef]
- Lima, P.S.; Oliveira, J.M.; Costa, V.A.F. Partial replacement of EPR by GTR in highly flowable PP/EPR blends: Effects on morphology and mechanical properties. J. Appl. Polym. Sci. 2015, 132, 42011. [Google Scholar] [CrossRef]
- Lima, P.S.; Oliveira, J.M.; Costa, V.A.F. Crystallization kinetics of thermoplastic elastomeric blends based on ground tyre rubber. J. Appl. Polym. Sci. 2015, 132, 42589. [Google Scholar] [CrossRef]
- Basso, A.; Zhang, Y.; Linnemann, L.; Hansen, H.N. Study of the distribution of rubber particles in ground tire rubber/polypropylene blends. Mater. Today Proc. 2021, 34, 311–316. [Google Scholar] [CrossRef]
- Egodage, S.M.; Harper, J.F.; Walpalage, S. Ground Tyre Rubber/Waste Polypropylene Blends—Effect of Composition on Mechanical Properties. Prog. Rubber Plast. Recycl. Technol. 2009, 25, 213–231. [Google Scholar] [CrossRef]
- Mujal-Rosas, R.; Orrit-Prat, J.; Ramis-Juan, X.; Marin-Genesca, M.; Rahhali, A. Study on dielectric, mechanical and thermal properties of polypropylene (PP) composites with ground tyre rubber (GTR). Polym. Polym. Compos. 2012, 20, 797–808. [Google Scholar] [CrossRef]
- Majewska-Laks, K.; Sykutera, D.; Osciak, A. Reuse of ground tire rubber (GTR) as a filler of TPE matrix. MATEC Web Conf. 2021, 332, 01003. [Google Scholar] [CrossRef]
- Lima, P.; da Silva, S.P.M.; Oliveira, J.; Costa, V. Rheological properties of ground tyre rubber based thermoplastics elastomeric blends. Polym. Test. 2015, 45, 58–67. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Rocha, J.S.; Pacheco, E.B.V.; Boucas, T. Mechanical and morphological properties of polypropylene and regenerated tire-rubber blends. Int. J. Polym. Mater. Polym. Biomater. 2008, 57, 555–568. [Google Scholar] [CrossRef]
- Elenien, K.F.A.; Abdel-Wahab, A.; El Gamsy, R.; Abdellatif, M.H. Assessment of the properties of PP composite with addition of recycled tire rubber. Ain Shams Eng. J. 2018, 9, 3271–3276. [Google Scholar] [CrossRef]
- Hernández Gámez José, F.; Hernández Ernesto, H.; Narro Céspedes Rosa, I.; Neira Velázquez María, G.; Solís Rosales Silvia, G.; Soriano Corral, F.; González Morones, P.; Fernández Tavizón, S.; Díaz de Leon, R.; Farías Cepeda, L. Mechanical reinforcement of thermoplastic vulcanizates using ground tyre rubber modified with sulfuric acid. Polym. Compos. 2016, 39, 229–237. [Google Scholar] [CrossRef]
- Hernandez, E.H.; Gamez, J.F.H.; Cepeda, L.F.; Munoz, E.J.C.; Corral, F.S.; Rosales, S.G.S.; Velazquez, G.N.; Morones, P.G.; Martinez, D.I.S. Sulfuric acid treatment of ground tire rubber and its effect on the mechanical and thermal properties of polypropylene composites. J. Appl. Polym. Sci. 2017, 134, 44858. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M.; Lee, H.; Jeong, H.; Park, Y.; Jhee, K.-H.; Bang, D. Effects of peroxides on the properties of reclaimed polypropylene/waste ground rubber tire composites prepared by twin screw extrusion. Elastomers Compos. 2016, 51, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Wagenknecht, U.; Wiessner, S.; Heinrich, G.; Michael, H.; Zichner, M. Effects of interface reactions in compatibilised ground tyre rubber polypropylene elastomeric alloys. Plast. Rubber Compos. 2006, 35, 393–400. [Google Scholar] [CrossRef]
- Coutinho, F.M.B.; Costa, T.H.S. Controlled degradation of polypropylene in solution by organic peroxide. Polym. Test. 1994, 13, 13363–13366. [Google Scholar] [CrossRef]
- Konieczka, R.; Kaluzny, W.; Sykutera, D. Fine rubber grinding by rotational cutting. KGK-Kautsch. Gummi Kunstst. 1997, 50, 641–644. [Google Scholar]
- Berzin, F.; Vergnes, B.; Delmare, L. Rheological behavior of controlled-rheology polypropylenes obtained by peroxide-promoted degradation during extrusion: Comparison between homopolimer and copolymer. J. Appl. Polym. Sci. 2001, 80, 1243–1252. [Google Scholar] [CrossRef]
- Herlambang, B.; Bramantika, S. Controlling polypropylene rheological properties by promoting organic peroxide during extrusion with improved properties for automotive applications. J. Phys. Conf. Ser. 2019, 1127, 012030. [Google Scholar] [CrossRef] [Green Version]
- Sykutera, D.; Czyżewski, P.; Kościuszko, A.; Szewczykowski, P.; Wajer, L.; Bieliński, M. Monitoring of the injection and holding phases by using a modular injection mold. J. Polym. Eng. 2018, 38, 63–71. [Google Scholar] [CrossRef]
- Sykutera, D.; Czyżewski, P.; Szewczykowski, P. The Microcellular Structure of Injection Molded 686 Thick-Walled Parts as Observed by In-Line Monitoring. Materials 2020, 13, 5464. [Google Scholar] [CrossRef]
- Yoon, L.K.; Choi, C.H.; Kim, B.K. Reactive extrusion of PP/natural rubber blends. J. Appl. Polym. Sci. 1995, 56, 239–246. [Google Scholar] [CrossRef]
- Rooj, S.; Basak, G.C.; Maji, P.K.; Bhowmick, A.K. New route for devulcanization of natural rubber and the properties of devulcanizated rubber. J. Polym. Environ. 2011, 19, 382–390. [Google Scholar] [CrossRef]

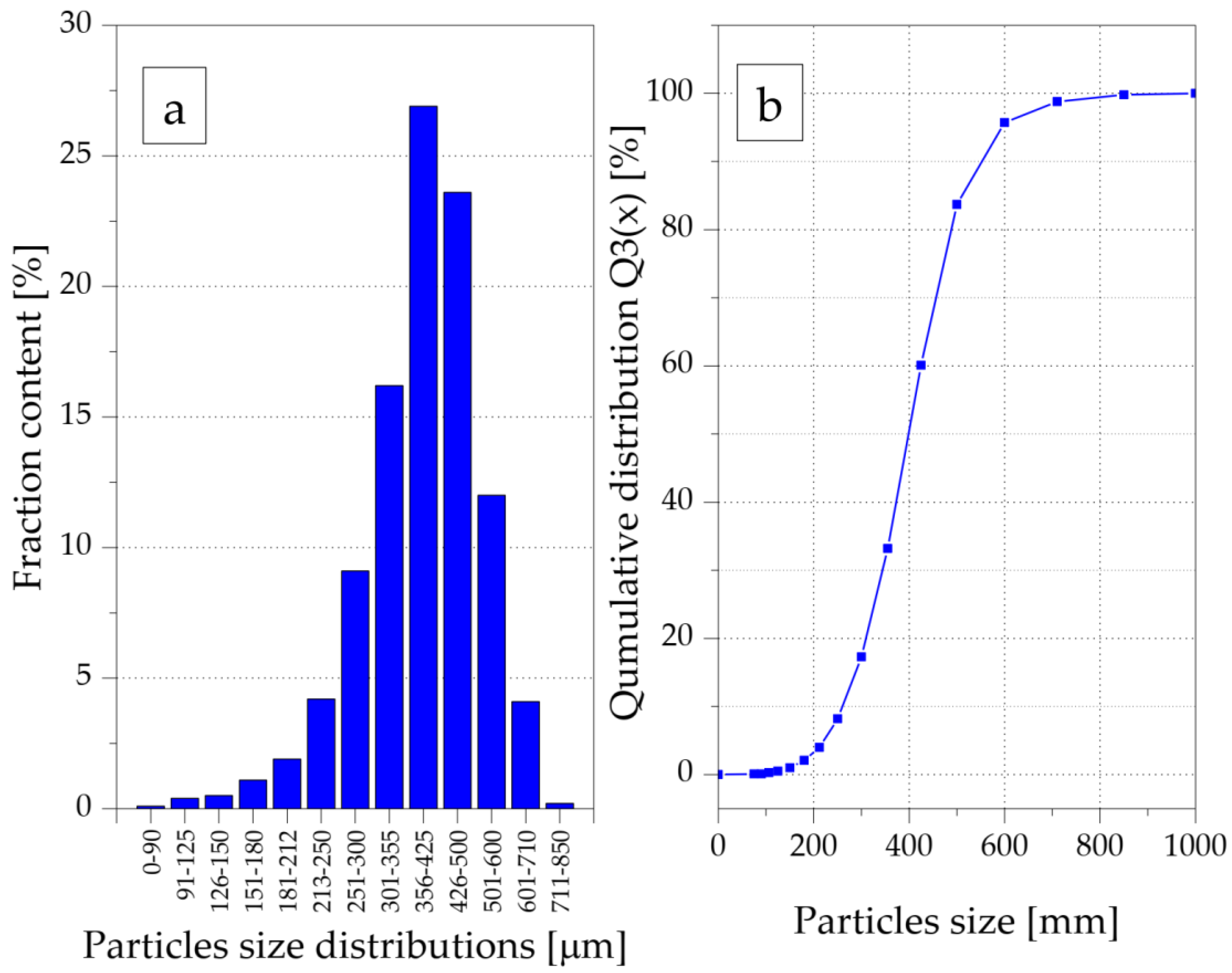
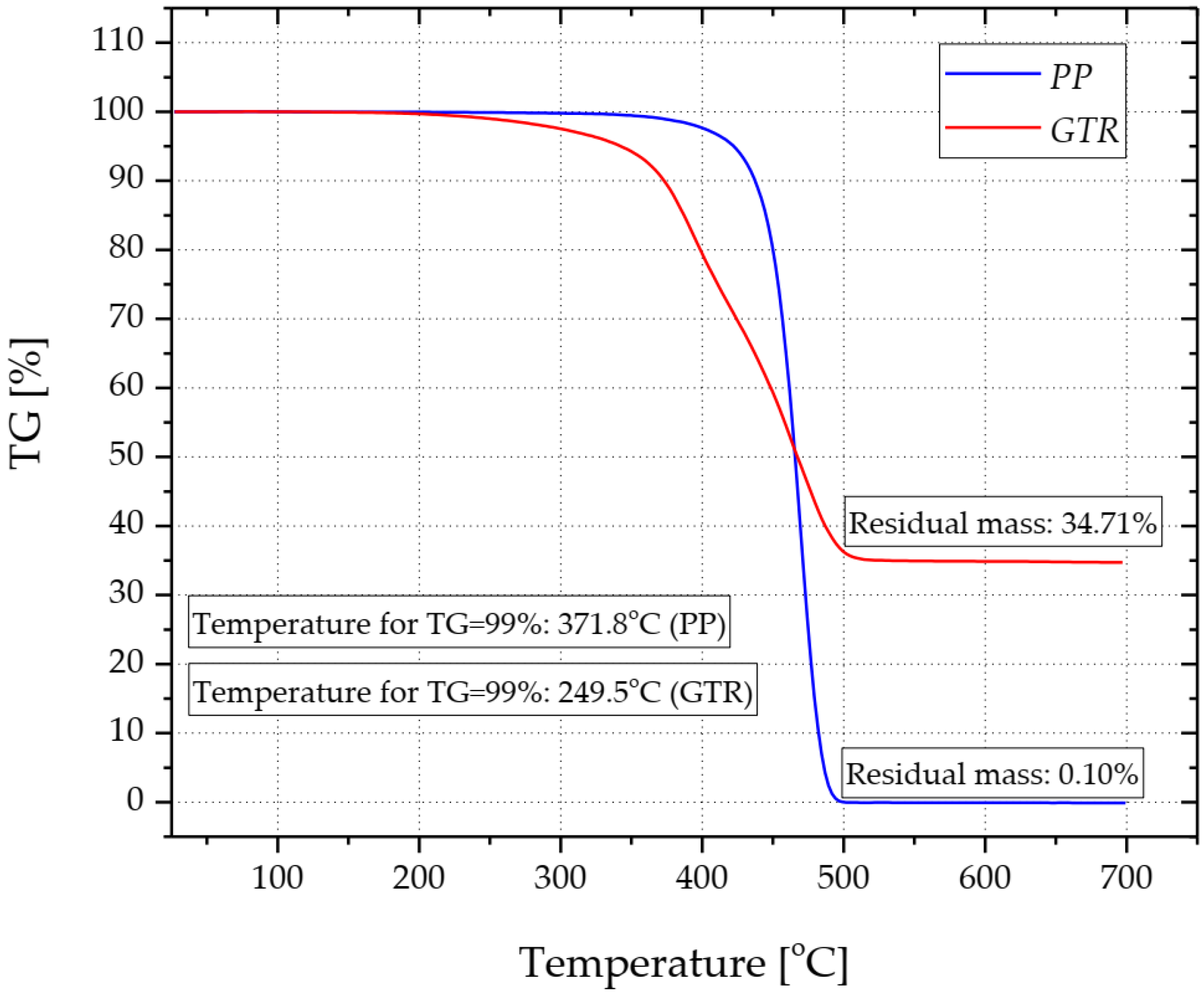
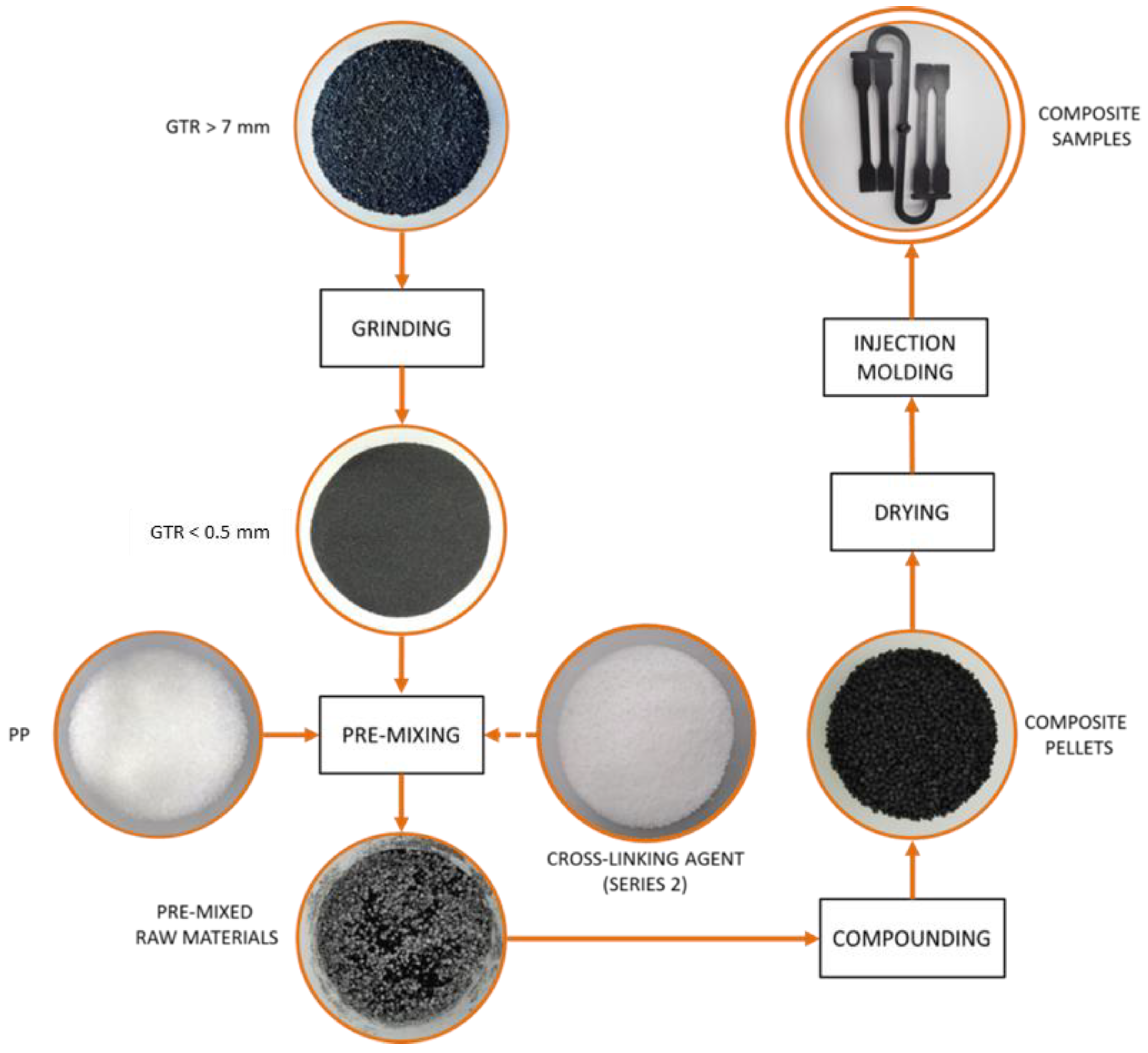
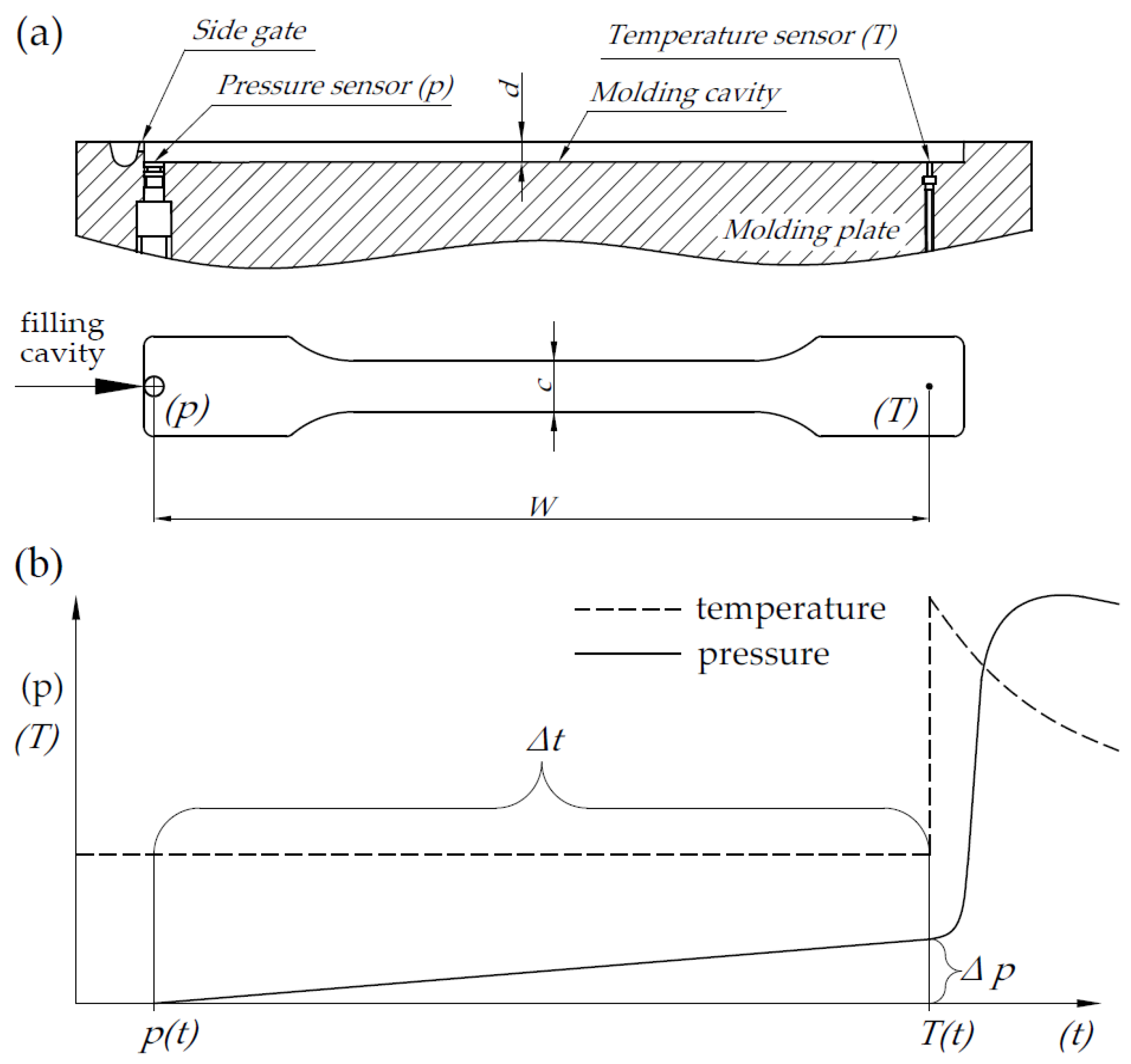



| Series | Signature | Content (wt.%) | ||
|---|---|---|---|---|
| PP | GTR | Organic Peroxide | ||
| 1 (PP/GTR) | PP | 100 | 0 | 0 |
| PP/GTR30 | 70 | 30 | 0 | |
| PP/GTR40 | 60 | 40 | 0 | |
| PP/GTR50 | 50 | 50 | 0 | |
| PP/GTR60 | 40 | 60 | 0 | |
| PP/GTR70 | 30 | 70 | 0 | |
| PP/GTR80 | 20 | 80 | 0 | |
| PP/GTR90 | 10 | 90 | 0 | |
| 2 (PP/GTR-P) | PP-P | 98.5 | 0 | 1.5 |
| PP/GTR30-P | 68.5 | 30 | 1.5 | |
| PP/GTR40-P | 58.5 | 40 | 1.5 | |
| PP/GTR50-P | 48.5 | 50 | 1.5 | |
| PP/GTR60-P | 38.5 | 60 | 1.5 | |
| PP/GTR70-P | 28.5 | 70 | 1.5 | |
| PP/GTR80-P | 18.8 | 80 | 1.5 | |
| PP/GTR90-P | 8.5 | 90 | 1.5 | |
| Samples | Share Stress (Pa) | Share Rate (1/s) | Samples | Share Stress (Pa) | Share Rate (1/s) |
|---|---|---|---|---|---|
| PP | 84,355 | 1221 | PP-P | 16,008 | 1266 |
| PP/GTR30 | 164,270 | 1438 | PP/GTR30-P | 165,630 | 1575 |
| PP/GTR50 | 191,159 | 1456 | PP/GTR50-P | 231,241 | 1648 |
| PP/GTR70 | 254,190 | 1553 | PP/GTR70-P | 283,768 | 1655 |
| PP/GTR90 | 378,003 | 1567 | PP/GTR90-P | 414,083 | 1486 |
| GTR Content, wt.% | Injection Pressure, MPa | Pressure in Mold Cavity, MPa | ||
|---|---|---|---|---|
| PP/GTR | PP/GTR-P | PP/GTR | PP/GTR-P | |
| 0 | 36.9 | 8.0 | 18.7 | 20.5 |
| 30 | 44.6 | 54.3 | 24.0 | 44.8 |
| 50 | 51.3 | 66.2 | 23.9 | 48.9 |
| 70 | 62.4 | 79.9 | 31.9 | 51.8 |
| 90 | 94.0 | 117.7 | 42.5 | 41.5 |
| Sample | Tc, °C | Tm, °C | Determined Content of GTR, wt.% |
|---|---|---|---|
| PP | 119.5 | 150.0 | - |
| PP/GTR30 | 107.6 | 144.9 | 33.96 |
| PP/GTR40 | 107.1 | 145.1 | 43.51 |
| PP/GTR50 | 107.3 | 144.9 | 47.37 |
| PP/GTR60 | 106.6 | 145.5 | 59.44 |
| PP/GTR70 | 105.5 | 145.6 | 71.54 |
| PP/GTR80 | 105.4 | 144.5 | 78.60 |
| PP/GTR90 | 105.2 | 143.2 | 89.41 |
| PP-P | 119.0 | 146.0 | - |
| PP/GTR30-P | 103.1 | 139.8 | 30.64 |
| PP/GTR40-P | 103.3 | 139.9 | 40.51 |
| PP/GTR50-P | 104.5 | 140.5 | 51.42 |
| PP/GTR60-P | 105.7 | 141.2 | 61.56 |
| PP/GTR70-P | 104.7 | 142.0 | 70.84 |
| PP/GTR80-P | 105.3 | 140.8 | 79.35 |
| PP/GTR90-P | 106.3 | 142.2 | 89.68 |
| Filler Content (wt.%) | Charpy Impact Strength (kJ/m2) | |
|---|---|---|
| Temperature 23 °C | ||
| PP/GTR | PP/GTR-P | |
| 0 | nb | nb |
| 30 | 23.8 | 24.8 |
| 40 | 25.3 | 34.3 |
| 50 | 27.8 | nb |
| 60 | 34.0 | nb |
| 70 | nb | nb |
| 80 | nb | nb |
| 90 | nb | nb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kościuszko, A.; Sykutera, D.; Czyżewski, P.; Hoyer, S.; Kroll, L.; Szczupak, B. Processing and Mechanical Properties of Highly Filled PP/GTR Compounds. Materials 2022, 15, 3799. https://doi.org/10.3390/ma15113799
Kościuszko A, Sykutera D, Czyżewski P, Hoyer S, Kroll L, Szczupak B. Processing and Mechanical Properties of Highly Filled PP/GTR Compounds. Materials. 2022; 15(11):3799. https://doi.org/10.3390/ma15113799
Chicago/Turabian StyleKościuszko, Artur, Dariusz Sykutera, Piotr Czyżewski, Stefan Hoyer, Lothar Kroll, and Bogusław Szczupak. 2022. "Processing and Mechanical Properties of Highly Filled PP/GTR Compounds" Materials 15, no. 11: 3799. https://doi.org/10.3390/ma15113799






