Molecular Dynamics Simulation Study on the Interactions of Mixed Cationic/Anionic Collectors on Muscovite (001) Surface in Aqueous Solution
Abstract
:1. Introduction
2. Computational Details
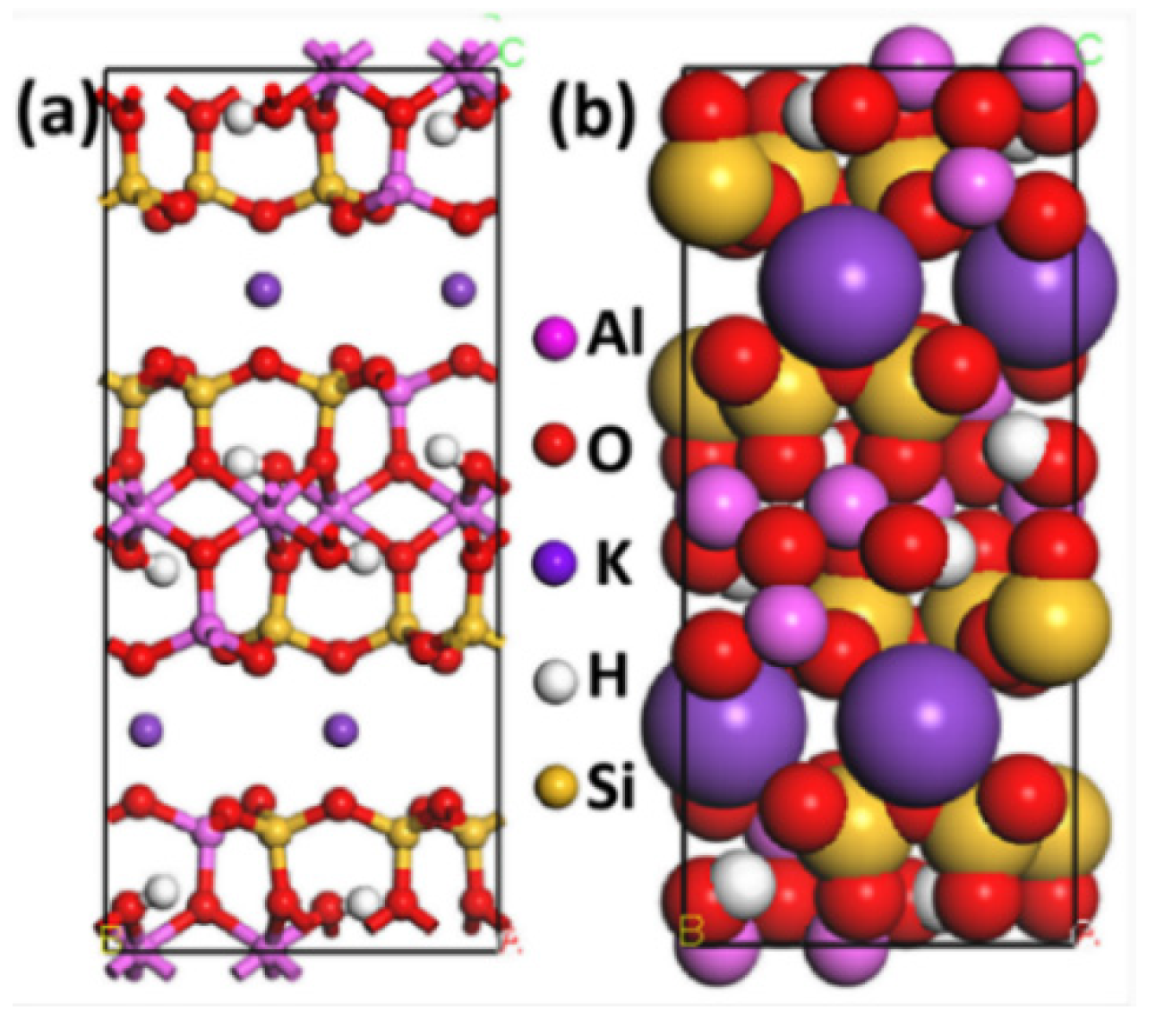
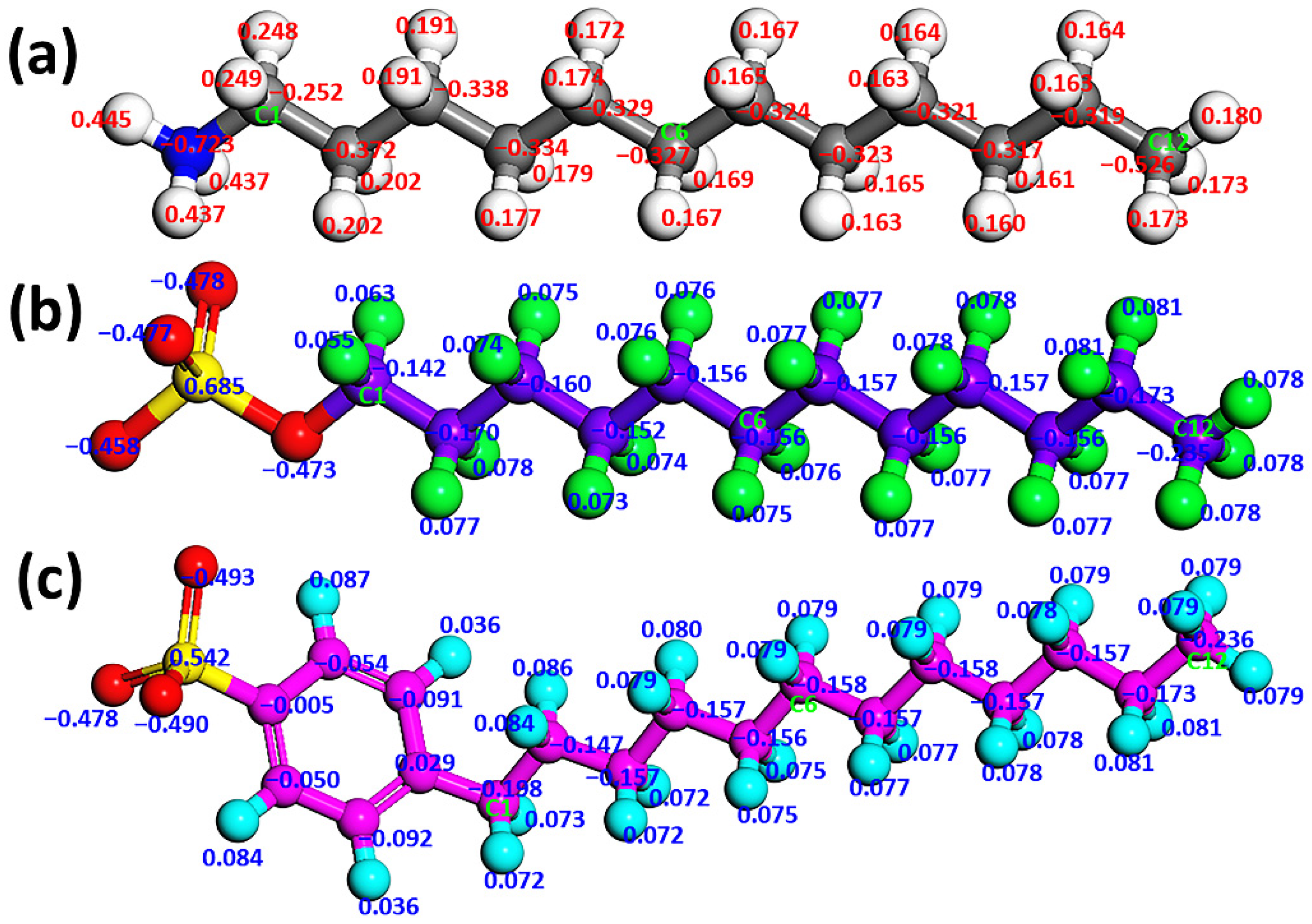
2.1. Models
2.2. Simulation Method
3. Model Methodology
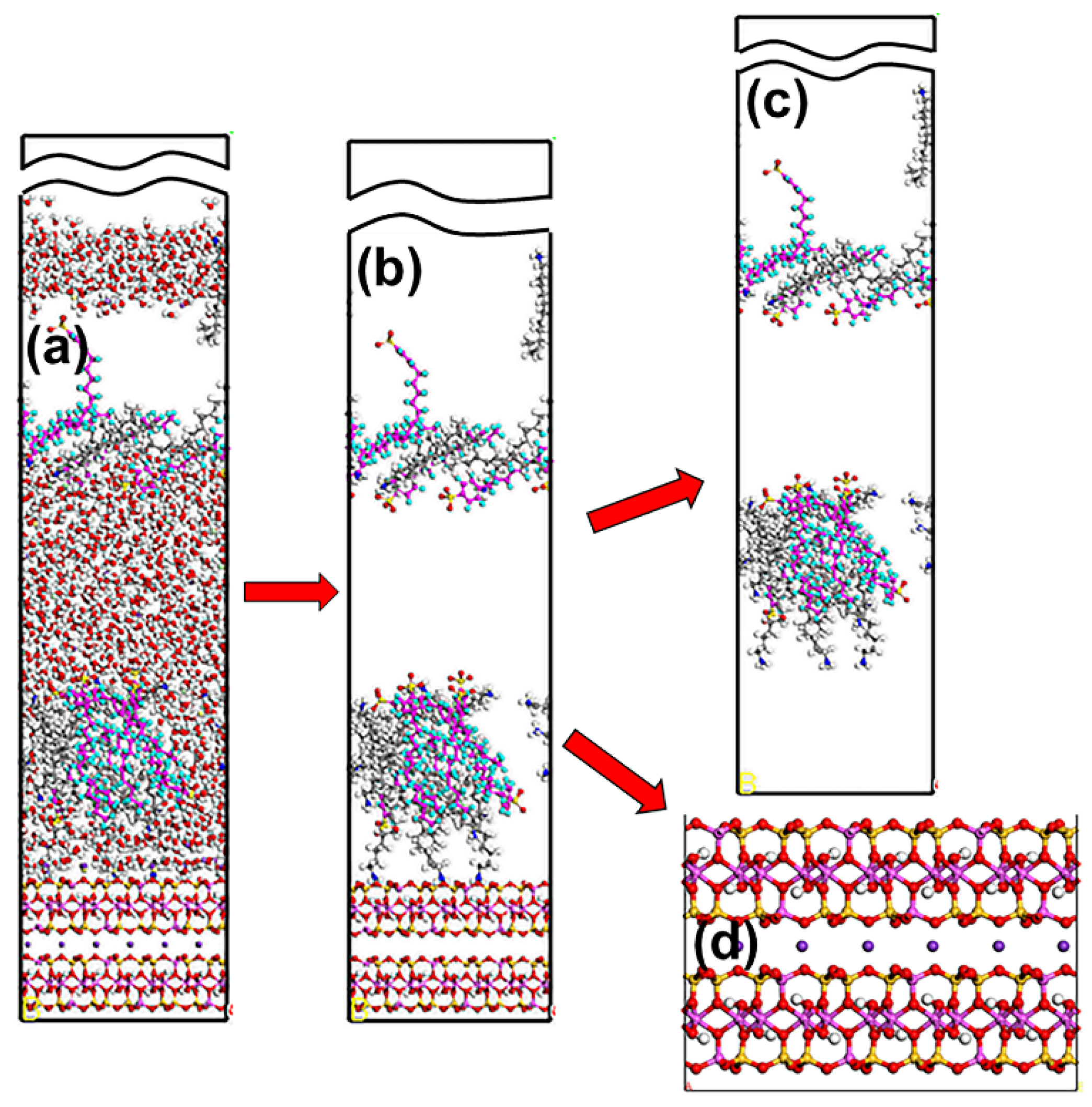
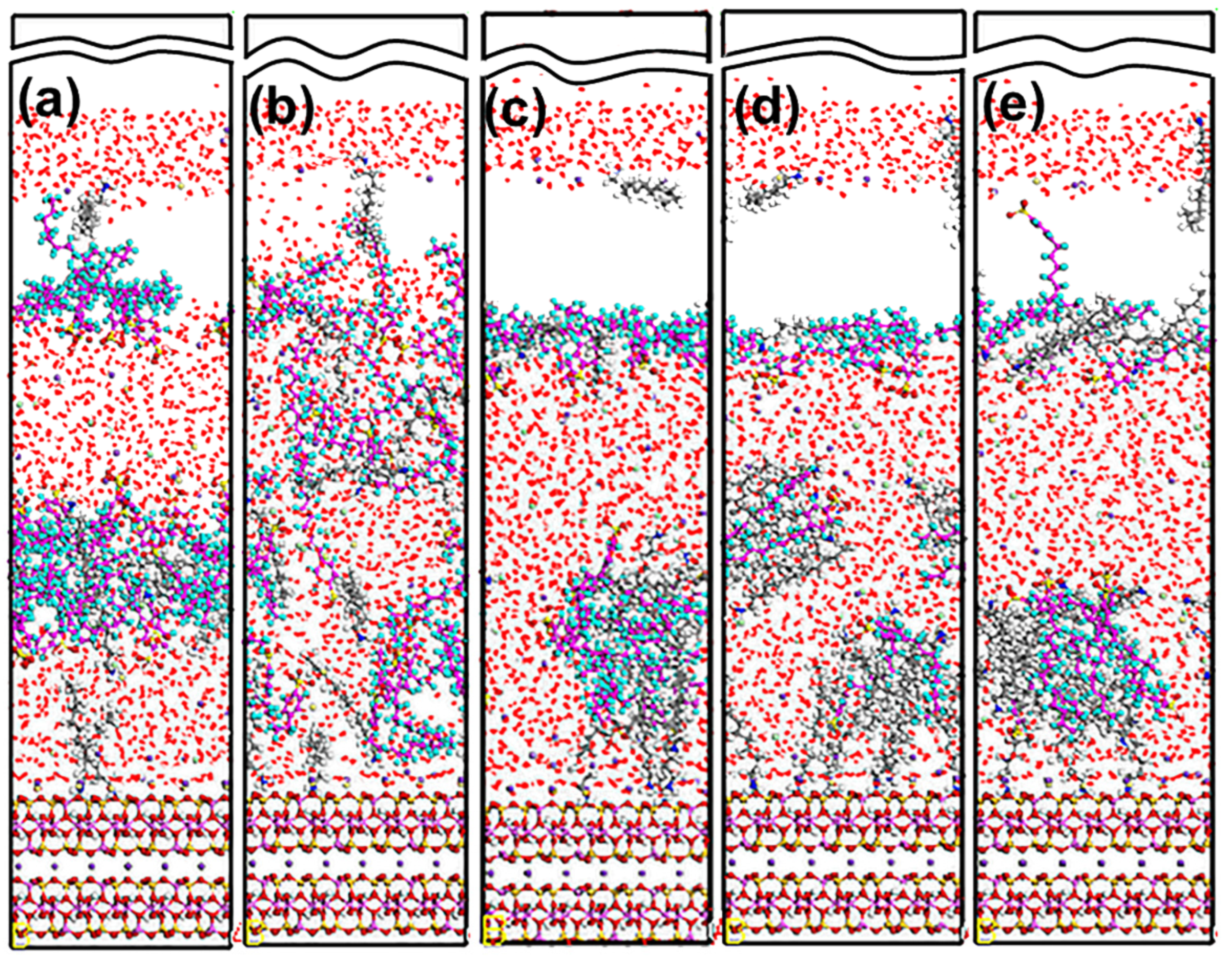
3.1. Snapshot of Structure
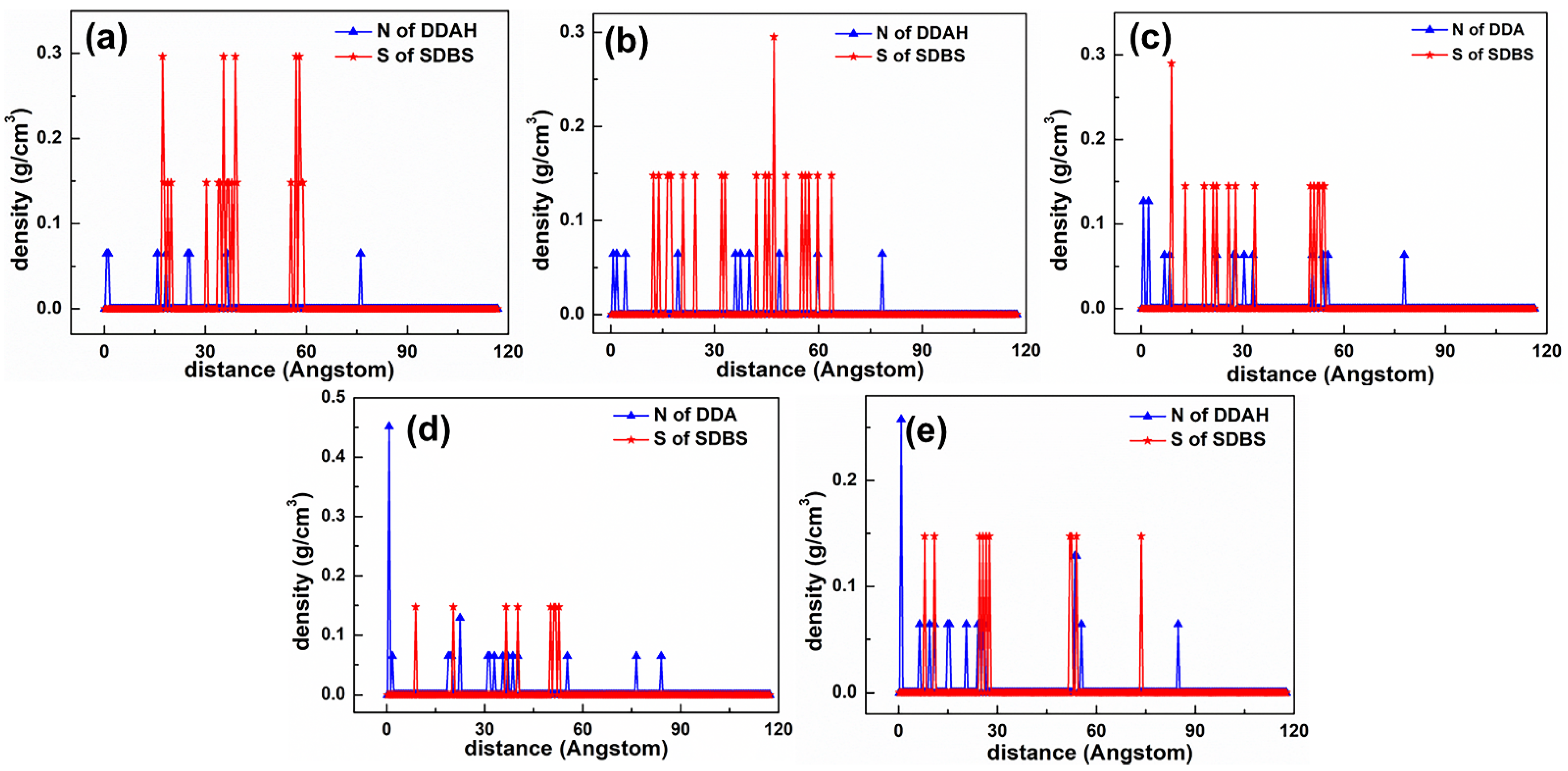
3.2. Density Profile
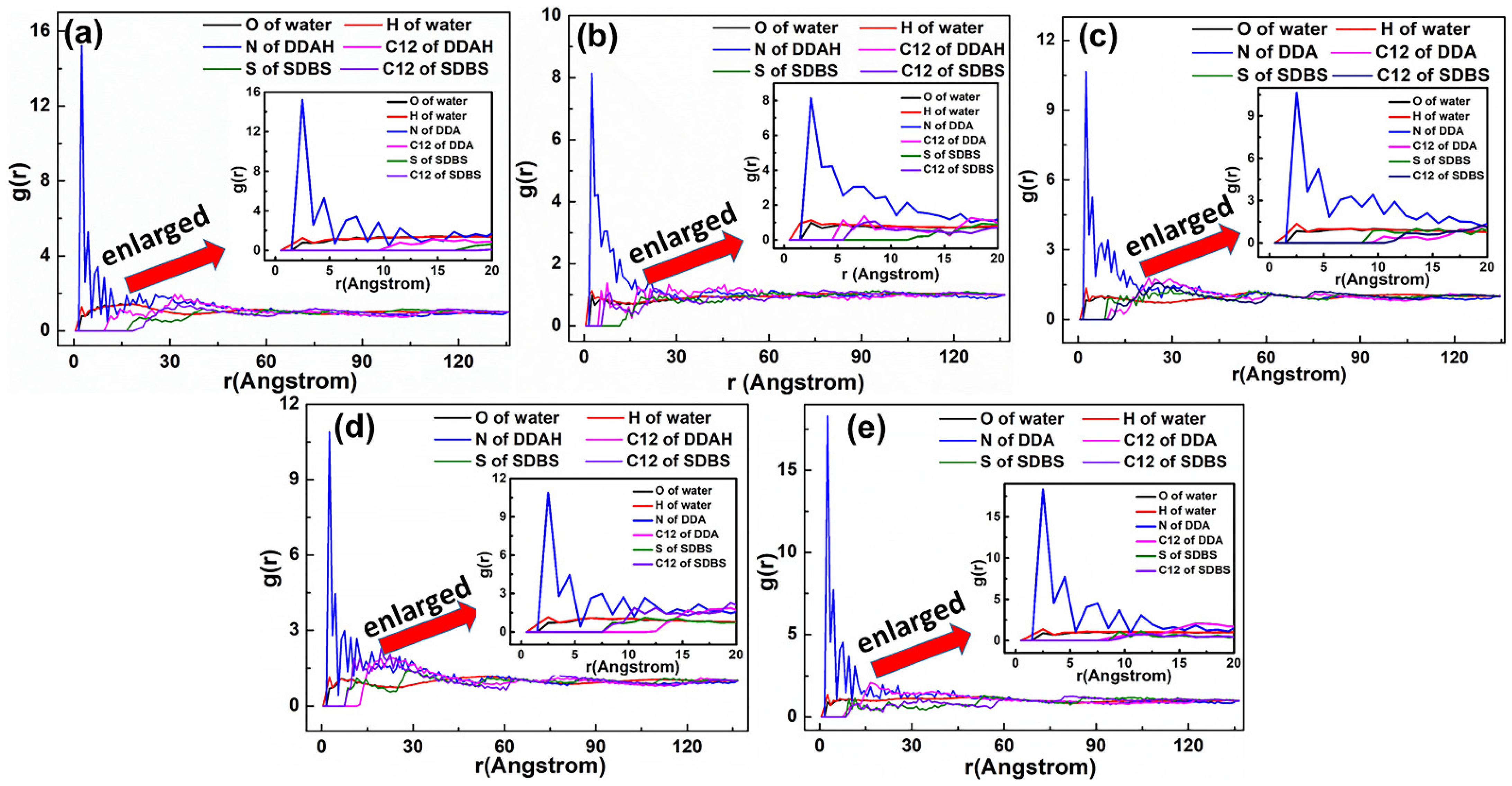
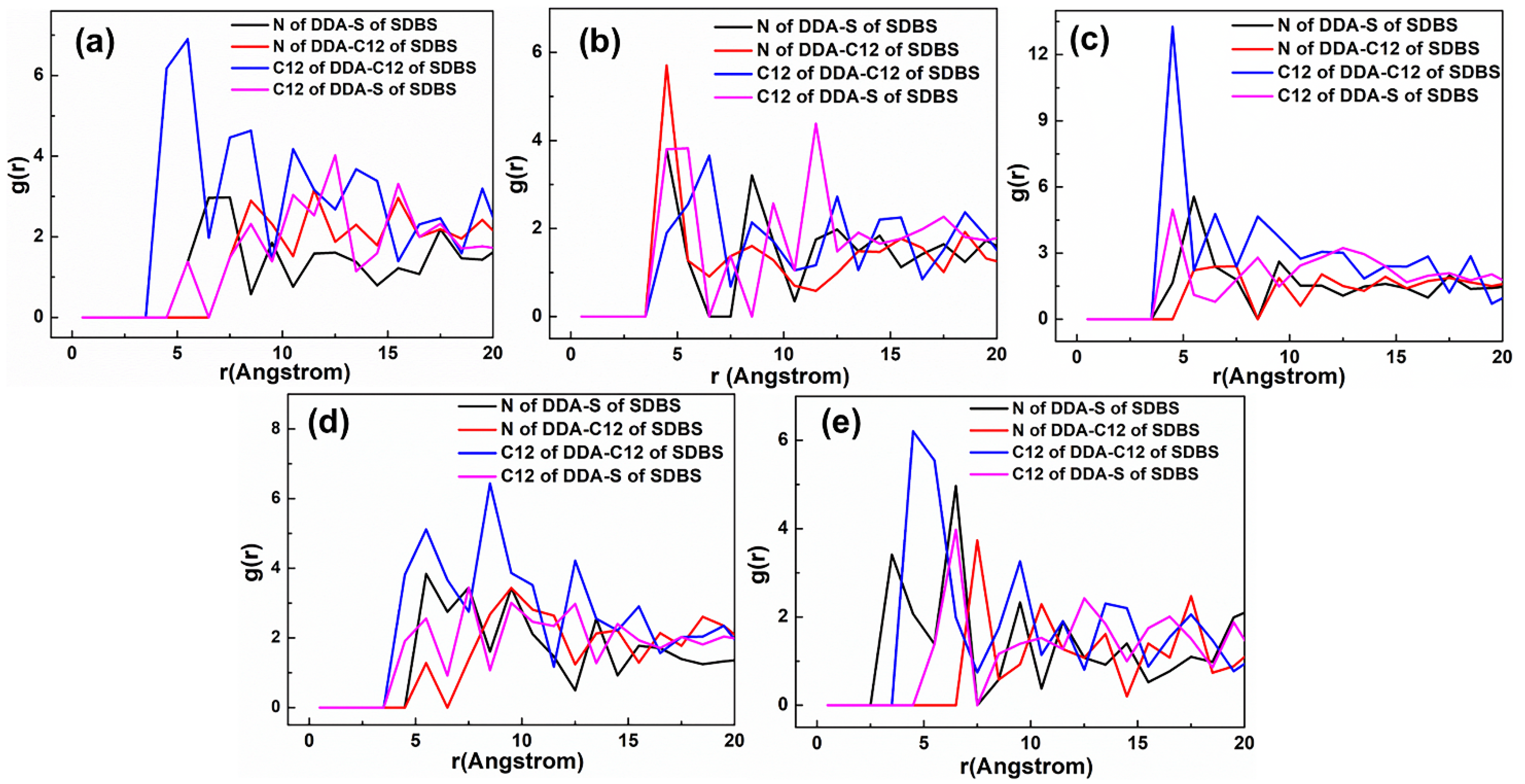
3.3. Radial Distribution Function (RDF)
3.4. Interaction Energy
4. Micro-Flotation Experiments
5. Results and Discussion
5.1. Adsorption Behavior of Single Collector on Mcv (001) Surface
5.1.1. DDAHC Collector
5.1.2. SDS Collector
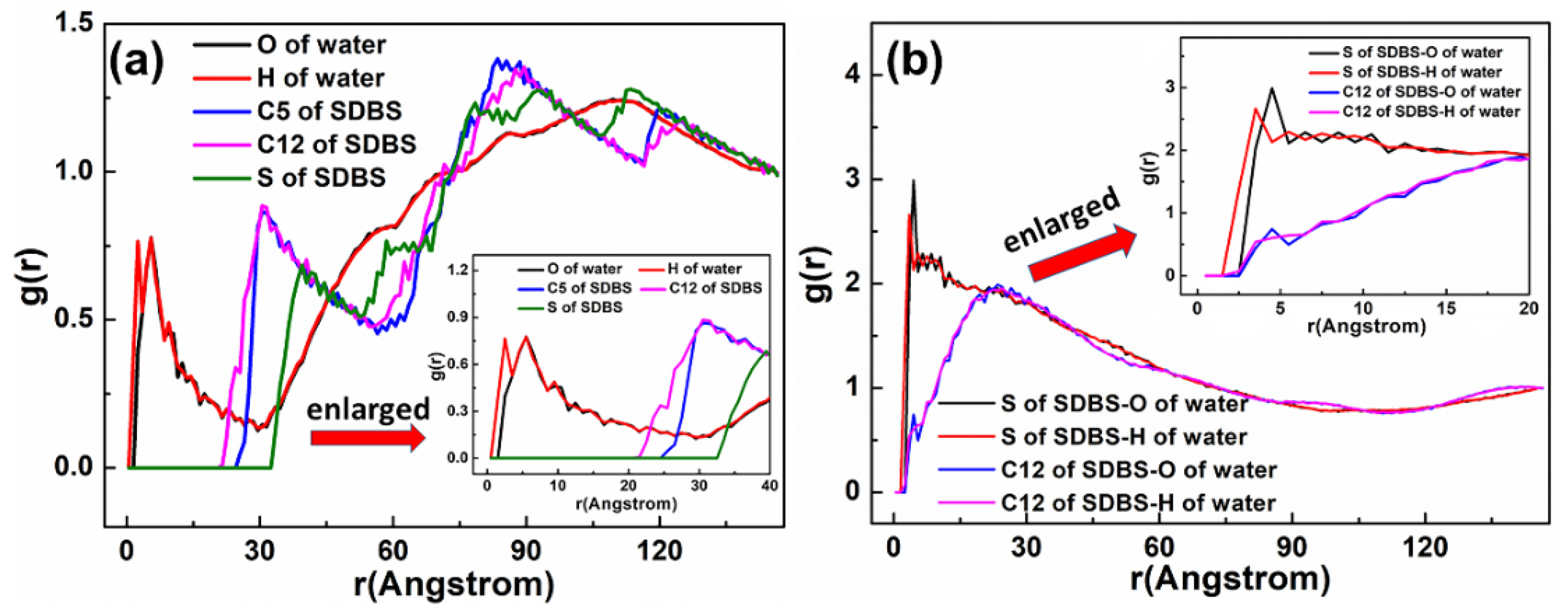
5.1.3. SDBS Collector
5.2. Adsorption Behavior of Mixed Collectors on Mcv (001) Surface
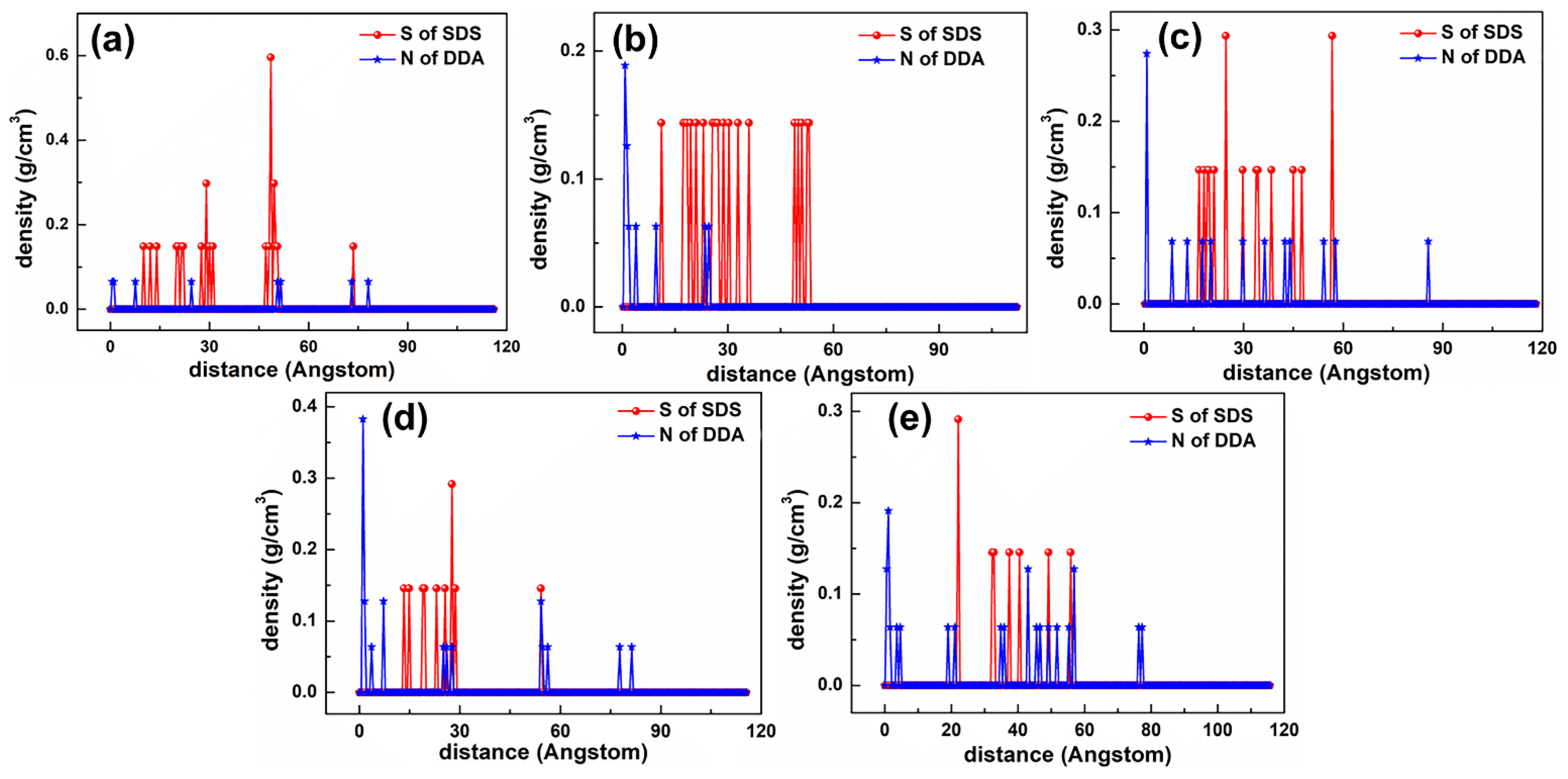
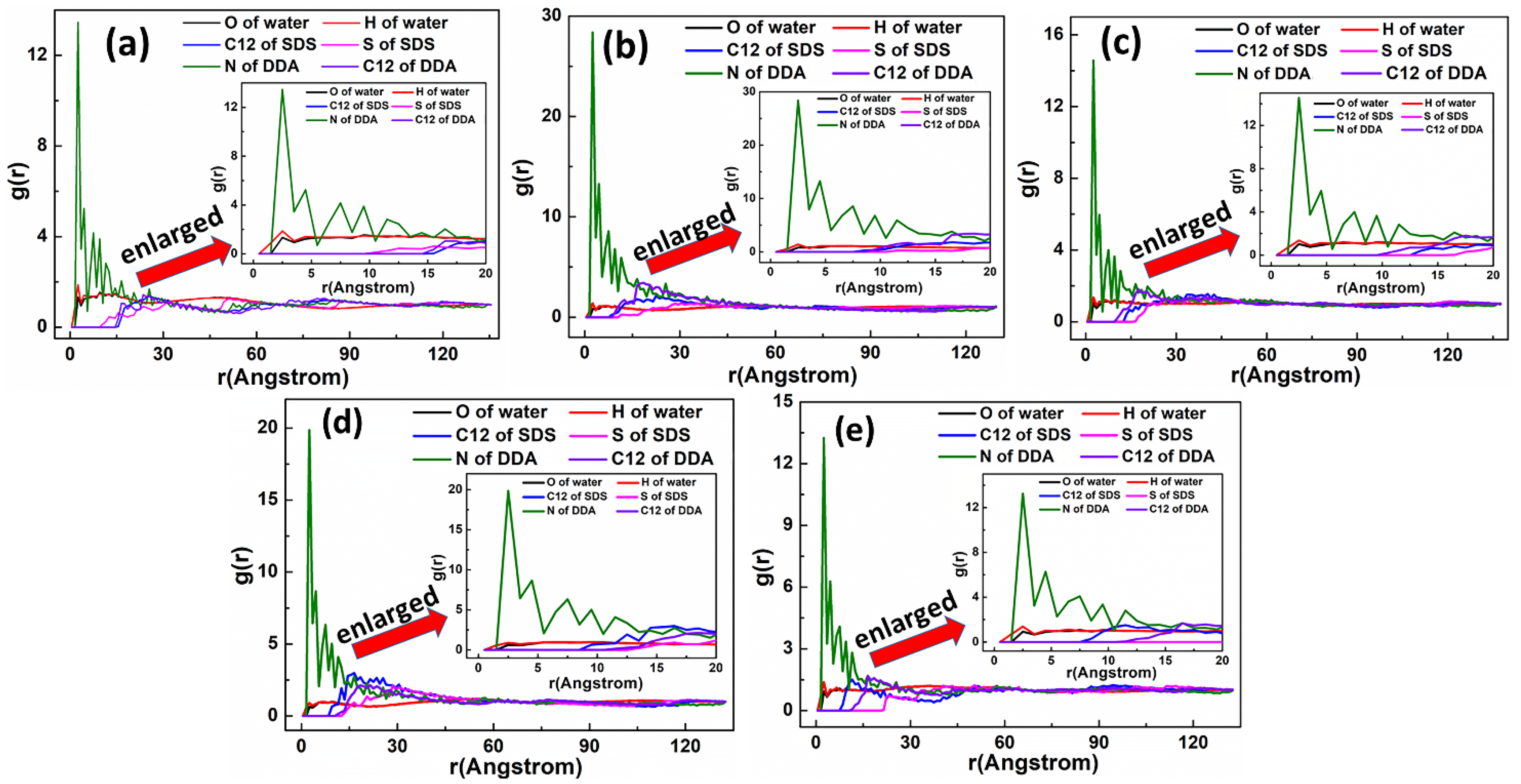
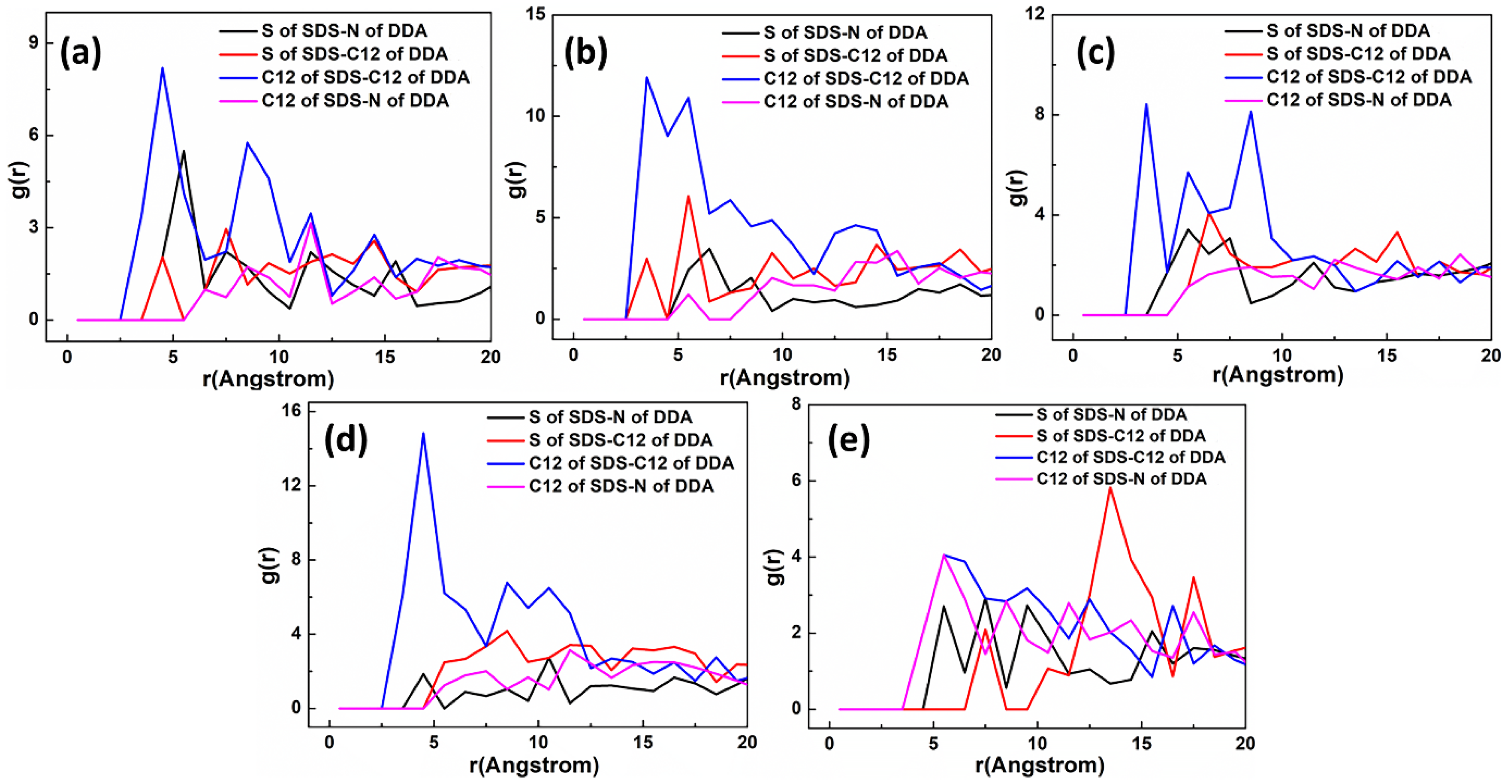
5.2.1. DDAHC/SDS Collectors
5.2.2. DDAHC/SDBS Collectors
5.3. Interaction Energy
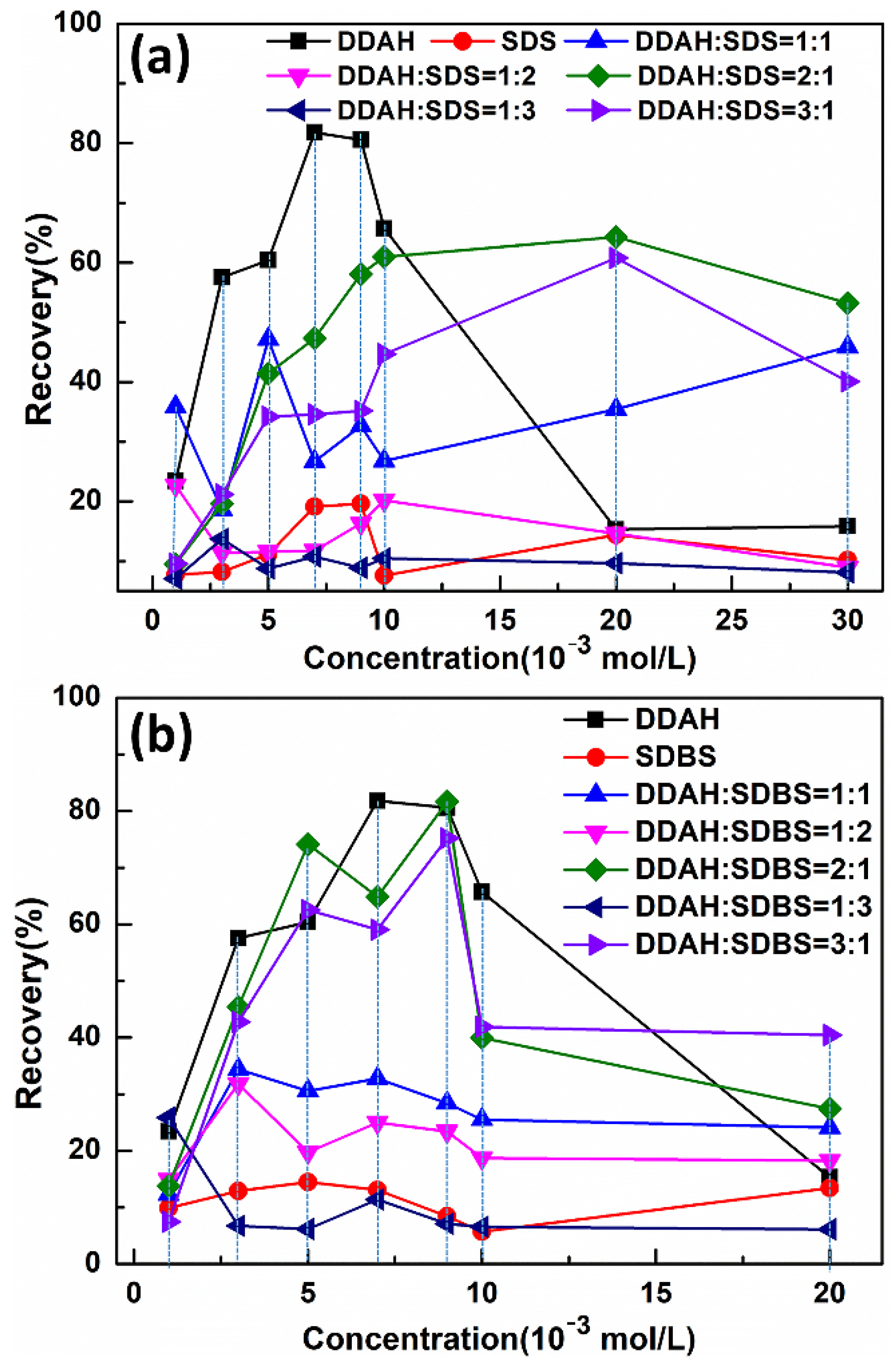
5.4. Flotation Recovery of Mcv with Cationic, Anionic and Mixed Collectors
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abukhadra, M.R.; Mostafa, M. Effective decontamination of phosphate and ammonium utilizing novel muscovite/phillipsite composite; equilibrium investigation and realistic application. Sci. Total Environ. 2019, 667, 101–111. [Google Scholar] [CrossRef]
- Chen, S.; Chen, F.; Di, Y.; Han, S.; Zhu, X. Preparation and characterisation of exfoliated muscovite/poly(2,3-dimethylaniline) nanocomposites with an enhanced anticorrosive performance. Micro Nano Lett. 2020, 15, 509–513. [Google Scholar] [CrossRef]
- Zhou, S.; Lv, J.; Guo, L.K.; Xu, G.Q.; Wang, D.M.; Zheng, Z.X.; Wu, Y.C. Preparation and photocatalytic properties of N-doped nano-TiO2/muscovite composites. Appl. Surf. Sci. 2012, 258, 6136–6141. [Google Scholar] [CrossRef]
- Jiang, H.; Ji, W.; Yang, Q.; Xu, L.; Zhao, C.; Hu, Y. Synergistic adsorption and flotation of new mixed cationic/nonionic collectors on muscovite. Minerals 2017, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Masliyah, J.H.; Xu, Z. Interaction of divalent cations with basal planes and edge surfaces of phyllosilicate minerals: Muscovite and talc. J. Colloid Interf. Sci. 2013, 404, 183–191. [Google Scholar] [CrossRef]
- Pugh, R.J.; Rutland, M.W.; Manev, E.; Claesson, P.M. Dodecylamine collector -pH effect on mica flotation and correlation with thin aqueous foam film and surface force measurements. Int. J. Miner. Process. 1996, 46, 245–262. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Liu, R.-Q. Mechanism of separating muscovite and quartz by flotation. J. Cent. South Univ. 2014, 21, 3596–3602. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.-L.; He, D.-D.; Liu, G.-S. Adsorption of cationic collectors and water on muscovite (001) surface: A molecular dynamics simulation study. Miner. Eng. 2013, 53, 101–107. [Google Scholar] [CrossRef]
- Rao, K.H.; Forssberg, K.S.E. Mixed collector systems in flotation. Int. J. Miner. Process. 1997, 51, 67–79. [Google Scholar]
- Vidyadhar, A.; Rao, K.H. Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system. J. Colloid Interf. Sci. 2007, 306, 195–204. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Li, Y.; Han, Y. Flotation behavior and mechanism of a new mixed collector on separation of spodumene from feldspar. Colloids Surf. A 2020, 599, 124932. [Google Scholar] [CrossRef]
- Kume, G.; Gallotti, M.; Nunes, G. Review on anionic/cationic surfactant mixtures. J. Surfactants Deterge 2007, 11, 1–11. [Google Scholar] [CrossRef]
- Yang, Z.; Teng, Q.; Liu, J.; Yang, W.; Hu, D.; Liu, S. Use of NaOL and CTAB mixture as collector in selective flotation separation of enstatite and magnetite. Colloids Surf. A 2019, 570, 481–486. [Google Scholar] [CrossRef]
- Shen, L.; Zhu, J.; Liu, L.; Wang, H. Flotation of fine kaolinite using dodecylamine chloride/fatty acids mixture as collector. Powder Technol. 2017, 312, 159–165. [Google Scholar] [CrossRef]
- Orhan, E.C.; Bayraktar, İ. Amine-oleate interactions in feldspar flotation. Miner. Eng. 2006, 19, 48–55. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, L.; Wu, H.; Wang, Z.; Shu, K.; Fang, S.; Zhang, Z. Flotation and co-adsorption of mixed collectors octanohydroxamic acid/sodium oleate on bastnaesite. J. Alloys Compd. 2020, 819, 152948. [Google Scholar] [CrossRef]
- Vidyadhar, A.; Kumari, N.; Bhagat, R.P. Adsorption mechanism of mixed collector systems on hematite flotation. Miner. Eng. 2012, 26, 102–104. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Sun, W.; Hu, Y. The flotation and adsorption of mixed collectors on oxide and silicate minerals. Adv. Colloid Interface Sci. 2017, 250, 1–14. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Hu, Y.; Liu, J.; Sun, W. Adsorption behavior of mixed cationic/anionic surfactants and their depression mechanism on the flotation of quartz. Powder Technol. 2016, 302, 15–20. [Google Scholar] [CrossRef]
- Jin, J.; Gao, H.; Chen, X.; Peng, Y.; Min, F. The flotation of aluminosilicate polymorphic minerals with anionic and cationic collectors. Miner. Eng. 2016, 99, 123–132. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Deng, W.; Jiang, H.; Gao, Z.; Hu, Y. Adsorption mechanism of new mixed anionic/cationic collectors in a spodumene-feldspar flotation system. Chem. Eng. Sci. 2017, 164, 99–107. [Google Scholar] [CrossRef]
- Rao, K.H.; Antti, B.-M.; Forssberg, K.S.E. Flotation of mica minerals and selectivity between muscovite and biotite while using mixed anionic/cationic collectors. Min. Metall. Explor. 1990, 7, 127–132. [Google Scholar]
- Jiang, H.; Gao, Y.; Khoso, S.A.; Ji, W.; Hu, Y. A new approach for characterization of hydrophobization mechanisms of surfactants on muscovite surface. Sep. Purif. Technol. 2019, 209, 936–945. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.-H.; Xu, L.-H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite—Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Wang, L.; Yang, Y.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloids Surf. A 2016, 492, 181–189. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Sun, W.; Sun, Y. Molecular dynamics simulation study of the interaction of mixed cationic/anionic surfactants with muscovite. Appl. Surf. Sci. 2015, 327, 364–370. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.-L.; Liu, G.-S. Molecular dynamics simulation of primary ammonium ions with different alkyl chains on the muscovite (001) surface. Int. J. Miner. Process. 2015, 145, 48–56. [Google Scholar] [CrossRef]
- Bai, Y.; Li, C.; An, H.; Wang, G.; Zhao, X.; Zhang, J. Flotation and molecular dynamics simulation of muscovite with mixed anionic/cationic collectors. Physicochem. Probl. Miner. Process. 2020, 56, 313–324. [Google Scholar]
- Blom, A.; Warr, G.G. Structure and composition of cationic-nonionic surfactant mixed adsorbed layeers on mica. Langmuir 2006, 22, 6787–6795. [Google Scholar] [CrossRef]
- Jordens, A.; Marion, C.; Grammatikopoulos, T.; Waters, K.E. Understanding the effect of mineralogy on muscovite flotation using QEMSCAN. Int. J. Miner. Process. 2016, 155, 6–12. [Google Scholar] [CrossRef]
- Marion, C.; Jordens, A.; McCarthy, S.; Grammatikopoulos, T.; Waters, K.E. An investigation into the flotation of muscovite with an amine collector and calcium lignin sulfonate depressant. Sep. Purif. Technol. 2015, 149, 216–227. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Wu, H.; Fang, S.; Deng, W.; Peng, T.; Sun, W.; Hu, Y. A novel approach for flotation recovery of spodumene, mica and feldspar from a lithium pegmatite ore. J. Clean. Prod. 2018, 174, 625–633. [Google Scholar] [CrossRef]
- Di, Y.; Jiang, A.; Huang, H.; Luo, Q.; Wei, W.; Wang, R.; Chen, S. Molecular dynamics simulations of adsorption behavior of DDAH, NaOL and mixed DDAH/NaOL surfactants on muscovite (001) surface in aqueous solution. J. Mol. Graph. Modell. 2022, 113, 108161. [Google Scholar] [CrossRef] [PubMed]
- Rrchardson, S.M.; Richardson, J.W. Crystal structure of a pink muscoyite from Archer’s Post, Kenya: Implications for reverse pleochroism in dioctahedral micas. Am. Mineral. 1982, 67, 69–75. [Google Scholar]
- Heinz, H.; Koerner, H.; Anderson, K.L.; Vaia, R.A.; Farmer, B.L. Force field for mica-type silicates and dynamics of octadecylammonium chains grafted to montmorillonite. Chem. Mater. 2005, 17, 5658–5669. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Liu, R.; Liu, J.; Sun, W. Synergistic adsorption of DDA/alcohol mixtures at the air/water interface: A molecular dynamics simulation. J. Mol. Liq. 2017, 243, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Liu, J.; Sun, Y.; Sun, W. Flotation and adsorption of muscovite using mixed cationic–nonionic surfactants as collector. Powder Technol. 2015, 276, 26–33. [Google Scholar] [CrossRef]
- Zhou, P.; Hou, J.; Yan, Y.; Wang, J. The effect of surfactant adsorption on surface wettability and flow resistance in slit nanopore: A molecular dynamics study. J. Colloid Interface Sci. 2018, 513, 379–388. [Google Scholar] [CrossRef]
- Li, L.; Hao, H.; Yuan, Z.; Liu, J. Molecular dynamics simulation of siderite-hematite-quartz flotation with sodium oleate. Appl. Surf. Sci. 2017, 419, 557–563. [Google Scholar] [CrossRef]
- Rai, B.; Sathish, P.; Tanwar, J.; Pradip; Moon, K.S.; Fuerstenau, D.W. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals. J. Colloid Interface Sci. 2011, 362, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, Z.; Zhang, R.; Xing, Y.; Gui, X. Enhancement of the surface hydrophobicity of low-rank coal by adsorbing DTAB: An experimental and molecular dynamics simulation study. Fuel 2019, 239, 145–152. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, S.; Wang, X.; Guo, M.; Wang, Y.; Wang, D. Molecular dynamics simulation of oil detachment from calcite surface in aqueous surfactant solution. Comput. Theor. Chem. 2016, 1092, 82–89. [Google Scholar] [CrossRef]
- Tang, Y.; Kelebek, S.; Yin, W. Surface chemistry of magnesite and calcite flotation and molecular dynamics simulation of their cetyl phosphate adsorption. Colloids Surf. A 2020, 603, 125246. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, W.; Kelebek, S. Molecular dynamics simulation of magnesite and dolomite in relation to flotation with cetyl phosphate. Colloids Surf. A 2021, 610, 125928. [Google Scholar] [CrossRef]

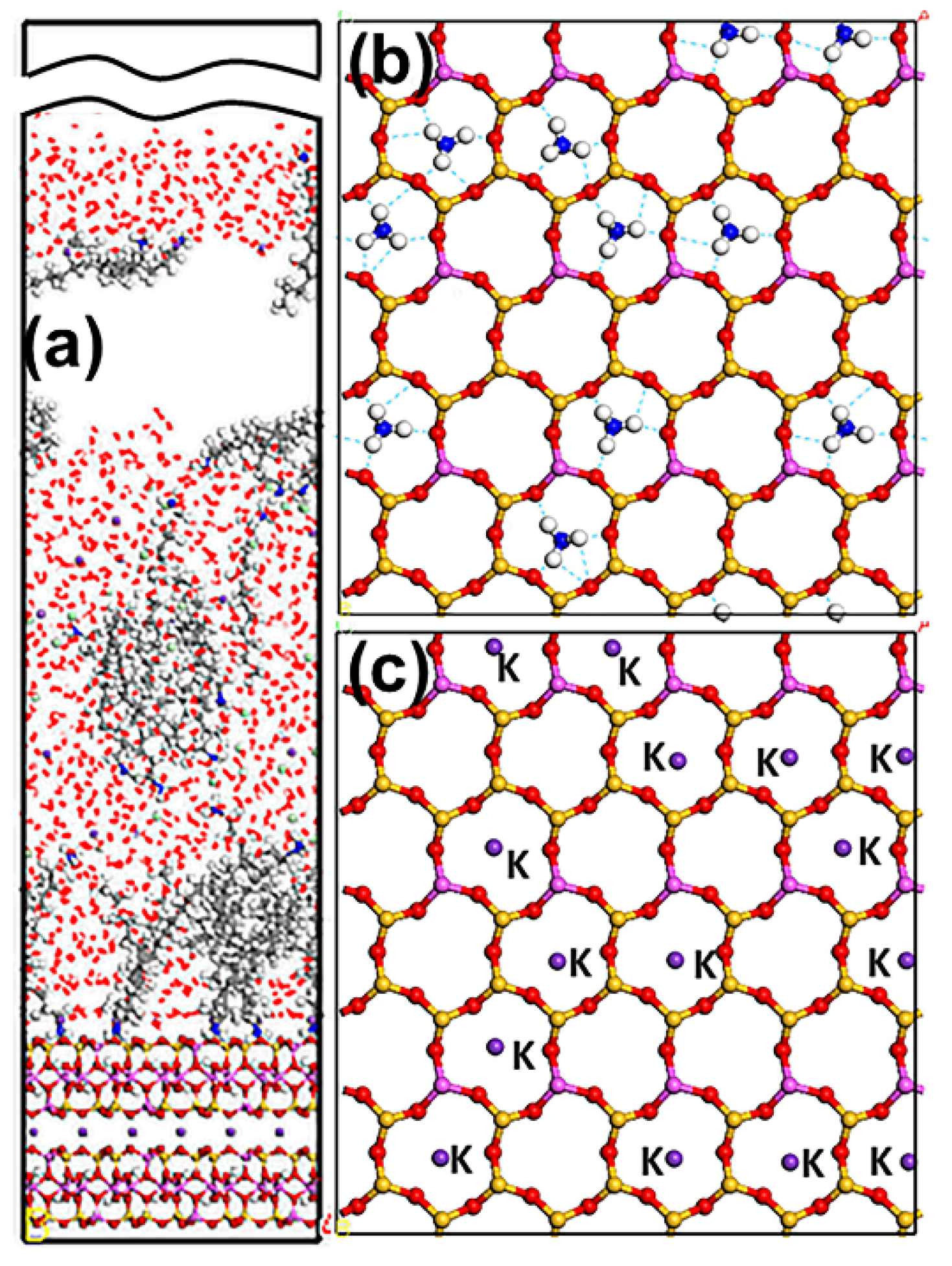
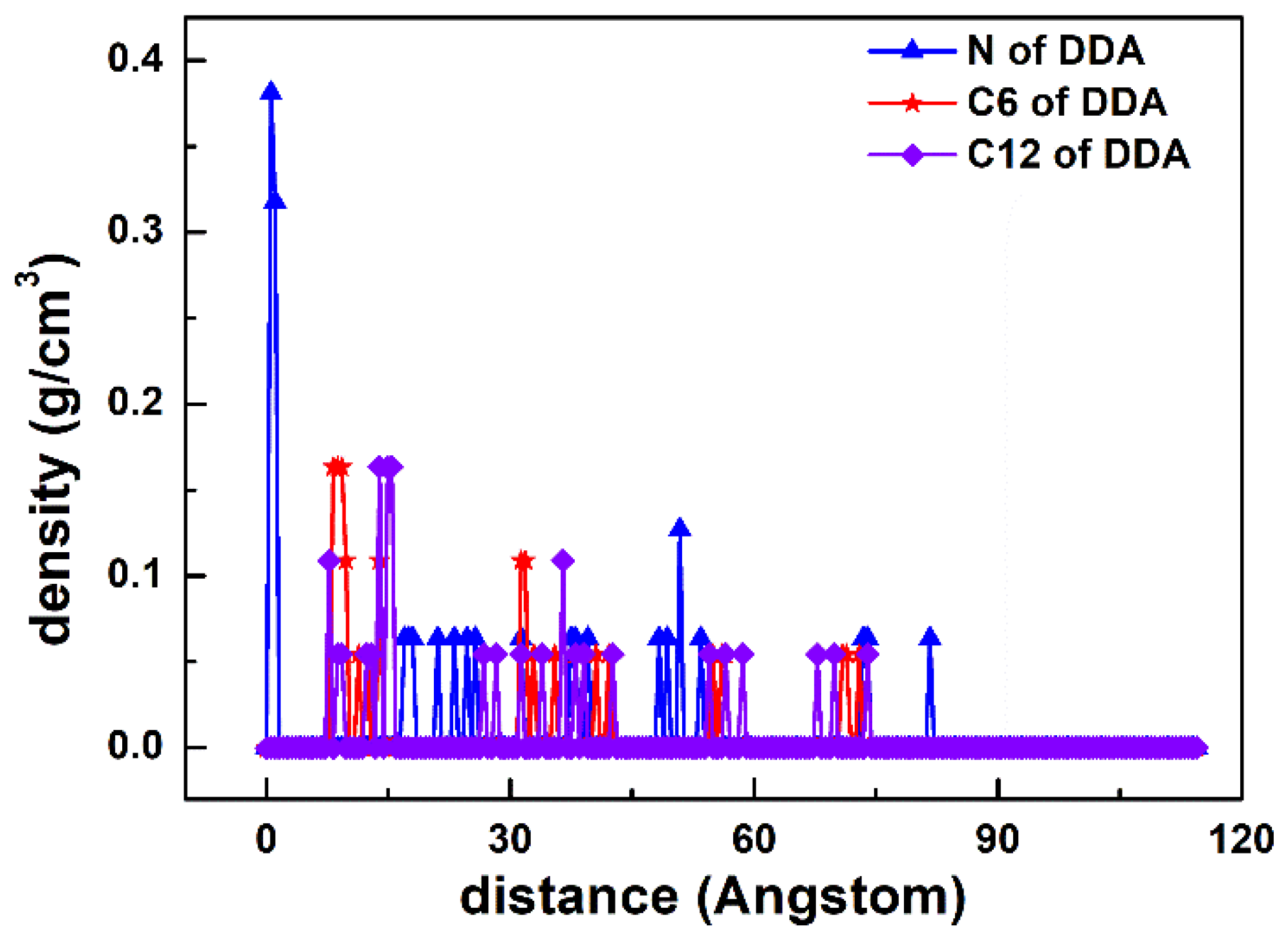
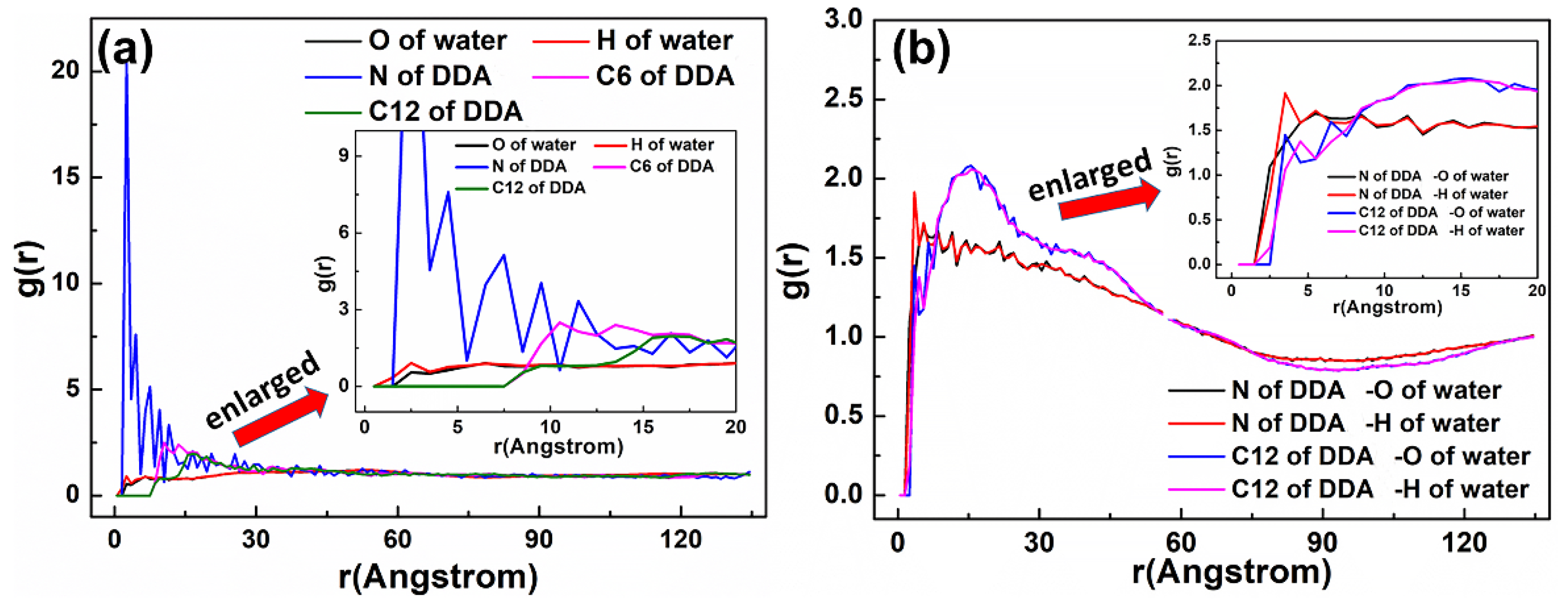

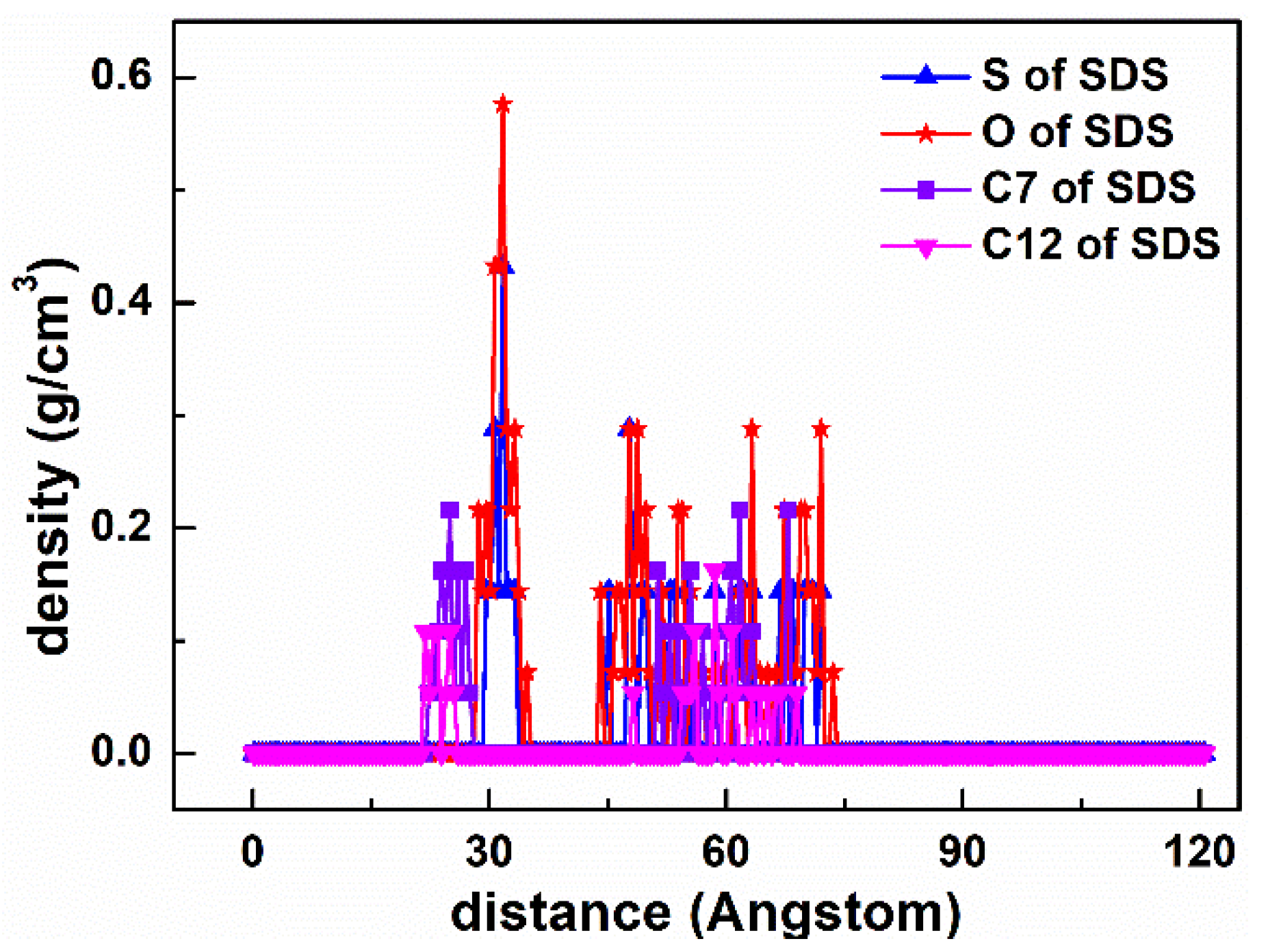




| Systems | Molar Ratios (D:S or D:SD) | DDA+ | SDS− | SDBS− | Cl− | Na+ |
|---|---|---|---|---|---|---|
| D30 | - | 30 | - | - | 30 | - |
| S30 | - | - | 30 | - | - | 30 |
| SD30 | - | - | - | 30 | - | 30 |
| D8S23 | 1:3 | 8 | 23 | - | 8 | 23 |
| D10S20 | 1:2 | 10 | 20 | - | 10 | 20 |
| D16S15 | 1:1 | 16 | 15 | - | 16 | 15 |
| D20S10 | 2:1 | 20 | 10 | - | 20 | 10 |
| D23S8 | 3:1 | 23 | 8 | - | 23 | 8 |
| D8SD23 | 1:3 | 8 | - | 23 | 8 | 23 |
| D10SD20 | 1:2 | 10 | - | 20 | 10 | 20 |
| D15SD15 | 1:1 | 15 | - | 15 | 15 | 15 |
| D20SD10 | 2:1 | 20 | - | 10 | 20 | 10 |
| D23SD8 | 3:1 | 23 | - | 8 | 23 | 8 |
| Al2O3 | K2O | MgO | SiO2 | Fe2O3 |
|---|---|---|---|---|
| 31.496 | 10.843 | 1.264 | 46.013 | 6.802 |
| Collectors | System | Etotal | Emus | Ecol | Einter |
|---|---|---|---|---|---|
| Single collector | D30 | −460,712.9139 | −442,048.0052 | 6881.007 | −851.53 |
| S30 | −469,886.5041 | −473,503.4898 | 2132.7143 | 49.47 | |
| SD30 | −468,925.1094 | −473,767.5963 | 3875.312 | 32.24 | |
| Mixed collectors DDAHC + SDS | D8S23 | −459,997.1125 | −456,393.1465 | 2752.9275 | −205.65 |
| D10S20 | −469,017.6415 | −463,698.4841 | 2380.5639 | −256.657 | |
| D16S15 | −458,419.7227 | −450,551.3448 | 3032.0479 | −351.63 | |
| D20S10 | −459,932.9465 | −447,300.8663 | 5073.0874 | −590.17 | |
| D23S8 | −454,045.5905 | −445,243.15 | 3620.148 | −400.73 | |
| Mixed collectors DDAHC + SDBS | D8SD23 | −463,780.6320 | −457,085.4462 | 2149.4245 | −285.31 |
| D10SD20 | −460,565.1272 | −450,882.7433 | 2139.3061 | −394.06 | |
| D15SD15 | −451,808.8386 | −440,744.5416 | 3442.0143 | −483.54 | |
| D20SD10 | −455,002.4834 | −440,724.5883 | 5677.6587 | −665.19 | |
| D23SD8 | −457,303.2624 | −445,861.5949 | 4729.0245 | −521.64 |
| Number | Minerals | Plane | Collectors | ΔE (kcal/mol) |
|---|---|---|---|---|
| [8] | muscovite | 001 | DDA+ | −123.400 |
| [14] | kaolinite | 001 | 10 DDAHC | −277.6000 |
| 10 DDAHC + 5 OA | −183.5333 | |||
| [20] | kyanite | 100 | 1 Octadecylamine (OA) | −305.5186 |
| 1 SHS | −493.2314 | |||
| [20] | andalusite | 110 | 1 OA | −253.1644 |
| 1 SHS | −1361.4364 | |||
| [20] | sillimanite | 010 | 1 OA | −263.0784 |
| [27] | muscovite | 001 | NaOL | 10.3754 |
| DDA | −27.7817 | |||
| DDA-NaOL | −79.2860 | |||
| [28] | muscovite | 001 | 15 DDA | −1049.10 |
| [40] | siderite | 101 | 10 NaOL | −2350.9587 |
| hematite | 001 | −42.2757 | ||
| quartz | 101 | −6259.5622 | ||
| [41] | spodumene | 110 | Oleate | −235.80 |
| anorthite | 001 | −141.90 | ||
| muscovite | 001 | 127.00 | ||
| [42] | low-rank coal | - | 9 dodecyltrimethylammonium bromide (DTAB) | −634.8016 |
| [43] | calcite | - | 50 Dodecane (C12) | −215.6400 |
| 50 C12 + 6 sodium hexadecyl sulfonate (SHS) | −180.0714 | |||
| [44] | magnesite | 101 | 20 cetyl phosphate adsorption (PCP) | −2301.0468 |
| calcite | 104 | 20 PCP | −162.4522 | |
| [45] | magnesite | 104 | 20 PCP | −97.18 |
| dolomite | 104 | 20 PCP | −22.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, Y.; Jiang, A.; Huang, H.; Deng, L.; Zhang, D.; Deng, W.; Wang, R.; Luo, Q.; Chen, S. Molecular Dynamics Simulation Study on the Interactions of Mixed Cationic/Anionic Collectors on Muscovite (001) Surface in Aqueous Solution. Materials 2022, 15, 3816. https://doi.org/10.3390/ma15113816
Di Y, Jiang A, Huang H, Deng L, Zhang D, Deng W, Wang R, Luo Q, Chen S. Molecular Dynamics Simulation Study on the Interactions of Mixed Cationic/Anionic Collectors on Muscovite (001) Surface in Aqueous Solution. Materials. 2022; 15(11):3816. https://doi.org/10.3390/ma15113816
Chicago/Turabian StyleDi, Yuli, Ao Jiang, Haiyan Huang, Lin Deng, Dafu Zhang, Wenwei Deng, Rui Wang, Qian Luo, and Shanhua Chen. 2022. "Molecular Dynamics Simulation Study on the Interactions of Mixed Cationic/Anionic Collectors on Muscovite (001) Surface in Aqueous Solution" Materials 15, no. 11: 3816. https://doi.org/10.3390/ma15113816





