Relationship between Structure and Antibacterial Activity of α-Aminophosphonate Derivatives Obtained via Lipase-Catalyzed Kabachnik−Fields Reaction
Abstract
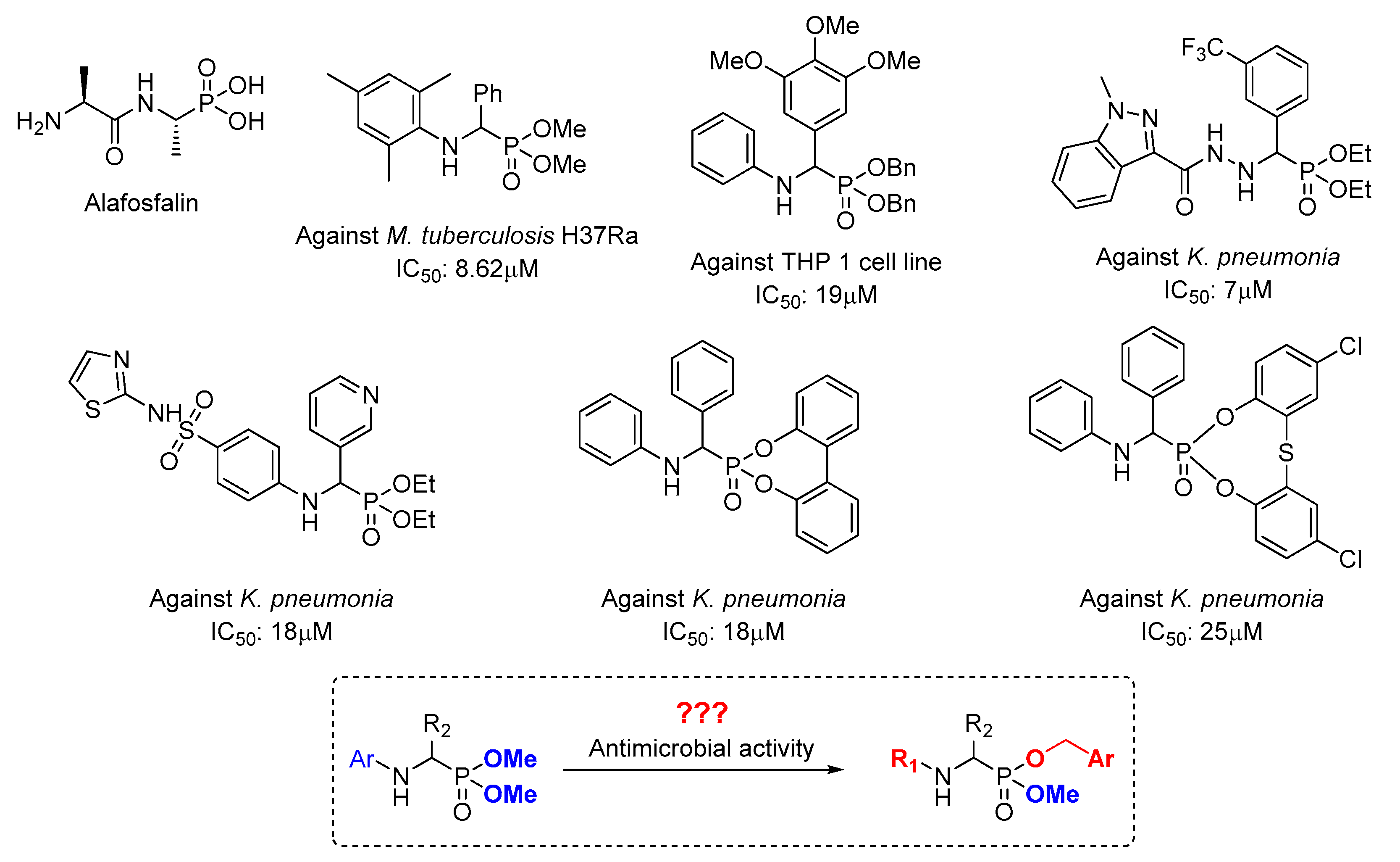
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Media
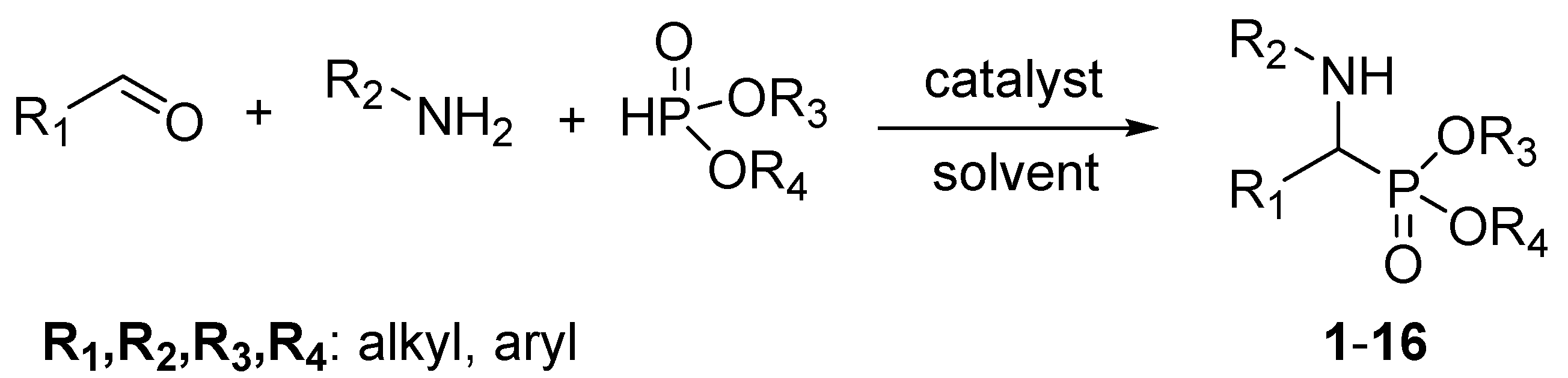
2.2. General Methods of Synthesis α-Aminophosphonate Derivatives
3. Results
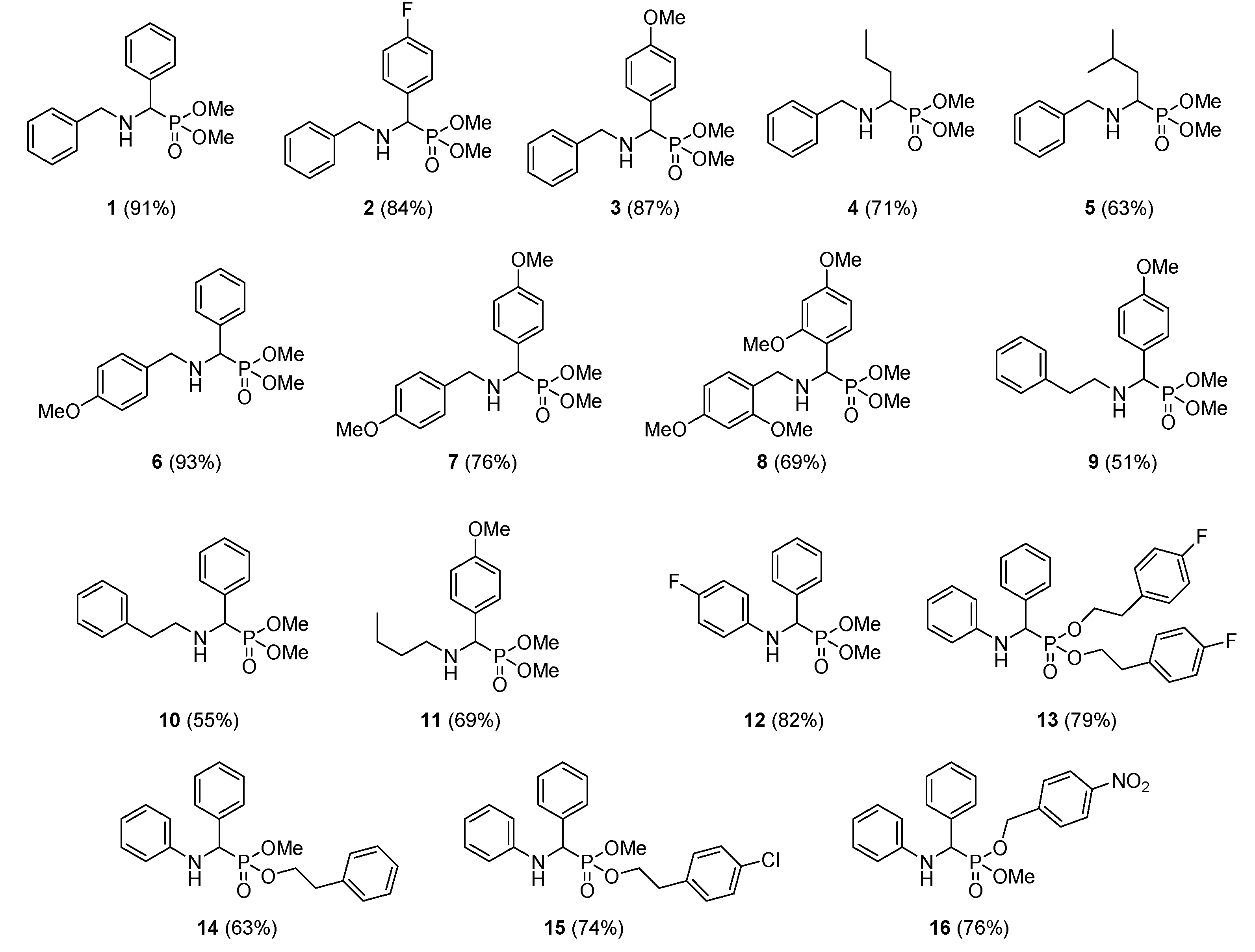
3.1. Chemistry
3.2. Cytotoxic Studies of the Library of α-Hydroxy Phosphonate Derivatives
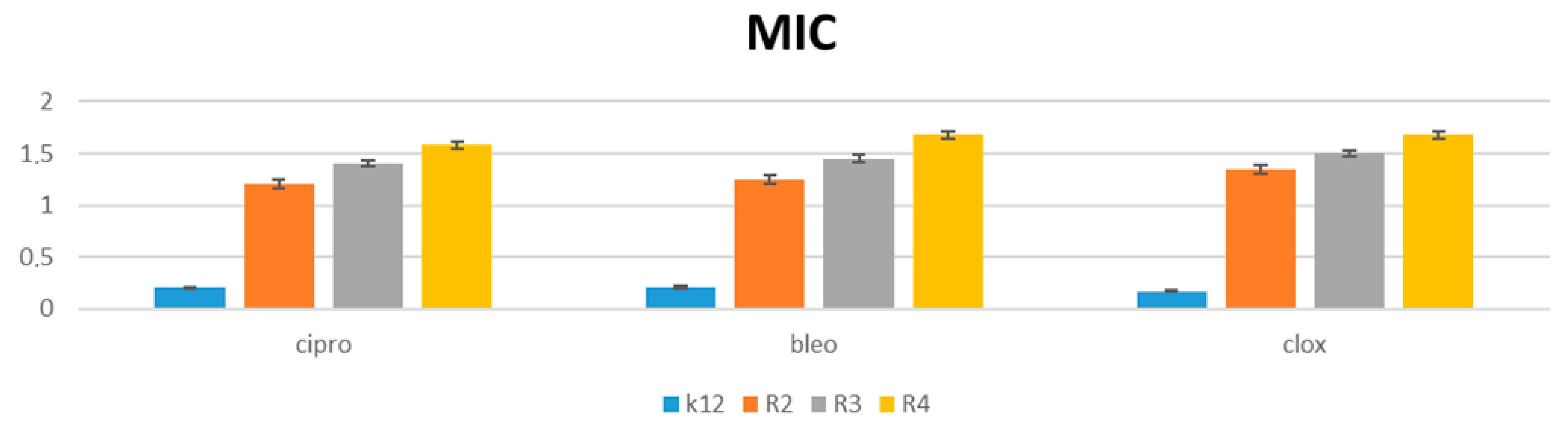
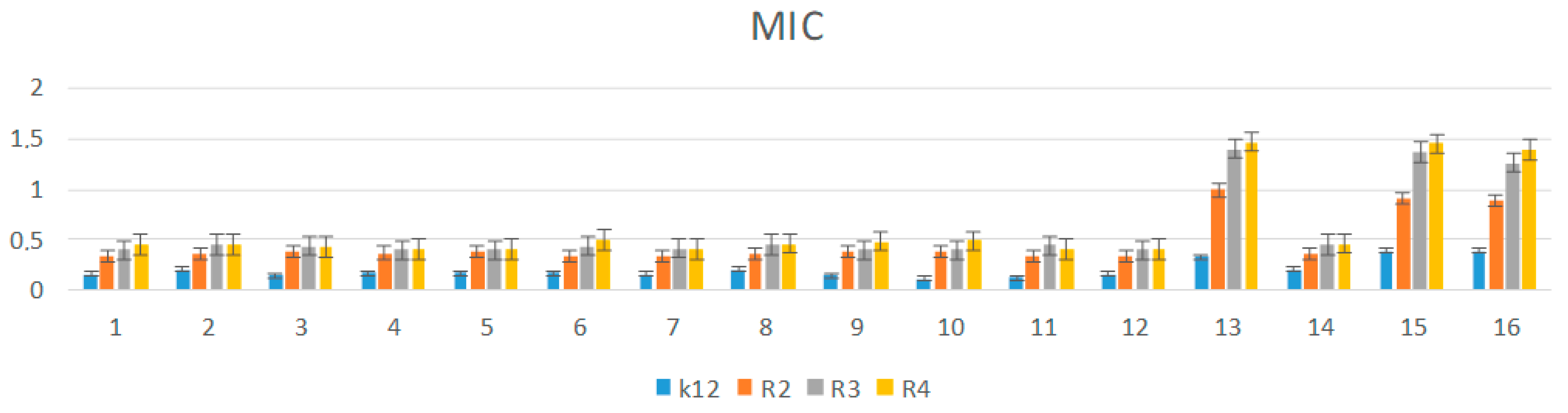
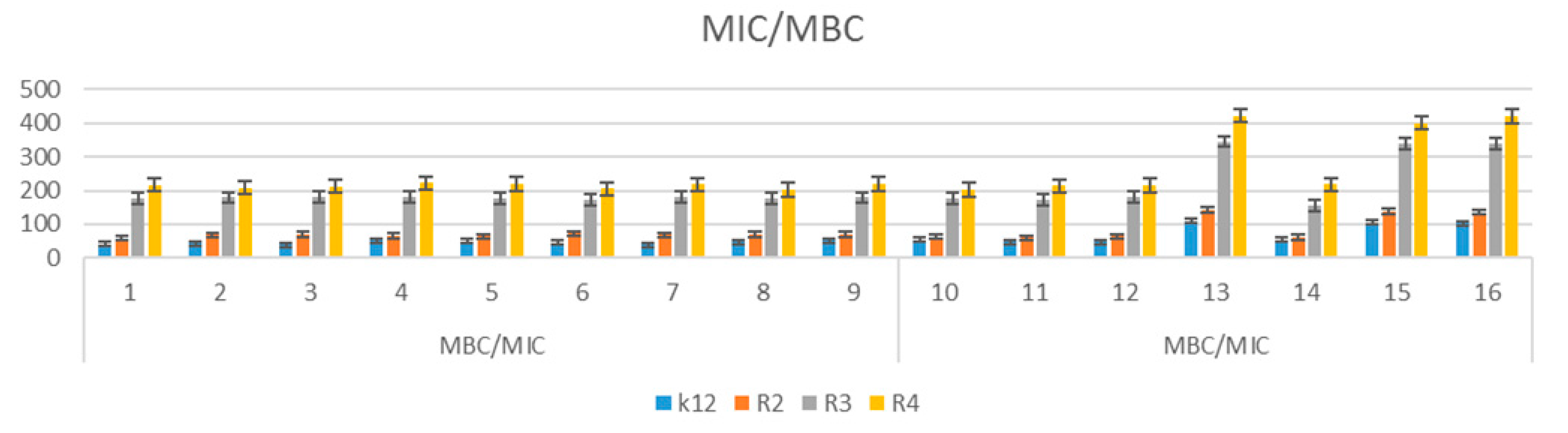
3.3. Analysis of R2–R4 E. coli Strains Modified with α-Aminophosphonate Derivatives
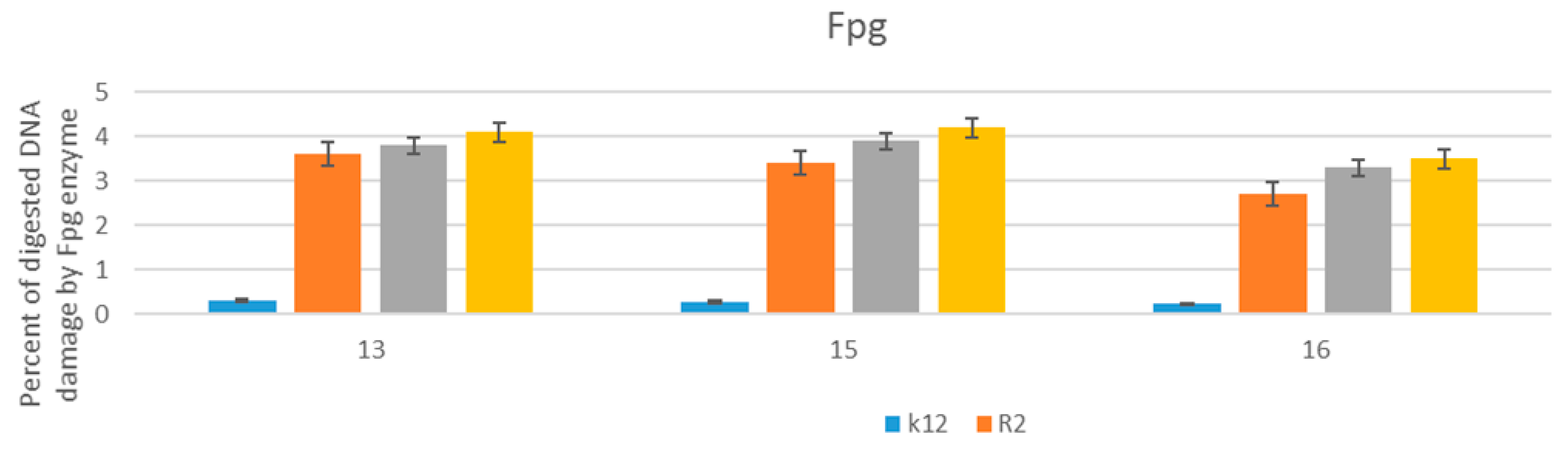
3.4. R2-R4 E. coli Strains with Tested α-Aminophosphonate Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIC | minimum inhibitory concentration |
| MBC | minimum bactericidal concentration |
| Oc | open circle |
| Ccc | covalently closed circle |
| BER | base excision repair |
| Fpg | DNA-formamidopyrimidine glycosylase |
References
- Albrecht, L.; Albrecht, A.; Krawczyk, H.; Jorgensen, K.A. Organocatalytic Asymmetric Synthesis of Organophosphorus Compounds. Chem. Eur. J. 2010, 16, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, R. Recent developments in metal catalyzed asymmetric addition of phosphorus nucleophiles. Chem. Soc. Rev. 2012, 41, 2095–2108. [Google Scholar] [CrossRef] [PubMed]
- Hiratake, J.; Oda, J. Aminophosphonic and Aminoboronic Acids as Key Elements of a Transition State Analogue Inhibitor of Enzymes. Biosci. Biotechnol. Biochem. 1997, 61, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Kafarski, P.; Lejczak, B. Biological activity of aminophosphonic acids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 63, 193–215. [Google Scholar] [CrossRef]
- Nassan, M.A.; Aldhahrani, A.; Amer, H.H.; Elhenawy, A.; Swelum, A.A.; Ali, O.M.; Zaki, Y.H. Investigation of the Anticancer Effect of α-Aminophosphonates and Arylidine Derivatives of 3-Acetyl-1-aminoquinolin-2(1H)-one on the DMBA Model of Breast Cancer in Albino Rats with In Silico Prediction of Their Thymidylate Synthase Inhibitory Effect. Molecules 2022, 27, 756. [Google Scholar] [CrossRef]
- Rezaei, Z.; Khabnadideh, S.; Zomorodian, K.; Pakshir, K.; Nadali, S.; Mohtashami, N.; Mirzaei, E.F. Design, Synthesis, and Antifungal Activity of New α-Aminophosphonates, Design, Synthesis, and Antifungal Activity of New α-Aminophosphonates. Int. J. Med. Chem. 2011, 2011, 678101. [Google Scholar] [CrossRef] [Green Version]
- Varga, P.R.; Keglevich, G. Synthesis of α-Aminophosphonates and Related Derivatives; The Last Decade of the Kabachnik–Fields Reaction. Molecules 2021, 26, 2511. [Google Scholar] [CrossRef]
- Iwanejko, J.; Samadaei, M.; Pinter, M.; Senfter, D.; Madlener, S.; Kochel, A.; Rohr-Udilova, N.; Wojaczyńska, E. Cytotoxic Activity of Piperazin-2-One-Based Structures: Cyclic Imines, Lactams, Aminophosphonates, and Their Derivatives. Materials 2021, 14, 2138. [Google Scholar] [CrossRef]
- Packialakshmi, P.; Gobinath, P.; Ali, D.; Alarifi, S.; Alsaiari, N.S.; Idhayadhulla, A.; Surendrakumar, R. Synthesis and Characterization of Aminophosphonate Containing Chitosan Polymer Derivatives: Investigations of Cytotoxic Activity and in Silico Study of SARS-CoV-19. Polymers 2021, 13, 1046. [Google Scholar] [CrossRef]
- López-Francés, A.; del Corte, X.; de Marigorta, E.M.; Palacios, F.; Vicario, J. Ugi Reaction on α-Phosphorated Ketimines for the Synthesis of Tetrasubstituted α-Aminophosphonates and Their Applications as Antiproliferative Agents. Molecules 2021, 26, 1654. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, K.; Song, B.; Xu, G.; Yang, S.; Xue, W.; Hu, D.; Lu, P.; Ouyang, G.; Jin, L.; et al. Synthesis and Antiviral Bioactivities of α-Aminophosphonates Containing Alkoxyethyl Moieties. Molecules 2006, 11, 666–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.-A.; Wu, Y.-L.; Yang, S.; Hu, D.-Y.; He, X.-Q.; Jin, L.-H. Synthesis and Bioactivity of a-Aminophosphonates Containing Fluorine. Molecules 2003, 8, 186–192. [Google Scholar] [CrossRef]
- Onita, N.; Şisu, I.; Penescu, M.; Purcarea, V.L.; Kurunczi, L. Synthesis Characterization and Biological activity of some α-aminophoshonates. Farmcia 2010, 58, 531–545. [Google Scholar]
- Bhagat, S.; Shah, P.; Garg, S.K.; Mishra, S.; Kaur, P.K.; Singh, S.; Chakraborti, A.K. α-Aminophosphonates as novel anti-leishmanialchemotypes: Synthesis, biological evaluation, and CoMFA studies. Med. Chem. Commun. 2014, 5, 665–669. [Google Scholar] [CrossRef]
- Lewkowski, J.; Malinowski, Z.; Matusiak, A.; Morawska, M.; Rogacz, D.; Rychter, P. The Effect of New Thiophene-Derived Aminophosphonic Derivatives on Growth of Terrestrial Plants: A Seedling Emergence and Growth Test. Molecules 2016, 21, 694. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.-H.; Park, Y.-K.; Nishiwaki, H.; Hammock, B.D.; Nishi, K. Structure–activity relationships of amide–phosphonate derivatives as inhibitors of the human soluble epoxide hydrolase. Bioorg. Med. Chem. 2015, 23, 7199–7210. [Google Scholar] [CrossRef]
- Klimczak, A.A.; Matusiak, A.; Lewkowski, J.; Bitner, J.; Szemraj, J.; Kontek, R. Dimethyl (2-Furyl)-N-(2-Methoxyphenyl)Aminomethylphosphonate Induces Apoptosis in Esophageal Squamous Cancer Cells. Structure Versus Activity of its Selected Analogs. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 1088–1099. [Google Scholar] [CrossRef]
- Klimczak, A.A.; Kuropatwa, A.; Lewkowski, J.; Szemraj, J. Synthesis of new N-arylamino(2-furyl)methylphosphonic acid diesters, and in vitro evaluation of their cytotoxicity against esophageal cancer cells. Med. Chem. Res. 2012, 22, 852–860. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Aminophosphonic acids of potential medical importance. Curr. Med. Chem. Anti-Cancer Agents 2001, 1, 301–312. [Google Scholar] [CrossRef]
- Li, C.; Song, B.; Yan, K.; Xu, G.; Hu, D.; Yang, S.; Jin, L.; Xue, W.; Lu, P. One Pot Synthesis of α-Aminophosphonates Containing Bromo and 3,4,5-Trimethoxybenzyl Groups under Solvent-free Conditions. Molecules 2007, 12, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Sonar, S.S.; Sadaphal, S.A.; Labade, V.B.; Shingate, B.B.; Shingare, M.S. An Efficient Synthesis and Antibacterial Screening of Novel Oxazepine α-Aminophosphonates by Ultrasound Approach. Phosphorus Sulfur Silicon Relat. Elem. 2009, 185, 65–73. [Google Scholar] [CrossRef]
- Sayed, I.E.T.E.; Fathy, G.; Ahmed, A.A.S. Synthesis and Antibacterial Activity of Novel Cyclic α-Aminophsophonates. Biomed. J. Sci. Tech. Res. 2019, 23, 17609–17614. [Google Scholar] [CrossRef]
- Abdel-Megeed, M.F.; Badr, B.E.; Azaam, M.M.; El–Hiti, G.A. Antimicrobial Activities of a Series of Diphenyl (4′-(Aryldiazenyl)Biphenyl-4-Ylamino)(Pyridin-3-YL)Methylphosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 1202–1207. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J.; Stevens, C.V. Aminophosphonic Acids—Phosphorus Analogues of Natural Amino Acids. Part 1: Syntheses of α-Aminophosphonic Acids. Curr. Org. Synth. 2011, 15, 2015–2071. [Google Scholar] [CrossRef]
- Allen, J.G.; Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Holmes, S.W.; Lambert, R.W.; Nisbet, L.J.P.; Ringrose, S. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature 1978, 272, 56–58. [Google Scholar] [CrossRef]
- Shaik, M.S.; Nadiveedhi, M.R.; Gundluru, M.; Katike, U.; Obulam, V.S.R.; Cirandur, S.R. Efficient catalyst free green synthesis and in vitro antimicrobial, antioxidant and molecular docking studies of α-substituted aromatic/heteroaromatic aminomethylene bisphosphonates. Synth. Commun. 2021, 51, 747–764. [Google Scholar] [CrossRef]
- Mulla, S.A.R.; Pathan, M.Y.; Chavan, S.S.; Gample, S.P.; Sarkar, D. Highly efficient one-pot multi-component synthesis of α-aminophosphonates and bis-α-aminophosphonates catalyzed by heterogeneous reusable silica supported dodecatungstophosphoric acid (DTP/SiO2) at ambient temperature and their antitubercular evaluation against Mycobactrium Tuberculosis. RSC Adv. 2014, 4, 7666–7672. [Google Scholar] [CrossRef]
- Ali, N.S.; Zakir, S.; Patel, M.; Farooqui, M. Synthesis of new α aminophosphonate system bearing Indazole moiety and their biological activity. Eur. J. Med. Chem. 2012, 50, 39–43. [Google Scholar] [CrossRef]
- Sampath, C.; Vani, K.V.; Kotaiah, Y.; Krishna, N.H.; Raju, C.N.; Rao, C.V. A facile and efficient One-pot Three Component Reaction (Kabachinik-Fields Reaction) for the Synthesis of Novel α-Aminophosphonates by 1, 4-Dimethylpiperazine as a new catalyst. J. Chem. Pharm. Res. 2012, 4, 1375–1382. [Google Scholar]
- Maruyama, H.B.; Arisawa, M.; Sawada, T. Alafosfalin, a new inhibitor of cell wall biosynthesis: In vitro activity against urinary isolates in Japan and potentiation with beta-lactams. Antimicrob. Agents Chemother. 1979, 16, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Kasthuraiah, M.; Kumar, K.A.; Reddy, C.S.; Reddy, C.D. Syntheses, spectral property, and antimicrobial activities of 6-α-amino dibenzo [d,f][1,3,2]dioxaphosphepin 6-oxides. Heteroat. Chem. 2007, 18, 2–8. [Google Scholar] [CrossRef]
- Kasthuraiah, M.; Balakrishna, A.; Reddy, K.R.K.K.; Kumar, B.S.; Reddy, C.S.; Nagaraju, C. Synthesis and Antimicrobial Activity of New 2,10-Dichloro-6-phenylaminobenzyl-dibenzo[d,g] [1,3,6,2]dioxathiaphosphocin 6-Oxides. Heterocycl. Chem. 2008, 45, 103–107. [Google Scholar] [CrossRef]
- Tajtihttps, Á.; Bálinthttps, E.; Keglevich, G. Microwave-assisted synthesis of α-aminophosphonates and related derivatives by the Kabachnik-Fields reaction. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 379–381. [Google Scholar] [CrossRef]
- Maestro, A.; del Corte, X.; López-Francés, A.; de Marigorta, E.M.; Palacios, F.; Vicario, J. Asymmetric Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives. Molecules 2021, 26, 3202. [Google Scholar] [CrossRef] [PubMed]
- Viveros-Ceballos, J.L.; Ordóñez, M.; Sayago, F.J.; Cativiela, C. Stereoselective Synthesis of α-Amino-C-phosphinic Acids and Derivatives. Molecules 2016, 21, 1141. [Google Scholar] [CrossRef] [Green Version]
- Bálint, E.; Tajti, Á.; Kalocsai, D.; Mátravölgyi, B.; Karaghiosoff, K.; Czugler, M.; Keglevich, G. Synthesis and utilization of optically active α-aminophosphonate derivatives by Kabachnik-Fields reaction. Tetrahedron 2017, 73, 5659–5667. [Google Scholar] [CrossRef]
- Tajti, Á.; Bálint, E.; Keglevich, G. Synthesis of Ethyl Octyl-α-Aminophosphonate Derivatives. Curr. Org. Synth. 2016, 13, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Philippe, N.; Denivet, F.; Vasse, J.; Santos, J.; Levacher, V.; Dupas, G. Highly stereoselective Friedel-Crafts type cyclization. Facile access to enantiopure 1,4-dihydro-4-phenyl isoquinolinones. Tetrahedron 2003, 59, 8049–8056. [Google Scholar] [CrossRef]
- Bálint, E.; Tajti, Á.; Drahos, L.; Ilia, G.; Keglevich, G. Alcoholysis of Dialkyl Phosphites Under Microwave Conditions. Curr. Org. Chem. 2013, 17, 555–562. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, Z.; Zhuang, R.; Xu, J.; Zhang, P.; Tang, G.; Zhao, Y. Direct Transformation of Amides into α-Amino Phosphonates via a Reductive Phosphination Process. Org. Lett. 2013, 15, 4214–4217. [Google Scholar] [CrossRef]
- Maier, L.; Diel, P.J. Organic Phosphorus compounds 941 preparation, physical and biological properties of amino-arylmethylphosphonic and phoshonous acids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 57, 57–64. [Google Scholar] [CrossRef]
- Bhagat, S.; Chakraborti, A.K. Zirconium(IV) Compounds As Efficient Catalysts for Synthesis of α-Aminophosphonates. J. Org. Chem. 2008, 73, 6029–6032. [Google Scholar] [CrossRef] [PubMed]
- Bedolla-Medrano, M.; Hernández-Fernández, E.; Ordóñez, M. Phenylphosphonic Acid as Efficient and Recyclable Catalyst in the Synthesis of α-Aminophosphonates under Solvent-Free Conditions. Synlett 2014, 25, 1145–1149. [Google Scholar] [CrossRef]
- Tibhe, G.D.; Bedolla-Medrano, M.; Cativiela, C.; Ordóñez, M. Phenylboronic Acid as Efficient and Eco-Friendly Catalyst for the One-Pot, Three-Component Synthesis of α-Aminophosphonates under Solvent-Free Conditions. Synlett 2012, 23, 1931–1936. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, S.; Hu, L.; Hussain, M.I.; Zhang, X.; Xiong, Y. Cross-dehydrogenative coupling strategy for phosphonation and cyanation of secondary N-alkyl anilines by employing 2,3-dichloro-5,6-dicyanobenzoquinone. Tetrahedron 2018, 74, 7209–7217. [Google Scholar] [CrossRef]
- Lee, O.-Y.; Law, K.-L.; Yang, D. Secondary Amine Formation from Reductive Amination of Carbonyl Compounds Promoted by Lewis Acid Using the InCl3/Et3SiH System. Org. Lett. 2009, 11, 3302–3305. [Google Scholar] [CrossRef]
- Hall, R.G. The design and synthesis of biologically active organophosphorus compounds--the role of a central research laboratory. Chimia 2010, 64, 34–36. [Google Scholar] [CrossRef]
- Orsini, F.; Sello, G.; Sisti, M. Aminophosphonic Acids and Derivatives. Synthesis and Biological Applications. Curr. Med. Chem. 2010, 17, 264–289. [Google Scholar] [CrossRef]
- Ahmadi, F.; Assadi, Y.; Hosseini, S.M.R.M.; Rezaee, M. Determination of organophosphorus pesticides in water samples by single drop microextraction and gas chromatography-flame photometric detector. J. Chromatogr. A 2006, 1101, 307–312. [Google Scholar] [CrossRef]
- Amira, A.; Aouf, Z.; K’tir, H.; Chemam, Y.; Ghodbane, R.; Zerrouki, R.; Aouf, N.-E. Recent Advances in the Synthesis of α-Aminophosphonates: A Review. ChemistrySelect 2021, 6, 6137–6149. [Google Scholar] [CrossRef]
- Shastri, R.A. Review on the Synthesis of α-Aminophosphonate Derivatives. Chem. Sci. Trans. 2019, 8, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Basha, M.H.; Subramanyam, C.; Rao, K.P. Ultrasound-promoted solvent-free synthesis of some new α-aminophosphonates as potential antioxidants. Main Group Met. Chem. 2020, 43, 147–153. [Google Scholar] [CrossRef]
- Eyckensa, D.J.; Henderson, L.C. Synthesis of α-aminophosphonates using solvate ionic liquids. RSC Adv. 2017, 7, 27900–27904. [Google Scholar] [CrossRef] [Green Version]
- Babak, K. Surface-Mediated Solid Phase Reactions: A Simple and New Method for the Synthesis of α-Aminophosphonates under Solvent-Free Conditions. Chem. Lett. 2001, 30, 880–881. [Google Scholar] [CrossRef]
- Basha, S.K.T.; Kalla, R.M.N.; Varalakshmi, M.; Sudhamani, H.; Appa, R.M.; Hong, S.C.; Raju, C.N. Heterogeneous catalyst SiO2–LaCl3·7H2O: Characterization and microwave-assisted green synthesis of α-aminophosphonates and their antimicrobial activity. Mol. Divers 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Albrecht, Ł. An organocatalytic biomimetic approach to α-aminophosphonates. Chem. Commun. 2015, 51, 3981–3984. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Liu, Y.; Lin, T.; Sun, H.; Yang, D.; Jiang, C. Identification of novel and selective non-peptide inhibitors targeting the polo-box domain of polo-like kinase 1. Bioorg. Chem. 2018, 81, 278–288. [Google Scholar] [CrossRef]
- Sravya, G.; Suresh, G.; Zyryanov, G.V.; Balakrishna, A.; Reddy, N.B. K2CO3/Al2O3: An Efficient and Recyclable Catalyst for One-Pot, Three Components Synthesis of α-Aminophosphonates and Bioactivity Evaluation. Asian J. Org. Chem. 2019, 31, 2383–2388. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Prakash, S.J.; Jagadeshwar, V.; Narsihmula, C. Three component coupling catalyzed by TaCl5–SiO2: Synthesis of α-amino phosphonates. Tetrahedron Lett. 2001, 42, 5561–5563. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Raj, K.S.; Reddy, K.B.; Prasad, A.R. Zr4+-Catalyzed Efficient Synthesis of α-Aminophosphonates. Synthesis 2001, 15, 2277–2280. [Google Scholar] [CrossRef]
- Manjula, A.; Rao, B.V.; Neelakantan, P. One-Pot Synthesis of α-Aminophosphonates: An Inexpensive Approach. Synth. Comun. 2003, 33, 2963–2969. [Google Scholar] [CrossRef]
- Milen, M.; Ábrányi-Balogh, P.; Dancsó, A.; Frigyes, D.; Pongó, L.; Keglevich, G. T3P®-promoted Kabachnik–Fields reaction: An efficient synthesis of α-aminophosphonates. Tetrahedron Lett. 2013, 54, 5430–5433. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, J.K.; Song, C.-E.; Kim, D.C. Microwave-assisted Kabachnik-Fields Reaction in Ionic Liquid. Bull. Korean Chem. Soc. 2002, 23, 667–668. [Google Scholar] [CrossRef] [Green Version]
- Janicki, I.; Łyżwa, P.; Kiełbasiński, P. The first enzyme-promoted addition of nitromethane to imines (aza-Henry reaction). Bioorg. Chem. 2020, 94, 103377. [Google Scholar] [CrossRef]
- Cai, J.-F.; Guan, Z.; He, Y.-Y. The lipase-catalyzed asymmetric C-C Michael addition. J. Mol. Catal. B Enzym. 2011, 68, 240–244. [Google Scholar] [CrossRef]
- Li, C.; Feng, X.-W.; Wang, N.; Zhou, Y.-J.; Yu, X.-Q. Biocatalytic promiscuity: The first lipase-catalysed asymmetric aldol reaction. Green Chem. 2008, 10, 616–618. [Google Scholar] [CrossRef]
- Albuquerque, T.B.; da Silva, C.D.G.; de Oliveira, A.R.; Santos, B.F.d.; da Silva, B.A.L.; Katla, R.; Rochaa, M.P.D.; Domingues, N.L.C. Lipase catalyzed 1,2-addition of thiols to imines under mild conditions. New J. Chem. 2018, 42, 1642–1645. [Google Scholar] [CrossRef]
- Reetz, M.T.; Mondière, R.; Carballeira, J.D. Enzyme promiscuity: First protein-catalyzed Morita–Baylis–Hillman reaction. Tetrahedron Lett. 2007, 48, 1679–1681. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Proc. Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Dwivedee, B.P.; Soni, S.; Sharma, M.; Bhaumik, J.; Laha, J.K.; Banerjee, U.C. Promiscuity of Lipase-Catalyzed Reactions for Organic Synthesis: A Recent Update. ChemistrySelect 2018, 3, 2441–2466. [Google Scholar] [CrossRef]
- Guezane-Lakoud, S.; Toffano, M.; Aribi-Zouioueche, L. Promiscuous lipase catalyzed a new P–C bond formation: Green and efficient protocol for one-pot synthesis of α-aminophosphonates. Heteroat. Chem. 2017, 28, e21408. [Google Scholar] [CrossRef] [Green Version]
- Aissa, R.; Guezane-Lakoud, S.; Kolodziej, E.; Toffano, M.; Aribi-Zouioueche, L. Diastereoselective synthesis of bis(α-aminophosphonates) by lipase catalytic promiscuity. New J. Chem. 2019, 43, 8153–8159. [Google Scholar] [CrossRef]
- Chavan, A.S.; Kharat, A.S.; Bhosle, R.; Dhumal, S.T.; Mane, R.A. CAL-B accelerated novel synthetic protocols for 3,3’-arylidenebis-4-hydroxycoumarins and dimethyl ((substituted phenyl) (phenylamino)methyl) phosphonates. Res. Chem. Intermed. 2021, 47, 4497–4512. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Koszelewski, D.; Gawdzik, B.; Samsonowicz-Górski, J.; Kramkowski, K.; Wypych, A.; Lizut, R.; Ostaszewski, R. Promiscuous Lipase-Catalyzed Markovnikov Addition of H-Phosphites to Vinyl Esters for the Synthesis of Cytotoxic α-Acyloxy Phosphonate Derivatives. Materials 2022, 15, 1975. [Google Scholar] [CrossRef] [PubMed]
- Koszelewski, D.; Brodzka, A.; Madej, A.; Trzepizur, D.; Ostaszewski, R. Evaluation of gem-Diacetates as Alternative Reagents for Enzymatic Regio- and Stereoselective Acylation of Alcohols. J. Org. Chem. 2021, 86, 6331–6342. [Google Scholar] [CrossRef]
- Koszelewski, D.; Ostaszewski, R.; Śmigielski, P.; Hrunyk, A.; Kramkowski, K.; Laskowski, Ł.; Laskowska, M.; Lizut, R.; Szymczak, M.; Michalski, J.; et al. Pyridine Derivatives—A New Class of Compounds That Are Toxic to E. coli K12, R2–R4 Strains. Materials 2021, 14, 5401. [Google Scholar] [CrossRef] [PubMed]
- Koszelewski, D.; Ostaszewski, R. The studies on chemoselective promiscuous activity of hydrolases on acylals transformations. Bioorg. Chem. 2019, 93, 102825. [Google Scholar] [CrossRef]
- Koszelewski, D.; Ostaszewski, R. Enzyme promiscuity as a remedy for the common problems with Knoevenagel condensation. Chem. Eur. J. 2019, 25, 10156–10164. [Google Scholar] [CrossRef]
- Koszelewski, D.; Ostaszewski, R. Biocatalytic Promiscuity of Lipases in Carbon-Phosphorus Bond Formation. ChemCatChem 2019, 11, 2554–2558. [Google Scholar] [CrossRef]
- Albanese, D.C.M.; Gaggero, N. Albumin as a promiscuous biocatalyst in organic synthesis. RSC Adv. 2015, 5, 10588–10598. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Xie, X.; Hong, B.; Zhao, Y.; Fang, M. Copper (I) Iodide-Catalyzed Solvent-Free Synthesis of α-Aminophosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 2145–2155. [Google Scholar] [CrossRef]
- Kandula, M.K.R.; Gundluru, M.; Nemallapudi, B.R.; Gundala, S.; Kotha, P.; Zyryanov, G.V.; Chadive, S.; Cirandur, S.R. Synthesis, antioxidant activity, and α-glucosidase enzyme inhibition of α-aminophosphonate derivatives bearing piperazine-1,2,3-triazole moiety. J. Heterocycl. Chem. 2021, 58, 172–181. [Google Scholar] [CrossRef]
- Azaam, M.M.; Kenawy, E.R.; El-din, A.S.B.; Khamis, A.A.; El-Magd, M.A. Antioxidant and anticancer activities of α-aminophosphonates containing thiadiazole moiety. J. Saudi. Chem. Soc. 2018, 22, 34–41. [Google Scholar] [CrossRef]
- Bollinger, A.; Molitor, R.; Thies, S.; Koch, R.; Coscolín, C.; Ferrer, M.; Jaeger, K.-E. Organic-Solvent-Tolerant Carboxylic Ester Hydrolases for Organic Synthesis. Appl. Environ. Microbiol. 2020, 86, e00106-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalczyk, P.; Wilk, M.; Parul, P.; Szymczak, M.; Kramkowski, K.; Raj, S.; Skiba, G.; Sulejczak, D.; Kleczkowska, P.; Ostaszewski, R. The Synthesis and Evaluation of Aminocoumarin Peptidomimetics as Cytotoxic Agents on Model Bacterial E. coli Strains. Materials 2021, 14, 5725. [Google Scholar] [CrossRef] [PubMed]
- Samsonowicz-Górski, J.; Kowalczyk, P.; Koszelewski, D.; Brodzka, A.; Szymczak, M.; Kramkowski, K.; Ostaszewski, R. The Synthesis and Evaluation of Amidoximes as Cytotoxic Agents on Model Bacterial E. coli Strains. Materials 2021, 14, 7577. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Trzepizur, D.; Szymczak, M.; Skiba, G.; Kramkowski, K.; Ostaszewski, R. 1,2-Diarylethanols—A New Class of Compounds That Are Toxic to E. coli K12, R2–R4 Strains. Materials 2021, 14, 1025. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Madej, A.; Szymczak, M.; Ostaszewski, R. α-Amidoamids as New Replacements of Antibiotics—Research on the Chosen K12, R2–R4 E. coli Strains. Materials 2020, 13, 5169. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Borkowski, A.; Czerwonka, G.; Cłapa, T.; Cieśla, J.; Misiewicz, A.; Borowiec, M.; Szala, M. The microbial toxicity of quaternary ammonium ionic liquids is dependent on the type of lipopolysaccharide. J. Mol. Liq. 2018, 266, 540–547. [Google Scholar] [CrossRef]
- Borkowski, A.; Kowalczyk, P.; Czerwonka, G.; Cieśla, J.; Cłapa, T.; Misiewicz, A.; Szala, M.; Drabik, M. Interaction of quaternary ammonium ionic liquids with bacterial membranes—Studies with Escherichia coli R1–R4-type lipopolysaccharides. J. Mol. Liq. 2017, 246, 282–289. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Gawdzik, B.; Trzepizur, D.; Szymczak, M.; Skiba, G.; Raj, S.; Kramkowski, K.; Lizut, R.; Ostaszewski, R. δ-Lactones—A New Class of Compounds That Are Toxic to E. coli K12 and R2–R4 Strains. Materials 2021, 14, 2956. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, A.; Kaszowska, M.; Jachymek, W.; Lugowski, C.; Lukasiewicz, J. Lipopolysaccharide-linked Enterobacterial Common Antigen (ECALPS) Occurs in Rough Strains of Escherichia coli R1, R2, and R4. Int. J. Mol. Sci. 2020, 21, 6038. [Google Scholar] [CrossRef] [PubMed]
- Prost, M.E.; Prost, R. Basic parameters of evaluation of the effectiveness of antibiotic therapy. OphthaTherapy 2017, 4, 233–236. [Google Scholar] [CrossRef]
- Kabachnik, M.I.; Medved, T.Y. Нoвый метoд синтеза сс-аминoфoсфинoвых кислoт [A new method for the synthesis of α-amino phosphoric acids]. Doklady Akademii Nauk SSSR 1952, 83, 689ff. [Google Scholar]
- Fields, E.K. The synthesis of esters of substituted amino phosphonic acids. J. Am. Chem. Soc. 1952, 74, 1528–1531. [Google Scholar] [CrossRef]



| Entry | Catalyst | T (°C) | Solvent | Yield [%] f |
|---|---|---|---|---|
| 1 | None | 25 | neat | <5 |
| 2 | Porcine pancreas lipase (PpL) | 25 | neat | 73 |
| 3 | Porcine pancreas lipase (PpL) | 25 | Toluene | 64 |
| 4 | Porcine pancreas lipase (PpL) | 25 | EtOAc | 18 |
| 5 | Porcine pancreas lipase (PpL) | 25 | THF | 52 |
| 6 | Porcine pancreas lipase (PpL) | 25 | 2-Me THF | 55 |
| 7 | Porcine pancreas lipase (PpL) | 25 | TBME | 88 |
| 8 | Porcine pancreas lipase (PpL) | 30 | TBME | 91 |
| 9 | Porcine pancreas lipase (PpL) | 40 | TBME | 87 |
| 10 | Porcine pancreas lipase (PpL) b | 30 | TBME | 93 |
| 11 | Wheat germ lipase | 20 | neat | 29 |
| 12 | Pseudomonas cepacia lipase (PfL) | 20 | neat | 44 |
| 13 | Candida cylindracea lipase (CcL) | 20 | neat | 57 |
| 14 | Candida rugosa lipase (CrL) | 20 | neat | 35 |
| 15 | Novozym 435 | 20 | neat | 67 |
| 16 | Bovine serum albumin (BSA) | 30 | TBME | 9 |
| 17 | Bovine liver acetone powder (BLAP) c | 20 | neat | 43 |
| 18 | Denatured PpL d | 30 | TBME | <1 |
| 19 | CuI e | 25 | neat | 39 |
| 20 | Cu2O e | 25 | neat | 24 |
| 21 | Cu(OAc)2 e | 25 | neat | 33 |
| 22 | PhB(OH)2 e | 25 | neat | 14 |
| No. of Samples | 13 | 15 | 16 | Type of Test |
|---|---|---|---|---|
| K12 | *** | *** | *** | MIC |
| R2 | *** | *** | *** | MIC |
| R3 | *** | *** | *** | MIC |
| R4 | *** | *** | *** | MIC |
| K12 | ** | * | ** | MBC |
| R2 | ** | * | ** | MBC |
| R3 | ** | * | ** | MBC |
| R4 | ** | * | ** | MBC |
| K12 | *** | ** | ** | MBC/MIC |
| R2 | *** | ** | ** | MBC/MIC |
| R3 | *** | ** | ** | MBC/MIC |
| R4 | *** | ** | ** | MBC/MIC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koszelewski, D.; Kowalczyk, P.; Śmigielski, P.; Samsonowicz-Górski, J.; Kramkowski, K.; Wypych, A.; Szymczak, M.; Ostaszewski, R. Relationship between Structure and Antibacterial Activity of α-Aminophosphonate Derivatives Obtained via Lipase-Catalyzed Kabachnik−Fields Reaction. Materials 2022, 15, 3846. https://doi.org/10.3390/ma15113846
Koszelewski D, Kowalczyk P, Śmigielski P, Samsonowicz-Górski J, Kramkowski K, Wypych A, Szymczak M, Ostaszewski R. Relationship between Structure and Antibacterial Activity of α-Aminophosphonate Derivatives Obtained via Lipase-Catalyzed Kabachnik−Fields Reaction. Materials. 2022; 15(11):3846. https://doi.org/10.3390/ma15113846
Chicago/Turabian StyleKoszelewski, Dominik, Paweł Kowalczyk, Paweł Śmigielski, Jan Samsonowicz-Górski, Karol Kramkowski, Aleksandra Wypych, Mateusz Szymczak, and Ryszard Ostaszewski. 2022. "Relationship between Structure and Antibacterial Activity of α-Aminophosphonate Derivatives Obtained via Lipase-Catalyzed Kabachnik−Fields Reaction" Materials 15, no. 11: 3846. https://doi.org/10.3390/ma15113846






