Effect of Candida albicans Suspension on the Mechanical Properties of Denture Base Acrylic Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.1.1. Sample Preparation
2.1.2. Incubation in the Suspension of Candida albicans
2.2. Methods
2.2.1. Tensile Strength Test
2.2.2. Flexural Properties
2.2.3. Charpy Impact Strength
2.2.4. Surface Vickers Hardness
2.2.5. Ball Hardness
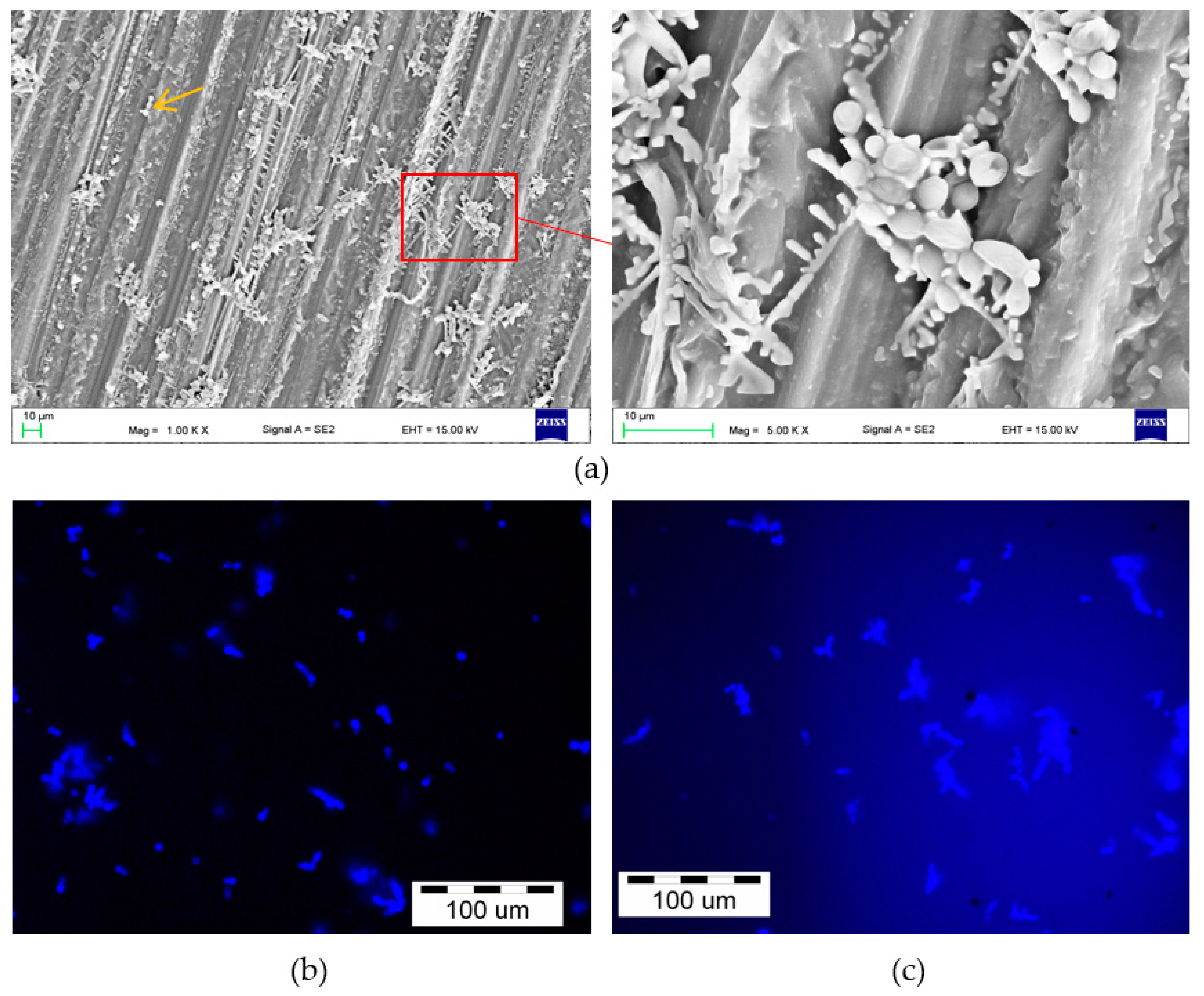
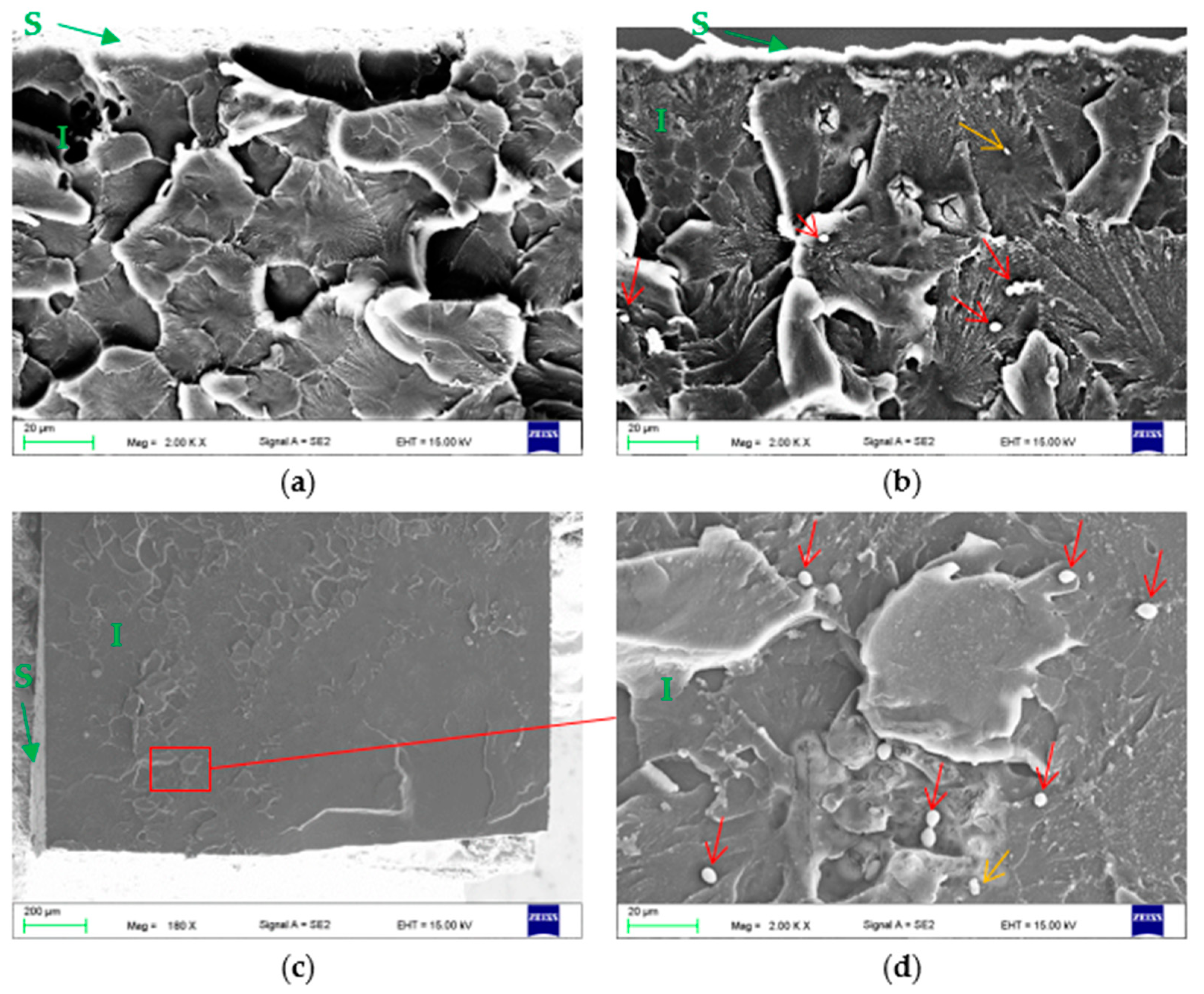
2.2.6. Microscopic Evaluation—Colonization and Penetration Evaluation
2.2.7. Statistical Analysis
3. Results
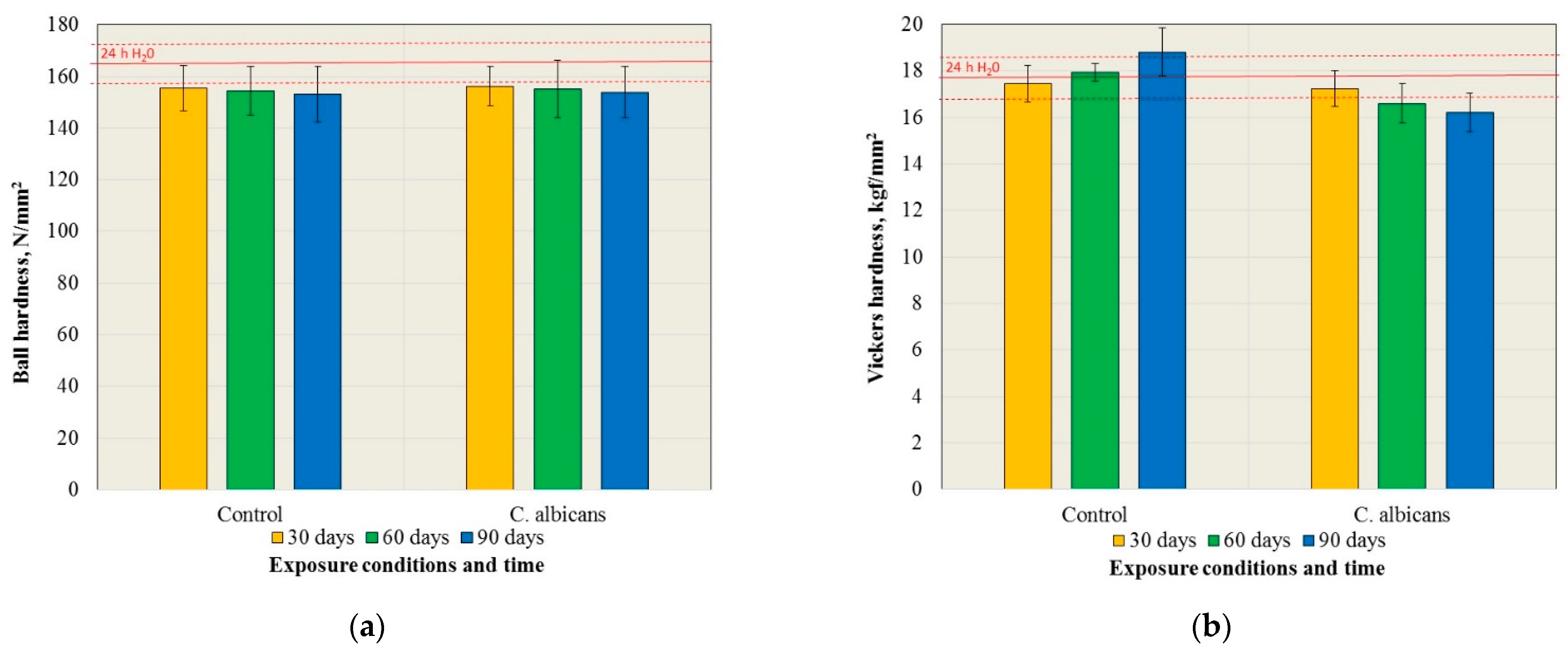
3.1. Mechanical Properties
3.2. Microscopic Evaluation of Surfaces and Fractures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Dwairi, Z.N. Isolation of candida species from the oral cavity and fingertips of complete and partial dentures wearers. J. Dent. Health Oral Disord. Ther. 2014, 1, 420–423. [Google Scholar] [CrossRef] [Green Version]
- Spiechowicz, E.; Mierzwińska-Nastalska, E. Fungal Infections of Oral Cavity, 1st ed.; Med Tour Press International: Warsaw, Poland, 1998; p. 34. [Google Scholar]
- Smith, A.; Williams, D.; Bradshaw, D.; Milward, P.; Kutubi, S.A.; Rowe, W. The effect of residual food stain on candida albicans colonisation of denture acrylics. Dent. Oral Biol. Craniofacial Res. 2020, 3, 2–5. [Google Scholar] [CrossRef]
- Uzel, N.G.; Teles, F.R.; Teles, R.P.; Song, X.Q.; Torresyap, G.; Socransky, S.S.; Haffajee, A.D. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J. Clin. Periodontol. 2011, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Evren, B.A.; Uludamar, A.; Işeri, U.; Ozkan, Y.K. The association between socioeconomic status, oral hygiene practice, denture stomatitis and oral status in elderly people living different residential homes. Arch. Gerontol. Geriatr. 2011, 53, 252–257. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Chladek, G.; Pakieła, K.; Pakieła, W.; Żmudzki, J.; Adamiak, M.; Krawczyk, C. Effect of antibacterial silver-releasing filler on the physicochemical properties of poly(methyl methacrylate) denture base material. Materials 2019, 12, 4146. [Google Scholar] [CrossRef] [Green Version]
- Kinkela Devcic, M.; Simonic-Kocijan, S.; Prpic, J.; Paskovic, I.; Cabov, T.; Kovac, Z.; Glazar, I. Oral candidal colonization in patients with different prosthetic appliances. J. Fungi 2021, 7, 662. [Google Scholar] [CrossRef]
- Scully, C. Oral and Maxillofacial Medicine—E-Book: The Basis of Diagnosis and Treatment, 3rd ed.; Churchill Livingstone Elsevier: Edinburgh, UK, 2013; ISBN 978-0-7020-5205-7. [Google Scholar]
- Gauch, L.M.R.; Pedrosa, S.S.; Silveira-Gomes, F.; Esteves, R.A.; Marques-da-Silva, S.H. Isolation of candida spp. from denture-related stomatitis in Pará, Brazil. Braz. J. Microbiol. 2017, 49, 148–151. [Google Scholar] [CrossRef]
- De Freitas Oliveira Paranhos, H.; Peracini, A.; Pisani, M.X.; Oliveira, V.D.C.; de Souza, R.F.; Silva-Lovato, C.H. Color stability, surface roughness and flexural strength of an acrylic resin submitted to simulated overnight immersion in denture cleansers. Braz. Dent. J. 2013, 24, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Peracini, A.; Davi, L.R.; de Queiroz Ribeiro, N.; de Souza, R.F.; Lovato da Silva, C.H.; de Freitas Oliveira Paranhos, H. Effect of denture cleansers on physical properties of heat-polymerized acrylic resin. J. Prosthodont. Res. 2010, 54, 78–83. [Google Scholar] [CrossRef]
- Nakahara, T.; Harada, A.; Yamada, Y.; Odashima, Y.; Nakamura, K.; Inagaki, R.; Kanno, T.; Sasaki, K.; Niwano, Y. Influence of a new denture cleaning technique based on photolysis of H2O2 the mechanical properties and color change of acrylic denture base resin. Dent. Mater. J. 2013, 32, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Chu, L.; Rawls, H.R.; Norling, B.K.; Cardenas, H.L.; Whang, K. Development of an antimicrobial resin—A pilot study. Dent. Mater. 2011, 27, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Zidan, S.; Silikas, N.; Alhotan, A.; Haider, J.; Yates, J. Investigating the mechanical properties of ZrO2-impregnated PMMA nanocomposite for denture-based applications. Materials 2019, 12, 1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Flexural strength and hardness of filler-reinforced PMMA targeted for denture base application. Materials 2021, 14, 2659. [Google Scholar] [CrossRef]
- Bajunaid, S.O. How effective are antimicrobial agents on preventing the adhesion of candida albicans to denture base acrylic resin materials? A systematic review. Polymers 2022, 14, 908. [Google Scholar] [CrossRef]
- Cruz, P.C.; de Andrade, I.M.; Peracini, A.; de Souza-Gugelmin, M.C.M.; Silva-Lovato, C.H.; de Souza, R.F.; de Freitas Oliveira Paranhos, H. The effectiveness of chemical denture cleansers and ultrasonic device in biofilm removal from complete dentures. J. Appl. Oral Sci. 2011, 19, 668–673. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Walls, A.W.G.; Jakubovics, N.S.; Moynihan, P.J.; Jepson, N.J.A.; Loewy, Z. Association of removable partial denture use with oral and systemic health. J. Dent. 2011, 39, 711–719. [Google Scholar] [CrossRef]
- Van Reenen, J.F. Microbiologic studies on denture stomatitis. J. Prosthet. Dent. 1973, 30, 493–505. [Google Scholar]
- Bulad, K.; Taylor, R.L.; Verran, J.; McCord, J.F. Colonization and penetration of denture soft lining materials by candida albicans. Dent. Mater. 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Hallikerimath, R.B. An in-vitro evaluation of retention, colonization and penetration of commonly used denture lining materials by candida albicans. J. Clin. Diagn. Res. 2016, 10, ZC84–ZC88. [Google Scholar] [CrossRef]
- Rodger, G.; Taylor, R.L.; Pearson, G.J.; Verran, J. In vitro colonization of an experimental silicone by candida albicans. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Kedjarune, U.; Charoenworaluk, N.; Koontongkaew, S. Release of methyl methacrylate from heat-curved and autopolymerized resins: Cytotoxicity testing related to residual monomer. Aust. Dent. J. 1999, 44, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Brooks, S.C.; Walker, D.M. The release of residual monomeric methyl methacrylate from acrylic appliances in the human mouth: An assay for monomer in saliva. J. Dent. Res. 1988, 67, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Shirkavand, S.; Moslehifard, E. Effect of TiO2 nanoparticles on tensile strength of dental acrylic resins. J. Dent. Res. Dent. Clin. Dent. Prospects 2014, 8, 197–203. [Google Scholar] [CrossRef]
- ISO 20795-1:2013; Dentistry—Base Polymers—Part 1: Denture Base Polymers. International Organization for Standardization: London, UK, 2013.
- ISO 179-1:2010; Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test 2010. International Organization for Standardization: London, UK, 2010.
- Farina, A.P.; Cecchin, D.; Soares, R.G.; Botelho, A.L.; Takahashi, J.M.F.K.; Mazzetto, M.O.; Mesquita, M.F. Evaluation of vickers hardness of different types of acrylic denture base resins with and without glass fibre reinforcement. Gerodontology 2012, 29, e155–e160. [Google Scholar] [CrossRef]
- ISO 2039-1:2001; Plastics—Determination of hardness—Part 1: Ball indentation method. International Organization for Standardization: London, UK, 2001.
- Ozyilmaz, O.Y.; Akin, C. Effect of cleansers on denture base resins’ structural properties. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019827797. [Google Scholar] [CrossRef] [Green Version]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J.; Özcan, M. A comparison of the surface properties of CAD/CAM and conventional polymethylmethacrylate (PMMA). J. Prosthodont. 2019, 28, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Arnold, A.M.; Vargas, M.A.; Shaull, K.L.; Laffoon, J.E.; Qian, F. Flexural and fatigue strengths of denture base resin. J. Prosthet. Dent. 2008, 100, 47–51. [Google Scholar] [CrossRef]
- Ajaj-ALKordy, N.M.; Alsaadi, M.H. Elastic modulus and flexural strength comparisons of high-impact and traditional denture base acrylic resins. Saudi Dent. J. 2014, 26, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Wadachi, J.; Sato, M.; Igarashi, Y. Evaluation of the rigidity of dentures made of injection-molded materials. Dent. Mater. J. 2013, 32, 508–511. [Google Scholar] [CrossRef] [Green Version]
- Polychronakis, N.; Sarafianou, A.; Zissis, A.; Papadopoulos, T. The influence of thermocycling on the flexural strength of a polyamide denture base material. Acta Stomatol. Croat. 2017, 51, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Taczała, J.; Fu, C.; Sawicki, J.; Pietrasik, J. Influence different amount of cellulose on the mechanical strength of dental acrylic resin. IOP Conf. Ser. Mater. Sci. Eng. 2020, 743, 012044. [Google Scholar] [CrossRef] [Green Version]
- Jagini, A.S.; Marri, T.; Jayyarapu, D.; Kumari, R. Effect of long-term immersion in water and artificial saliva on the flexural strength of two heat cure denture base resins. J. Contemp. Dent. Pract. 2019, 20, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Chai, J.; Kawaguchi, M. Equilibrium strengths of denture polymers subjected to long-term water immersion. Int. J. Prosthodont. 1999, 12, 348–352. [Google Scholar] [PubMed]
- Chandrahari, N.; Kumar, C.R.; Salgar, A.R.; Singh, M.; Singh, S. Comparison of fracture resistance of heat cure resins polymerized by conventional and microwave methods after immersion in artificial saliva. J. Contemp. Dent. Pract. 2019, 20, 71–77. [Google Scholar]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The influence of graphene in improvement of physico-mechanical properties in PMMA denture base resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Cavalcante, M.R.S.; Orsi, I.A.; de Freitas Oliveira Paranhos, H.; Zaniquelli, O. Assessment of flexural strength and color alteration of heat-polymerized acrylic resins after simulated use of denture cleansers. Braz. Dent. J. 2005, 16, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.L.; Yunus, N.; Abu-Hassan, M.I. Hardness, flexural strength, and flexural modulus comparisons of three differently cured denture base systems. J. Prosthodont. 2008, 17, 545–549. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J. A comparison of the flexural and impact strengths and flexural modulus of CAD/CAM and conventional heat-cured polymethyl methacrylate (PMMA). J. Prosthodont. 2020, 29, 341–349. [Google Scholar] [CrossRef]
- Chladek, G.; Żmudzki, J.; Basa, K.; Pater, A.; Krawczyk, C.; Pakieła, W. Effect of silica filler on properties of PMMA resin. Arch. Mater. Sci. Eng. 2015, 71, 10. [Google Scholar]
- Mishra, J.; Kumar, D.; Samanta, S.; Vishwakarma, M.K. A comparative study of ethanol production from various agro residues by using saccharomyces cerevisiae and candida albicans. J. Yeast Fungal Res. 2012, 3, 12–17. [Google Scholar]
- Aruna, A.; Nagavalli, M.; Girijashankar, V.; Ponamgi, S.P.D.; Swathisree, V.; Rao, L.V. Direct bioethanol production by amylolytic yeast candida albicans. Lett. Appl. Microbiol. 2015, 60, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef]
- Yajima, D.; Motani, H.; Kamei, K.; Sato, Y.; Hayakawa, M.; Iwase, H. Ethanol production by candida albicans in postmortem human blood samples: Effects of blood glucose level and dilution. Forensic Sci. Int. 2006, 164, 116–121. [Google Scholar] [CrossRef]
- Jang, D.-E.; Lee, J.-Y.; Jang, H.-S.; Lee, J.-J.; Son, M.-K. Color stability, water sorption and cytotoxicity of thermoplastic acrylic resin for non metal clasp denture. J. Adv. Prosthodont. 2015, 7, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Samaranayake, L.P.; Geddes, D.A.; Weetman, D.A.; MacFarlane, T.W. Growth and acid production of candida albicans in carbohydrate supplemented media. Microbios 1983, 37, 105–115. [Google Scholar]
- Samaranayake, L.P.; Hughes, A.; Weetman, D.A.; MacFarlane, T.W. Growth and acid production of candida species in human saliva supplemented with glucose. J. Oral Pathol. Med. 1986, 15, 251–254. [Google Scholar] [CrossRef]
- Kapica, L.; Shaw, C.E.; Bartlett, G.W. Inhibition of histoplasma capsulatum by candida albicans and other yeasts on Sabouraud’s agar media. J. Bacteriol. 1968, 95, 2171–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, D.D.A.; Bertoldo, C.E.D.S.; Aguiar, F.H.B.; Lima, D.A.N.L.; Lovadino, J.R. Effects of mouthwashes on Knoop hardness and surface roughness of dental composites after different immersion times. Braz. Oral Res. 2011, 25, 168–173. [Google Scholar] [CrossRef]
- Dayan, C.; Kiseri, B.; Gencel, B.; Kurt, H.; Tuncer, N. Wear resistance and microhardness of various interim fixed prosthesis materials. J. Oral Sci. 2019, 61, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, D.R.; Burns, D.A.; DiPietro, G.J.; Gregory, R.L. Response of processed resilient denture liners to Candida albicans. J. Prosthet. Dent. 1987, 57, 507–512. [Google Scholar] [CrossRef]
- Anusavice, K.; Shen, C.; Rawls, H.R. Phillips’ Science of Dental Materials, 12th ed.; Saunders: St. Louis, MO, USA, 2013; ISBN 978-0-323-24205-9. [Google Scholar]
- Chladek, G.; Basa, K.; Mertas, A.; Pakieła, W.; Żmudzki, J.; Bobela, E.; Król, W. Effect of storage in distilled water for three months on the antimicrobial properties of poly(methyl methacrylate) denture base material doped with inorganic filler. Materials 2016, 9, 328. [Google Scholar] [CrossRef] [Green Version]
- Radford, D.R.; Challacombe, S.J.; Walter, J.D. Denture Plaque and Adherence of Candida Albicans to Denture-Base Materials in Vivo and in Vitro. Crit. Rev. Oral. Biol. Med. 1999, 10, 99–116. [Google Scholar] [CrossRef]
- Dniluk, T.; Fiedoruk, K.; Ściepuk, M. Aerobic bacteria in the oral cavity of patients with removable dentures. Adv. Med. Sci. 2006, 51, 86–90. [Google Scholar]
- Degirmenci, K.; Hayati Atala, M.; Sabak, C.; Degirmenci, K.; Hayati Atala, M.; Sabak, C. Effect of different denture base cleansers on surface roughness of heat polymerised acrylic materials with different curing process. Odovtos Int. J. Dent. Sci. 2020, 22, 145–153. [Google Scholar] [CrossRef]
- Sorgini, D.B.; da Silva-Lovato, C.H.; de Souza, R.F.; Davi, L.R.; de Freitas Oliveira Paranhos, H. Abrasiveness of conventional and specific denture-cleansing dentifrices. Braz. Dent. J. 2012, 23, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Atalay, S.; Çakmak, G.; Fonseca, M.; Schimmel, M.; Yilmaz, B. Effect of thermocycling on the surface properties of CAD-CAM denture base materials after different surface treatments. J. Mech. Behav. Biomed. Mater. 2021, 121, 104646. [Google Scholar] [CrossRef]
- Taylor, R.L.; Bulad, K.; Verran, J.; McCord, J.F. Colonization and deterioration of soft denture lining materials in vivo. Eur. J. Prosthodont. Restor. Dent. 2008, 16, 50–55. [Google Scholar] [CrossRef]

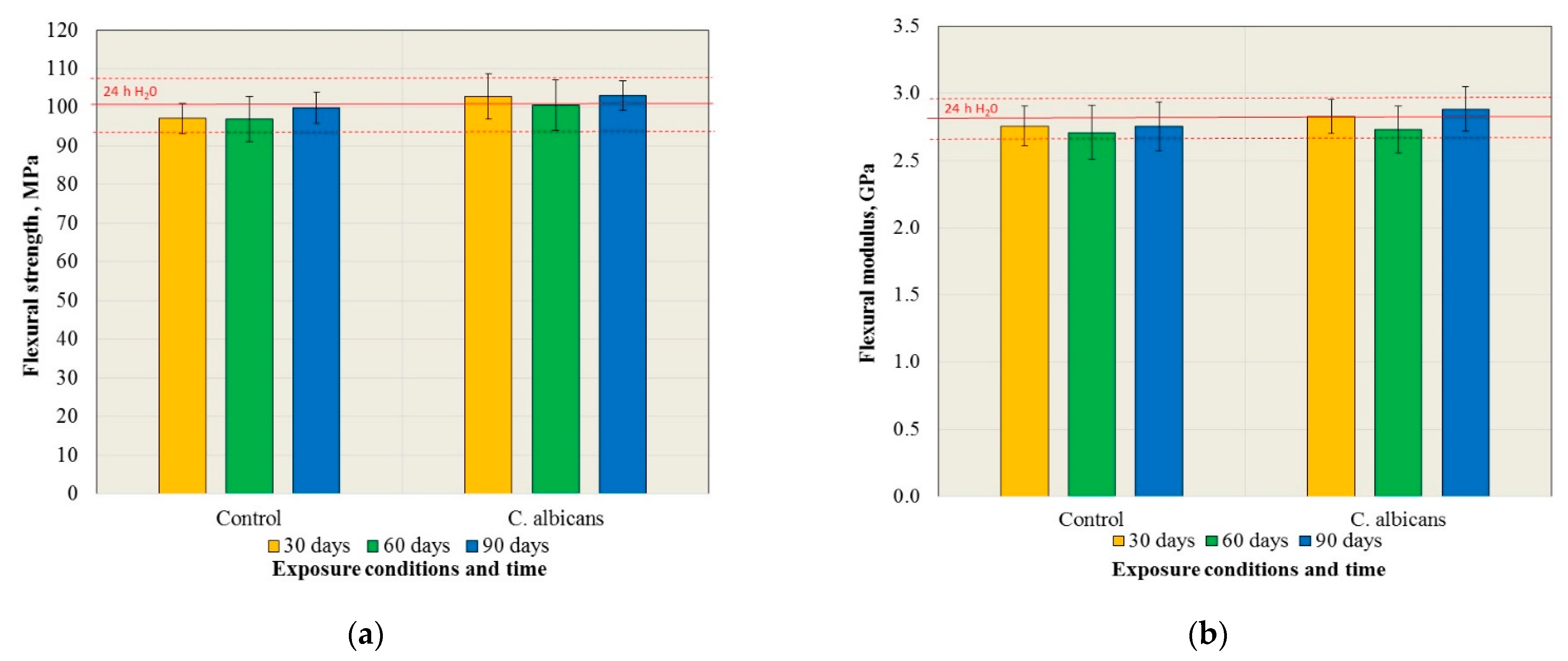


| Exposure Time | Control Medium | C. albicans |
|---|---|---|
| 24 h H2O | A | A |
| 30 days | B; a | B; a |
| 60 days | B; a | B; a |
| 90 days | B; a | B; a |
| Exposure Time | Control Medium | C. albicans |
|---|---|---|
| 24 h H2O | A | A |
| 30 days | A; a | A,B; a |
| 60 days | A; a | B,C; b |
| 90 days | B; a | C; b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chladek, G.; Nowak, M.; Pakieła, W.; Mertas, A. Effect of Candida albicans Suspension on the Mechanical Properties of Denture Base Acrylic Resin. Materials 2022, 15, 3841. https://doi.org/10.3390/ma15113841
Chladek G, Nowak M, Pakieła W, Mertas A. Effect of Candida albicans Suspension on the Mechanical Properties of Denture Base Acrylic Resin. Materials. 2022; 15(11):3841. https://doi.org/10.3390/ma15113841
Chicago/Turabian StyleChladek, Grzegorz, Michał Nowak, Wojciech Pakieła, and Anna Mertas. 2022. "Effect of Candida albicans Suspension on the Mechanical Properties of Denture Base Acrylic Resin" Materials 15, no. 11: 3841. https://doi.org/10.3390/ma15113841








