Sulfidized Nanoscale Zerovalent Iron Supported by Oyster Powder for Efficient Removal of Cr (VI): Characterization, Performance, and Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagent
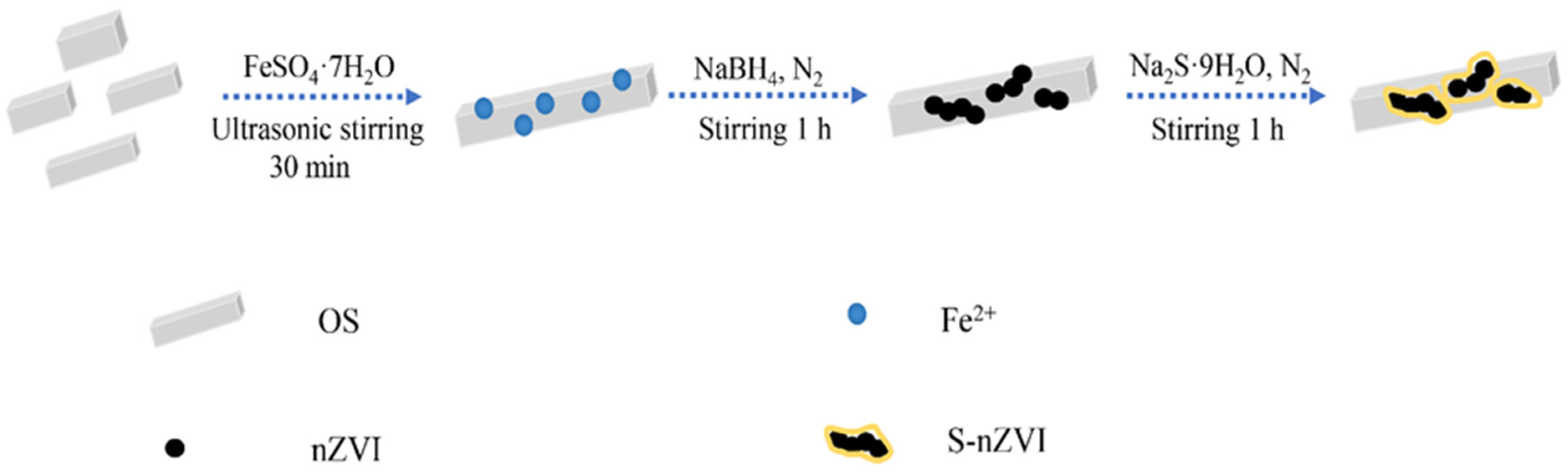
2.2. The Preparation of S-nZVI@OS
2.3. Characterization and Analysis Methods
2.4. Removal Process
2.5. Kinetic Study
2.6. Isotherms and Thermodynamics
3. Results and Discussion
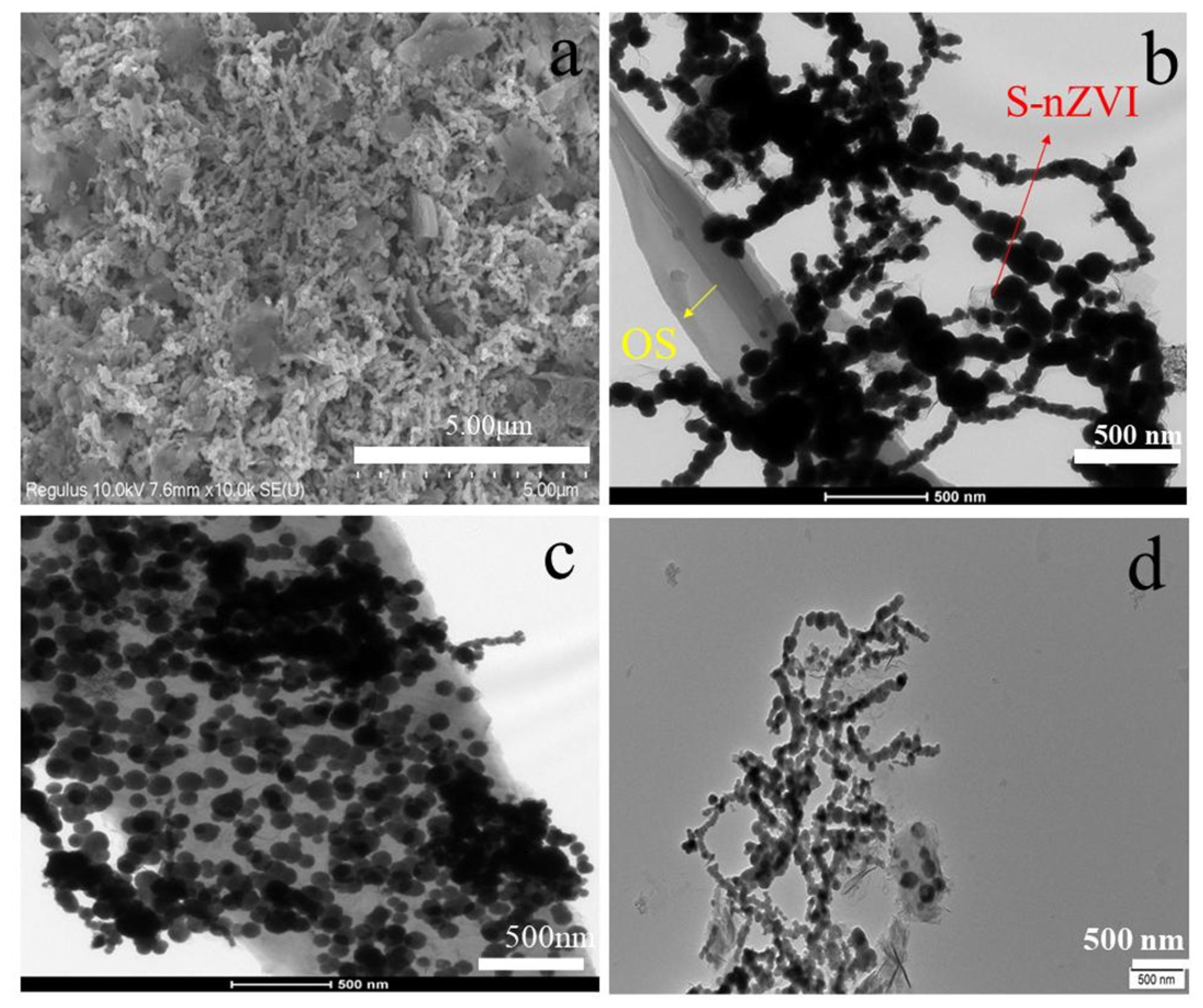
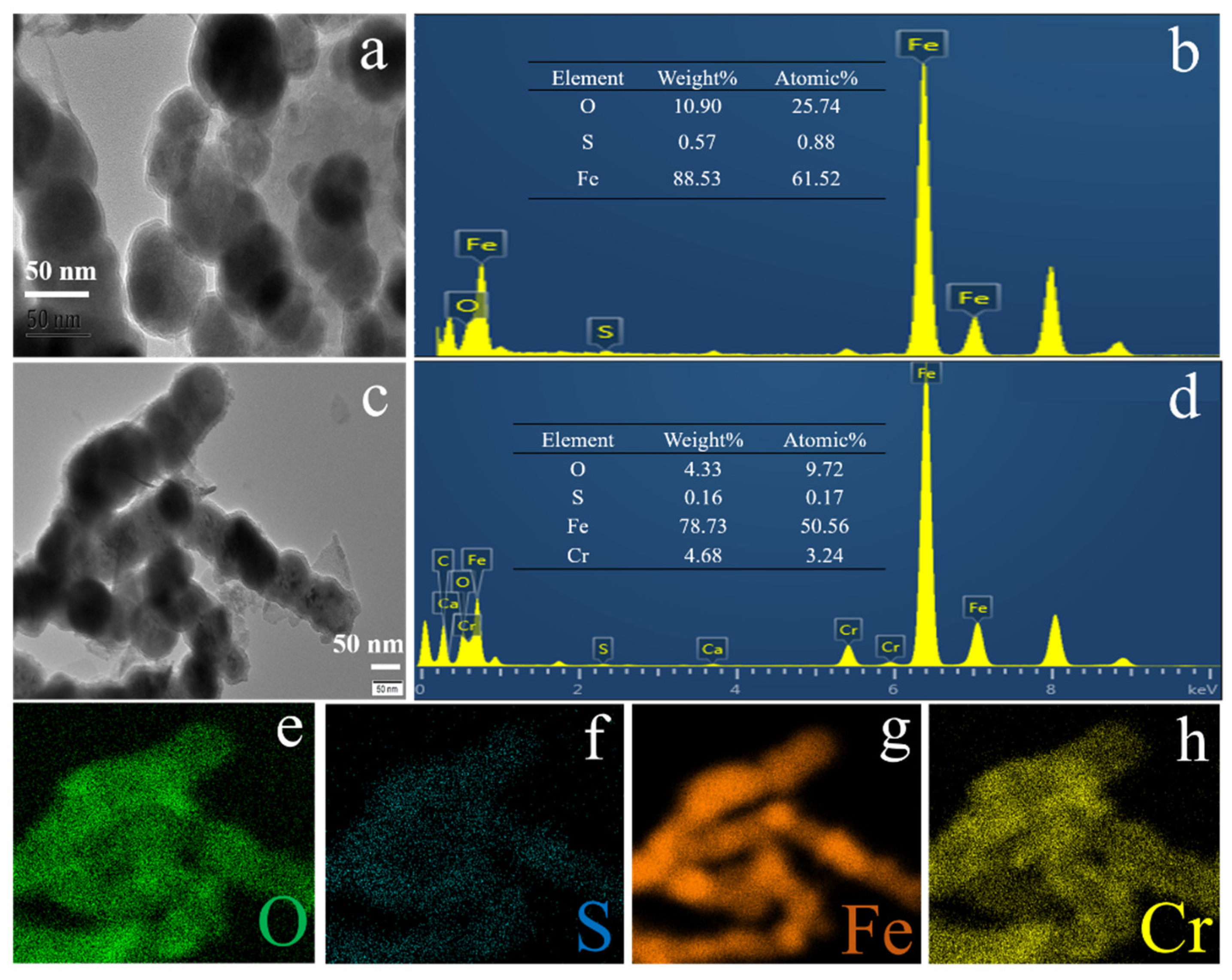
3.1. Characterization
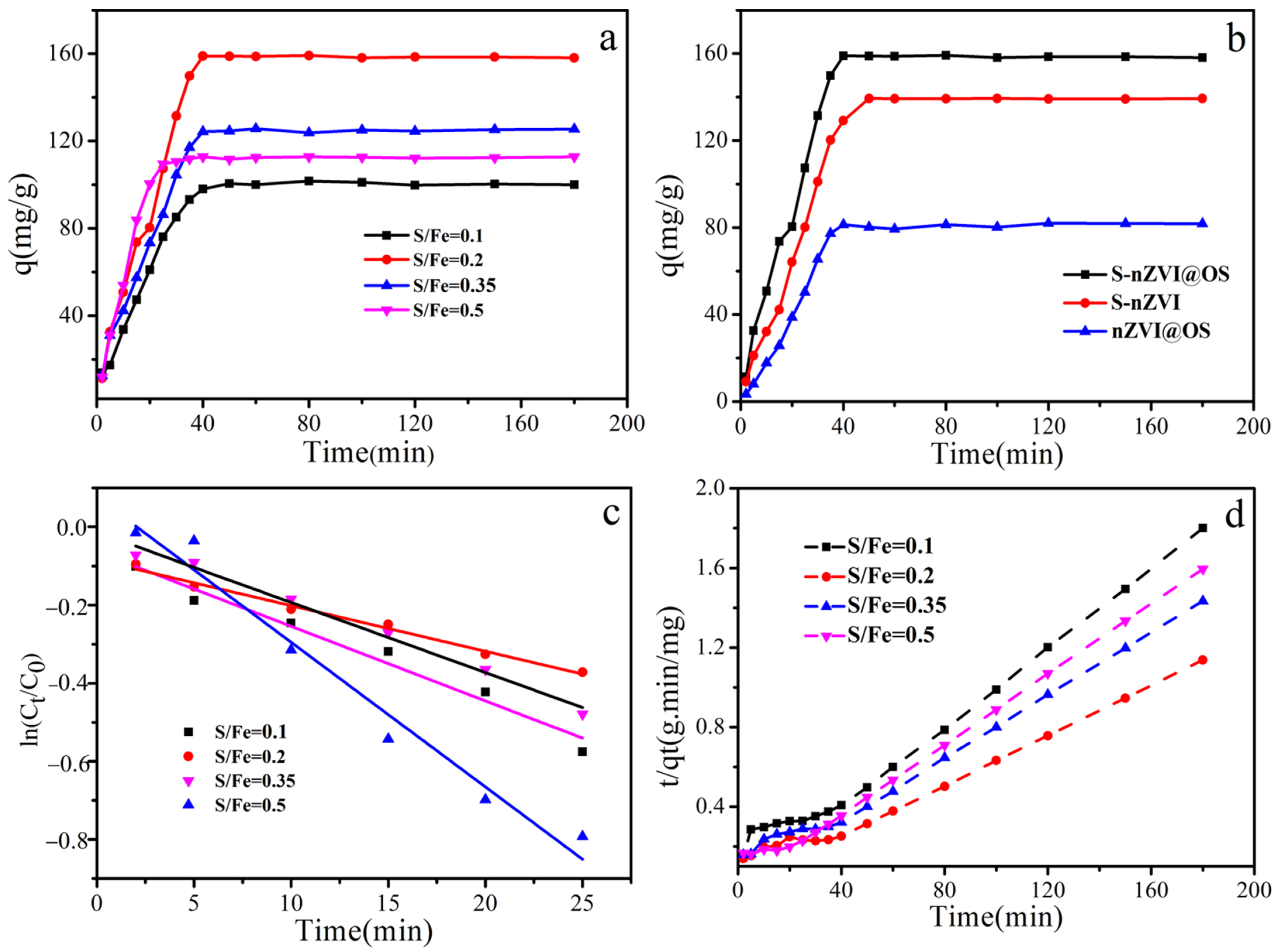
3.2. Adsorption Kinetics for S-nZVI@OS with Different S/Fe Ratio
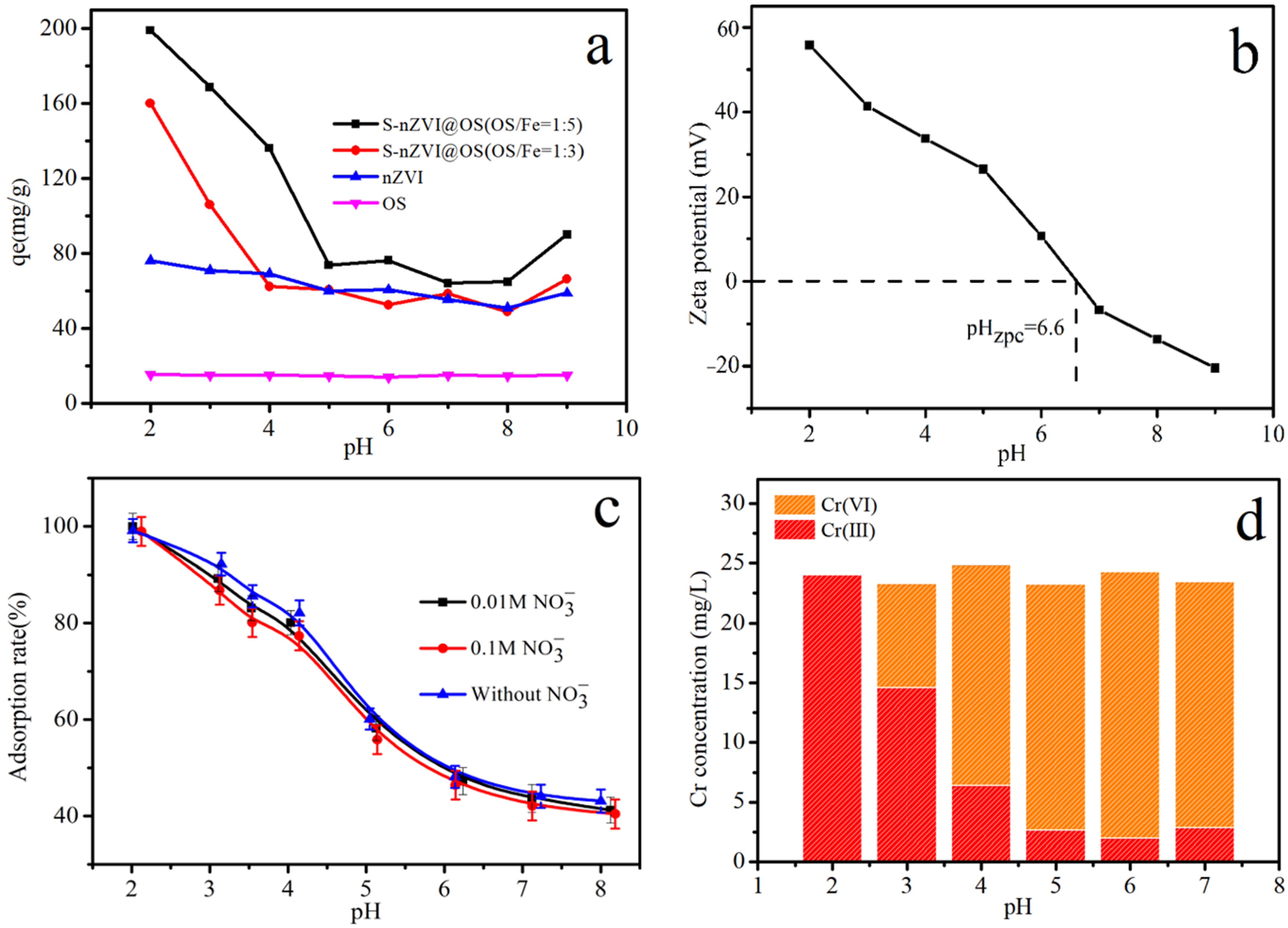
3.3. Effect of Initial Solution pH and Ionic Strength
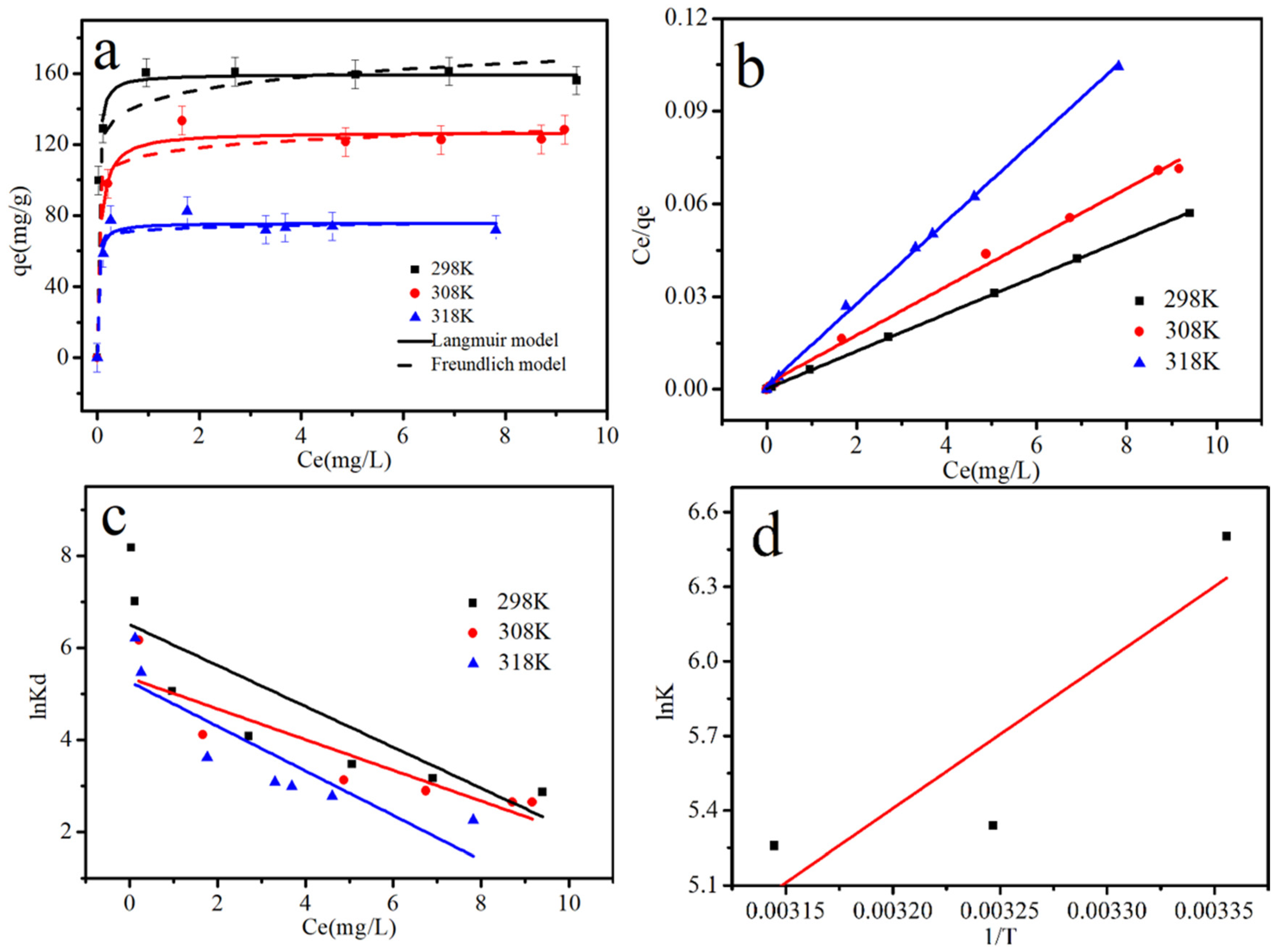
3.4. Adsorption Isotherms and Thermodynamics
3.5. Effect of Material Ageing on Removal Efficiency
4. Mechanism Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.X.; Zhao, G.X.; Chen, C.L.; Chai, Z.F.; Alsaedi, A.; Hayat, T.; Wang, X.K. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.R.; Chen, C.L.; Lu, S.H.; Zhang, X.D.; Alsaedi, A.; Hayat, T. MOFs-induced encapsulation of ultrafine Ni nanoparticles into 3D N-doped graphene-CNT frameworks as a recyclable catalyst for Cr (VI) reduction with formic acid. Carbon 2019, 148, 52–63. [Google Scholar] [CrossRef]
- Li, S.L.; Wang, W.; Liu, Y.Y.; Zhang, W.X. Zero-valent iron nanoparticles (nZVI) for the treatment of smelting wastewater: A pilot-scale demonstration. Chem. Eng. J. 2014, 254, 115–123. [Google Scholar] [CrossRef]
- Sarin, V.; Singh, T.S.; Pant, K.K. Thermodynamic and breakthrough column studies for the selective sorption of chromium from industrial effluent on activated eucalyptus bark. Bioresour. Technol. 2006, 97, 1986–1993. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, Y.C.; Xie, Y.; Zhu, K.R.; Alsaedi, A.; Hayat, T.; Chen, C.L. Construction of novel graphene-based materials GO@SiO2@C@Ni for Cr (VI) removal from aqueous solution. J. Colloid Interface Sci. 2019, 557, 254–269. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Srinivasu, V.V.; Onyango, M.S. Enhanced removal of Cr (VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite. J. Hazard. Mater. 2011, 190, 381–390. [Google Scholar] [CrossRef]
- Gong, K.D.; Hu, Q.; Xiao, Y.Y.; Cheng, X.; Liu, H.; Wang, N.; Qiu, B.; Guo, Z.H. Triple layered core-shell ZVI@carbon@polyaniline composite enhanced electron utilization in Cr (VI) reduction. J. Mater. Chem. 2018, A6, 11119–11128. [Google Scholar] [CrossRef]
- Lei, C.S.; Zhu, X.F.; Zhu, B.C.; Jiang, C.J.; Le, Y.; Yu, J.G. Superb adsorption capacity of hierarchical calcined Ni/Mg/Al layered double hydroxides for Congo red and Cr(VI) ions. J. Hazard. Mater. 2017, 321, 801–811. [Google Scholar] [CrossRef]
- Tan, Y.; Zou, Q.; Liu, X.F. Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresour. Technol. 2015, 190, 388–394. [Google Scholar] [CrossRef]
- Liu, C.; Fiol, N.; Villaescusa, I.; Poch, J. New approach in modeling Cr (VI) sorption onto biomass from metal binary mixtures solutions. Sci. Total Environ. 2016, 541, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, S.; Joo, C.K.; Kim, Y.; Yi, J. Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins: 1200H, 1500H and IRN97H. J. Hazard. Mater. 2003, 102, 257–275. [Google Scholar] [CrossRef]
- Kaya, A.; Onac, C.; Alpoguz, H.K.; Yilmaz, A.; Atar, N. Removal of Cr(VI) through calixarene based polymer inclusion membrane from chrome plating bath water. Chem. Eng. J. 2016, 283, 141–149. [Google Scholar] [CrossRef]
- Putz, A.; Ciopec, M.; Negrea, A.; Grad, O.; Iană¸si, C.; Ivankov, O.; Milanovi’c, M.; Stijepovi’c, I.; Almásy, L. Comparison of Structure and Adsorption Properties of Mesoporous Silica Functionalized with Aminopropyl Groups by the Co-Condensation and the Post Grafting Methods. Materials 2021, 14, 628. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Yong, S.O. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267C, 194–205. [Google Scholar] [CrossRef]
- Wan, Z.H.; Chu, D.W.; Tsang, D.C.W.; Li, M.; Sun, T.; Verpoort, F. Concurrent adsorption and micro-electrolysis of Cr (VI) by nanoscale zerovalent iron/biochar/Ca-alginate composite. Environ. Pollut. 2019, 247, 410–420. [Google Scholar] [CrossRef]
- Han, Y.; Yan, W. Reductive dechlorination of trichloroethene by zero-valent iron nanoparticles: Reactivity enhancement through sulfidation treatment. Environ. Sci. Technol. 2016, 50, 2992–13001. [Google Scholar] [CrossRef]
- Fang, Y.; Wen, J.; Zhang, H.; Wang, Q.; Hu, X. Enhancing Cr (VI) reduction and immobilization by magnetic core-shell structured NZVI@MOF derivative hybrids. Environ. Pollut. 2020, 260, 114021. [Google Scholar] [CrossRef]
- Fang, W.; Jiang, X.Y.; Luo, H.J.; Geng, J.J. Synthesis of graphene/SiO2@polypyrrole nanocomposites and their application for Cr (VI) removal in aqueous solution. Chemosphere 2018, 197, 594–602. [Google Scholar] [CrossRef]
- Wang, Y.H.; Lin, S.H.; Juang, R.S. Removal of heavy metal ions from aqueous solutions using various low-cost adsorbents. J. Hazard. Mater. 2003, 102, 291–302. [Google Scholar] [CrossRef]
- Al-Othman, Z.A.; Ali, R.; Naushad, M. Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: Adsorption kinetics, equilibrium and thermodynamic studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar] [CrossRef]
- Yusof, A.M.; Malek, N.A.N.N. Removal of Cr(VI) and As(V) from aqueous solutions by HDTMA-modified zeolite Y. J. Hazard. Mater. 2009, 162, 1019–1024. [Google Scholar] [CrossRef]
- Dong, X.L.; Ma, L.Q.; Li, Y.C. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem. Eng. J. 2015, 259, 438–448. [Google Scholar] [CrossRef]
- Hu, L.Y.; Chen, L.X.; Liu, M.T.; Wu, L.J.; Feng, J.J. Theophylline-assisted, eco-friendly synthesis of PtAu nanospheres at reduced graphene oxide with enhanced catalytic activity towards Cr(VI) reduction. J. Colloid Interface Sci. 2017, 493, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Sun, Y.; Tsang, D.C.W.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2018, 232, 10–30. [Google Scholar] [CrossRef]
- Gao, J.; Yang, L.; Liu, Y.; Shao, F.; Liao, Q.; Shang, J. Scavenging of Cr (VI) from aqueous solutions by sulfide-modified nanoscale zero-valent iron supported by biochar. J. Taiwan Inst. 2018, 91, 449–456. [Google Scholar] [CrossRef]
- Qian, L.B.; Zhang, W.Y.; Yan, J.C.; Han, L.; Chen, Y.; Ouyang, D.; Chen, M.F. Nanoscale zero-valent iron supported by biochars produced at different temperatures: Synthesis mechanism and effect on Cr (VI) removal. Environ. Pollut. 2017, 223, 153–160. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, Y.M.; Li, J.F.; Sheng, G.D.; Zhang, Y.; Zheng, X.M. Enhanced Cr (VI) removal by using the mixture of pillared bentonite and zero-valent iron. Chem. Eng. J. 2012, 185–186, 243–249. [Google Scholar] [CrossRef]
- Bhaumik, M.; McCrindle, R.I.; Maity, A. Enhanced adsorptive degradation of Congo red in aqueous solutions using polyaniline/Fe0 composite nanofibers. Chem. Eng. J. 2015, 260, 716–729. [Google Scholar] [CrossRef]
- Huang, R.F.; Ma, X.G.; Li, X.; Guo, L.H.; Xie, X.W.; Zhang, M.Y.; Li, J. A novel ion imprinted polymer based on graphene oxide-mesoporous silica nanosheet for fast and efficient removal of chromium (VI) from aqueous solution. J. Colloid Interface Sci. 2018, 514, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.L.; Zhang, R.; Wang, X.K. Nanoscale zero-valent iron particles supported on reduced graphene oxides by using a plasma technique and their application for removal of heavy-metal ions. Chem. Asian J. 2015, 10, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Kim, S.Y.; Owens, V.N.; Park, S.; Kim, J.; Hong, C.O. How does oyster shell immobilize cadmium? Arch. Environ. Contam. Toxicol. 2018, 74, 114–120. [Google Scholar] [CrossRef]
- Ok, Y.S.; Lim, J.E.; Moon, D.H. Stabilization of Pb and Cd contaminated soils and soil quality improvements using waste oyster shells. Environ. Geochem. Health 2011, 33, 83–91. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Oh, M.; Park, J. Oyster shell as a low-cost adsorbent for removing heavy metal ions from wastewater. Pol. J. Environ. Stud. 2019, 28, 2949–2959. [Google Scholar] [CrossRef]
- Zhao, D.L.; Gao, X.; Chen, S.H.; Xie, F.Z.; Feng, S.J.; Alsaedi, A.; Hayat, T.; Chen, C.L. Interaction between U(VI) with sulfhydryl groups functionalized graphene oxides investigated by batch and spectroscopic techniques. J. Colloid Interface Sci. 2018, 524, 129–138. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, D.L.; Feng, S.J.; Wang, Y.Y.; Jin, J.; Alsaedi, A.; Hayat, T.; Chen, C.L. Synthesis of nanoscale zero-valent iron loaded chitosan for synergistically enhanced removal of U(VI) based on adsorption and reduction. J. Colloid Interface Sci. 2019, 552, 735–743. [Google Scholar] [CrossRef]
- Singh, P.; Raizada, P.; Kunari, S. Solar-Fenton removal of malachite green with novelFe-0-activated carbon nanocomposite. Appl. Catal. A Gen. 2014, 476, 9–18. [Google Scholar] [CrossRef]
- Luo, S.L.; Xu, X.L.; Zhou, G.Y. Amino siloxane oligomer-linked graphene oxide as an efficient adsorbent for removal of Pb(II) from wastewater. J. Hazard. Mater. 2014, 274, 145–155. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Ma, J.; Xia, P.; Zhao, J. Removal of cadmium (II) from aqueous solution: A comparative study of raw attapulgite clay and a reusable waste-struvite/attapulgite obtained from nutrient-rich wastewater. J. Hazard. Mater. 2017, 329, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Wang, B.; Li, Z.; Jia, X.; Zhou, Q. Biosorption of Acid Black 172 and Congo Red from aqueous solution by nonviable Penicillium YW 01: Kinetic study, equilibrium isotherm and artificial neural network modeling. Bioresour. Technol. 2011, 102, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Gong, Y.; Tang, J.; Huang, Y.; Wang, Q. Immobilization of heavy metals in electroplating sludge by biochar and iron sulfide. Environ. Sci. Pollut. Res. Int. 2016, 23, 14472–14488. [Google Scholar] [CrossRef]
- Ai, Y.; Liu, Y.; Lan, W.; Jin, J.; Xing, J.; Zou, Y.; Zhao, C.; Wang, X. The effect of pH on the U(VI) sorption on graphene oxide (GO): A theoretical study. Chem. Eng. J. 2018, 343, 460–466. [Google Scholar] [CrossRef]
- Zhang, S.; Lyu, H.H.; Tang, J.C.; Song, B.; Zhen, M.; Liu., X. A novel biochar supported CMC stabilized nano zero-valent iron composite for hexavalent chromium removal from water. Chemosphere 2019, 217, 686–694. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, S.Y.; Liu, T.; Deng, X.Q. Green synthesis of nano zero-valent iron/Cu by green tea to remove hexavalent chromium from groundwater. J. Clean. Prod. 2017, 174, 184–190. [Google Scholar] [CrossRef]
- Dong, H.R.; Deng, J.M.; Xie, Y.K.; Zhang, C.; Jiang, Z.; Cheng, Y.J.; Hou, K.J.; Zeng, G.G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Jiao, X.Q.; Liu, N.; Lv, J.; Yang, Y.D. Enhanced removal of aqueous Cr(VI) by a green synthesized nanoscale zero-valent iron supported on oak wood biochar. Chemosphere 2020, 245, 125542. [Google Scholar] [CrossRef]
- Wu, B.; Peng, D.H.; Hou, S.Y.; Tang, B.C.; Wang, C.; Xu, H. Dynamic study of Cr(VI) removal performance and mechanism from water using multilayer material coated nanoscale zerovalent iron. Environ. Pollut. 2018, 240, 717–724. [Google Scholar] [CrossRef]
- Li, X.; Ai, L.; Jing, J. Nanoscale zerovalent iron decorated on graphene nanosheets for Cr(VI) removal from aqueous solution: Surface corrosion retard induced the enhanced performance. Chem. Eng. J. 2016, 288, 789–797. [Google Scholar] [CrossRef]
- Fu, R.B.; Yang, Y.P.; Xu, Z.; Zhang, X.; Guo, X.P.; Bi, D.S. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015, 138, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Ren, H.; Ma, X.; Zhou, S.; Huang, J.; Jiao, W.; Qi, G.; Liu, Y. High-gravity continuous preparation of chitosan-stabilized nanoscale zero-valent iron towards Cr (VI) removal. Chem. Eng. J. 2020, 390, 124639. [Google Scholar] [CrossRef]
- Su, Y.M.; Adeleye, Y.M.; Keller, A.A.; Huang, Y.X.; Dai, C.M.; Zhou, X.F.; Zhang, Y.L. Magnetic sulfide-modified nanoscale zerovalent iron (S-nZVI) for dissolved metal ion removal. Water Res. 2015, 74, 47–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.R.; Zhou, Z.M.; Zhao, X.D.; Jing, G.H. Enhanced Cr(VI) removal from simulated electroplating rinse wastewater by amino-functionalized vermiculite-supported nanoscale zero-valent iron. Chemosphere 2019, 218, 458–467. [Google Scholar] [CrossRef]
- Fontecha-Cámara, M.A.; Álvarez-Merino, M.A.; Carrasco-Marín, F.; Lopez-Ramon, M.V.; Moreno-Castilla, C. Heterogeneous and homogeneous Fenton processes using activated carbon for the removal of the herbicide amitrole from water. Appl. Catal. B Environ. 2011, 101, 425–430. [Google Scholar] [CrossRef]
- Montesinos, V.N.; Quici, N.; Halac, E.B.; Leyva, A.G.; Custo, G.; Bengio, S. Highly efficient removal of Cr (VI) from water with nanoparticulated zerovalent iron: Understanding the Fe(III)-Cr(III) passive outer layer structure. Chem. Eng. J. 2014, 244, 569–575. [Google Scholar] [CrossRef]
- Lyu, H.H.; Tang, J.C.; Huang, Y.; Gai, L.S.; Zeng, E.Y.; Liber, K.; Gong, Y.Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 2017, 322, 516–524. [Google Scholar] [CrossRef]




| S/Fe Ratio | Langmuir-Hinshelwood First-Order Kinetics Model | Pseudo-Second Order Model | |||
|---|---|---|---|---|---|
| Kobs (min−1) | R2 | k2 (g/mg min) | Qe (mg/g) | R2 | |
| 0.100 | 0.018 | 0.990 | 0.647 × 10−3 | 112.996 | 0.985 |
| 0.200 | 0.012 | 0.990 | 0.352 × 10−3 | 178.473 | 0.982 |
| 0.350 | 0.019 | 0.975 | 0.514 × 10−3 | 139.538 | 0.988 |
| 0.500 | 0.037 | 0.974 | 1.240 × 10−3 | 119.764 | 0.992 |
| T(K) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qmax (mg/g) | b (L/mg) | R2 | k (mg·g−1) | n | R2 | |
| 298 | 164.745 | 21.328 | 0.999 | 143.314 | 12.402 | 0.908 |
| 308 | 126.582 | 4.463 | 0.994 | 104.921 | 14.760 | 0.760 |
| 318 | 75.075 | 11.684 | 0.998 | 66.567 | 17.077 | 0.925 |
| Adsorbents | pH | Qmax (mg/g) | References |
|---|---|---|---|
| S-nZVI@OS | 3.5 | 164.7 | This work |
| Biochar-CMC-nZVI | 5.6 | 112.5 | [45] |
| nZVI/Cu | 5.0 | 18.8 | [46] |
| nZVI@HCl-BC | 5.0 | 17.8 | [47] |
| TP-nZVI-OB | 2.0 | 95.5 | [48] |
| SBC-nZVI | 3.0 | 84.4 | [49] |
| nGO-nZVI | 7.0 | 21.7 | [50] |
| Sepiolite/nZVI | 6.0 | 43.9 | [51] |
| CS-nZVI | 4.0 | 101.8 | [52] |
| T (K) | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/mol/K) |
|---|---|---|---|
| 298 | −15.695 | ||
| 308 | −14.562 | −49.457 | −113.294 |
| 318 | −13.429 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Zhao, D.; Wu, C.; Xie, R. Sulfidized Nanoscale Zerovalent Iron Supported by Oyster Powder for Efficient Removal of Cr (VI): Characterization, Performance, and Mechanisms. Materials 2022, 15, 3898. https://doi.org/10.3390/ma15113898
Hu H, Zhao D, Wu C, Xie R. Sulfidized Nanoscale Zerovalent Iron Supported by Oyster Powder for Efficient Removal of Cr (VI): Characterization, Performance, and Mechanisms. Materials. 2022; 15(11):3898. https://doi.org/10.3390/ma15113898
Chicago/Turabian StyleHu, Hao, Donglin Zhao, Changnian Wu, and Rong Xie. 2022. "Sulfidized Nanoscale Zerovalent Iron Supported by Oyster Powder for Efficient Removal of Cr (VI): Characterization, Performance, and Mechanisms" Materials 15, no. 11: 3898. https://doi.org/10.3390/ma15113898
APA StyleHu, H., Zhao, D., Wu, C., & Xie, R. (2022). Sulfidized Nanoscale Zerovalent Iron Supported by Oyster Powder for Efficient Removal of Cr (VI): Characterization, Performance, and Mechanisms. Materials, 15(11), 3898. https://doi.org/10.3390/ma15113898






