Adverse Effects of Using Metallurgical Slags as Supplementary Cementitious Materials and Aggregate: A Review
Abstract
:1. Introduction
| Compound | SS | CS | LZS | EFS | ||||
|---|---|---|---|---|---|---|---|---|
| Average | Range | Average | Range | Average | Range | Average | Range | |
| SiO2 | 16.40 | 11.04–23.30 | 29.09 | 22.63–33.62 | 25.30 | 18.89–30.76 | 41.22 | 32.74–53.29 |
| Al2O3 | 3.56 | 1.61–6.10 | 4.76 | 2.79–8.25 | 5.08 | 3.44–7.28 | 8.23 | 2.67–16.52 |
| Fe2O3 | 24.52 | 16.85–31.60 | 54.26 | 49.88–61.70 | 34.64 | 28.10–46.81 | 26.04 | 9.57–43.83 |
| CaO | 40.54 | 30.80–45.26 | 3.42 | 1.87–6.06 | 15.12 | 10.53–23.11 | 4.20 | 0.42–11.49 |
| MgO | 7.55 | 5.98–12.00 | 1.49 | 0.99–1.81 | 2.80 | 1.28–5.44 | 16.07 | 2.76–32.88 |
| Na2O | 0.33 | 0.20–0.45 | 0.41 | 0.14–0.96 | 1.44 | 0.27–4.12 | 0.33 | 0.09–0.80 |
| K2O | 0.07 | 0.07 | 1.08 | 0.61–2.28 | 0.59 | 0.26–0.96 | 0.28 | 0.18–0.37 |
| SO3 | 0.15 | 0.11–0.19 | 1.67 | 1.12–1.99 | 3.97 | 2.41–6.64 | 0.58 | 0.52–0.64 |
| TiO2 | 0.89 | 0.50–1.57 | 0.00 | 0.00 | 0.56 | 0.19–0.90 | 0.18 | 0.12–0.24 |
| P2O5 | 1.49 | 0.01–3.24 | 0.05 | 0.05–0.05 | 0.22 | 0.15–0.36 | 1.86 | 1.86–1.86 |
| MnO | 2.26 | 1.50–3.04 | 0.06 | 0.06–0.06 | 1.83 | 0.66–2.97 | 0.58 | 0.44–0.81 |
| CuO | - | - | 1.60 | 0.57–2.63 | 0.53 | 0.42–0.62 | - | - |
| Cr2O3 | 0.03 | 0.15 | - | - | 0.14 | 0.11–0.19 | 1.97 | 0.70–3.07 |
| PbO | - | - | - | - | 1.86 | 0.03–4.06 | - | - |
| ZnO | - | - | 2.31 | 2.31–2.31 | 8.25 | 5.01–13.95 | - | - |
| LOI | 1.25 | 0.64–1.86 | 6.09 | 6.09–6.09 | 4.52 | 0.46–7.48 | 3.44 | 3.44 |
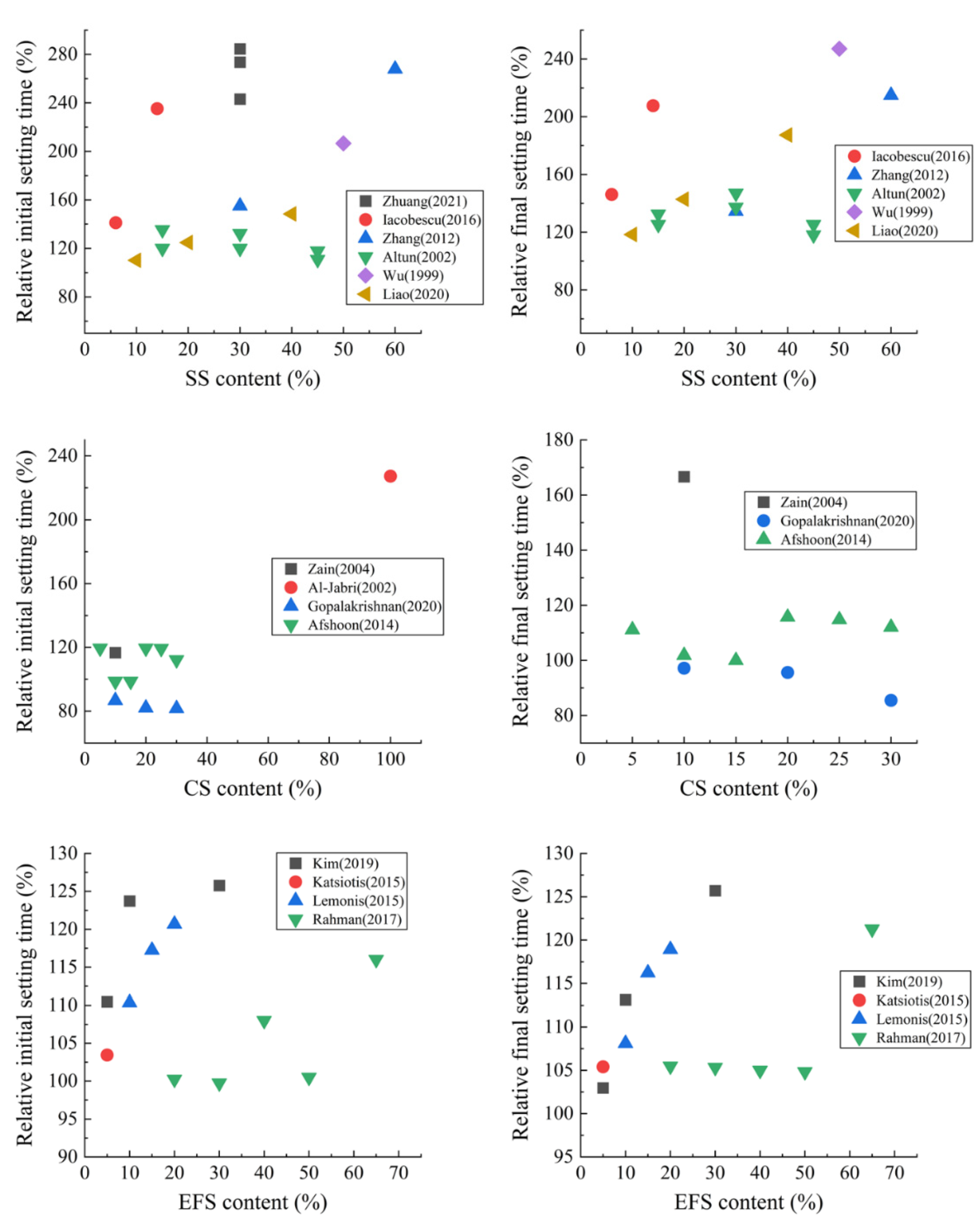
2. Fresh Properties
2.1. Metallurgical Slags as SCMs
2.2. Metallurgical Slags as Aggregates
| Slag | Ref | Specific Gravity (g/cm3) | Water Absorption (%) | |
|---|---|---|---|---|
| Slag | Natural Aggregate | |||
| SS | Lim et al. [90] | 3.56 | 2.65 | 1.5 |
| Palankar et al. [91] | 3.35 | 2.69 | 2 | |
| Qasrawi, [92] | 3.19 | 2.57 | 0.8 | |
| CS | Sim et al. [93] | 3.53 | 2.51 | 0.16 |
| Sharma et al. [23] | 3.51 | 2.6 | 0.36 | |
| Patil et al. [94] | 3.3 | 2.65 | 0.65 | |
| LZS | Penpolcharoen [66] | 3.62 | 2.71 | - |
| Buzatu et al. [32] | 3.79 | 2.65 | 1.29 | |
| Saikia et al. [89] | 3.76 | 2.62 | 1.5 | |
| EFS | Nguyen et al. [37] | 2.88 | 2.65 | 0.8 |
| Sun et al. [44] | 2.99 | 2.65 | 0.94 | |
| Saha et al. [69] | 2.78 | 2.16 | 0.42 | |
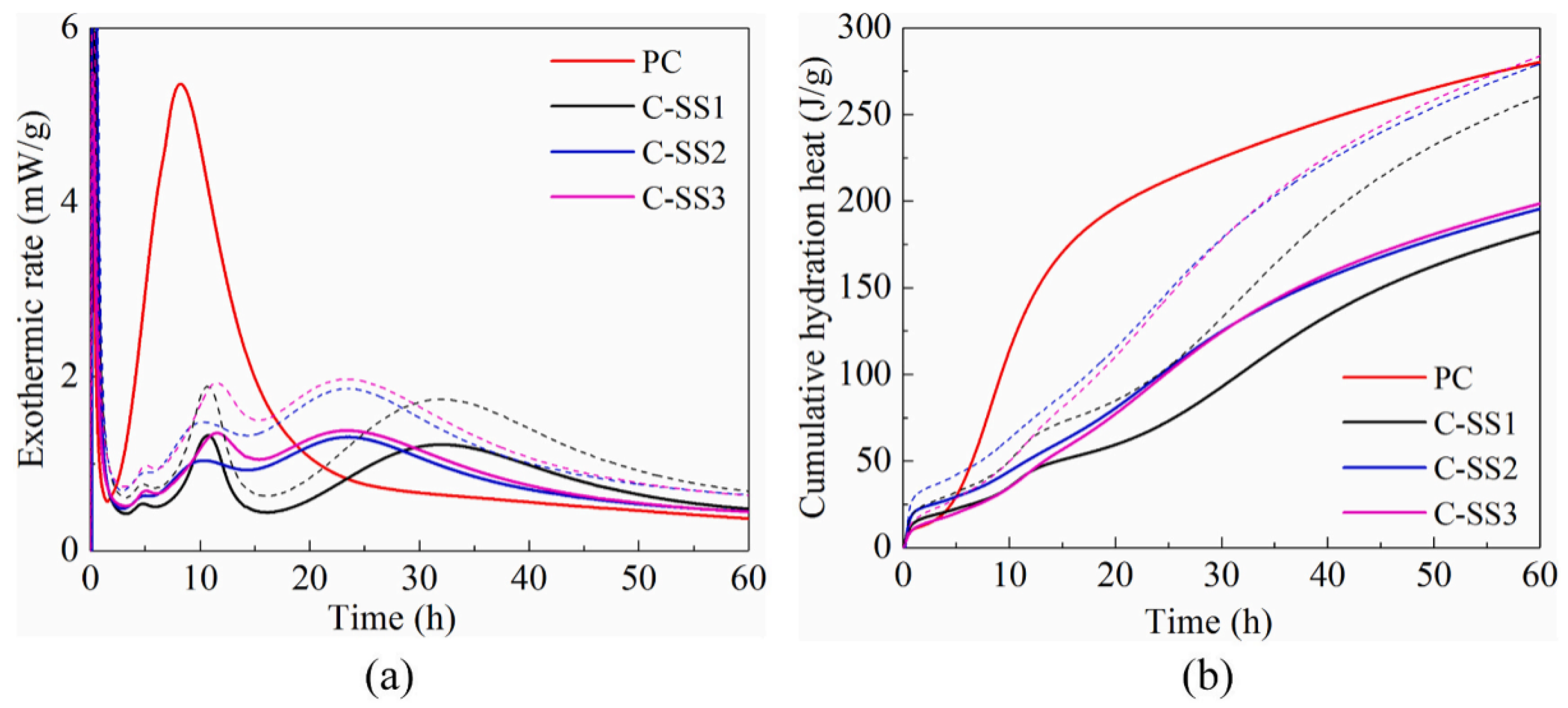
3. Hydration Process
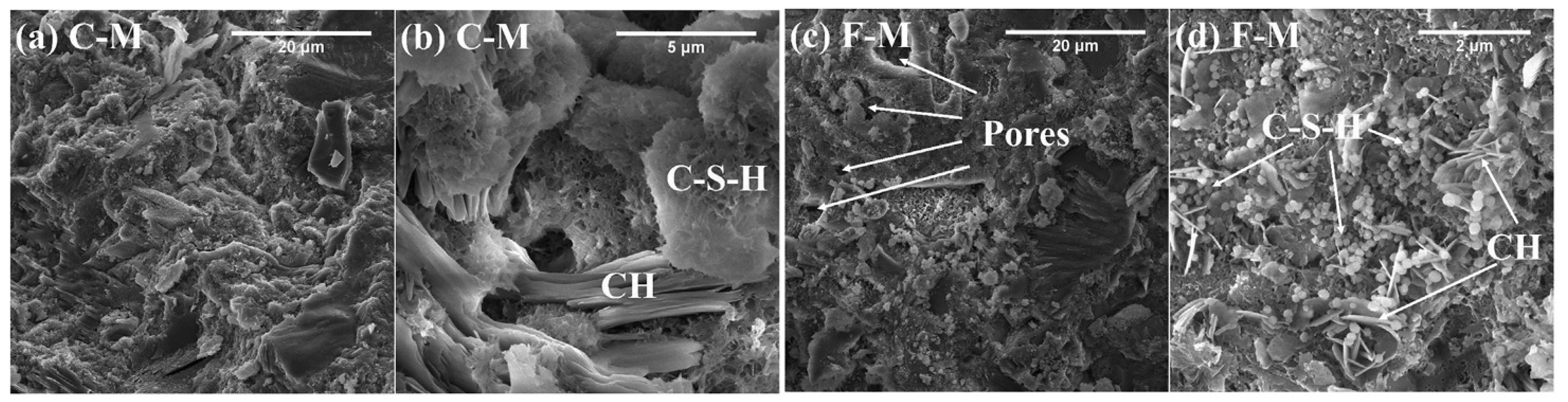
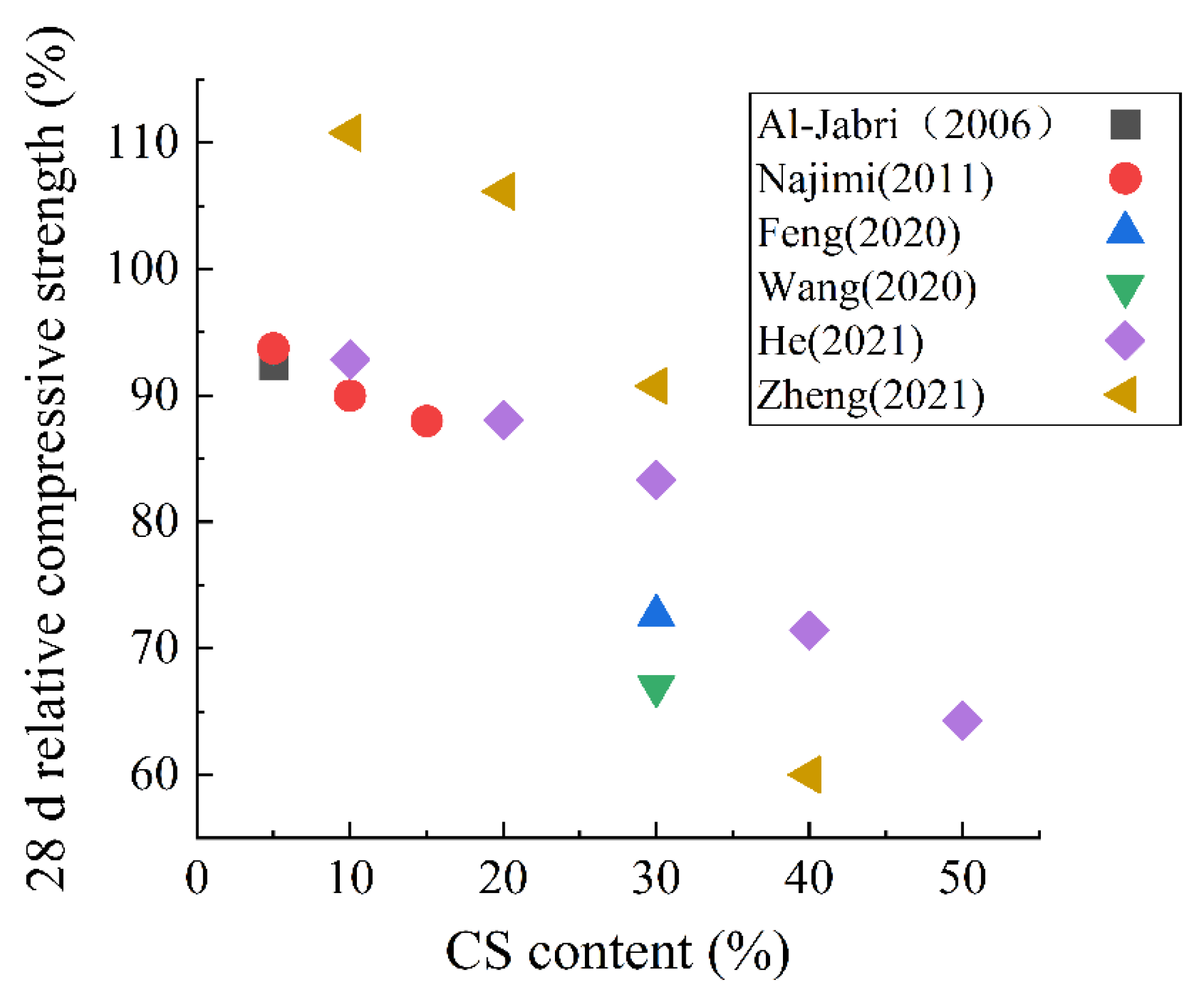
4. Microstructure, Mechanical Properties and Durability
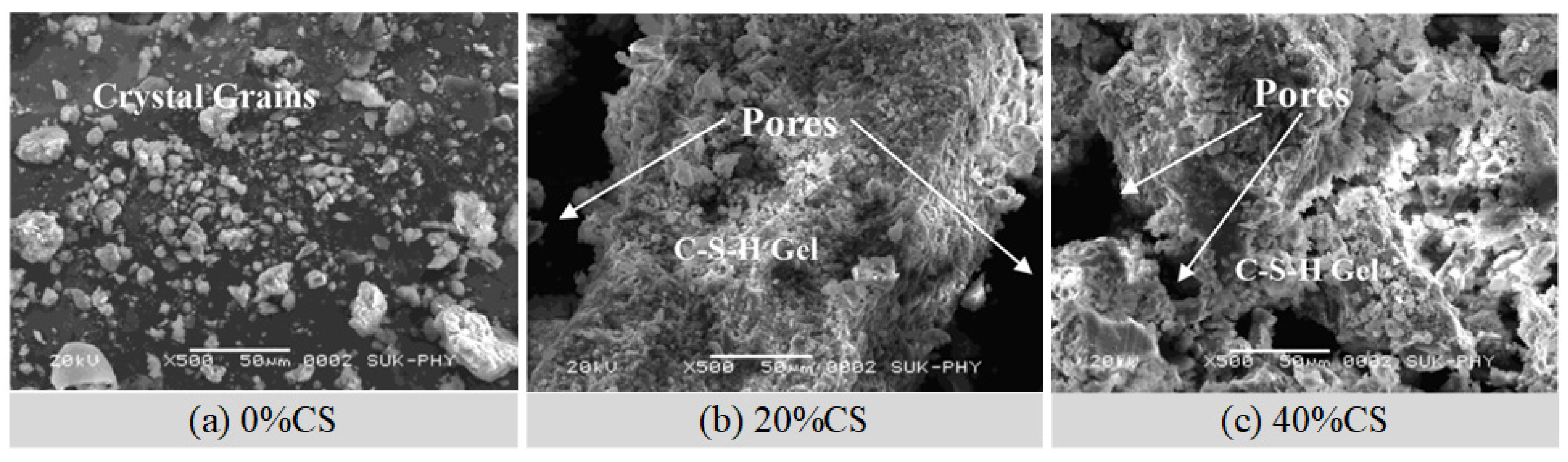
4.1. Steel Slag

4.2. Copper Slag
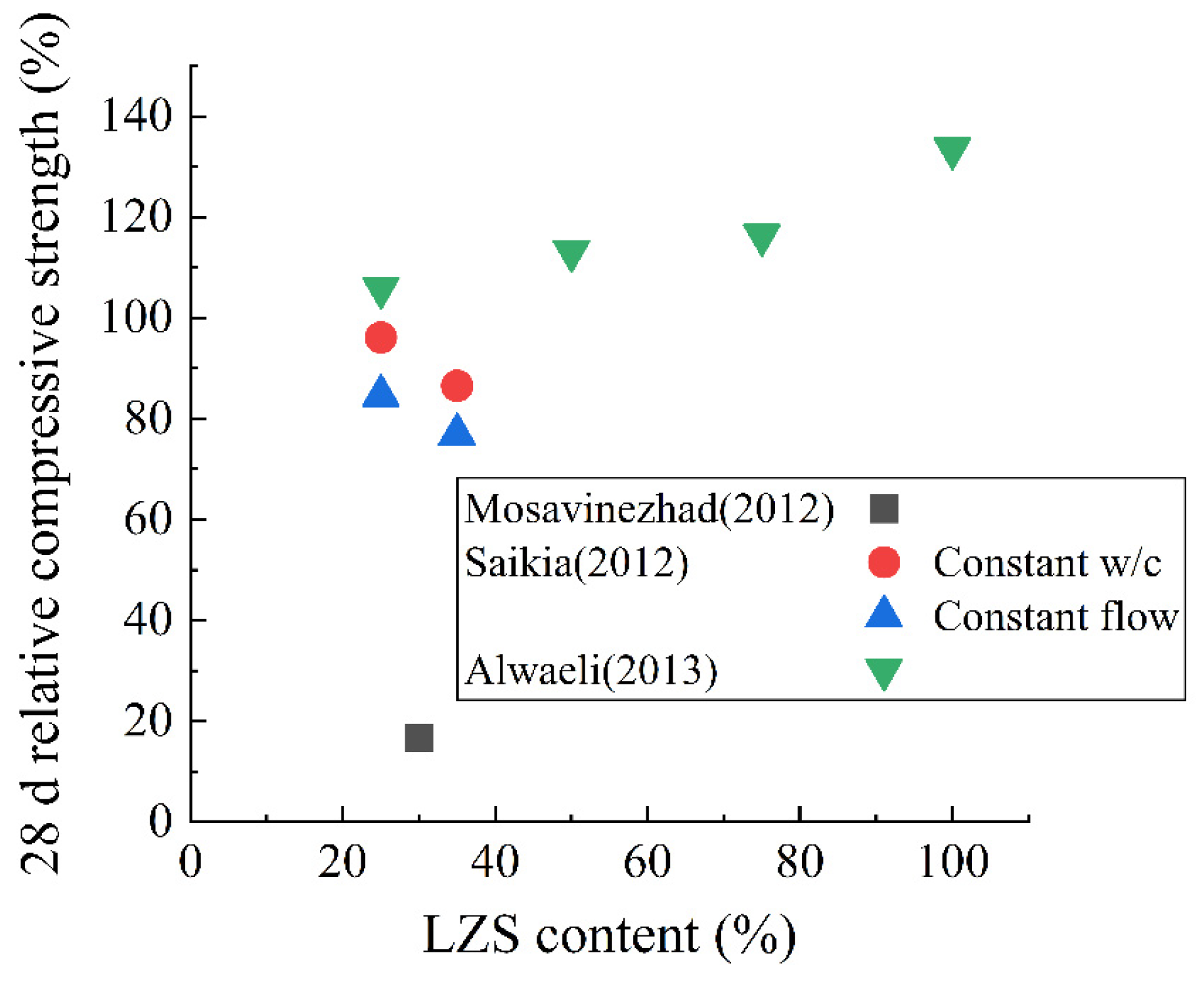
4.3. Lead-Zinc Slag
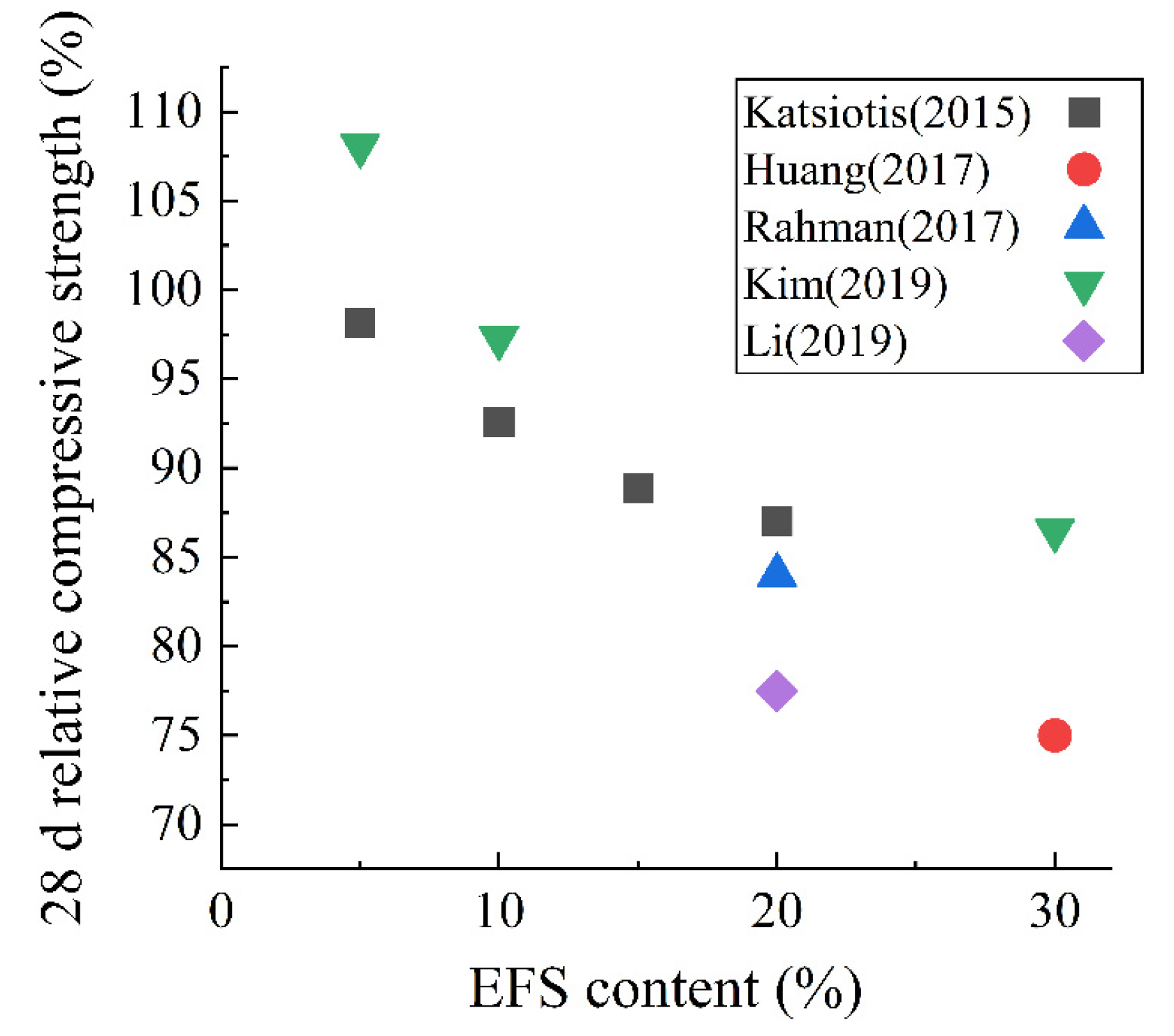
4.4. Electric Furnace Ferronickel Slag
5. Safety
5.1. Soundness
5.2. Alkali-Silica Reaction
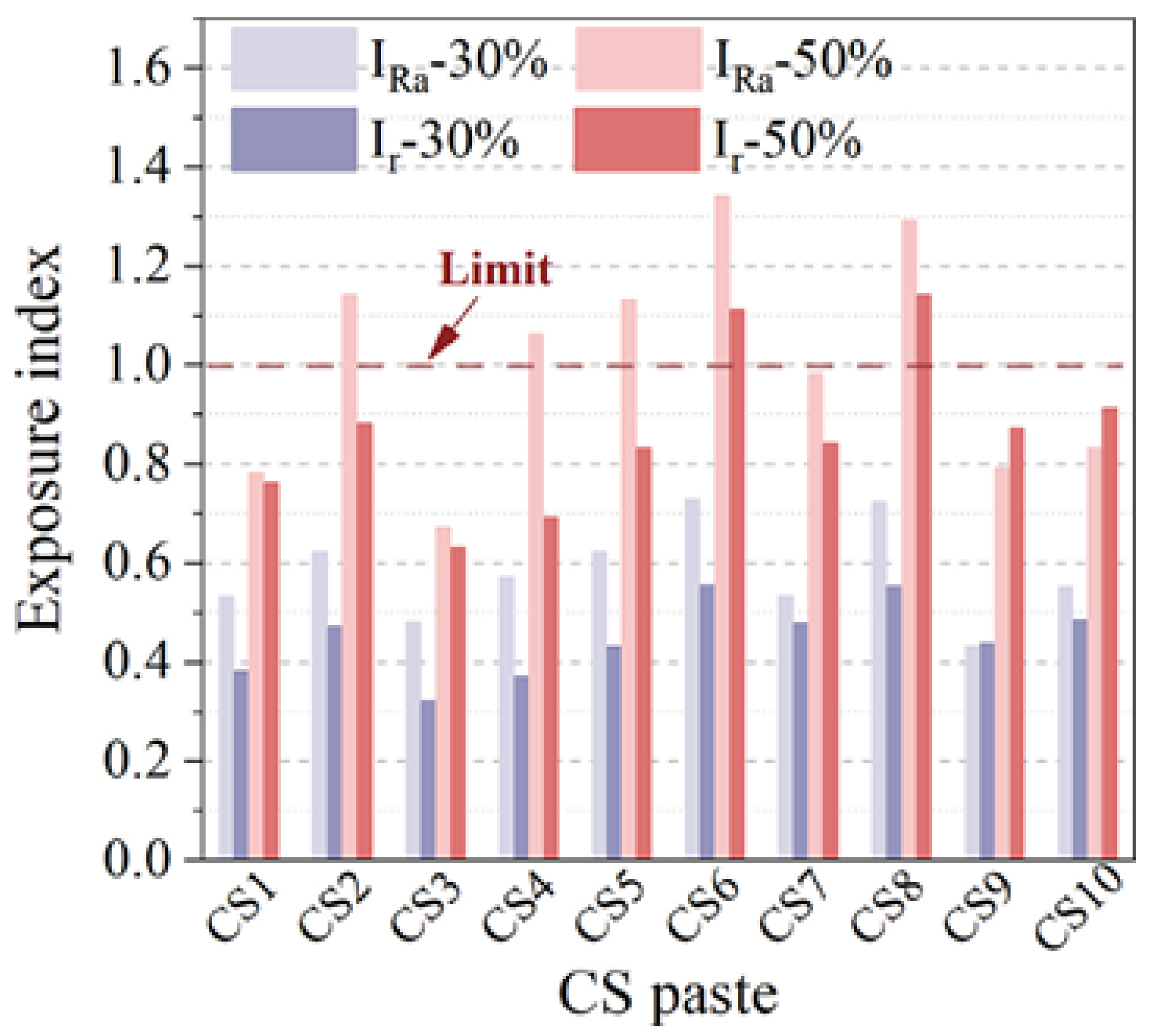
5.3. Environmental Safety
6. Conclusions
- Metallurgical slags can affect the fresh properties of mortar or concrete. The delaying effect of SS as an SCM on setting time is the most obvious. When SS content is set at 30%, the initial setting time and final setting time are prolonged by about 60% and 40%, respectively. CS has the least impact on setting time.
- When used as SCMs, these metallurgical slags can inhibit the hydration of cement. SS will inhibit the formation of hydration products in cement. Compounds that contain heavy metals in CS or LZS can seal clinker particles, thus limiting the hydration of cement. EFS powder will change the morphology and composition of hydration products in composite paste.
- The microstructure of hardened paste determines the mechanical properties and durability. Adding some metallurgical slags will degrade the microstructure and eventually decreases the strength and durability. SS as an SCM has the most obvious effect on the porosity, and the porosity increases by nearly 20% when the SS content is 30%. As for mechanical properties, when SS or CS is used as an SCM, the influence on strength is the most obvious. The 28 d compressive strength was reduced by about 20% for both SS and CS at 30% content. For EFS and LZS, they had relatively less effect on mechanical properties.
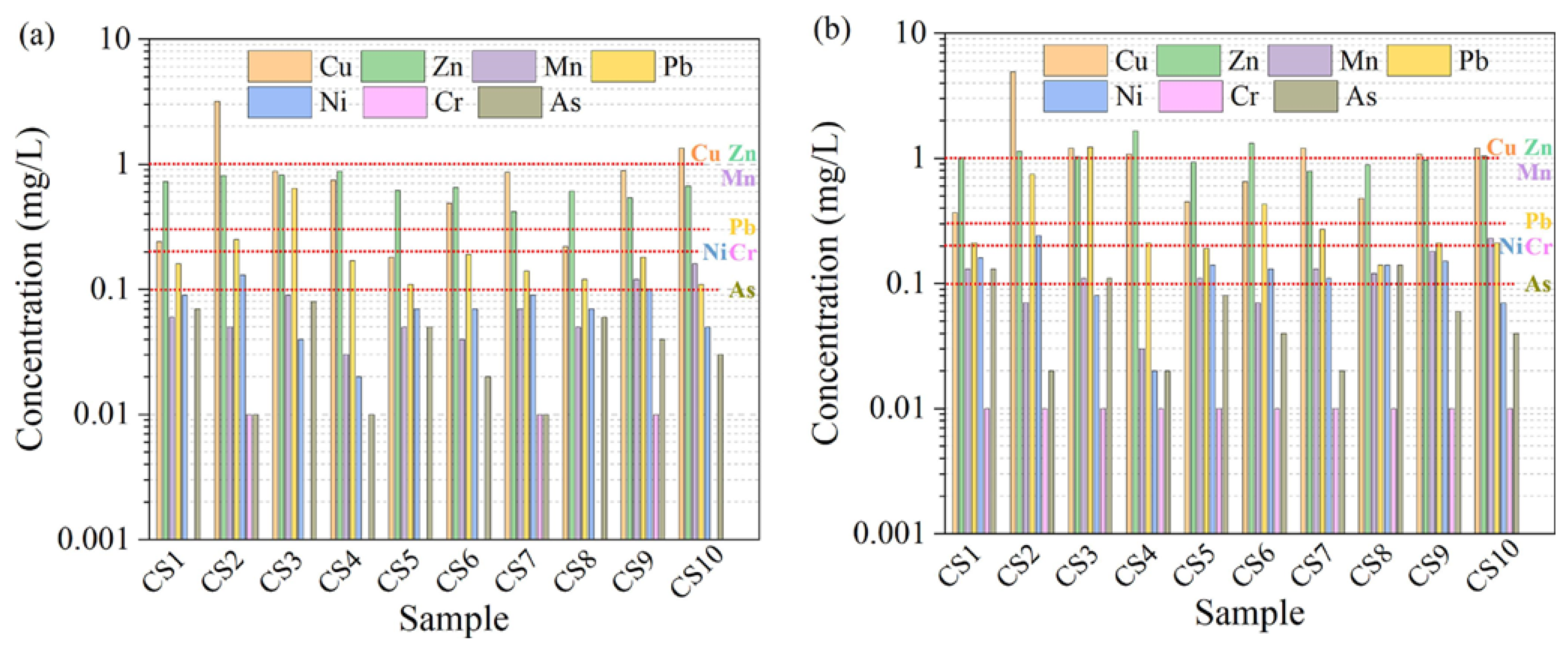
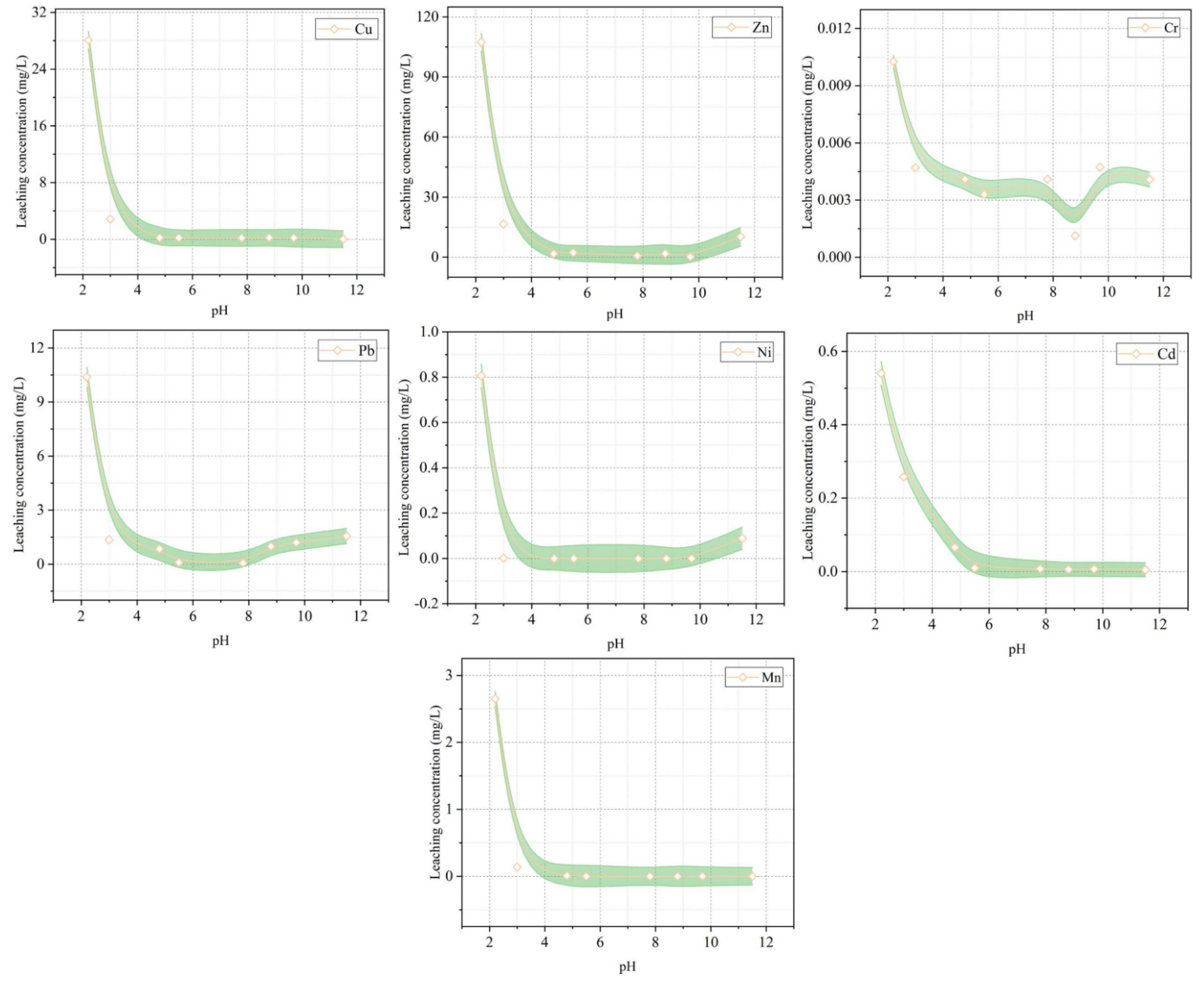
- There are some issues that are related to engineering safety when metallurgical slags are used in cement-based materials. The SS aggregates will cause poor soundness. The amorphous silica that exists in EFS will cause volume expansion as a result of the alkali-silica reaction. CS can lead to environmental problems because of the leaching of heavy metals, such as Cu, Pb, Mn, Zn, Ni, Cd, and Cr. At the same time, CS can pose great radiological risks to the surrounding environment. LZS has a leaching risk of harmful elements, such as Pb, Zn, and Cu, especially when used to prepare road materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cement Production Top Countries 2020|Statista. Available online: https://www.statista.com/statistics/267364/world-cement-production-by-country/ (accessed on 28 December 2021).
- Liu, Z.; Ciais, P.; Deng, Z.; Davis, S.J.; Zheng, B.; Wang, Y.; Cui, D.; Zhu, B.; Dou, X.; Ke, P.; et al. Carbon Monitor, a near-Real-Time Daily Dataset of Global CO2 Emission from Fossil Fuel and Cement Production. Sci. Data 2020, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhu, X.; Wang, J.; Mu, M.; Wang, Y. Comparison of CO2 Emissions from OPC and Recycled Cement Production. Constr. Build. Mater. 2019, 211, 965–973. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-Efficient Cements: Potential Economically Viable Solutions for a Low-CO2 Cement-Based Materials Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Hitch, M.; Pascual, R. Carbonation of Steel Slag and Gypsum for Building Materials and Associated Reaction Mechanisms. Cem. Concr. Res. 2019, 125, 105893. [Google Scholar] [CrossRef]
- Shi, C. Steel Slag—Its Production, Processing, Characteristics, and Cementitious Properties. ChemInform 2005, 36, 230–236. [Google Scholar] [CrossRef]
- Yang, C.; Xie, J.; Wu, S.; Amirkhanian, S.; Zhou, X.; Kong, D.; Ye, Q.; Hu, J. Revelation and Characterization of Selective Absorption Behavior of Bitumen to Basic Oxygen Furnace Slag. Constr. Build. Mater. 2020, 253, 119210. [Google Scholar] [CrossRef]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Verdeja, L.F. The Treatment of Basic Oxygen Furnace (BOF) Slag with Concentrated Solar Energy. Sol. Energy 2019, 180, 372–382. [Google Scholar] [CrossRef]
- Zod, N.; Mucci, A.; Bahn, O.; Provençal, R.; Shao, Y. Steel Slag-Bonded Strand Board as a Carbon-Negative Building Product. Constr. Build. Mater. 2022, 340, 127695. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel Slag in China: Treatment, Recycling, and Management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Liu, G.; Schollbach, K.; Li, P.; Brouwers, H.J.H. Valorization of Converter Steel Slag into Eco-Friendly Ultra-High Performance Concrete by Ambient CO2 Pre-Treatment. Constr. Build. Mater. 2021, 280, 122580. [Google Scholar] [CrossRef]
- Qiang, W.; Mengxiao, S.; Jun, Y. Influence of Classified Steel Slag with Particle Sizes Smaller than 20 Μm on the Properties of Cement and Concrete. Constr. Build. Mater. 2016, 123, 601–610. [Google Scholar] [CrossRef]
- Liao, Y.; Jiang, G.; Wang, K.; Al Qunaynah, S.; Yuan, W. Effect of Steel Slag on the Hydration and Strength Development of Calcium Sulfoaluminate Cement. Constr. Build. Mater. 2020, 265, 120301. [Google Scholar] [CrossRef]
- Duan, S.; Liao, H.; Song, H.; Cheng, F.; Yang, H. Performance Improvement to Ash-Cement Blocks by Adding Ultrafine Steel Slag Collected from a Supersonic Steam-Jet Smasher. Constr. Build. Mater. 2019, 212, 140–148. [Google Scholar] [CrossRef]
- Motz, H.; Geiseler, J. Products of Steel Slags an Opportunity to Save Natural Resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef]
- Zheng, W.; He, D.; Wang, Y.; Chen, J.; Xue, M.; Li, H. Preparation of Cement-Based Color Facing Mortar by Copper Pyrometallurgical Slag Modification: Efficient Utilization of High-Iron-Content Slag. J. Environ. Chem. Eng. 2021, 9, 105888. [Google Scholar] [CrossRef]
- Global Copper Production to Recover by 5.6% in 2021, after COVID-19 Hit Output in 2020, Says GlobalData. Available online: https://www.globaldata.com/global-copper-production-recover-5-6-2021-covid-19-hit-output-2020-says-globaldata/ (accessed on 4 January 2022).
- Zhang, S.; Zhu, N.; Mao, F.; Zhang, J.; Huang, X.; Li, F.; Li, X.; Wu, P.; Dang, Z. A Novel Strategy for Harmlessness and Reduction of Copper Smelting Slags by Alkali Disaggregation of Fayalite (Fe2SiO4) Coupling with Acid Leaching. J. Hazard. Mater. 2021, 402, 123791. [Google Scholar] [CrossRef]
- Martins, A.C.P.; Franco de Carvalho, J.M.; Costa, L.C.B.; Andrade, H.D.; de Melo, T.V.; Ribeiro, J.C.L.; Pedroti, L.G.; Peixoto, R.A.F. Steel Slags in Cement-Based Composites: An Ultimate Review on Characterization, Applications and Performance. Constr. Build. Mater. 2021, 291, 123265. [Google Scholar] [CrossRef]
- Dhir, R.K.; de Brito, J.; Mangabhai, R.; Lye, C.Q. Production and Properties of Copper Slag. In Sustainable Construction Materials: Copper Slag; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–86. ISBN 978-0-08-100986-4. [Google Scholar]
- Nainwal, A.; Negi, P.; Kumar Emani, P.; Chandra Shah, M.; Negi, A.; Kumar, V. An Experimental Investigation to Substitute Copper Slag in Concrete with Beas River Fine Aggregate. Mater. Today Proc. 2021, 46, 10339–10343. [Google Scholar] [CrossRef]
- Nainwal, A.; Emani, P.K.; Shah, M.C.; Negi, A.; Kumar, V.; Negi, P. The Influence of Metakaolin on the Copper Slag Substituted Concrete with the Fine Aggregate of Beas River. Mater. Today Proc. 2021, 46, 10425–10432. [Google Scholar] [CrossRef]
- Sharma, R.; Khan, R.A. Sulfate Resistance of Self Compacting Concrete Incorporating Copper Slag as Fine Aggregates with Mineral Admixtures. Constr. Build. Mater. 2021, 287, 122985. [Google Scholar] [CrossRef]
- Sambangi, A.; Arunakanthi, E. Fresh and Mechanical Properties of SCC with Fly Ash and Copper Slag as Mineral Admixtures. Mater. Today Proc. 2021, 45, 6687–6693. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Z.; An, D.; Huo, J. Collaborative Design of Cement-Based Composites Incorporated with Cooper Slag in Considerations of Engineering Properties and Microwave-Absorbing Characters. J. Clean. Prod. 2021, 283, 124614. [Google Scholar] [CrossRef]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, Í.; González-Gasca, C.; Gómez-Rodríguez, C.; Verdeja, L.F. Recovery of Copper and Magnetite from Copper Slag Using Concentrated Solar Power (CSP). Metals 2021, 11, 1032. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Huang, Z. Reuse of Copper Slag as a Supplementary Cementitious Material: Reactivity and Safety. Resour. Conserv. Recycl. 2020, 162, 105037. [Google Scholar] [CrossRef]
- Al-Jabri, K.S.; Hisada, M.; Al-Oraimi, S.K.; Al-Saidy, A.H. Copper Slag as Sand Replacement for High Performance Concrete. Cem. Concr. Compos. 2009, 31, 483–488. [Google Scholar] [CrossRef]
- Najimi, M.; Sobhani, J.; Pourkhorshidi, A.R. Durability of Copper Slag Contained Concrete Exposed to Sulfate Attack. Constr. Build. Mater. 2011, 25, 1895–1905. [Google Scholar] [CrossRef]
- Pan, D.; Li, L.; Tian, X.; Wu, Y.; Cheng, N.; Yu, H. A Review on Lead Slag Generation, Characteristics, and Utilization. Resour. Conserv. Recycl. 2019, 146, 140–155. [Google Scholar] [CrossRef]
- Zhang, P.; Muhammad, F.; Yu, L.; Xia, M.; Lin, H.; Huang, X.; Jiao, B.; Shiau, Y.; Li, D. Self-Cementation Solidification of Heavy Metals in Lead-Zinc Smelting Slag through Alkali-Activated Materials. Constr. Build. Mater. 2020, 249, 118756. [Google Scholar] [CrossRef]
- Buzatu, T.; Talpoş, E.; Petrescu, M.I.; Ghica, V.G.; Iacob, G.; Buzatu, M. Utilization of Granulated Lead Slag as a Structural Material in Roads Constructions. J. Mater. Cycles Waste Manag. 2015, 17, 707–717. [Google Scholar] [CrossRef]
- Li, H.; An, J.; Yuan, H.; Wang, S. Study on Comprehensive Utilization of Historical Left Lead and Zinc Smelting Slag. Environ. Eng. 2016, 34, 661–665. [Google Scholar]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Geopolymerisation of Low Calcium Ferronickel Slags. J. Mater. Sci. 2007, 42, 3073–3082. [Google Scholar] [CrossRef]
- Saha, A.K.; Sarker, P.K. Expansion Due to Alkali-Silica Reaction of Ferronickel Slag Fine Aggregate in OPC and Blended Cement Mortars. Constr. Build. Mater. 2016, 123, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Xi, B.; Li, R.; Zhao, X.; Dang, Q.; Zhang, D.; Tan, W. Constraints and Opportunities for the Recycling of Growing Ferronickel Slag in China. Resour. Conserv. Recycl. 2018, 139, 15–16. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Castel, A.; Kim, T.; Khan, M.S.H. Performance of Fly Ash Concrete with Ferronickel Slag Fine Aggregate against Alkali-Silica Reaction and Chloride Diffusion. Cem. Concr. Res. 2021, 139, 106265. [Google Scholar] [CrossRef]
- Cao, R.; Jia, Z.; Zhang, Z.; Zhang, Y.; Banthia, N. Leaching Kinetics and Reactivity Evaluation of Ferronickel Slag in Alkaline Conditions. Cem. Concr. Res. 2020, 137, 106202. [Google Scholar] [CrossRef]
- Han, F.; Zhang, H.; Pu, S.; Zhang, Z. Hydration Heat and Kinetics of Composite Binder Containing Blast Furnace Ferronickel Slag at Different Temperatures. Thermochim. Acta 2021, 702, 178985. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Qi, A.; Lin, W.; Wu, H. Flexural Performance of Reinforced Beams Made with Composite Ferronickel Slag Concrete. Constr. Build. Mater. 2021, 310, 125251. [Google Scholar] [CrossRef]
- Qi, A.; Liu, X.; Xu, R.; Huang, Y. Bond Behavior of Steel Reinforcement in Concrete Containing Ferronickel Slag and Blast Furnace Slag Powder. Constr. Build. Mater. 2020, 262, 120884. [Google Scholar] [CrossRef]
- Qi, A.; Liu, X.; Wang, Z.; Chen, Z. Mechanical Properties of the Concrete Containing Ferronickel Slag and Blast Furnace Slag Powder. Constr. Build. Mater. 2020, 231, 117120. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Zhuang, S.; Yang, J. Evaluation of Alkali-Activated Blast Furnace Ferronickel Slag as a Cementitious Material: Reaction Mechanism, Engineering Properties and Leaching Behaviors. Constr. Build. Mater. 2018, 188, 860–873. [Google Scholar] [CrossRef]
- Sun, J.; Feng, J.; Chen, Z. Effect of Ferronickel Slag as Fine Aggregate on Properties of Concrete. Constr. Build. Mater. 2019, 206, 201–209. [Google Scholar] [CrossRef]
- Andrade, H.D.; de Carvalho, J.M.F.; Costa, L.C.B.; da Fonseca Elói, F.P.; do Carmo e Silva, K.D.; Peixoto, R.A.F. Mechanical Performance and Resistance to Carbonation of Steel Slag Reinforced Concrete. Constr. Build. Mater. 2021, 298, 123910. [Google Scholar] [CrossRef]
- Alwaeli, M. Application of Granulated Lead–Zinc Slag in Concrete as an Opportunity to Save Natural Resources. Radiat. Phys. Chem. 2013, 83, 54–60. [Google Scholar] [CrossRef]
- Gupta, N.; Siddique, R. Strength and Micro-Structural Properties of Self-Compacting Concrete Incorporating Copper Slag. Constr. Build. Mater. 2019, 224, 894–908. [Google Scholar] [CrossRef]
- Prem, P.R.; Verma, M.; Ambily, P.S. Sustainable Cleaner Production of Concrete with High Volume Copper Slag. J. Clean. Prod. 2018, 193, 43–58. [Google Scholar] [CrossRef]
- Mavroulidou, M. Mechanical Properties and Durability of Concrete with Water Cooled Copper Slag Aggregate. Waste Biomass Valoriz. 2017, 8, 1841–1854. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, T.; Zhou, L. Investigation on Electromagnetic and Microwave Absorption Properties of Copper Slag-Filled Cement Mortar. Cem. Concr. Compos. 2016, 74, 174–181. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M. Mechanical Performance and Microstructure of the Calcium Carbonate Binders Produced by Carbonating Steel Slag Paste under CO2 Curing. Cem. Concr. Res. 2016, 88, 217–226. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, H.; Wang, J.; Feng, Q. Preliminary Investigation on the Pozzolanic Activity of Superfine Steel Slag. Constr. Build. Mater. 2015, 82, 227–234. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Han, S. The Influence of Steel Slag on the Hydration of Cement during the Hydration Process of Complex Binder. Sci. China Technol. Sci. 2011, 54, 388–394. [Google Scholar] [CrossRef]
- Muhmood, L.; Vitta, S.; Venkateswaran, D. Cementitious and Pozzolanic Behavior of Electric Arc Furnace Steel Slags. Cem. Concr. Res. 2009, 39, 102–109. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Huang, Z. Value-Added Utilization of Copper Slag to Enhance the Performance of Magnesium Potassium Phosphate Cement. Resour. Conserv. Recycl. 2022, 180, 106212. [Google Scholar] [CrossRef]
- Ahirwar, S.K.; Mandal, J.N. Experimental Study on Bamboo Grid Reinforced Copper Slag Overlying Soft Subgrade. Constr. Build. Mater. 2021, 306, 124758. [Google Scholar] [CrossRef]
- Siddique, R.; Singh, M.; Jain, M. Recycling Copper Slag in Steel Fibre Concrete for Sustainable Construction. J. Clean. Prod. 2020, 271, 122559. [Google Scholar] [CrossRef]
- Lan, W.; Wu, A.; Yu, P. Development of a New Controlled Low Strength Filling Material from the Activation of Copper Slag: Influencing Factors and Mechanism Analysis. J. Clean. Prod. 2020, 246, 119060. [Google Scholar] [CrossRef]
- Gupta, N.; Siddique, R. Durability Characteristics of Self-Compacting Concrete Made with Copper Slag. Constr. Build. Mater. 2020, 247, 118580. [Google Scholar] [CrossRef]
- Xia, M.; Muhammad, F.; Zeng, L.; Li, S.; Huang, X.; Jiao, B.; Shiau, Y.; Li, D. Solidification/Stabilization of Lead-Zinc Smelting Slag in Composite Based Geopolymer. J. Clean. Prod. 2019, 209, 1206–1215. [Google Scholar] [CrossRef]
- Mao, Y.; Muhammad, F.; Yu, L.; Xia, M.; Huang, X.; Jiao, B.; Shiau, Y.; Li, D. The Solidification of Lead-Zinc Smelting Slag through Bentonite Supported Alkali-Activated Slag Cementitious Material. Int. J. Environ. Res. Public. Health 2019, 16, 1121. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Huang, X.; Muhammad, F.; Yu, L.; Xia, M.; Zhao, J.; Jiao, B.; Shiau, Y.; Li, D. Waste Solidification/Stabilization of Lead–Zinc Slag by Utilizing Fly Ash Based Geopolymers. RSC Adv. 2018, 8, 32956–32965. [Google Scholar] [CrossRef] [Green Version]
- Lima, L.R.P.D.A.; Bernardez, L.A. Evaluation of the Chemical Stability of a Landfilled Primary Lead Smelting Slag. Environ. Earth Sci. 2013, 68, 1033–1040. [Google Scholar] [CrossRef]
- Saikia, N.; Cornelis, G.; Mertens, G.; Elsen, J.; Van Balen, K.; Van Gerven, T.; Vandecasteele, C. Assessment of Pb-Slag, MSWI Bottom Ash and Boiler and Fly Ash for Using as a Fine Aggregate in Cement Mortar. J. Hazard. Mater. 2008, 154, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Weeks, C.; Hand, R.J.; Sharp, J.H. Retardation of Cement Hydration Caused by Heavy Metals Present in ISF Slag Used as Aggregate. Cem. Concr. Compos. 2008, 30, 970–978. [Google Scholar] [CrossRef]
- Penpolcharoen, M. Utilization of Secondary Lead Slag as Construction Material. Cem. Concr. Res. 2005, 35, 1050–1055. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, Y.; Mu, W.; Liu, J.; He, J.; Zhou, X. Magnesium Phosphate Cement Prepared with Electric Furnace Ferronickel Slag: Properties and Its Hydration Mechanism. Constr. Build. Mater. 2021, 300, 123991. [Google Scholar] [CrossRef]
- Cao, R.; Li, B.; You, N.; Zhang, Y.; Zhang, Z. Properties of Alkali-Activated Ground Granulated Blast Furnace Slag Blended with Ferronickel Slag. Constr. Build. Mater. 2018, 192, 123–132. [Google Scholar] [CrossRef]
- Saha, A.K.; Sarker, P.K. Sustainable Use of Ferronickel Slag Fine Aggregate and Fly Ash in Structural Concrete: Mechanical Properties and Leaching Study. J. Clean. Prod. 2017, 162, 438–448. [Google Scholar] [CrossRef]
- Rahman, M.A.; Sarker, P.K.; Shaikh, F.U.A.; Saha, A.K. Soundness and Compressive Strength of Portland Cement Blended with Ground Granulated Ferronickel Slag. Constr. Build. Mater. 2017, 140, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Lemonis, N.; Tsakiridis, P.E.; Katsiotis, N.S.; Antiohos, S.; Papageorgiou, D.; Katsiotis, M.S.; Beazi-Katsioti, M. Hydration Study of Ternary Blended Cements Containing Ferronickel Slag and Natural Pozzolan. Constr. Build. Mater. 2015, 81, 130–139. [Google Scholar] [CrossRef]
- Katsiotis, N.S.; Tsakiridis, P.E.; Velissariou, D.; Katsiotis, M.S.; Alhassan, S.M.; Beazi, M. Utilization of Ferronickel Slag as Additive in Portland Cement: A Hydration Leaching Study. Waste Biomass Valoriz. 2015, 6, 177–189. [Google Scholar] [CrossRef]
- Maragkos, I.; Giannopoulou, I.P.; Panias, D. Synthesis of Ferronickel Slag-Based Geopolymers. Miner. Eng. 2009, 22, 196–203. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Effect of Synthesis Parameters on the Compressive Strength of Low-Calcium Ferronickel Slag Inorganic Polymers. J. Hazard. Mater. 2009, 161, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Wang, Q. Inhibition Mechanisms of Steel Slag on the Early-Age Hydration of Cement. Cem. Concr. Res. 2021, 140, 106283. [Google Scholar] [CrossRef]
- Zain, M.F.M.; Islam, M.N.; Radin, S.S.; Yap, S.G. Cement-Based Solidification for the Safe Disposal of Blasted Copper Slag. Cem. Concr. Compos. 2004, 26, 845–851. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Nithiyanantham, S. Microstructural, Mechanical, and Electrical Properties of Copper Slag Admixtured Cement Mortar. J. Build. Eng. 2020, 31, 101375. [Google Scholar] [CrossRef]
- Kim, H.; Lee, C.H.; Ann, K.Y. Feasibility of Ferronickel Slag Powder for Cementitious Binder in Concrete Mix. Constr. Build. Mater. 2019, 207, 693–705. [Google Scholar] [CrossRef]
- Iacobescu, R.I.; Angelopoulos, G.N.; Jones, P.T.; Blanpain, B.; Pontikes, Y. Ladle Metallurgy Stainless Steel Slag as a Raw Material in Ordinary Portland Cement Production: A Possibility for Industrial Symbiosis. J. Clean. Prod. 2016, 112, 872–881. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Q.; Wei, J.; Li, J. Investigation on Mechanical Properties, Durability and Micro-Structural Development of Steel Slag Blended Cements. J. Therm. Anal. Calorim. 2012, 110, 633–639. [Google Scholar] [CrossRef]
- Akın Altun, İ.; Yılmaz, İ. Study on Steel Furnace Slags with High MgO as Additive in Portland Cement. Cem. Concr. Res. 2002, 32, 1247–1249. [Google Scholar] [CrossRef]
- Xuequan, W.; Hong, Z.; Xinkai, H.; Husen, L. Study on Steel Slag and Fly Ash Composite Portland Cement. Cem. Concr. Res. 1999, 29, 1103–1106. [Google Scholar] [CrossRef]
- Al-Jabri, K.; Taha, R.; Al-Ghassani, M. Use of Copper Slag and Cement By-Pass Dust as Cementitious Materials. Cem. Concr. Aggreg. 2002, 24, 7. [Google Scholar] [CrossRef]
- Afshoon, I.; Sharifi, Y. Ground Copper Slag as a Supplementary Cementing Material and Its Influence on the Fresh Properties of Self-Consolidating Concrete. IES J. Part A Civ. Struct. Eng. 2014, 7, 229–242. [Google Scholar]
- Wang, R.; Shi, Q.; Li, Y.; Cao, Z.; Si, Z. A Critical Review on the Use of Copper Slag (CS) as a Substitute Constituent in Concrete. Constr. Build. Mater. 2021, 292, 123371. [Google Scholar] [CrossRef]
- Saha, A.K.; Khan, M.N.N.; Sarker, P.K. Value Added Utilization of By-Product Electric Furnace Ferronickel Slag as Construction Materials: A Review. Resour. Conserv. Recycl. 2018, 134, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, P.; Ait-Aider, H. The Influence of Cement Source and Slag Additions on the Bleeding of Concrete. Cem. Concr. Res. 1995, 25, 1445–1456. [Google Scholar] [CrossRef]
- Mosavinezhad, S.H.G.; Nabavi, S.E. Effect of 30% Ground Granulated Blast Furnace, Lead and Zinc Slags as Sand Replacements on the Strength of Concrete. KSCE J. Civ. Eng. 2012, 16, 989–993. [Google Scholar] [CrossRef]
- Saikia, N.; Cornelis, G.; Cizer, Ö.; Vandecasteele, C.; Van Gemert, D.; Van Balen, K.; Van Gerven, T. Use of Pb Blast Furnace Slag as a Partial Substitute for Fine Aggregate in Cement Mortar. J. Mater. Cycles Waste Manag. 2012, 14, 102–112. [Google Scholar] [CrossRef]
- Lim, J.S.; Cheah, C.B.; Ramli, M.B. The Setting Behavior, Mechanical Properties and Drying Shrinkage of Ternary Blended Concrete Containing Granite Quarry Dust and Processed Steel Slag Aggregate. Constr. Build. Mater. 2019, 215, 447–461. [Google Scholar] [CrossRef]
- Palankar, N.; Ravi Shankar, A.U.; Mithun, B.M. Durability Studies on Eco-Friendly Concrete Mixes Incorporating Steel Slag as Coarse Aggregates. J. Clean. Prod. 2016, 129, 437–448. [Google Scholar] [CrossRef]
- Qasrawi, H. The Use of Steel Slag Aggregate to Enhance the Mechanical Properties of Recycled Aggregate Concrete and Retain the Environment. Constr. Build. Mater. 2014, 54, 298–304. [Google Scholar] [CrossRef]
- Sim, S.; Jeon, D.; Kim, D.H.; Yum, W.S.; Yoon, S.; Oh, J.E. Incorporation of Copper Slag in Cement Brick Production as a Radiation Shielding Material. Appl. Radiat. Isot. 2021, 176, 109851. [Google Scholar] [CrossRef]
- Patil, M.V.; Patil, Y.D. Effect of Copper Slag and Granite Dust as Sand Replacement on the Properties of Concrete. Mater. Today Proc. 2021, 43, 1666–1677. [Google Scholar] [CrossRef]
- Bhatty, J.I. Role of Minor Elements in Cement Manufacture and Use; Research and Development Bulletin RD109T; Portland Cement Association: Skokie, IL, USA, 1995. [Google Scholar]
- Arliguie, G.; Grandet, J. Influence de La Composition d’un Ciment Portland Sur Son Hydration En Presence de Zinc. Cem. Concr. Res. 1990, 20, 517–524. [Google Scholar] [CrossRef]
- Tashiro, C.; Oba, J. The Effects of Cr2O3, Cu(OH)2, ZnO and PbO on the Compressive Strength and the Hydrates of the Hardened C3A Paste. Cem. Concr. Res. 1979, 9, 253–258. [Google Scholar] [CrossRef]
- Kolovos, K.; Barafaka, S.; Kakali, G.; Tsivilis, S. CuO and ZnO Addition in the Cement Raw Mix: Effect on Clinkering Process and Cement Hydration and Properties. Ceramics 2005, 49, 205–212. [Google Scholar]
- Saca, N.; Radu, L.; Fugaru, V.; Gheorghe, M.; Petre, I. Composite Materials with Primary Lead Slag Content: Application in Gamma Radiation Shielding and Waste Encapsulation Fields. J. Clean. Prod. 2018, 179, 255–265. [Google Scholar] [CrossRef]
- Li, B.; Huo, B.; Cao, R.; Wang, S.; Zhang, Y. Sulfate Resistance of Steam Cured Ferronickel Slag Blended Cement Mortar. Cem. Concr. Compos. 2019, 96, 204–211. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.; Shi, M. Characteristics and Reactivity of Ferronickel Slag Powder. Constr. Build. Mater. 2017, 156, 773–789. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Yan, P. Cementitious Properties of Super-Fine Steel Slag. Powder Technol. 2013, 245, 35–39. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Yang, J.; Zhang, B. Influence of Steel Slag on Mechanical Properties and Durability of Concrete. Constr. Build. Mater. 2013, 47, 1414–1420. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Hou, G.; Yan, P. Preparation of Sustainable and Green Cement-Based Composite Binders with High-Volume Steel Slag Powder and Ultrafine Blast Furnace Slag Powder. J. Clean. Prod. 2021, 289, 125133. [Google Scholar] [CrossRef]
- Manso, J.M.; Polanco, J.A.; Losañez, M.; González, J.J. Durability of Concrete Made with EAF Slag as Aggregate. Cem. Concr. Compos. 2006, 28, 528–534. [Google Scholar] [CrossRef]
- Brand, A.S.; Roesler, J.R. Interfacial Transition Zone of Cement Composites with Steel Furnace Slag Aggregates. Cem. Concr. Compos. 2018, 86, 117–129. [Google Scholar] [CrossRef]
- Gupta, T.; Sachdeva, S.N. Laboratory Investigation and Modeling of Concrete Pavements Containing AOD Steel Slag. Cem. Concr. Res. 2019, 124, 105808. [Google Scholar] [CrossRef]
- Boakye, D.M.; Uzoegbo, H. Durability and Strength Assessment of Copper Slag Concrete. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2014. [Google Scholar]
- Dhir, R.K.; de Brito, J.; Mangabhai, R.; Lye, C.Q. Copper Slag in Cement Manufacture and as Cementitious Material. In Sustainable Construction Materials: Copper Slag; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–209. ISBN 978-0-08-100986-4. [Google Scholar]
- Al-Jabri, K.S.; Taha, R.A.; Al-Hashmi, A.; Al-Harthy, A.S. Effect of Copper Slag and Cement By-Pass Dust Addition on Mechanical Properties of Concrete. Constr. Build. Mater. 2006, 20, 322–331. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Q.; Zhou, Y.; Yang, Q.; Zhang, Q.; Jiang, L.; Guo, H. Modification of Glass Structure via CaO Addition in Granulated Copper Slag to Enhance Its Pozzolanic Activity. Constr. Build. Mater. 2020, 240, 117970. [Google Scholar] [CrossRef]
- He, R.; Zhang, S.; Zhang, X.; Zhang, Z.; Zhao, Y.; Ding, H. Copper Slag: The Leaching Behavior of Heavy Metals and Its Applicability as a Supplementary Cementitious Material. J. Environ. Chem. Eng. 2021, 9, 105132. [Google Scholar] [CrossRef]
- Maharishi, A.; Singh, S.P.; Gupta, L.K. Shehnazdeep Strength and Durability Studies on Slag Cement Concrete Made with Copper Slag as Fine Aggregates. Mater. Today Proc. 2021, 38, 2639–2648. [Google Scholar] [CrossRef]
- Al-Jabri, K.S.; Hisada, M.; Al-Saidy, A.H.; Al-Oraimi, S.K. Performance of High Strength Concrete Made with Copper Slag as a Fine Aggregate. Constr. Build. Mater. 2009, 23, 2132–2140. [Google Scholar] [CrossRef]
- Kubissa, W.; Jaskulski, R. Improving of Concrete Tightness by Using Surface Blast-Cleaning Waste as a Partial Replacement of Fine Aggregate. Period. Polytech. Civ. Eng. 2019, 63, 1193–1203. [Google Scholar] [CrossRef]
- Kubissa, W.; Jaskulski, R.; Simon, T. Surface Blast-Cleaning Waste as a Replacement of Fine Aggregate in Concrete. Archit. Civ. Eng. Environ. 2017, 3, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Sridharan, M.; Madhavi, T.C. Investigating the Influence of Copper Slag on the Mechanical Behaviour of Concrete. Mater. Today Proc. 2021, 46, 3225–3232. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, W.; Ma, G. Optimum Content of Copper Slag as a Fine Aggregate in High Strength Concrete. Mater. Des. 2010, 31, 2878–2883. [Google Scholar] [CrossRef]
- Kumar Tiwary, A.; Bhatia, S. A Study Incorporating the Influence of Copper Slag and Fly Ash Substitutions in Concrete. Mater. Today Proc. 2021, 48, 1476–1483. [Google Scholar] [CrossRef]
- Usha Kranti, J.; Naga Sai, A.; Rama Krishna, A.; Srinivasu, K. An Experimental Investigation on Effect of Durability on Strength Properties of M40 Grade Concrete with Partial Replacement of Sand with Copper Slag. Mater. Today Proc. 2021, 43, 1626–1633. [Google Scholar] [CrossRef]
- Sharma, R.; Khan, R.A. Durability Assessment of Self Compacting Concrete Incorporating Copper Slag as Fine Aggregates. Constr. Build. Mater. 2017, 155, 617–629. [Google Scholar] [CrossRef]
- Deb, P.S.; Sarker, P.K.; Barbhuiya, S. Sorptivity and Acid Resistance of Ambient-Cured Geopolymer Mortars Containing Nano-Silica. Cem. Concr. Compos. 2016, 72, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Wang, G.; Chen, X.; Tan, J.; Gu, X. Recycling of Steel Slag Aggregate in Portland Cement Concrete: An Overview. J. Clean. Prod. 2021, 282, 124447. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, X.; Gu, Y.Y.; Zhang, Y.Y. Effects of Pozzolanic Reaction on Carbonation Degree and Strength of Steel Slag Compacts Containing Zeolite. Constr. Build. Mater. 2021, 277, 122334. [Google Scholar] [CrossRef]
- Gencel, O.; Karadag, O.; Oren, O.H.; Bilir, T. Steel Slag and Its Applications in Cement and Concrete Technology: A Review. Constr. Build. Mater. 2021, 283, 122783. [Google Scholar] [CrossRef]
- Chen, Z.; Li, R.; Zheng, X.; Liu, J. Carbon Sequestration of Steel Slag and Carbonation for Activating RO Phase. Cem. Concr. Res. 2021, 139, 106271. [Google Scholar] [CrossRef]
- Choi, Y.C.; Choi, S. Alkali–Silica Reactivity of Cementitious Materials Using Ferro-Nickel Slag Fine Aggregates Produced in Different Cooling Conditions. Constr. Build. Mater. 2015, 99, 279–287. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. The Potential for Copper Slag Waste as a Resource for a Circular Economy: A Review—Part II. Miner. Eng. 2021, 172, 107150. [Google Scholar] [CrossRef]
- Kubissa, W.; Jaskulski, R.; Gil, D.; Wilińska, I. Holistic Analysis of Waste Copper Slag Based Concrete by Means of EIPI Method. Buildings 2019, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Quina, M.J.; Bordado, J.C.M.; Quinta-Ferreira, R.M. The Influence of PH on the Leaching Behaviour of Inorganic Components from Municipal Solid Waste APC Residues. Waste Manag. 2009, 29, 2483–2493. [Google Scholar] [CrossRef]
- Vítková, M.; Ettler, V.; Šebek, O.; Mihaljevič, M.; Grygar, T.; Rohovec, J. The PH-Dependent Leaching of Inorganic Contaminants from Secondary Lead Smelter Fly Ash. J. Hazard. Mater. 2009, 167, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.J.; van der Sloot, H.A.; Comans, R.N.J. The Leaching of Major and Trace Elements from MSWI Bottom Ash as a Function of PH and Time. Appl. Geochem. 2006, 21, 335–351. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An Assessment of PH-Dependent Release and Mobility of Heavy Metals from Metallurgical Slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef]
- Barna, R.; Moszkowicz, P.; Gervais, C. Leaching Assessment of Road Materials Containing Primary Lead and Zinc Slags. Waste Manag. 2004, 24, 945–955. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Pang, L.; Wang, D. Adverse Effects of Using Metallurgical Slags as Supplementary Cementitious Materials and Aggregate: A Review. Materials 2022, 15, 3803. https://doi.org/10.3390/ma15113803
Zhao Q, Pang L, Wang D. Adverse Effects of Using Metallurgical Slags as Supplementary Cementitious Materials and Aggregate: A Review. Materials. 2022; 15(11):3803. https://doi.org/10.3390/ma15113803
Chicago/Turabian StyleZhao, Qiang, Lang Pang, and Dengquan Wang. 2022. "Adverse Effects of Using Metallurgical Slags as Supplementary Cementitious Materials and Aggregate: A Review" Materials 15, no. 11: 3803. https://doi.org/10.3390/ma15113803







