Effect of Sulfate Ions on Galvanized Post-Tensioned Steel Corrosion in Alkaline Solutions and the Interaction with Other Ions
Abstract
1. Introduction
2. Experimental Work
2.1. Solutions
2.2. Corrosion Electrochemical Cells
2.3. Techniques
2.3.1. Electrochemical Tests
2.3.2. Electron Microscopy SEM/EDS
2.3.3. Optical Microscopy
3. Results and Discussion
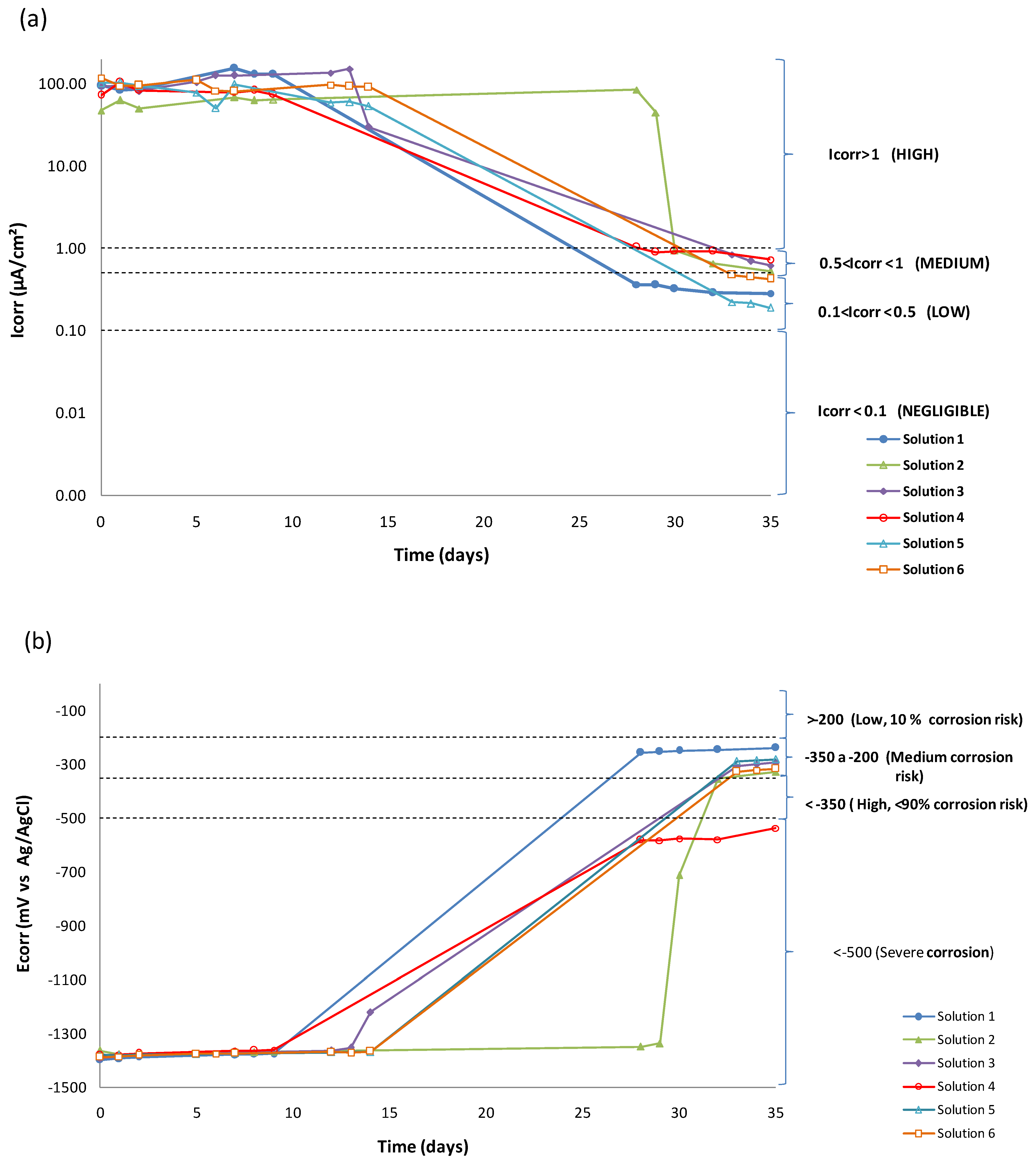
3.1. LPR Results
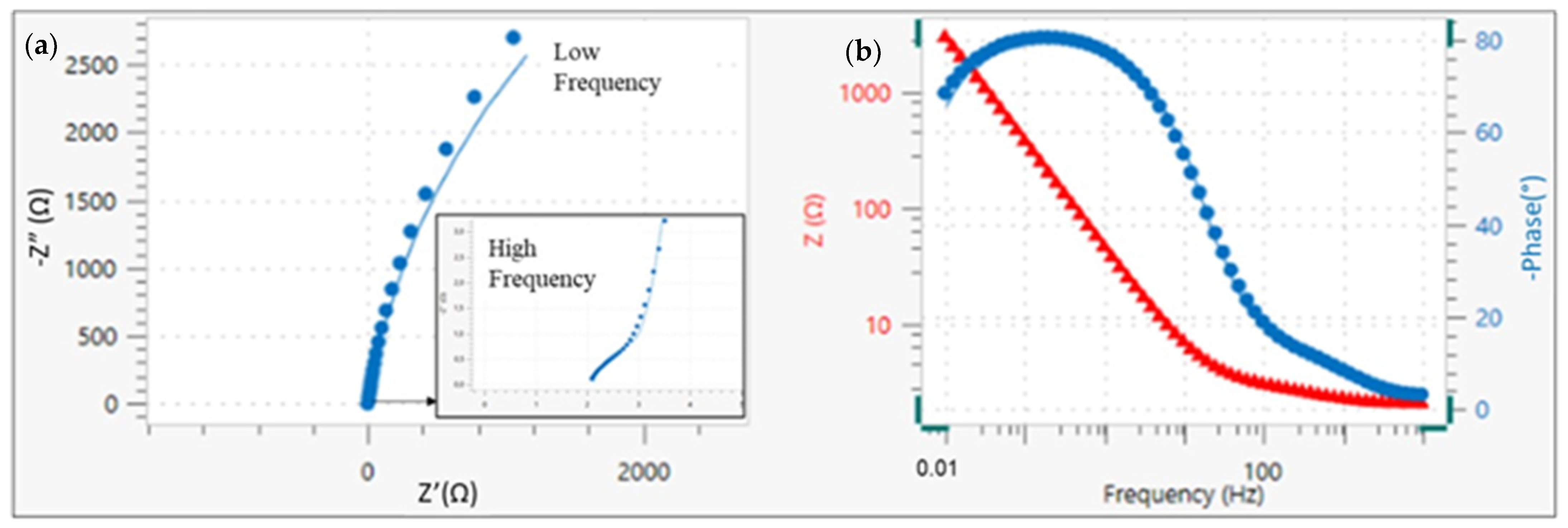
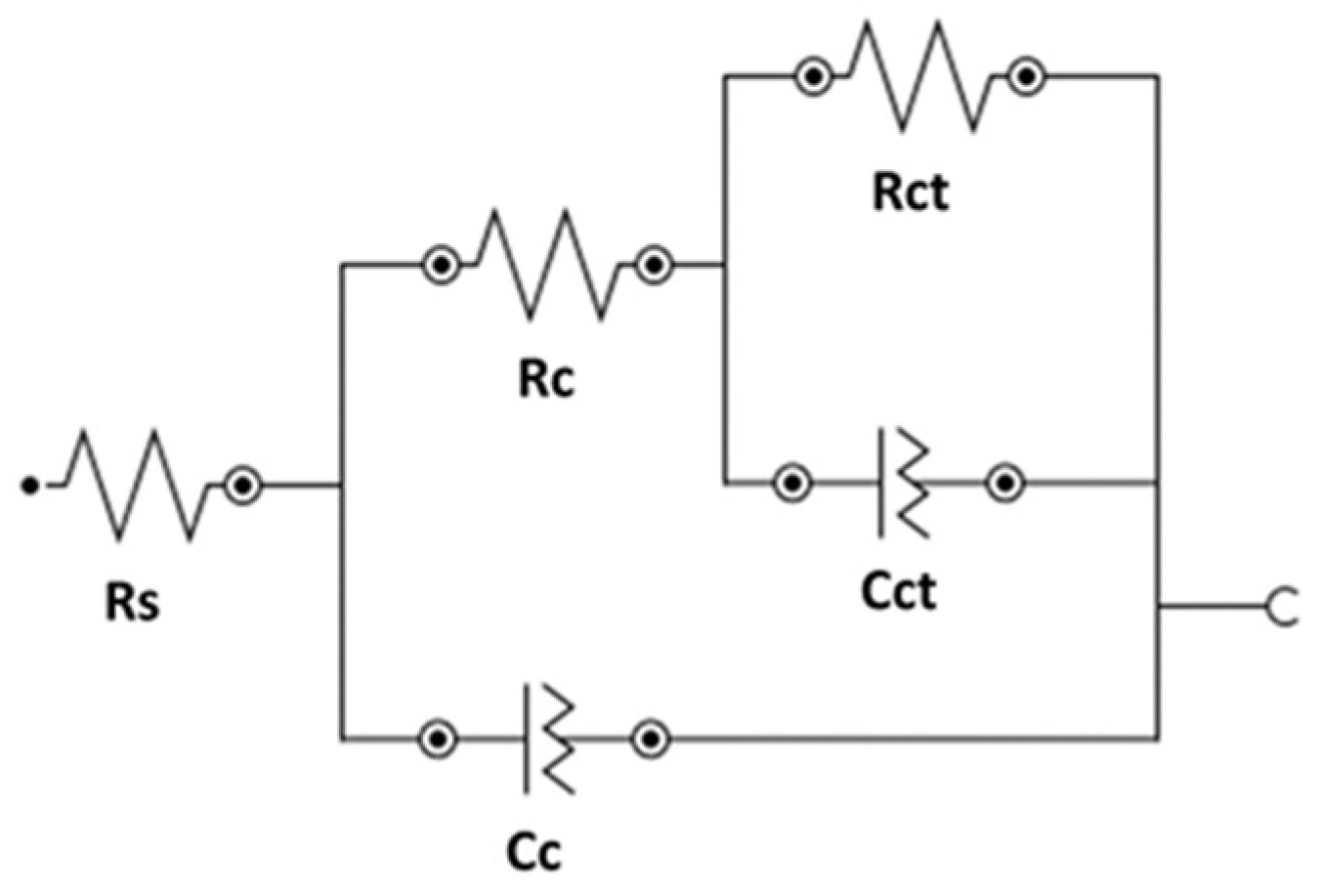
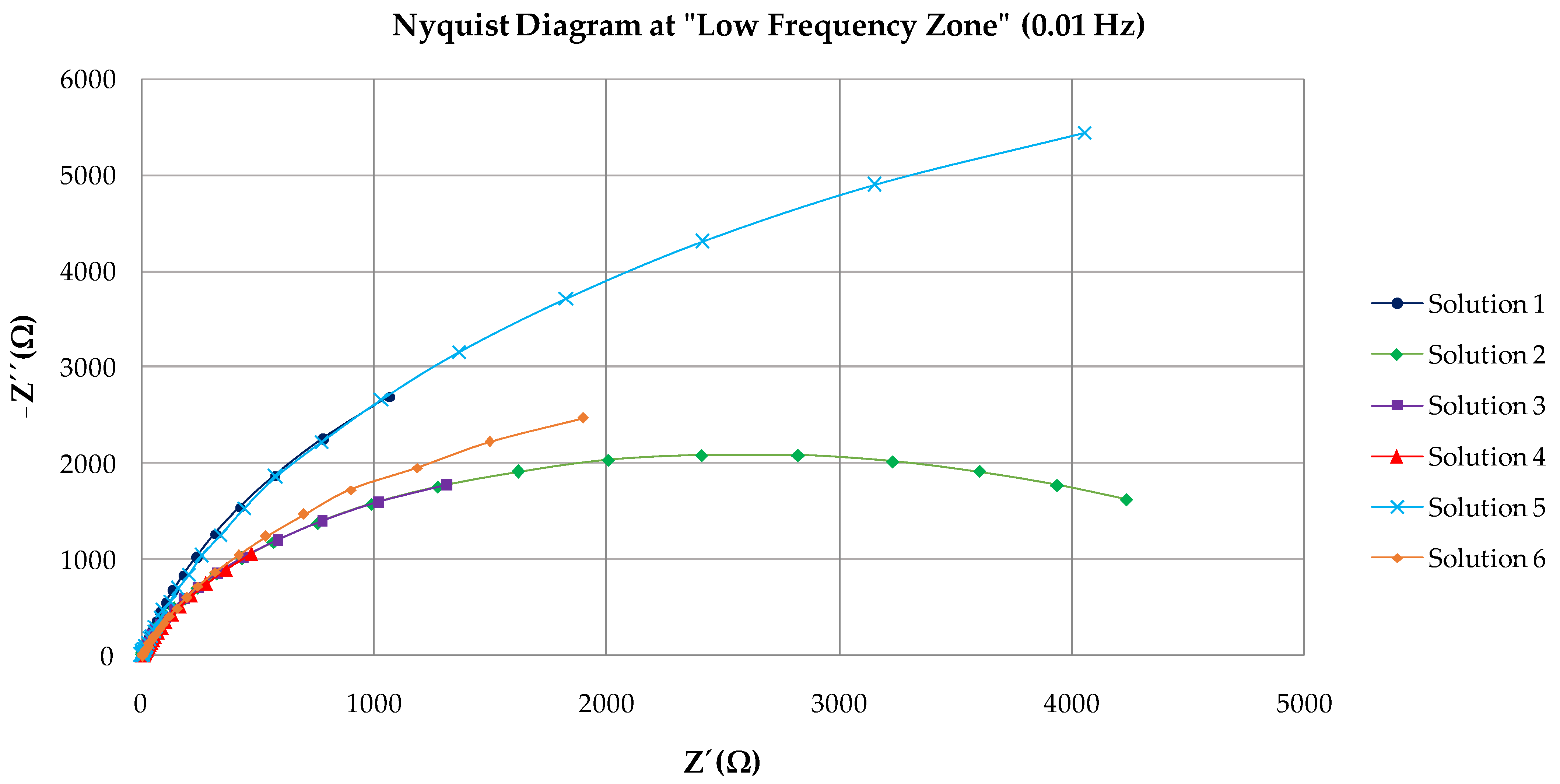
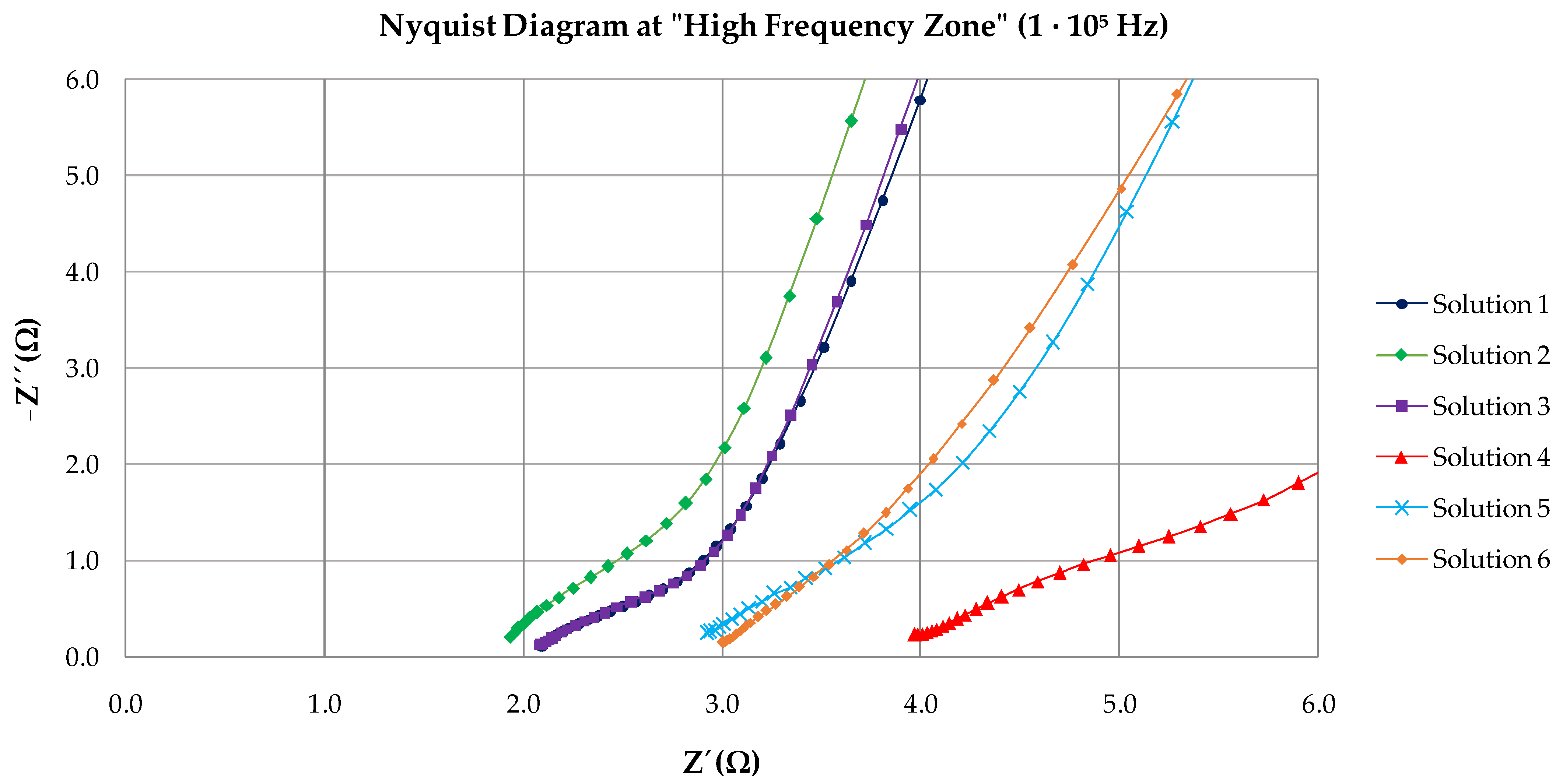
3.2. EIS Results
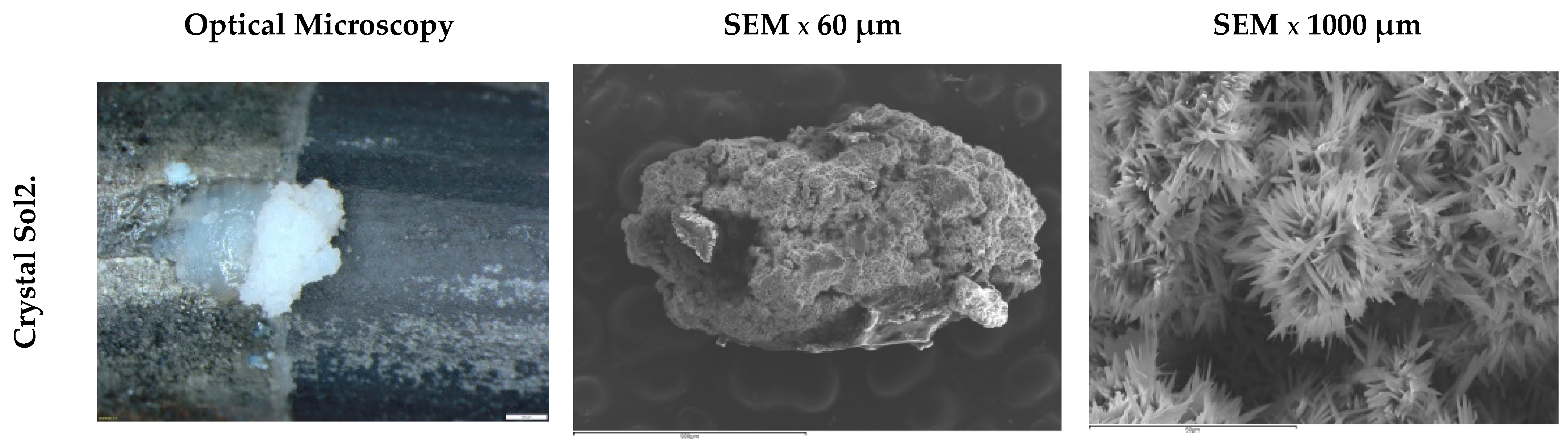
3.3. Morphology of Steel Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powers, R.W.; Breiter, M.W. The anodic dissolution and passivation of zinc in concentrated potassium hydroxide solutions. J. Electrochem. Soc. 1969, 116, 719. [Google Scholar] [CrossRef]
- Thirsk, H.R.; Armstrong, R.D.; Bell, M.F. Electrochemistry; The Royal Society of Chemistry: London, UK, 1974; Volume 4, ISBN 978-0-85186-037-4. [Google Scholar]
- Muralidharan, V.S.; Rajagopalan, K.S. Kinetics and mechanism of passivation of zinc in dilute sodium hydroxide solutions. Br. Corros. J. 1979, 14, 231–234. [Google Scholar] [CrossRef]
- Riecke, E. Investigations on the influence of zinc on the corrosion behavior of high strength steels. Mater. Corros. 1979, 30, 619–631. [Google Scholar] [CrossRef]
- Parsons, R. Atlas of electrochemical equilibria in aqueous solutions. J. Electroanal. Chem. Interfacial Electrochem. 1967, 13, 471. [Google Scholar] [CrossRef]
- Recio, F.; Alonso, C.; Gaillet, L.; Sánchez Moreno, M. Hydrogen embrittlement risk of high strength galvanized steel in contact with alkaline media. Corros. Sci. 2011, 53, 2853–2860. [Google Scholar] [CrossRef]
- Macías, A.; Andrade, C. Corrosion rate of galvanized steel immersed in saturated solutions of Ca (OH)2 in the PH range 12–13.8. Br. Corros. J. 1983, 18, 82–87. [Google Scholar] [CrossRef]
- Blanco, M.T.; Andrade, C.; Macías, A. Estudio por SEM de los productos de corrosión de armaduras galvanizadas sumergidas en disoluciones de PH comprendido entre 12,6 y 13,6. Mater. Constr. 1984, 34, 55–66. [Google Scholar] [CrossRef][Green Version]
- Macías, A.; Andrade, C. Corrosion of galvanized steel in dilute Ca (OH)2 solutions (PH 11.1–12.6). Br. Corros. J. 1987, 22, 162–171. [Google Scholar] [CrossRef]
- Acha Hurtado, M. Corrosión Bajo Tensión de Alambres de Acero Pretensado en Medios Neutros con HCO3− y alcalinos con SO42−. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, October 1993; p. 321. [Google Scholar]
- Liu, G.; Zhang, Y.; Ni, Z.; Huang, R. Corrosion behavior of steel submitted to chloride and sulphate ions in simulated concrete pore solution. Constr. Build. Mater. 2016, 115, 1–5. [Google Scholar] [CrossRef]
- Bertolini, L.; Carsana, M.; High, P.H. Corrosion of Prestressing Steel in Segregated Grout. In Proceedings of the Modelling of Corroding Concrete Structures, Madrid, Spain, 22–23 November 2010; Andrade, C., Mancini, G., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 147–158. [Google Scholar]
- Roventi, G.; Bellezze, T.; Barbaresi, E.; Fratesi, R. Effect of carbonation process on the passivating products of zinc in Ca(OH)2 Saturated Solution. Mater. Corros. 2013, 64, 1007–1014. [Google Scholar] [CrossRef]
- Farina, S.B.; Duffó, G.S. Corrosion of zinc in simulated carbonated concrete pore solutions. Electrochim. Acta 2007, 52, 5131–5139. [Google Scholar] [CrossRef]
- Zhu, F.; Persson, D.; Thierry, D.; Taxén, C. Formation of corrosion products on open and confined zinc surfaces exposed to periodic wet/dry conditions. Corrosion 2000, 56, 1256–1265. [Google Scholar] [CrossRef]
- Ramirez, E.; González, J.A.; Bautista, A. The protective efficiency of galvanizing against corrosion of steel in mortar and in Ca(OH)2 saturated solutions containing chlorides. Cem. Concr. Res. 1996, 26, 1525–1536. [Google Scholar] [CrossRef]
- Yadav, A.; Nishikata, A.; Tsuru, T. Degradation mechanism of galvanized steel in wet–dry cyclic environment containing chloride ions. Corros. Sci. 2004, 46, 361–376. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Sun, L.; Fan, H. Effects of PH and Cl− concentration on corrosion behavior of the galvanized steel in simulated rust layer solution. Corros. Sci. 2012, 65, 520–527. [Google Scholar] [CrossRef]
- Persson, D.; Thierry, D.; Karlsson, O. Corrosion and corrosion products of hot dipped galvanized steel during long term atmospheric exposure at different sites world-wide. Corros. Sci. 2017, 126, 152–165. [Google Scholar] [CrossRef]
- Xu, P.; Jiang, L.; Guo, M.-Z.; Zha, J.; Chen, L.; Chen, C.; Xu, N. Influence of sulfate salt type on passive film of steel in simulated concrete pore solution. Constr. Build. Mater. 2019, 223, 352–359. [Google Scholar] [CrossRef]
- Neupane, S.; Hastuty, S.; Yadav, N.; Singh, N.; Gupta, D.K.; Yadav, B.; Singh, S.; Karki, N.; Kumari Das, A.; Subedi, V.; et al. Effects of NH4+, Na+, and Mg2+ ions on the corrosion behavior of galvanized steel in wet–dry cyclic conditions. Mater. Corros. 2021, 72, 1388–1395. [Google Scholar] [CrossRef]
- Metálicos, A. UNE 112072; Determinación de la Velocidad de Corrosión de Armaduras en Laboratorio Mediante Medida de la Resistencia a la Polarización; Asociación Española de Normalización: Madrid, Spain, 2011. [Google Scholar]
- Jones, D.A. Principles and Prevention of Corrosion; Prentice-Hall, Inc.: Hoboken, NJ, USA, 1996; ISBN -0-13-359993-0. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical Polarization. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Corrosion rate monitoring in the laboratory and on-site. Constr. Build. Mater. 1996, 10, 315–328. [Google Scholar] [CrossRef]
- Andrade, C.; Castelo, V.; Alonso, C.; González, J.A. The determination of the corrosion rate of steel embedded in concrete by the polarization resistance and AC impedance methods. Corros. Eff. Stray Curr. Tech. Eval. Corros. Rebars Concr. 1986. [Google Scholar] [CrossRef]
- González, J.A.; Albéniz, J.; Feliu, S. Valores de la constante B del método de resistencia de polarización para veinte sistemas metal-medio diferentes. Rev. Metal. 1996, 32, 10–17. [Google Scholar] [CrossRef][Green Version]
- Hladky, K.; Callow, L.M.; Dawson, J.L. Corrosion rates from impedance measurements: An introduction. Br. Corros. J. 1980, 15, 20–25. [Google Scholar] [CrossRef]
- Bonk, S.; Wicinski, M.; Hassel, A.W.; Stratmann, M. Electrochemical characterizations of precipitates formed on zinc in alkaline sulphate solution with increasing PH values. Electrochem. Commun. 2004, 6, 800–804. [Google Scholar] [CrossRef]
- Krishna Vigneshwaran, K.K.; Permeh, S.; Echeverría, M.; Lau, K.; Lasa, I. Corrosion of post-tensioned tendons with deficient grout, part 1: Electrochemical behavior of steel in alkaline sulfate solutions. Corrosion 2017, 74, 362–371. [Google Scholar] [CrossRef]
- Pan, H.; Wang, L.; Lin, Y.; Ge, F.; Zhao, K.; Wang, X.; Cui, Z. Mechanistic study of ammonium-induced corrosion of AZ31 magnesium alloy in sulfate solution. J. Mater. Sci. Technol. 2020, 54, 1–13. [Google Scholar] [CrossRef]
- Hu, J.; Koleva, D.; Petrov, P.; Breugel, K. Polymeric vesicles for corrosion control in reinforced mortar: Electrochemical behavior, steel surface analysis and bulk matrix properties. Corros. Sci. 2012, 65, 414–430. [Google Scholar] [CrossRef]
- Ye, C.Q.; Hu, R.G.; Dong, S.G.; Zang, X.J.; Ho, R.Q.; Du, R.G.; Lin, C.J.; Pan, J.S. EIS analysis on chloride-induced corrosion behavior of reinforcement steel in simulated carbonated concrete pore solutions. J. Electrochem. Chem. 2013, 688, 275–288. [Google Scholar] [CrossRef]
- Garces, P.; Andrade, M.; Saez, A.; Alonso, C. Corrosion of reinforcing steel in neutral and acid solutions simulating the electrolytic environments in the micropores of concrete in the propagation period. Corros. Sci. 2005, 47, 289–306. [Google Scholar] [CrossRef]

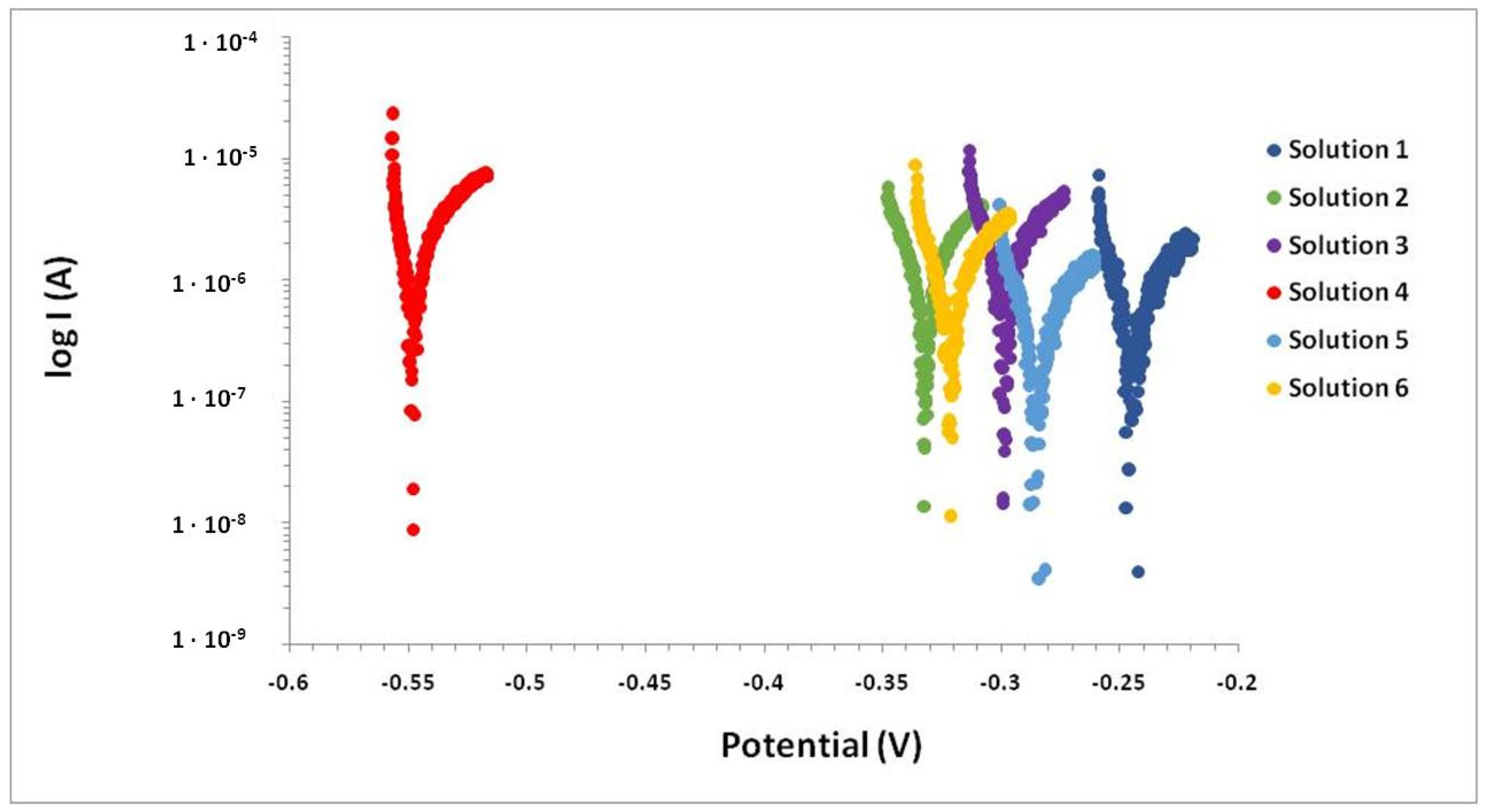


| Solution | SO42− [mol/L] 0.04 | Ca2+ [mol/L] 4 · 10−4 | NH4+ [mol/L] 5 · 10−3 | HCO3− [mol/L] 4 · 10−4 | Mg2+ [mol/L] 9.6 · 10−3 | Cl− [mol/L] 7.6 · 10−3 |
|---|---|---|---|---|---|---|
| 1 | X | |||||
| 2 | X | X | ||||
| 3 | X | X | X | |||
| 4 | X | X | X | X | ||
| 5 | X | X | X | X | X | |
| 6 | X | X | X | X | X | X |
| Day | Solution 1 | Solution 2 | Solution 3 | ||||||
| Ecorr (mV) | Rp (Ω) | Icorr (µA/cm2) | Ecorr (mV) | Rp (Ω) | Icorr (µA/cm2) | Ecorr (mV) | Rp (Ω) | Icorr (µA/cm2) | |
| 0 | −1403 | 27.33 | 97.24 | −1373 | 56.23 | 47.26 | −1396 | 28.75 | 92.45 |
| 1 | −1396 | 31.45 | 84.51 | −1386 | 42.26 | 62.89 | −1389 | 28.30 | 93.92 |
| 8 | −1379 | 20.57 | 129.20 | −1375 | 55.35 | 63.35 | −1375 | 21.17 | 125.54 |
| 28 | −261 | 7290.27 | 0.36 | −1351 | 31.50 | 84.37 | - | - | - |
| 35 | −246 | 9383.37 | 0.28 | −332 | 5039.88 | 0.53 | −300 | 4306.20 | 0.62 |
| Day | Solution 4 | Solution 5 | Solution 6 | ||||||
| Ecorr (mV) | Rp (Ω) | Icorr (µA/cm2) | Ecorr (mV) | Rp (Ω) | Icorr (µA/cm2) | Ecorr (mV) | Rp (Ω) | Icorr (µA/cm2) | |
| 0 | −1387 | 36.67 | 72.48 | −1384 | 26.07 | 101.94 | −1393 | 22.43 | 118.51 |
| 1 | −1382 | 25.64 | 103.66 | −1385 | 25.42 | 104.54 | −1389 | 28.15 | 94.40 |
| 8 | −1367 | 32.10 | 82.79 | −1379 | 26.97 | 98.54 | −1378 | 32.95 | 80.66 |
| 28 | −593 | 2595.41 | 1.02 | - | - | - | - | - | - |
| 35 | −548 | 3660.90 | 0.73 | −287 | 14,025.21 | 0.19 | −322 | 6303.55 | 0.42 |
| Solution | Rs | Rc | CPE | Rct | CPE | χ2 |
|---|---|---|---|---|---|---|
| (Ω·cm2) | (Ω·cm2) | Y0(Ω−1·cm−2·sn) | (kΩ·cm2) | Y0(Ω−1·cm−2·sn) | ||
| 1 | 2.11 | 1.55 | 1.92 · 10−3 | 10,169 | 6.36 · 10−4 | 0.036 |
| 2 | 1.84 | 2.18 | 9.20 · 10−5 | 5346 | 8.59 · 10−5 | 0.024 |
| 3 | 2.13 | 2.95 | 4.99 · 10−4 | 4777 | 3.99 · 10−4 | 0.034 |
| 4 | 3.87 | 4.48 | 3.86 ·10−4 | 4665 | 1.35 · 10−3 | 0.036 |
| 5 | 2.93 | 2.87 | 9.20 · 10−5 | 14,923 | 1.92 · 10−4 | 0.019 |
| 6 | 3.03 | 4.83 | 3.21 · 10−4 | 7240 | 2.51 · 10−4 | 0.016 |
| Solution | Rp (LPR) | Rct (EIS) | Difference (%) |
|---|---|---|---|
| (Ω) | (Ω) | ||
| 1 | 9383 | 10,169 | 8 |
| 2 | 5040 | 5346 | 6 |
| 3 | 4306 | 4777 | 10 |
| 4 | 3661 | 4665 | 24 |
| 5 | 14,025 | 14,923 | 6 |
| 6 | 6304 | 7240 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla, A.; Argiz, C.; Moragues, A.; Gálvez, J.C. Effect of Sulfate Ions on Galvanized Post-Tensioned Steel Corrosion in Alkaline Solutions and the Interaction with Other Ions. Materials 2022, 15, 3950. https://doi.org/10.3390/ma15113950
Bonilla A, Argiz C, Moragues A, Gálvez JC. Effect of Sulfate Ions on Galvanized Post-Tensioned Steel Corrosion in Alkaline Solutions and the Interaction with Other Ions. Materials. 2022; 15(11):3950. https://doi.org/10.3390/ma15113950
Chicago/Turabian StyleBonilla, Andrés, Cristina Argiz, Amparo Moragues, and Jaime C. Gálvez. 2022. "Effect of Sulfate Ions on Galvanized Post-Tensioned Steel Corrosion in Alkaline Solutions and the Interaction with Other Ions" Materials 15, no. 11: 3950. https://doi.org/10.3390/ma15113950
APA StyleBonilla, A., Argiz, C., Moragues, A., & Gálvez, J. C. (2022). Effect of Sulfate Ions on Galvanized Post-Tensioned Steel Corrosion in Alkaline Solutions and the Interaction with Other Ions. Materials, 15(11), 3950. https://doi.org/10.3390/ma15113950






