Recent Progress in Functionalized Coatings for Corrosion Protection of Magnesium Alloys—A Review
Abstract
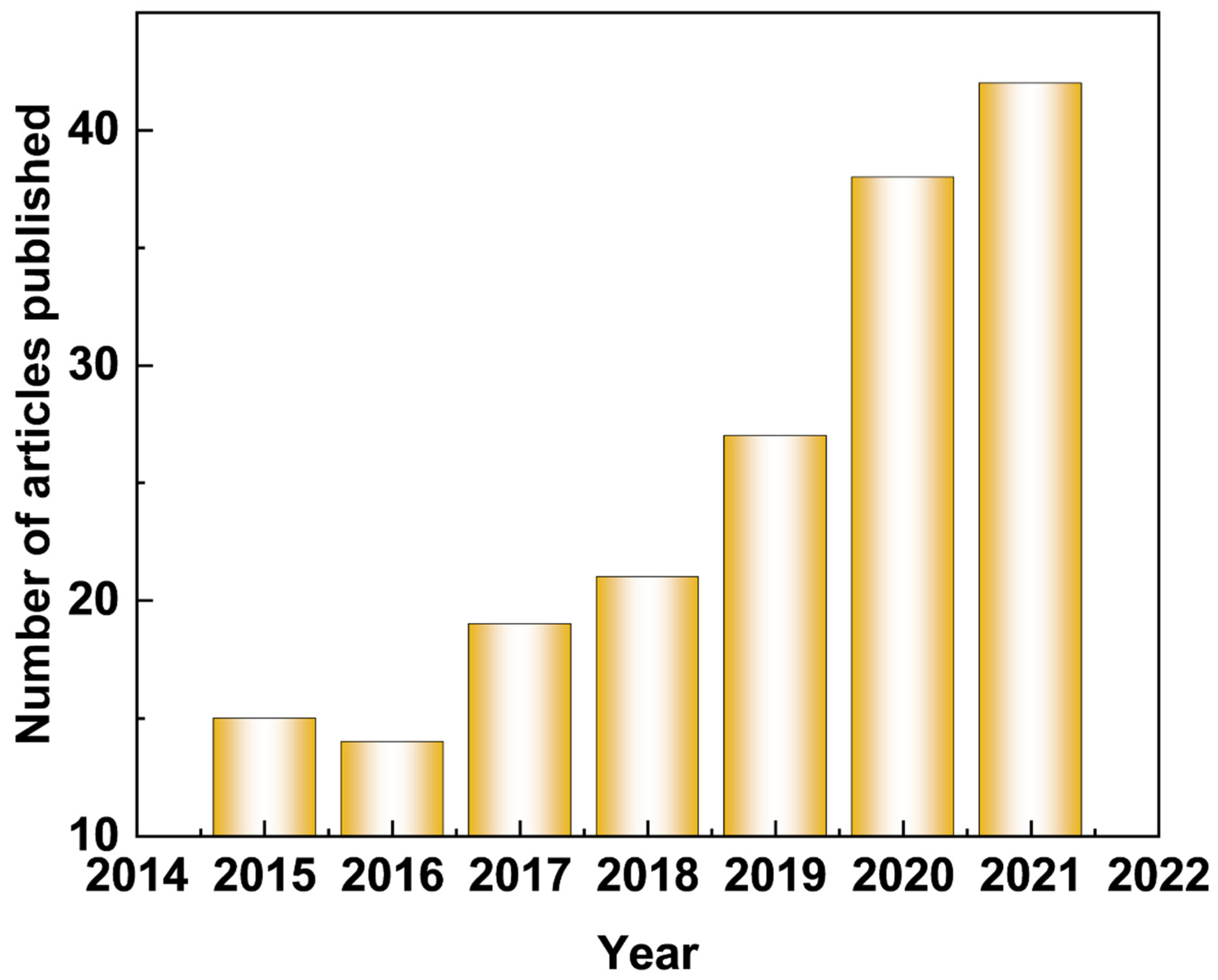
:1. Introduction
2. Superhydrophobic Coatings
2.1. Micro Arc Oxidation
2.2. Chemical Etching
2.3. Hydrothermal Synthesis
2.4. Electrodeposition
2.5. Spraying
2.6. Sol-Gel
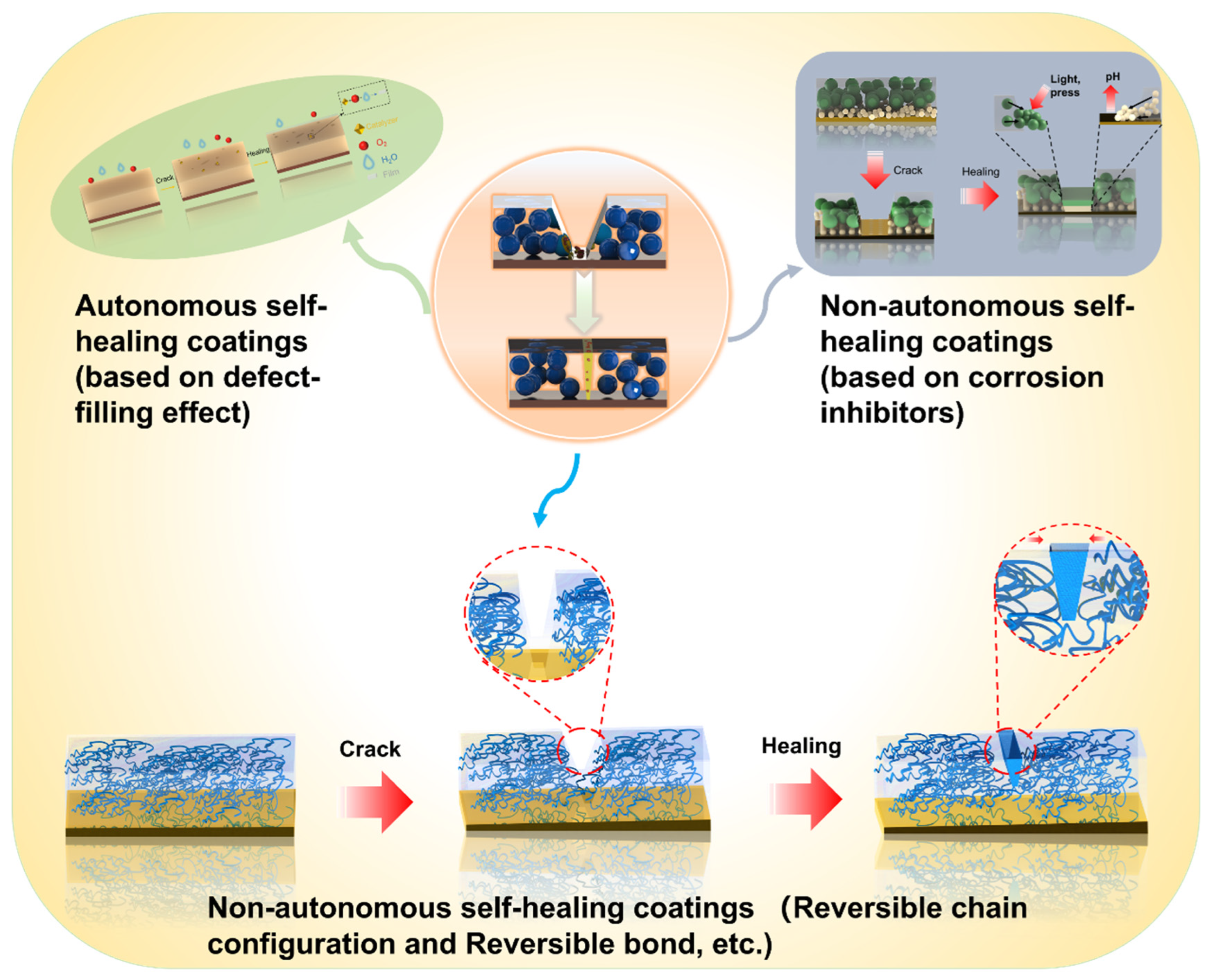
3. Self-Healing Coatings
3.1. Autonomous Self-Healing Coatings
3.2. Non-Autonomous Self-Healing Coatings
4. Comparison and Challenges in the Functional Coating on Mg Alloys
5. Conclusions and Future Perspectives
- (1)
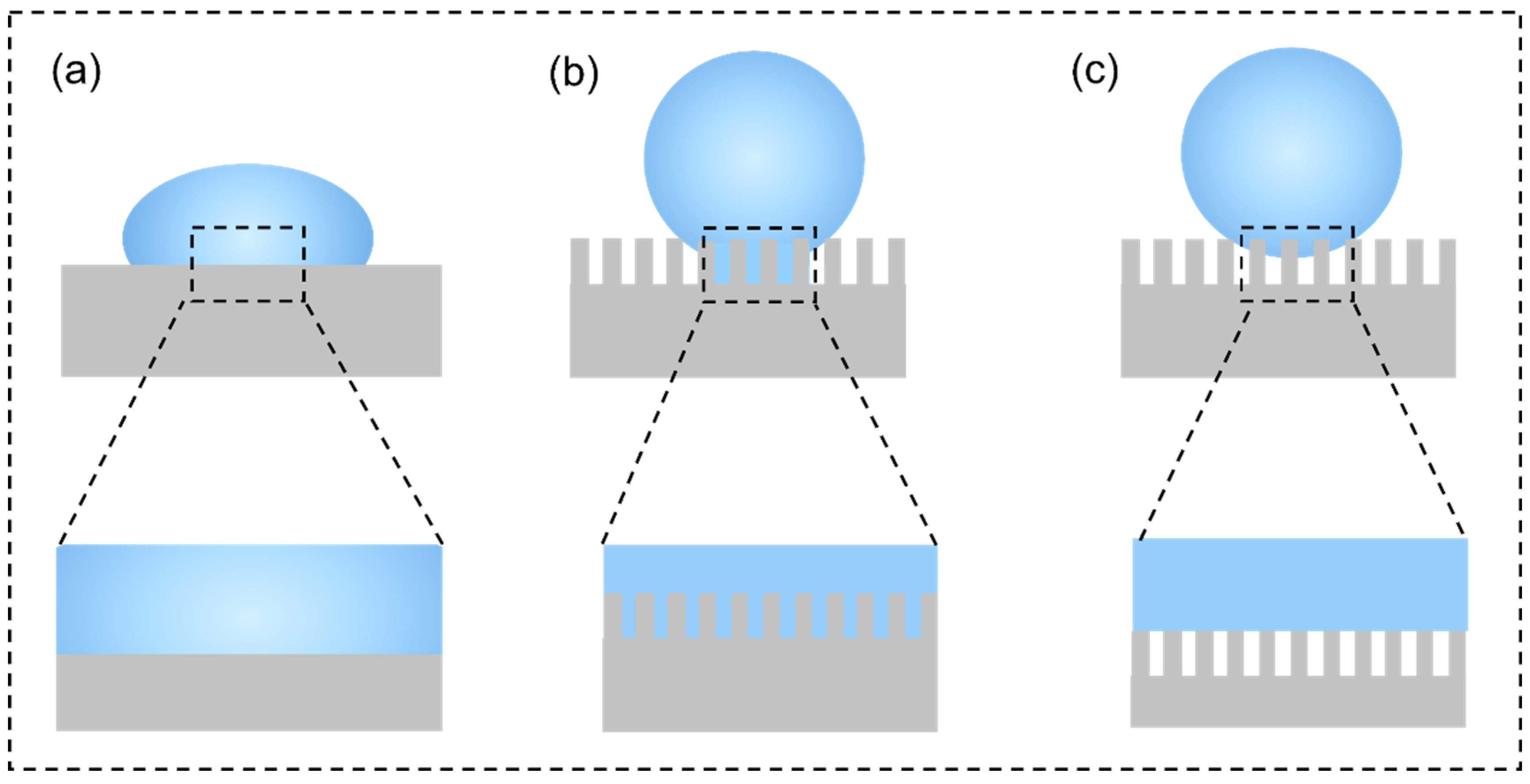
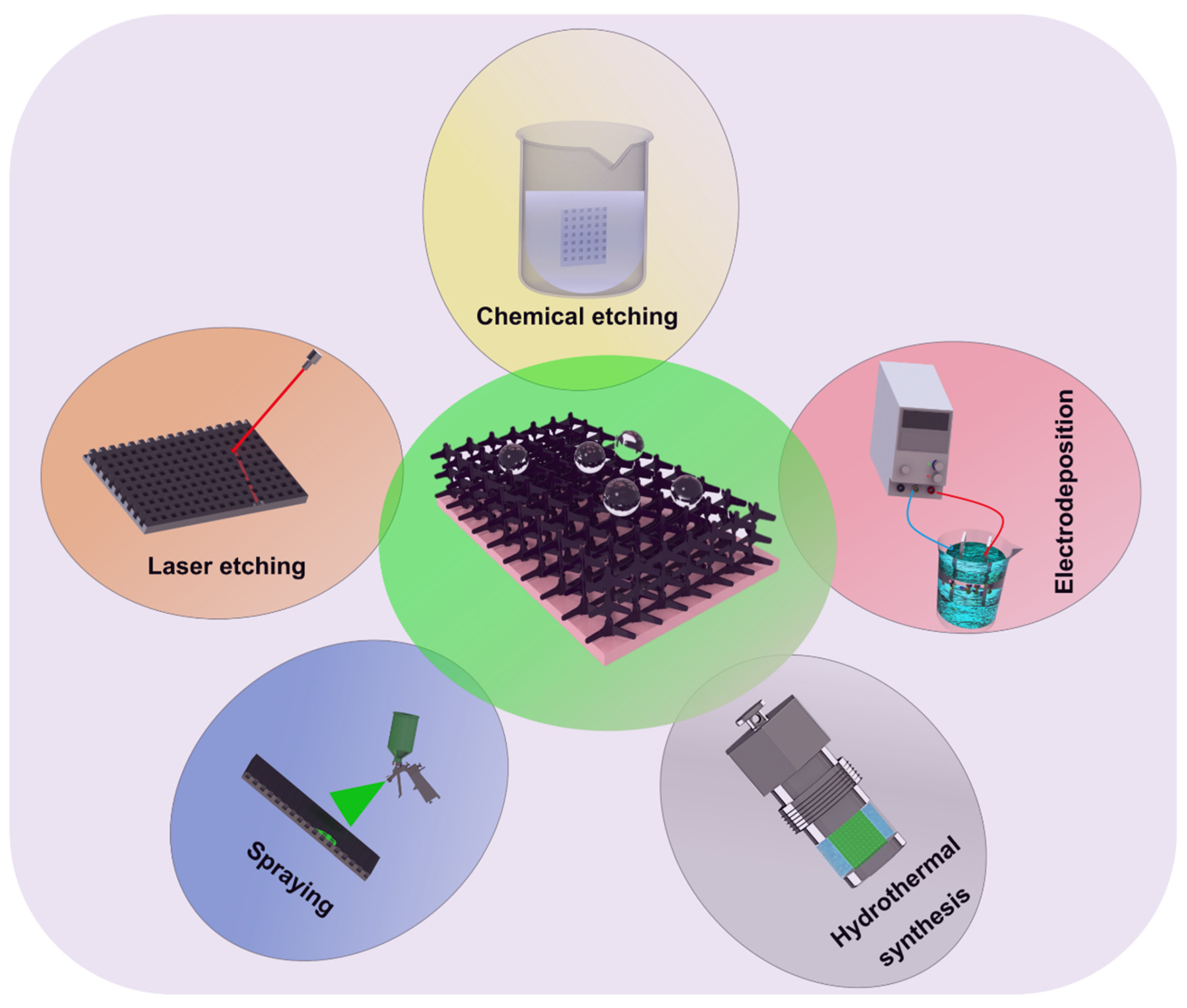
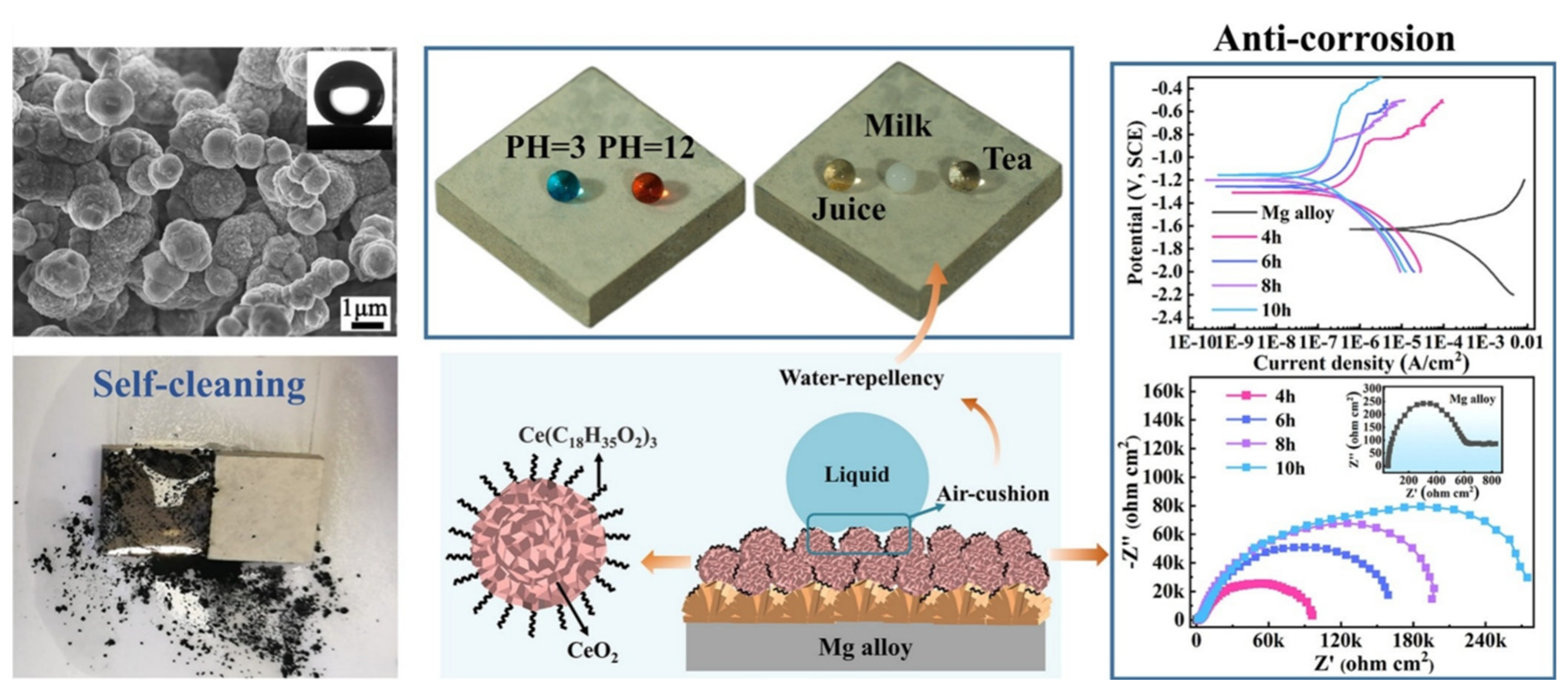
- Superhydrophobic coatings are projected as a suitable way to tackle the poor corrosion resistance of Mg alloys because the hydrophobic ability can usefully reduce the contact area between corrosive media and Mg alloys. However, the superhydrophobic coating is easily subjected to damage, and thus, loses superhydrophobicity. As a general rule, the prepared technology of superhydrophobic coatings on Mg alloys can be divided into one-step and two-step/multi-step methods. The two-step/multi-step method, such as micro-arc oxidation, chemical etching, and hydrothermal synthesis, first manufactures a rough structure on the Mg substrate, followed by modifying with the low surface-energy material; the one-step method, which consists of hydrothermal synthesis, electrodeposition, and sol-gel, is used to create a superhydrophobic substance that has both low surface energy and rough structure.
- (2)
- Self-healing coatings are judged as a reasonable tactic that can meet the requirements of corrosion resistance of Mg alloys in some extreme service environments, which is because the self-healing property of the coating can entirely, or partially, repair the damaged area during applications, thereby extending the lifetime of the coating. Conceptually, these self-healing coatings can be compartmentalized into autonomous and non-autonomous coatings. Autonomous coatings can quickly respond to environmental or mechanical attacks related to corrosion or other destructive processes and release corrosion inhibitors or polymerizable healing agents. Non-autonomous self-healing coating can be truly repaired when exposed to external heat or light stimulation, which closes the damage and reforms the chemical or physical cross-linking inherent in the coating matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogawa, Y.; Ando, D.; Sutou, Y.; Koike, J. A lightweight shape-memory magnesium alloy. Science 2016, 353, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy. 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Xu, T.; Yang, Y.; Peng, X.; Song, J.; Pan, F. Overview of advancement and development trend on magnesium alloy. J. Magnes. Alloy. 2019, 7, 536–544. [Google Scholar] [CrossRef]
- Bella, F.; De Luca, S.; Fagiolari, L.; Versaci, D.; Amici, J.; Francia, C.; Bodoardo, S. An Overview on Anodes for Magnesium Batteries: Challenges towards a Promising Storage Solution for Renewables. Nanomaterials 2021, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Zeng, R.-C.; Lin, C.-G.; Wang, L.; Chen, X.-B.; Chen, D.-C. Corrosion resistance of self-cleaning silane/polypropylene composite coatings on magnesium alloy AZ31. J. Mater. Sci. Technol. 2020, 41, 43–55. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.-L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of Recent Developments in the Field of Magnesium Corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Zheng, T.; Hu, Y.; Pan, F.; Zhang, Y.; Tang, A. Fabrication of corrosion-resistant superhydrophobic coating on magnesium alloy by one-step electrodeposition method. J. Magnes. Alloy. 2019, 7, 193–202. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Yang, Y.X.; Li, J.A.; Zeng, R.C.; Guan, S.K. Advances in coatings on magnesium alloys for cardiovascular stents—A review. Bioact. Mater. 2021, 6, 4729–4757. [Google Scholar] [CrossRef]
- Yin, Z.-Z.; Qi, W.-C.; Zeng, R.-C.; Chen, X.-B.; Gu, C.-D.; Guan, S.-K.; Zheng, Y.-F. Advances in coatings on biodegradable magnesium alloys. J. Magnes. Alloy. 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Tokunaga, T.; Ohno, M.; Matsuura, K. Coatings on Mg alloys and their mechanical properties: A review. J. Mater. Sci. Technol. 2018, 34, 1119–1126. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; You, Z.; Liu, S.; Guan, K.; Wu, R.; Wang, J.; Feng, J. Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying. J. Magnes. Alloy. 2021, 9, 41–56. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.; Sun, M.; Ding, W. Recent developments and applications on high-performance cast magnesium rare-earth alloys. J. Magnes. Alloy. 2021, 9, 1–20. [Google Scholar] [CrossRef]
- Südholz, A.D.; Birbilis, N.; Bettles, C.J.; Gibson, M.A. Corrosion behaviour of Mg-alloy AZ91E with atypical alloying additions. J. Alloy. Compd. 2009, 471, 109–115. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Emerging magnesium-based biomaterials for orthopedic implantation. Emerg. Mater. Res. 2019, 8, 305–319. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.; Cui, L.; Ke, W. Biomedical Magnesium Alloys: Composition, Microstructure and Corrosion. Acta Metall. Sin. 2018, 54, 1215–1235. [Google Scholar] [CrossRef]
- Gungor, A.; Incesu, A. Effects of alloying elements and thermomechanical process on the mechanical and corrosion properties of biodegradable Mg alloys. J. Magnes. Alloy. 2021, 9, 241–253. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy. 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Okido, M.; Ichino, R.; Kim, S.-J.; Jang, S.-K. Surface characteristics of chemical conversion coating for Mg-Al alloy. Trans. Nonferrous Met. Soc. China 2009, 19, 892–897. [Google Scholar] [CrossRef]
- Yang, H.; Guo, X.; Wu, G.; Ding, W.; Birbilis, N. Electrodeposition of chemically and mechanically protective Al-coatings on AZ91D Mg alloy. Corros. Sci. 2011, 53, 381–387. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, D.; Yu, J.; Di, S. Effects of Al2O3 Nano-additive on Performance of Micro-arc Oxidation Coatings Formed on AZ91D Mg Alloy. J. Mater. Sci. Technol. 2014, 30, 984–990. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Y.; Liang, M.; Hou, L.; Li, Y.; Guo, C. Fabrication of stable and corrosion-resisted super-hydrophobic film on Mg alloy. Colloids Surf. A 2016, 509, 351–358. [Google Scholar] [CrossRef]
- Kim, J.; Mousa, H.M.; Park, C.H.; Kim, C.S. Enhanced corrosion resistance and biocompatibility of AZ31 Mg alloy using PCL/ZnO NPs via electrospinning. Appl. Surf. Sci. 2017, 396, 249–258. [Google Scholar] [CrossRef]
- Yang, Y.; Ali, N.; Bilal, M.; Khan, A.; Ali, F.; Mao, P.; Ni, L.; Gao, X.; Hong, K.; Rasool, K.; et al. Robust membranes with tunable functionalities for sustainable oil/water separation. J. Mol. Liq. 2021, 321, 114701. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Krishnan, A.; Krishnan, A.V.; Ajith, A.; Shibli, S.M.A. Influence of materials and fabrication strategies in tailoring the anticorrosive property of superhydrophobic coatings. Surf. Interfaces 2021, 25, 101238. [Google Scholar] [CrossRef]
- Onda, T.; Shibuichi, S.; Satoh, N.; Tsujii, K. Super-Water-Repellent Fractal Surfaces. Langmuir 1996, 12, 2125–2127. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Wei, Z.; Song, Y.; Jiang, L. Bioinspired Design of a Superoleophobic and Low Adhesive Water/Solid Interface. Adv. Mater. 2009, 21, 665–669. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.H.; Tang, S.K.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Liu, T.L.; Kim, C.-J.C. Turning a surface superrepellent even to completely wetting liquids. Science 2014, 346, 1096–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saji, V.S. Recent progress in superhydrophobic and superamphiphobic coatings for magnesium and its alloys. J. Magnes. Alloy. 2021, 9, 748–778. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Zhang, W.; Yu, X.; Lin, L.; Guo, X.; Liu, M.; Jiang, L. Corrosion-Resistant Superhydrophobic Coatings on Mg Alloy Surfaces Inspired by Lotus Seedpod. Adv. Funct. Mater. 2017, 27, 1605446. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, D.; Liu, X.; Zhang, Y. Recent progress in superhydrophobic coating on Mg alloys: A general review. J. Magnes. Alloy. 2021, 1471–1486. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Wang, L.; Zeng, M.Q.; Zeng, R.C.; Kannan, M.B.; Lin, C.G.; Zheng, Y.F. Biodegradation behavior of micro-arc oxidation coating on magnesium alloy-from a protein perspective. Bioact. Mater. 2020, 5, 398–409. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Yu, X.; Sun, X.; Yang, H. Functionalization treatment of micro-arc oxidation coatings on magnesium alloys: A review. J. Alloy. Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- Kaseem, M.; Ramachandraiah, K.; Hossain, S.; Dikici, B. A Review on LDH-Smart Functionalization of Anodic Films of Mg Alloys. Nanomaterials 2021, 11, 536. [Google Scholar] [CrossRef]
- Guo, M.; Cao, L.; Lu, P.; Liu, Y.; Xu, X. Anticorrosion and cytocompatibility behavior of MAO/PLLA modified magnesium alloy WE42. J. Mater. Sci. Mater. Med. 2011, 22, 1735–1740. [Google Scholar] [CrossRef]
- Liu, P.; Pan, X.; Yang, W.; Cai, K.; Chen, Y. Improved anticorrosion of magnesium alloy via layer-by-layer self-assembly technique combined with micro-arc oxidation. Mater. Lett. 2012, 75, 118–121. [Google Scholar] [CrossRef]
- Liang, J.; Guo, Z.; Fang, J.; Hao, J. Fabrication of Superhydrophobic Surface on Magnesium Alloy. Chem. Lett. 2007, 36, 416–417. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Wang, L.; Zeng, M.-Q.; Zeng, R.-C.; Lin, C.-G.; Wang, Z.-L.; Chen, D.-C.; Zhang, Q. Corrosion resistance and superhydrophobicity of one-step polypropylene coating on anodized AZ31 Mg alloy. J. Magnes. Alloy. 2021, 9, 1443–1457. [Google Scholar] [CrossRef]
- Cui, X.J.; Lin, X.Z.; Liu, C.H.; Yang, R.S.; Zheng, X.W.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Kaseem, M.; Zehra, T.; Dikici, B.; Dafali, A.; Yang, H.W.; Ko, Y.G. Improving the electrochemical stability of AZ31 Mg alloy in a 3.5wt.% NaCl solution via the surface functionalization of plasma electrolytic oxidation coating. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, B.; Gao, S.; Cao, R. The sandwich-like structures of polydopamine and 8-hydroxyquinoline coated graphene oxide for excellent corrosion resistance of epoxy coatings. J. Colloid Interface Sci. 2020, 565, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Kaseem, M.; Ko, Y.G. Morphological modification and corrosion response of MgO and Mg3(PO4)2 composite formed on magnesium alloy. Compos. Part B Eng. 2019, 176, 107225. [Google Scholar] [CrossRef]
- Kaseem, M.; Hussain, T.; Zeeshan, U.R.; Yang, H.W.; Dikici, B.; Ko, Y.G. Fabrication of functionalized coating with a unique flowery-flake structure for an effective corrosion performance and catalytic degradation. Chem. Eng. J. 2021, 420, 129737. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Babaei, K. Impressive strides in amelioration of corrosion and wear behaviors of Mg alloys using applied polymer coatings on PEO porous coatings: A review. J. Magnes. Alloy. 2022. [Google Scholar] [CrossRef]
- Chaharmahali, R.; Fattah-alhosseini, A.; Nouri, M.; Babaei, K. Improving surface characteristics of PEO coatings of Mg and its alloys with zirconia nanoparticles: A review. Appl. Surf. Sci. Adv. 2021, 6, 100131. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Egorkin, V.S.; Sinebryukhov, S.L.; Vyaliy, I.E.; Pashinin, A.S.; Emelyanenko, A.M.; Boinovich, L.B. Formation and electrochemical properties of the superhydrophobic nanocomposite coating on PEO pretreated Mg–Mn–Ce magnesium alloy. Surf. Coat. Technol. 2013, 232, 240–246. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, B.; Qu, M.; Zhang, J.; He, D. Fabrication of superhydrophobic surfaces on engineering material surfaces with stearic acid. Appl. Surf. Sci. 2008, 254, 2009–2012. [Google Scholar] [CrossRef]
- Wan, P.; Wu, J.; Tan, L.; Zhang, B.; Yang, K. Research on super-hydrophobic surface of biodegradable magnesium alloys used for vascular stents. Mater. Sci. Eng. C 2013, 33, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wang, J.; Liu, Y.; Wang, D.; Li, S.; Wang, H. Controllable superhydrophobic surfaces with tunable adhesion on Mg alloys by a simple etching method and its corrosion inhibition performance. Chem. Eng. J. 2021, 404, 126444. [Google Scholar] [CrossRef]
- Ou, J.; Hu, W.; Xue, M.; Wang, F.; Li, W. Superhydrophobic surfaces on light alloy substrates fabricated by a versatile process and their corrosion protection. ACS Appl. Mater. Interfaces 2013, 5, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Zeng, R.-C.; Yan, W.; Lin, C.-G.; Wang, L.; Wang, Z.-L.; Chen, D.-C. Corrosion resistance of one-step superhydrophobic polypropylene coating on magnesium hydroxide-pretreated magnesium alloy AZ31. J. Alloy. Compd. 2020, 821, 153515. [Google Scholar] [CrossRef]
- Wang, Z.; Su, Y.; Li, Q.; Liu, Y.; She, Z.; Chen, F.; Li, L.; Zhang, X.; Zhang, P. Researching a highly anti-corrosion superhydrophobic film fabricated on AZ91D magnesium alloy and its anti-bacteria adhesion effect. Mater. Charact. 2015, 99, 200–209. [Google Scholar] [CrossRef]
- Zhou, M.; Pang, X.; Wei, L.; Gao, K. Insitu grown superhydrophobic Zn–Al layered double hydroxides films on magnesium alloy to improve corrosion properties. Appl. Surf. Sci. 2015, 337, 172–177. [Google Scholar] [CrossRef]
- Chu, J.H.; Sun, G.X.; Tong, L.B.; Jiang, Z.H. Facile one-step hydrothermal fabrication of Allium giganteum-like superhydrophobic coating on Mg alloy with self-cleaning and anti-corrosion properties. Colloids Surf. A 2021, 617, 126370. [Google Scholar] [CrossRef]
- Yin, Z.-Z.; Zhang, Z.-Q.; Tian, X.-J.; Wang, Z.-L.; Zeng, R.-C. Corrosion Resistance and Durability of Superhydrophobic Coating on AZ31 Mg Alloy via One-Step Electrodeposition. Acta Metall. Sin. (Engl. Lett.) 2020, 34, 25–38. [Google Scholar] [CrossRef]
- She, Z.; Li, Q.; Wang, Z.; Tan, C.; Zhou, J.; Li, L. Highly anticorrosion, self-cleaning superhydrophobic Ni–Co surface fabricated on AZ91D magnesium alloy. Surf. Coat. Technol. 2014, 251, 7–14. [Google Scholar] [CrossRef]
- Liu, J.; Fang, X.; Zhu, C.; Xing, X.; Cui, G.; Li, Z. Fabrication of superhydrophobic coatings for corrosion protection by electrodeposition: A comprehensive review. Colloids Surf. A 2020, 607, 125498. [Google Scholar] [CrossRef]
- Li, B.; Miller, R.H.B.; Zhang, H.; Ouyang, Y.; Qiu, R.; Hu, S.; Niu, H. A facile one-step route to fabricate bio-inspired superhydrophobic matrix with high corrosion inhibition to Cu metal. Surf. Interfaces 2021, 25, 101189. [Google Scholar] [CrossRef]
- Li, B.; Ouyang, Y.; Haider, Z.; Zhu, Y.; Qiu, R.; Hu, S.; Niu, H.; Zhang, Y.; Chen, M. One-step electrochemical deposition leading to superhydrophobic matrix for inhibiting abiotic and microbiologically influenced corrosion of Cu in seawater environment. Colloids Surf. A 2021, 616, 126337. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Yu, S.; Han, Z.; Ren, L. A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Wang, C.; Zhang, M.; Liu, P.; Wang, J. Fabrication of ZnO/epoxy resin superhydrophobic coating on AZ31 magnesium alloy. Chem. Eng. J. 2019, 368, 261–272. [Google Scholar] [CrossRef]
- Liu, L.; Lei, J.; Li, L.; Zhang, J.; Shang, B.; He, J.; Li, N.; Pan, F. Robust Rare-Earth-Containing Superhydrophobic Coatings for Strong Protection of Magnesium and Aluminum Alloys. Adv. Mater. Interfaces 2018, 5, 1800213. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Chen, J.; Liu, L.; Lei, J.; Li, N.; Liu, G.; Pan, F. One-step spraying method to construct superhydrophobic magnesium surface with extraordinary robustness and multi-functions. J. Magnes. Alloy. 2021, 9, 668–675. [Google Scholar] [CrossRef]
- Teisala, H.; Geyer, F.; Haapanen, J.; Juuti, P.; Makela, J.M.; Vollmer, D.; Butt, H.J. Ultrafast Processing of Hierarchical Nanotexture for a Transparent Superamphiphobic Coating with Extremely Low Roll-Off Angle and High Impalement Pressure. Adv. Mater. 2018, 30, e1706529. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Hu, J.; Lin, X.; Fang, L.; Wu, F.; Xie, J.; Meng, F. A robust superhydrophobic PPS-PTFE/SiO2 composite coating on AZ31 Mg alloy with excellent wear and corrosion resistance properties. J. Alloy. Compd. 2017, 721, 157–163. [Google Scholar] [CrossRef]
- Rojaee, R.; Fathi, M.; Raeissi, K. Controlling the degradation rate of AZ91 magnesium alloy via sol-gel derived nanostructured hydroxyapatite coating. Mater. Sci. Eng. C 2013, 33, 3817–3825. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Xie, Y.; Liu, L.; Yang, H.; Zhu, R.; Gong, J.; Peng, L.; Ding, W. Preparation of superhydrophobic silica film on Mg–Nd–Zn–Zr magnesium alloy with enhanced corrosion resistance by combining micro-arc oxidation and sol–gel method. Surf. Coat. Technol. 2012, 213, 192–201. [Google Scholar] [CrossRef]
- Jifang, S. Progress in research on sol-gel technology. Inorg. Chem. Ind. 2005, 37, 14–17. [Google Scholar]
- Liang, M.; Wei, Y.; Hou, L.; Wang, H.; Li, Y.; Guo, C. Fabrication of a super-hydrophobic surface on a magnesium alloy by a simple method. J. Alloy. Compd. 2016, 656, 311–317. [Google Scholar] [CrossRef]
- Wang, G.; Song, D.; Qiao, Y.; Cheng, J.; Liu, H.; Jiang, J.; Ma, A.; Ma, X. Developing super-hydrophobic and corrosion-resistant coating on magnesium-lithium alloy via one-step hydrothermal processing. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Cao, D.-L.; Xu, L.-K.; Tang, J.-K.; Meng, R.-Q.; Li, H.-D. Corrosion resistance of a superhydrophobic dodecyltrimethoxysilane coating on magnesium alloy AZ31 fabricated by one-step electrodeposition. New J. Chem. 2021, 45, 14665–14676. [Google Scholar] [CrossRef]
- Yin, X.; Mu, P.; Wang, Q.; Li, J. Superhydrophobic ZIF-8-Based Dual-Layer Coating for Enhanced Corrosion Protection of Mg Alloy. ACS Appl. Mater. Interfaces 2020, 12, 35453–35463. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yin, X.; Xue, S.; Mu, P.; Li, J. Facile fabrication of graphene oxide and MOF-based superhydrophobic dual-layer coatings for enhanced corrosion protection on magnesium alloy. Appl. Surf. Sci. 2022, 580, 152305. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Chulkova, E.V.; Semiletov, A.M.; Domantovsky, A.G.; Palacheva, V.V.; Emelyanenko, A.M.; Boinovich, L.B. The Potential of the Superhydrophobic State to Protect Magnesium Alloy against Corrosion. Coatings 2022, 12, 74. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, F.; Liu, X. Protection of magnesium alloys: From physical barrier coating to smart self-healing coating. J. Alloy. Compd. 2021, 853, 157010. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Yu, Q.; Qi, Y.; Peng, Z.; Liang, J. Dual self-healing composite coating on magnesium alloys for corrosion protection. Chem. Eng. J. 2021, 424, 130551. [Google Scholar] [CrossRef]
- García, S.J.; Fischer, H.R.; van der Zwaag, S. A critical appraisal of the potential of self healing polymeric coatings. Prog. Org. Coat. 2011, 72, 211–221. [Google Scholar] [CrossRef]
- Junaid Anjum, M. A Review on Self-Healing Coatings Applied to Mg Alloys and Their Electrochemical Evaluation Techniques. Int. J. Electrochem. Sci. 2020, 3040–3053. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
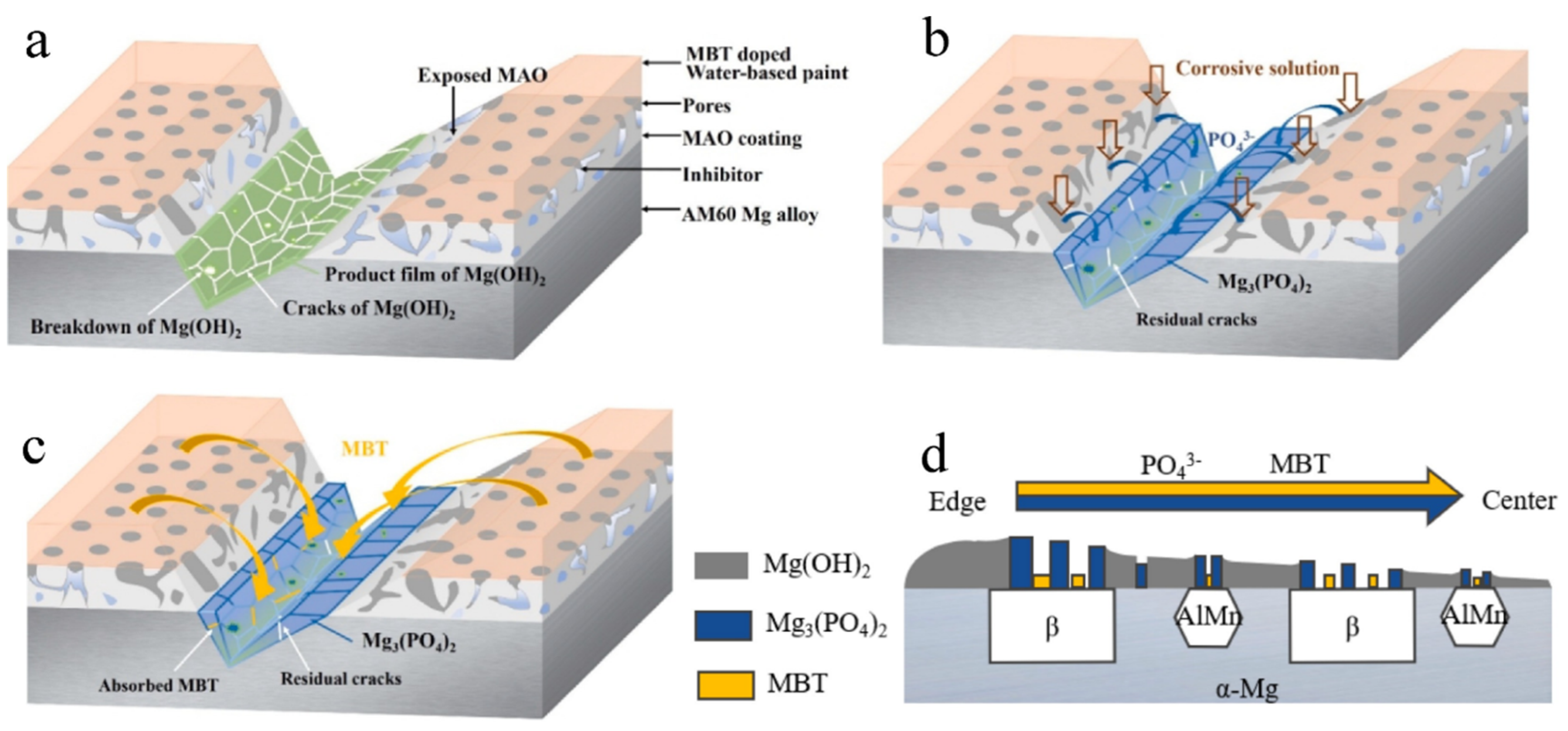
- Liu, D.; Han, E.H.; Song, Y.; Shan, D. Enhancing the self-healing property by adding the synergetic corrosion inhibitors of Na3PO4 and 2-mercaptobenzothiazole into the coating of Mg alloy. Electrochim. Acta 2019, 323, 134796. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef] [PubMed]
- Heakal, F.E.-T.; Shehata, O.S.; Tantawy, N.S. Enhanced corrosion resistance of magnesium alloy AM60 by cerium(III) in chloride solution. Corros. Sci. 2012, 56, 86–95. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Song, Y.; Liu, D.; Tang, W.; Dong, K.; Shan, D.; Han, E.H. Comparison of the corrosion behavior of AM60 Mg alloy with and without self-healing coating in atmospheric environment. J. Magnes. Alloy. 2021, 9, 1220–1232. [Google Scholar] [CrossRef]
- Dong, Q.; Zhou, X.; Feng, Y.; Qian, K.; Liu, H.; Lu, M.; Chu, C.; Xue, F.; Bai, J. Insights into self-healing behavior and mechanism of dicalcium phosphate dihydrate coating on biomedical Mg. Bioact. Mater. 2021, 6, 158–168. [Google Scholar] [CrossRef]
- Rudd, A.L.; Breslin, C.B.; Mansfeld, F. The corrosion protection aorded by rare earth conversion coatings applied to magnesium. Corros. Sci. 2000, 42, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Guo, R.; Jiang, S. Evaluation of self-healing ability of Ce–V conversion coating on AZ31 magnesium alloy. J. Magnes. Alloy. 2016, 4, 230–241. [Google Scholar] [CrossRef] [Green Version]
- Xun, S.X.; Junwei, S.; Qun, X.; Dan, C. Fabrication of Ca-P Coating on AZ31 Magnesium by Electrochemical-Assisted Chemical Conversion and Evaluation of Its Corrosion Resistance. Mater. Prot. 2018, 51, 18–24. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Localized corrosion of the Mg alloys with inhibitor-containing coatings: SVET and SIET studies. Corros. Sci. 2016, 102, 269–278. [Google Scholar] [CrossRef]
- Adsul, S.H.; Soma Raju, K.R.C.; Sarada, B.V.; Sonawane, S.H.; Subasri, R. Evaluation of self-healing properties of inhibitor loaded nanoclay-based anticorrosive coatings on magnesium alloy AZ91D. J. Magnes. Alloy. 2018, 6, 299–308. [Google Scholar] [CrossRef]
- Xiong, P.; Jia, Z.; Zhou, W.; Yan, J.; Wang, P.; Yuan, W.; Li, Y.; Cheng, Y.; Guan, Z.; Zheng, Y. Osteogenic and pH stimuli-responsive self-healing coating on biomedical Mg-1Ca alloy. Acta Biomater. 2019, 92, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, J.; Yuan, W.; Liu, X.; Li, Z.; Zheng, Y.; Liang, Y.; Zhu, S.; Cui, Z.; Yang, X.; et al. Near-infrared light controlled fast self-healing protective coating on magnesium alloy. Corros. Sci. 2020, 163, 108257. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Gao, R.; Wang, J.; Yang, W.; Liu, L. One-step method for the fabrication of superhydrophobic surface on magnesium alloy and its corrosion protection, antifouling performance. Corros. Sci. 2014, 80, 177–183. [Google Scholar] [CrossRef]
- Yang, N.; Li, Q.; Chen, F.; Cai, P.; Tan, C.; Xi, Z. A solving-reprecipitation theory for self-healing functionality of stannate coating with a high environmental stability. Electrochim. Acta 2015, 174, 1192–1201. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Tong, Y.; Yan, W.; Tu, J. A Smart Superhydrophobic Coating on AZ31B Magnesium Alloy with Self-Healing Effect. Adv. Mater. Interfaces 2016, 3, 1500694. [Google Scholar] [CrossRef]
- Xue, C.-H.; Ma, J.-Z. Long-lived superhydrophobic surfaces. J. Mater. Chem. A 2013, 1, 4146. [Google Scholar] [CrossRef]
- Esteves, A.C.C.; Luo, Y.; van de Put, M.W.P.; Carcouët, C.C.M.; de With, G. Self-Replenishing Dual Structured Superhydrophobic Coatings Prepared by Drop-Casting of an All-In-One Dispersion. Adv. Funct. Mater. 2014, 24, 986–992. [Google Scholar] [CrossRef]
- Jiang, D.; Xia, X.; Hou, J.; Zhang, X.; Dong, Z. Enhanced Corrosion Barrier of Microarc-Oxidized Mg Alloy by Self-Healing Superhydrophobic Silica Coating. Ind. Eng. Chem. Res. 2018, 58, 165–178. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Zhang, T.C.; Ouyang, L.; Zhang, Y.-X.; Yuan, S. Superhydrophobic and self-healing dual-function coatings based on mercaptabenzimidazole inhibitor-loaded magnesium silicate nanotubes for corrosion protection of AZ31B magnesium alloys. Chem. Eng. J. 2021, 404, 127106. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, B.; Wu, J.; Li, R.; Wang, P. Hydrophobic Light-to-Heat Conversion Membranes with Self-Healing Ability for Interfacial Solar Heating. Adv. Mater. 2015, 27, 4889–4894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H. Mechanically durable superhydrophobic surfaces. Adv. Mater. 2011, 23, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Kobina Sam, E.; Kobina Sam, D.; Lv, X.; Liu, B.; Xiao, X.; Gong, S.; Yu, W.; Chen, J.; Liu, J. Recent development in the fabrication of self-healing superhydrophobic surfaces. Chem. Eng. J. 2019, 373, 531–546. [Google Scholar] [CrossRef]





| References | Substrates | Synthesis Method | Wettability | Comments |
|---|---|---|---|---|
| [42] | AZ31B | Micro-arc oxidation | 152° | Stearic acid-modified MAO surface provided the protracted corrosion guard of the AZ31 Mg alloy. |
| [70] | Mg-3.0Nd-0.2Zn-0.4Zr | Micro-arc oxidation | 151° | The superhydrophobic film was successfully prepared by combining the MAO and the sol-gel method, which could effectively diminish the direct contact area between the sample and the aggressive medium. |
| [72] | AZ31 | Chemical etching | 154° | A simple method, involving etching with CuCl2, was used to fabricate a superhydrophobic surface on Mg alloys. |
| [52] | AZ31 | Chemical etching | 159° | Through a simple chemical etching (HCl aqueous solution) and surface modification (stearic acid-ethanol solution), a superhydrophobic coating with tunable water adhesion was prepared on the AZ31 alloy. |
| [33] | AZ91D | Hydrothermal synthesis | 154° | A lotus seedpod bioinspired superhydrophobic surface was developed on the Mg alloy via an in situ hydrothermal synthesis technique, which rendered effective corrosion protection for the Mg substrate. |
| [73] | Mg-9Li | Hydrothermal synthesis | 152° | One-step hydrothermal processing to develop the super-hydrophobic and corrosion-resistant coating on Mg-9Li alloy, using a mixed solution of stearic acid-ethanol-distilled. |
| [57] | Mg-2.5Y-1Ce-0.5 Mn | Hydrothermal synthesis | 164° | Superhydrophobic coating of the main composition of CeO2 and Ce(CH3(CH2)16COO)3 with an allium giganteum-like structure shows excellent performance of anti-corrosion. |
| [74] | AZ31 | Electrode-position | 158° | an excellent anti-corrosion superhydrophobic DTMS coating was successfully fabricated on Mg alloy AZ31 by one-step electrodeposition in a relatively neutral solution. |
| [75] | AZ31B | Electrode-position | 156° | The underlying LDH structure was prepared electrodeposition, which could form a passive-tion layer on the surface to protect the substrate as well as enhance the interface adhesion. |
| [7] | AZ31 | Electrode-position | 156° | A superhydrophobic coating with excellent corrosion resistance was successfully prepared on the AZ31 by one-step electrodeposition. |
| [68] | AZ31 | Spraying | 152° | PPS-PTFE/SiO2 superhydrophobic coatings with the fibrous-network structure were successfully fabricated by a simple spraying method, which has superior corrosion protection ability. |
| [76] | AZ31B | Spraying | 157° | A robust MOF-organic compound superhydrophobic coating was created on the AZ31B alloy by a spraying method, which is a sustainable method of delaying metal corrosion. |
| [77] | MA8 | Sol-gel | 171° | A simple route combining laser processing with a sol-gel route was utilized for preparing a superhydrophobic coating, which could improve the anti-corrosion. |
| References | Substrate | Functional Coatings | Corrosion Media (3.5 wt.% NaCl) | icorr (A·cm−2) | Ecorr (V/SCE) | Inhibition Efficiency (η) | Immersion Time (h) | ||
|---|---|---|---|---|---|---|---|---|---|
| Substrates | Coatings | Substrates | Coatings | ||||||
| [59] ♣ | AZ91D | Ni-Co/SA | - | 1.5 × 10−5 | 9.2 × 10−9 | −1.59 | −0.172 | 0.9994 | - |
| [96] ♣ | AZ31 | Ferric myristate | - | 2.075 × 10−5 | 2.579 × 10−8 | −1.167 | −1.377 | 0.9988 | 72 |
| [42] ♣ | AZ31 | MAO/SA | - | 1.606 × 10−4 | 0.14 × 10−7 | −1.524 | −1.353 | - | 264 |
| [97] ♥ | AZ91D | Stannate/SA | - | 1.104 × 10−5 | 1.132 × 10−6 | −1.524 | −0.803 | 0.3126 | 24 |
| [56] ♣ | AZ91D | Zn–Al LDHs/SA | - | 8.8 × 10−3 | 2.6 × 10−5 | −1.51 | −1.22 | 0.9970 | - |
| [72] ♣ | AZ31 | Etching with copper (II) chloride/SA | - | - | - | −1.59 | −1.16 | - | 36 |
| [98] ♣,♥ | AZ31B | Cr (III) CCC/SA | - | 6.09 × 10−4 | 0.643 × 10−6 | −0.151 | 0.146 | 0.9989 | - |
| [7] ♣ | AZ31 | Magnesium stearate | - | 7.02 × 10−5 | 5.80 × 10−8 | −1.542 | −1.525 | 0.9992 | 168 |
| [5] ♣ | AZ31 | PAPTMS/PP | - | 4.96 × 10−5 | 9.08 × 10−8 | −1.51 | −1.38 | 0.9982 | 250 |
| [76] ♣ | AZ31B | GO/PPy/ZIF-8 | - | 7.779 × 10−4 | 1.133 × 10−6 | −1.411 | −1.509 | 0.9985 | 120 |
| [54] ♣ | AZ31 | Mg(OH)2/PP | - | 6.15 × 10−5 | 3.12 × 10−9 | −1.46 | −1.18 | 0.9999 | 250 |
| [57] ♣ | Mg-2.5Y-1Ce-0.5 Mn | CeO2@cerium stearate | - | 4.22 × 10−5 | 8.06 × 10−8 | −1.63 | −1.16 | 0.9998 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Zhang, Z.; Liu, T.; Qiu, Z.; Su, Y.; Zhang, J.; Lin, C.; Wang, L. Recent Progress in Functionalized Coatings for Corrosion Protection of Magnesium Alloys—A Review. Materials 2022, 15, 3912. https://doi.org/10.3390/ma15113912
Li B, Zhang Z, Liu T, Qiu Z, Su Y, Zhang J, Lin C, Wang L. Recent Progress in Functionalized Coatings for Corrosion Protection of Magnesium Alloys—A Review. Materials. 2022; 15(11):3912. https://doi.org/10.3390/ma15113912
Chicago/Turabian StyleLi, Bingzhi, Zhaoqi Zhang, Tengteng Liu, Zhenghui Qiu, Yan Su, Jinwei Zhang, Cunguo Lin, and Li Wang. 2022. "Recent Progress in Functionalized Coatings for Corrosion Protection of Magnesium Alloys—A Review" Materials 15, no. 11: 3912. https://doi.org/10.3390/ma15113912
APA StyleLi, B., Zhang, Z., Liu, T., Qiu, Z., Su, Y., Zhang, J., Lin, C., & Wang, L. (2022). Recent Progress in Functionalized Coatings for Corrosion Protection of Magnesium Alloys—A Review. Materials, 15(11), 3912. https://doi.org/10.3390/ma15113912






