Remarkable Structural Modifications of Tialite Solid Solutions Obtained by Different Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reaction Sintering of Tialite AT and Solid-Solutions MAT
2.3. Simulations of the Structure of Tialite and MAT Solid Solutions
3. Results
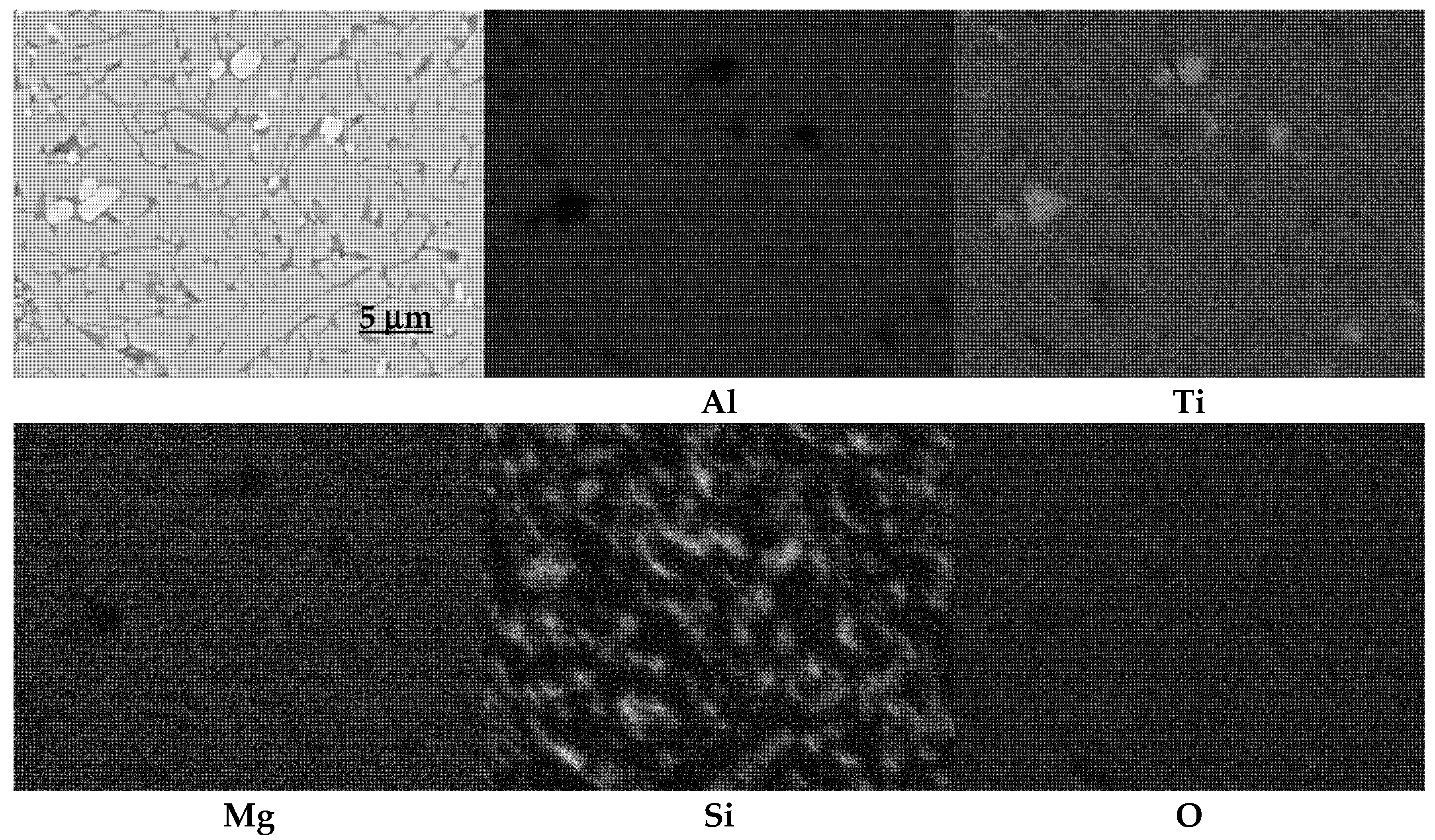
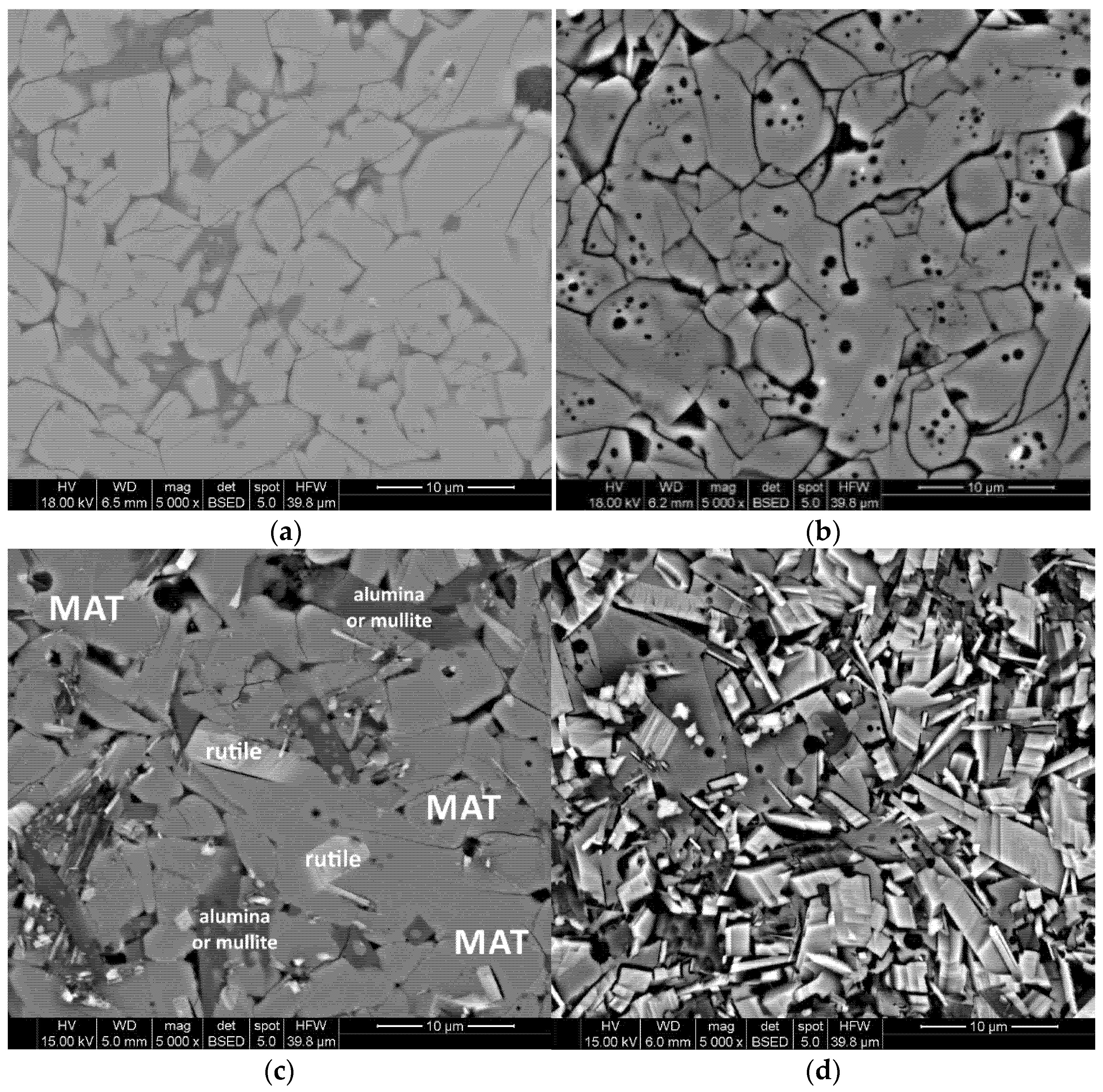
3.1. Phase Composition Analysis by X-ray Diffraction Method
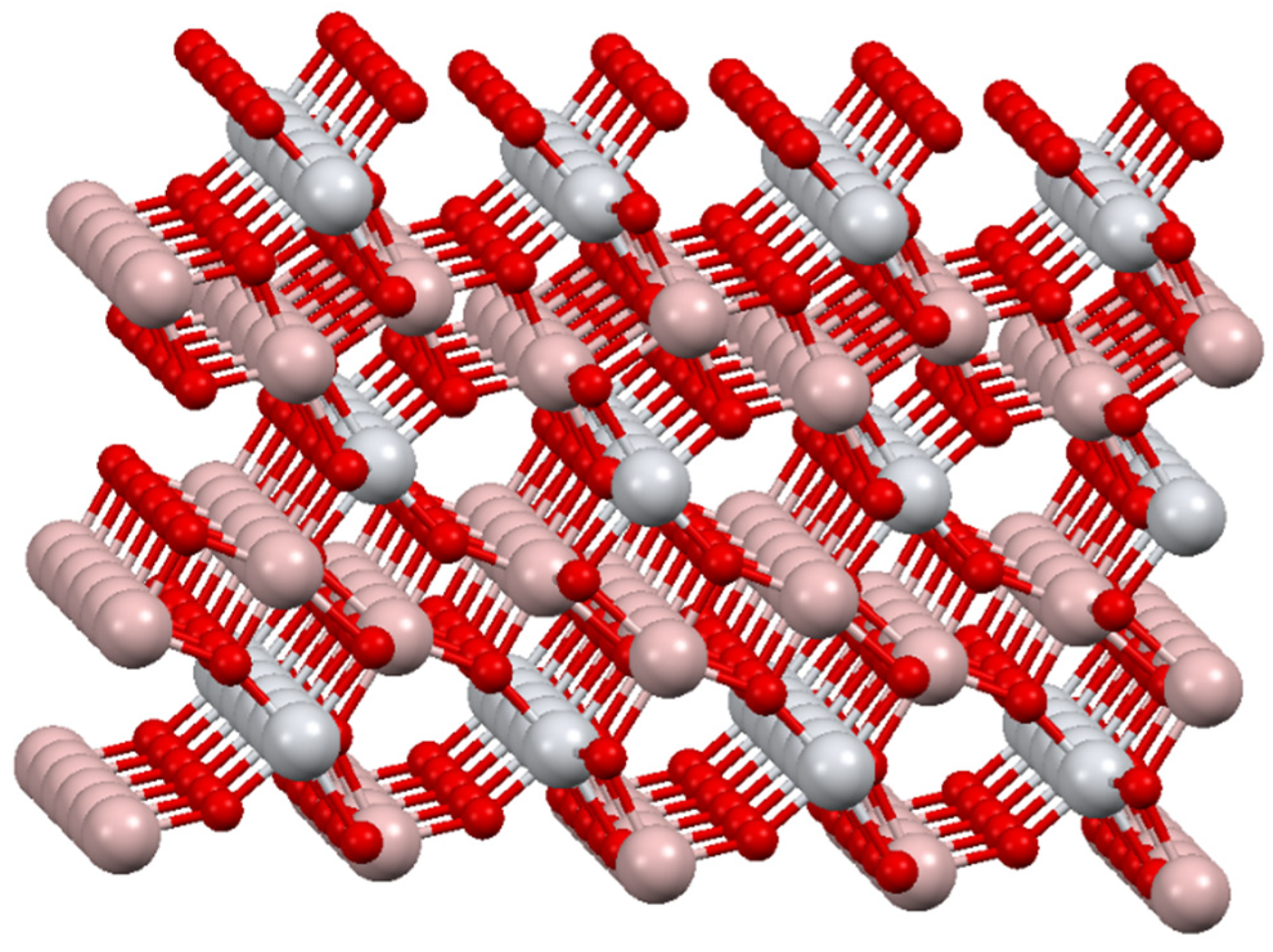
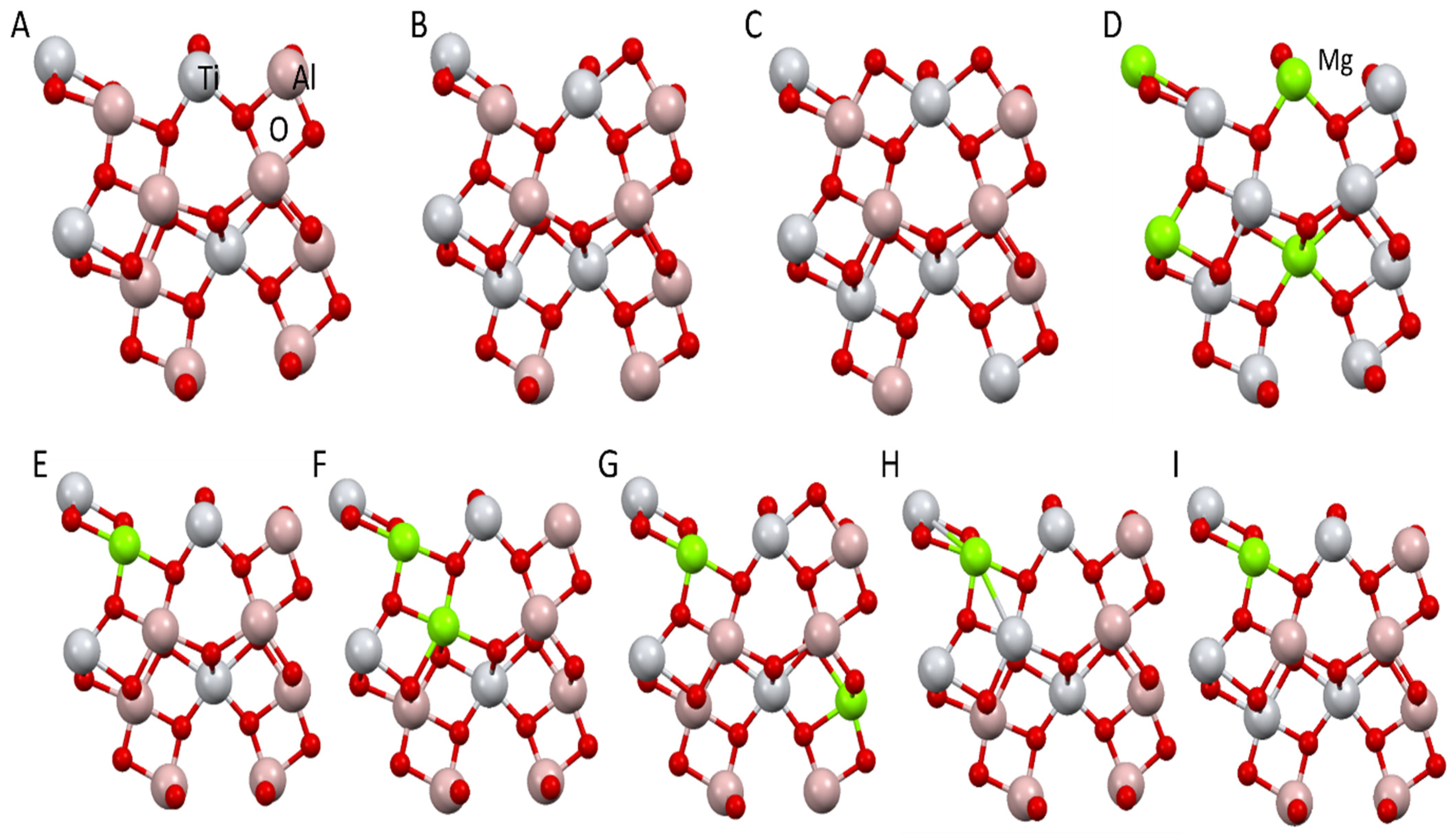
3.2. Results of Structure Simulation Studies of Tialite, Magnesium Titanate, and MAT Solid Solutions
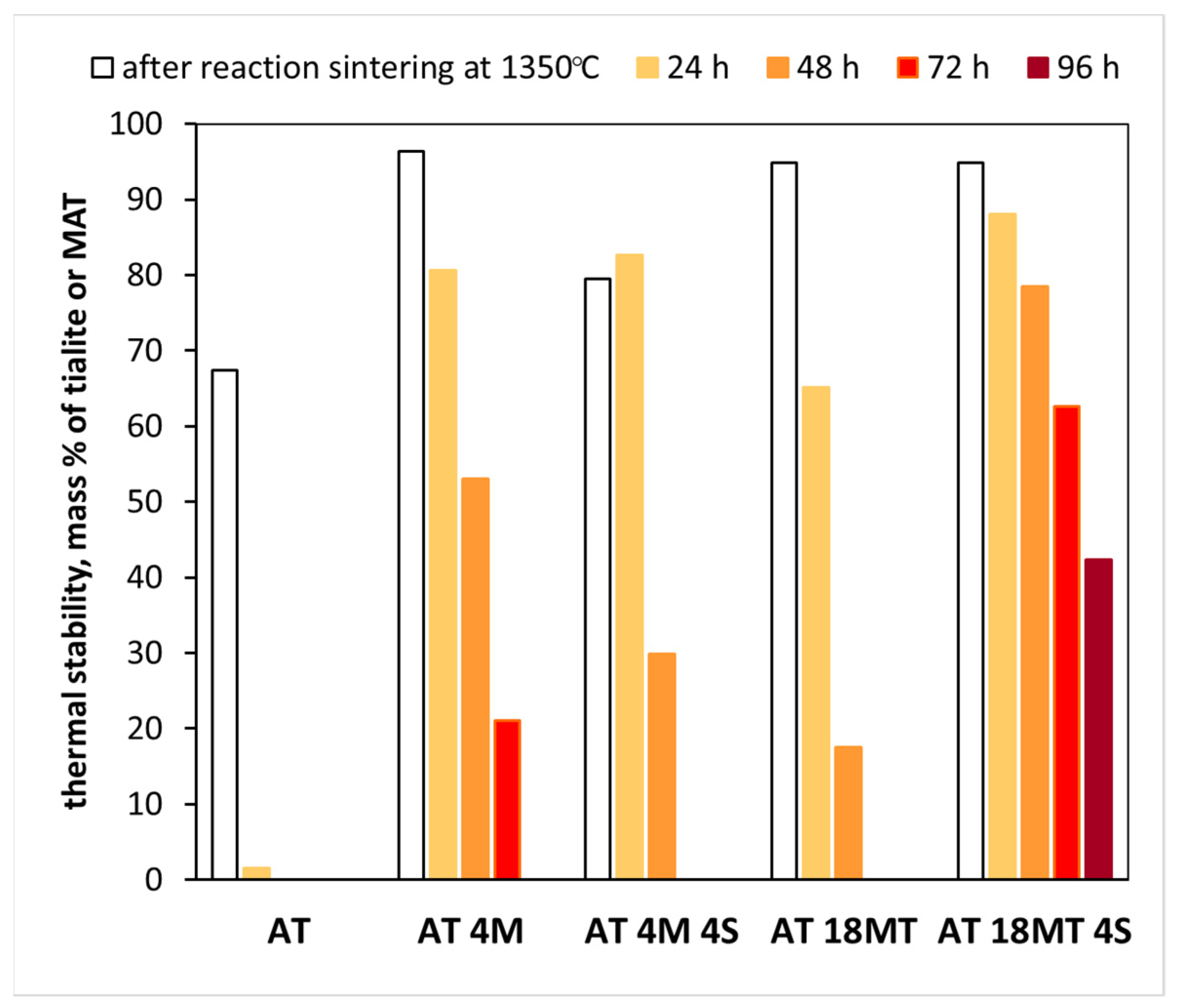
3.3. Thermal Stability
4. Discussion of Results
5. Conclusions
- (1)
- The values of the lattice constants, obtained from the theoretical calculations, confirmed the experimental data on the changes in the lattice constants under the influence of the substitution of the aluminum centers with magnesium and titanium centers. The differences between the calculated values of the lattice parameters and those determined from the X-ray images were statistically negligible, up to 0.5%.
- (2)
- The comparison of the obtained experimental data with the data from theoretical calculations indicates that the most probable mechanism of stabilization of tialite by magnesium cations comprises the simultaneous substitution of two non-adjacent and distant aluminum cations by magnesium and titanium cations (Al2TiO5 Mg1Ti1 system).
- (3)
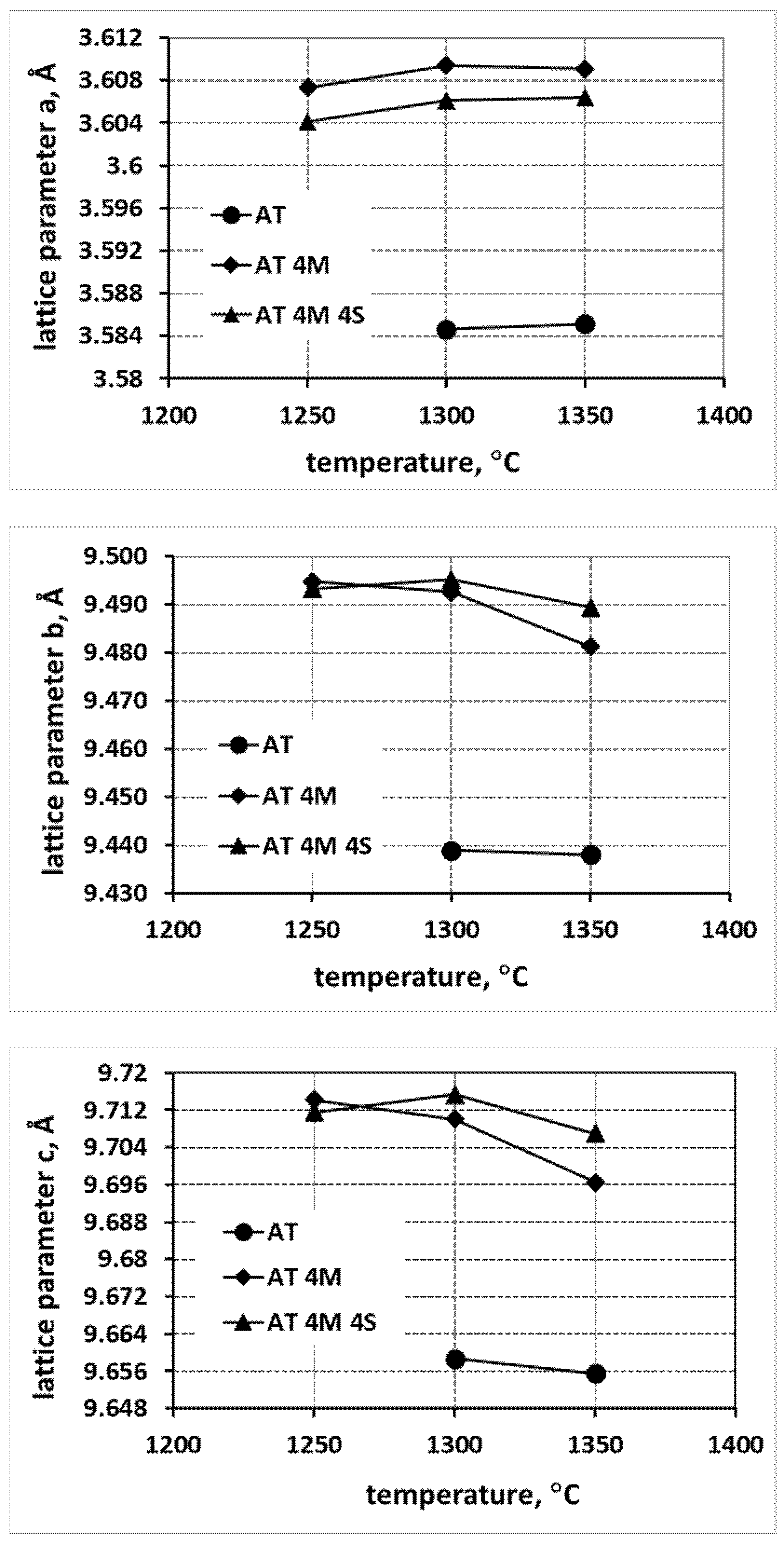
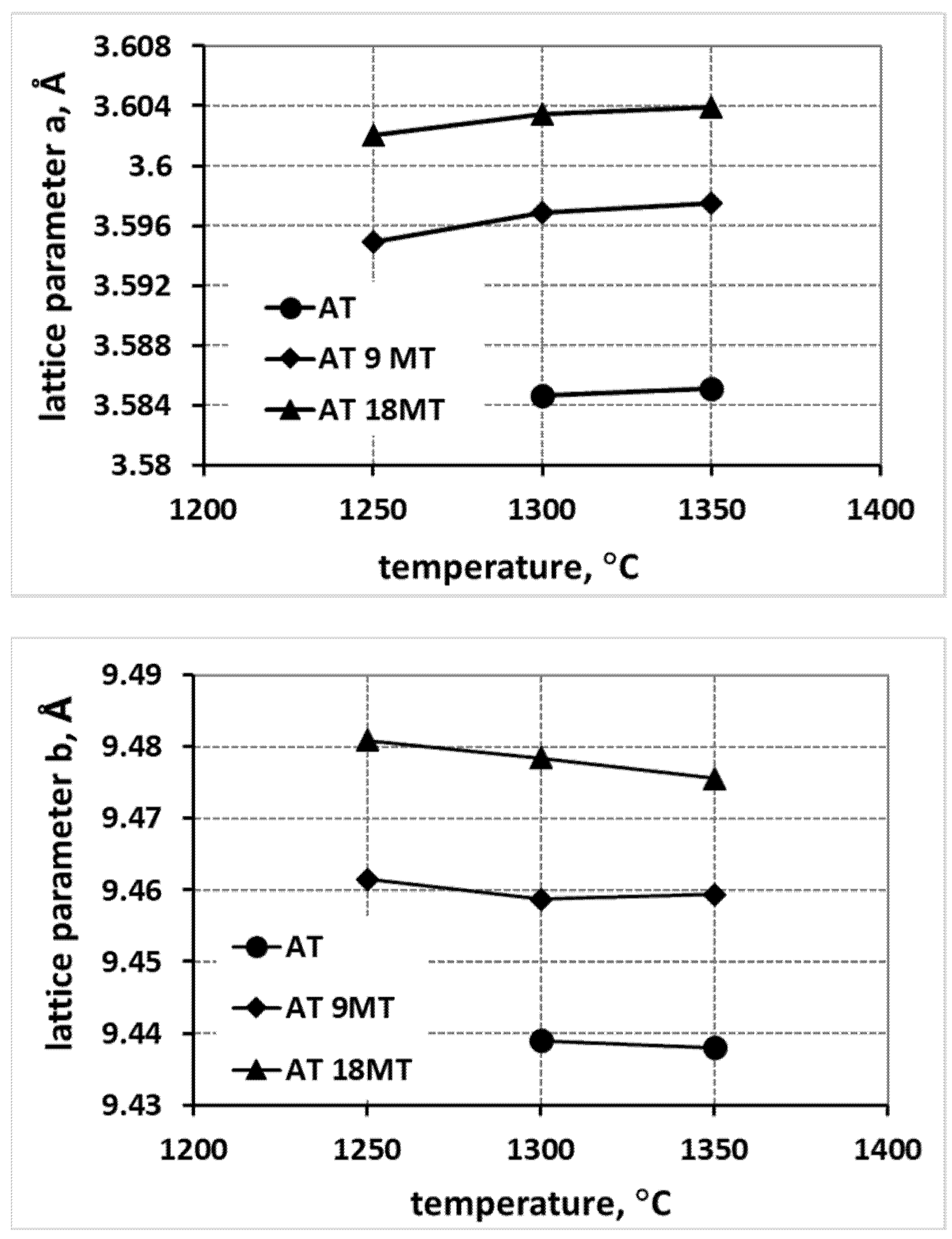
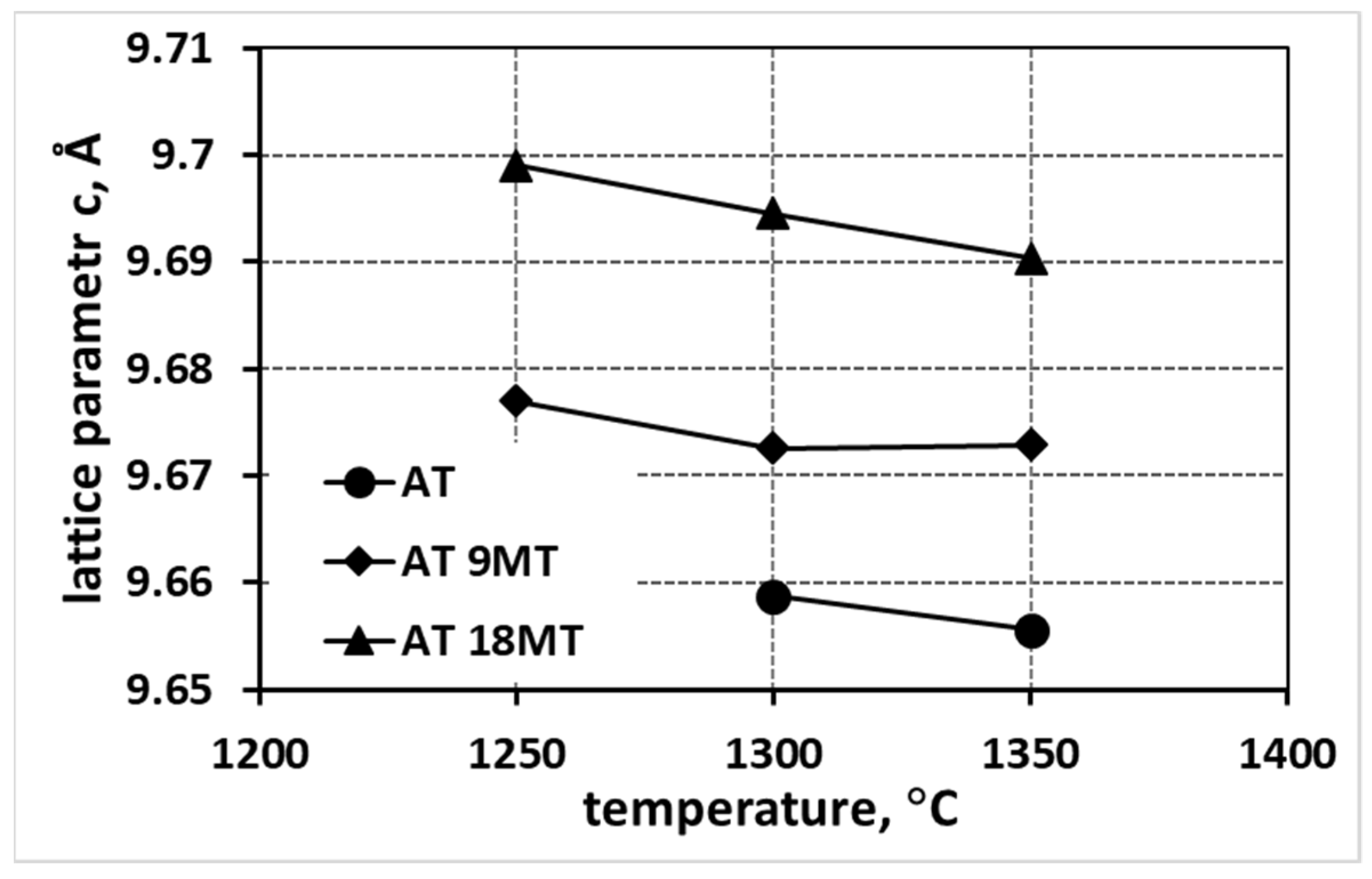
- An increase in the reaction sintering temperature of tialite leads to an increase in the lattice parameter a in all systems, both for pure tialite and MAT solid solutions, with a simultaneous decrease in the magnitude of the parameters b and c.
- (4)
- At the same time, the total volume of the elementary cell does not change significantly. Thus, the stresses in the material at the structural level are reduced, leading to an increase in the thermal stability of tialite. This indicates the significant effect of the reaction sintering temperature of tialite on its stability.
- (5)
- The necessity of using stabilizing additives structurally, e.g., Mg2+, and microstructurally, e.g., SiO2, is indisputable. The presence of magnesium substitutions in the tialite structure significantly reduces elementary cell deformation and the combination of structural and microstructural stabilization by means of nano-silica addition leads to thermally stable polycrystals with desirable properties.
- (6)
- The AT 18MT 4S system to which both stabilizing additives, i.e., magnesium cations in the form of isostructured MgTi2O5 phase and nanosilica, were added showed high thermal stability. After 96 h of annealing at 1100 °C, more than 40mass % of MAT solid solutions were present in the sample.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AT | tialite |
| MT | magnesium titanite |
| MAT | solid-solution Al2TiO5-MgTi2O5 |
| AT 4M | reaction system with additive of 4 mass % of MgO |
| AT 4M 4S | reaction system with addition of 4 mass % of MgO and 4 mass % of nanosilica |
| AT 9MT | reaction system with addition of 9 mol % of MgTi2O5 |
| AT 18MT | reaction system with addition of 18 mol % of MgTi2O5 |
| AT 18MT 4S | reaction system with addition of 9 mol % of MgTi2O5 and 4 mass % of nanosilica |
References
- Maitra, S.; Bhattacharya, S.; Sil, G.; Mondal, S. Aluminium Titanate Ceramics—A Review. Trans. Indian Ceram. Soc. 2002, 61, 69–98. [Google Scholar] [CrossRef]
- Thomas, H. Aluminium Titanate—A Literature Review. I. Microcracking Phenomena. Br. Ceram. Trans. J. 1988, 88, 144–151. [Google Scholar]
- Thomas, H. Aluminium Titanate—A Literature Review. II. Engineering Properties and Thermal Stability. Br. Ceram. Trans. J. 1988, 88, 184–190. [Google Scholar]
- Austin, A.E.; Schwartz, C.M. The crystal structure of aluminium titanate. Acta Crystallogr. 2002, 6, 812–813. [Google Scholar] [CrossRef]
- Sequoia, E.; Bayer, G. Thermal expansion pseudobrookite—Type compunds Me3O5. J. Less Comm. Met. 1971, 24, 129–138. [Google Scholar]
- Skala, R.D.; Li, D.; Low, I.M. Diffraction, structure and phase stability studies on aluminium titanate. J. Eur. Ceram. Soc. 2009, 29, 67–75. [Google Scholar] [CrossRef]
- Fukuda, M.; Yoko, T.; Takahashi, M. Decomposition Free Al2TiO5-MgTi2O5 Ceramics with Low-Thermal Expansion Coefficient. New J. Glas. Ceram. 2013, 3, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Korim, T. Effect of Mg2+-and Fe3+-Ions on Formation Mechanism of Aluminium Titanate. Ceram. Int. 2009, 35, 1671–1675. [Google Scholar] [CrossRef]
- Krstic, V.D. On the fracture of brittle-matrix/ductile-particle composites. Philos. Mag. A Phys. Condens. Matter. Struct. Defects Mech. Prop. 1983, 48, 695–708. [Google Scholar] [CrossRef]
- Krstic, V.D.; Vlajic, M.D. Conditions for spontaneous cracking of a brittle matrix due to the presence of thermoelastic stresses. Acta Metall. 1983, 31, 139–144. [Google Scholar] [CrossRef]
- Krstic, V.D. Fracture of Brittle Solids in the Presence of Thermoelastic Stresses. J. Am. Ceram. Soc. 1984, 3, 589–593. [Google Scholar] [CrossRef]
- Krstic, V.D.; Komac, M.; Vlajic, M.D. Crack extension and arrest within the particle subjected to tensile thermoelastic stresses in brittle matrix composites. J. Mater. Sci. 1984, 19, 4119–4124. [Google Scholar] [CrossRef]
- Evans, H.E.; Knowles, G. A model of creep in pure materials. Acta Metall. 1977, 25, 963–975. [Google Scholar] [CrossRef]
- Fu, Y.; Evans, A.G. Microcrack zone formation in single phase polycrystals. Acta Metall. 1982, 30, 1619–1625. [Google Scholar] [CrossRef]
- Fu, Y.; Evans, A.G. Some effects of microcracks on the mechanical properties of brittle solids—I. Stress, strain relations. Acta Metall. 1985, 33, 1515–1523. [Google Scholar] [CrossRef]
- Evans, A.G.; Fu, Y. Some effects of microcracks on the mechanical properties of brittle solids—II. Microcrack toughening A. Acta Metall. 1985, 133, 1525–1531. [Google Scholar] [CrossRef]
- Rice, R.W.; Freiman, S.W.; Becher, P.F. Grain-Size Dependence of Fracture Energy in Ceramics: I, Experiment. J. Am. Ceram. Soc. 1981, 64, 345–350. [Google Scholar] [CrossRef]
- Sobhani, M.; Ebadzadeh, T.; Rahimipour, M.R. A comparison study on the R-curve behavior of alumina/aluminum titanate composites prepared with different TiO2 powders. Theor. Appl. Fract. Mech. 2016, 85, 159–163. [Google Scholar] [CrossRef]
- Heidary, P.; Shokuhfar, A.; Samani, M.N.; Naserifar, N.; Naderi, G. Prediction of physical properties of Al2TiO5-based ceramics containing micro and nano size oxide additives by using artificial neural network. Materwiss. Werksttech. 2009, 40, 169–177. [Google Scholar] [CrossRef]
- Huang, X.; Song, X.; Yang, N.; Chang, Y. Sintering and characterization of aluminum titanate ceramics with enhanced thermomechanical properties. Int. J. Appl. Ceram. Technol. 2022, 19, 1939–1948. [Google Scholar] [CrossRef]
- Lian, J.; Shan, Q.; Chen, W.; Ma, Q.; Zeng, S.; Xiong, H.; Wang, Y.; Xu, Q.; Shui, A. Influence of Cooling Rates on the Microstructure and Mechanical Properties of Aluminum Titanate Flexible Ceramic. Adv. Eng. Mater. 2021, 23, 2100170. [Google Scholar] [CrossRef]
- Buessem, R.; Thielke, W.R.; Sarakauskas, N.R. Thermal expansion hysteresis of aluminum titanate. Ceram. Age. 1952, 60, 38–40. [Google Scholar]
- Papitha, R.; Suresh, M.; Das, D.; Johnson, R. Effect of micro-cracking on the thermal conductivity and thermal expansion of tialite (Al2TiO5) ceramics. Process. Appl. Ceram. 2013, 7, 143–146. [Google Scholar] [CrossRef]
- Vu, M.N.; Nguyen, S.T.; Vu, M.H.; Tang, A.M.; To, V.T. Heat conduction and thermal conductivity of 3D cracked media. Int. J. Heat Mass Transf. 2015, 89, 1119–1126. [Google Scholar] [CrossRef]
- Bueno, S.; Moreno, R.; Baudín, C. Reaction sintered Al2O3/Al2TiO5 microcrack-free composites obtained by colloidal filtration. J. Eur. Ceram. Soc. 2004, 24, 2785–2791. [Google Scholar] [CrossRef]
- Shen, Q.; Hu, Y.; Bin Wang, C.; Li, M.Z.; Zhang, L.M. Effect of MgTi2O5 Addition on Thermal Shock Resistance of Al2TiO5. Key Eng. Mater. 2009, 280–283, 1187–1190. [Google Scholar] [CrossRef]
- Zhao, J.; Hao, X.; Wang, S.; Zhang, X.; Wang, Z.; Luo, X.; Xie, Z.; Luo, J. Sintering behavior and thermal shock resistance of aluminum titanate (Al2TiO5)-toughened MgO-based ceramics. Ceram. Inter. 2021, 47, 26643–26650. [Google Scholar] [CrossRef]
- Hamano, K.; Ohya, Y.; Nakagawa, Z.E. Crack propagation resistance of aluminium titanate ceramics. Int. J. High Technol. Ceram. 1985, 1, 129–137. [Google Scholar] [CrossRef]
- Perera, F.H.; Pajares, A.; Meléndez, J.J. Strength of aluminium titanate/mullite composites containing thermal stabilizers. J. Eur. Ceram. Soc. 2011, 31, 1695–1701. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, X.Y.; Han, G.M.; Meng, Y. Effect of additives on properties of aluminium titanate ceramics. Trans. Nonferrous Met. Soc. China English 2011, 21, 1574–1579. [Google Scholar] [CrossRef]
- Cleveland, J.J.; Bradt, R.C. Grain Size/Microcracking Relations for Pseudobrookite Oxides. J. Am. Ceram. Soc. 1978, 61, 478–481. [Google Scholar] [CrossRef]
- Da Silva Aragao, V.T.; Silva, V.S.; de Carvalho, R.R.; Matos, R.S.; Ferreira, N.S.; de Araújo Melo, D.M.; Oliveira, R.M.P.B. Analyzing coarsening versus densification phenomenon present in partial sintering of Al2O3/TiO2 composites. J. Mater. Res. Technol. 2021, 15, 2711–2724. [Google Scholar] [CrossRef]
- Kunin, A.V.; Prokof’ev, V.Y.; Il’in, A.P. Synthesis of aluminum titanate using stabilizing additives. Glass Ceram. 1999, 56, 20–23. [Google Scholar] [CrossRef]
- Kumagai, M.; Messing, G.L. Enhanced Densification of Boehmite Sol-Gels by α-Alumina Seeding. J. Am. Ceram. Soc. 1984, 67, 230–231. [Google Scholar] [CrossRef]
- Nordahl, C.S.; Messing, G.L. Sintering of α-Al2O3-seeded nanocrystalline γ-Al2O3 powders. J. Eur. Ceram. Soc. 2002, 22, 415–422. [Google Scholar] [CrossRef]
- Nordahl, C.S.; Messing, G.L. Thermal analysis of phase transformation kinetics in α—Al2O3 seeded boehmite and γ -Al2O3. Thermochim. Acta 1998, 318, 187–199. [Google Scholar] [CrossRef]
- Kumagai, M.; Messing, G.L. Controlled Transformation and Sintering of a Boehmite Sol-Gel by α-Alumina Seeding. J. Am. Ceram. Soc. 1985, 68, 500–505. [Google Scholar] [CrossRef]
- Kornaus, K.; Lach, R.; Szumera, M.; Świerczek, K.; Gubernat, A. Synthesis of aluminium titanate by means of isostructural heterogeneous nucleation. J. Eur. Ceram. Soc. 2019, 39, 2535–2544. [Google Scholar] [CrossRef]
- Kornaus, K.; Rutkowski, P.; Lach, R.; Gubernat, A. Effect of microstructure on thermal and mechanical properties of solid solutions Al2TiO5—MgTi2O5. J. Eur. Ceram. Soc. 2021, 41, 1498–1505. [Google Scholar] [CrossRef]
- Morosin, B.; Lynch, R.W. Structure studies on Al2TiO5 at room temperature and at 600 °C. Acta Crystallogr. B. 1972, 28, 1040–1046. [Google Scholar] [CrossRef]
- Kresse, G. and Furthmller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Shinoda, Y. Magnesium dititanate (MgTi2O5) with pseudobrookite structure: A review. Sci. Technol. Adv. Mater. 2011, 12, 34301. [Google Scholar] [CrossRef]
- Buscaglia, V.; Nanni, P. Decomposition of Al2TiO5 and Al2(1−x)MgxTi(1+x)O5 Ceramics. J. Am. Ceram. Soc. 1998, 81, 2645–2653. [Google Scholar] [CrossRef]
- Azarniya, A.; Hosseini, H.R.M.; Amutha, C.; Ramakrishna, S. Effect of nanostructuring on thermal stability and decomposition of aluminium titanate (Al2TiO5): A phase transformation study. Mater. Character. 2021, 173, 110764. [Google Scholar] [CrossRef]



| Reaction System | Symbol |
|---|---|
| Al2O3 + TiO2 | AT |
| Al2O3 + TiO2 + 4 mass % MgO | AT 4M |
| Al2O3 + TiO2 + 4 mass % MgO + 4 mass % SiO2 | AT 4M 4S |
| Al2O3 + TiO2 + 9% mol. MgTi2O5 | AT 9MT |
| Al2O3 + TiO2 + 18% mol. MgTi2O5 | AT 18MT |
| Al2O3 + TiO2 + 18% mol. MgTi2O5 + 4 mass % SiO2 | AT 18MT 4S |
| Reaction System | Phase Composition, Mass % | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature of Reaction Sintering 1250 °C | Temperature of Reaction Sintering 1300 °C | Temperature of Reaction Sintering 1350 °C | ||||||||||||||||
| AT | MAT | K | R | M | S | AT | MAT | K | R | M | S | AT | MAT | K | R | M | S | |
| AT | 0 | - | 59.7 | 38.9 | - | - | 62.9 | - | 29.9 | 7.2 | - | - | 67.4 | - | 26.6 | 6.0 | - | - |
| AT 4M | - | 89.2 | 5.4 | 4.3 | - | 1.1 | 96.1 | 2.0 | 0.9 | - | 1.0 | - | 96.4 | 0.7 | 1.0 | - | 1.9 | |
| AT 4M 4S | - | 73.9 | 18.1 | 5.9 | 2.1 | - | - | 82.4 | 3.1 | 5.1 | 7.3 | 2.2 | - | 94.4 | - | 2.0 | 3.6 | - |
| AT 9MT | - | 81.4 | 16.7 | 1.9 | - | - | - | 87.9 | 11.8 | 0.3 | - | - | - | 90.5 | 9.5 | - | - | - |
| AT 18MT | - | 86.7 | 12.7 | 0.6 | - | - | - | 91.2 | 8.8 | - | - | - | - | 94.8 | 5.2 | - | - | - |
| AT 18MT 4S | - | 82.6 | 9.8 | 2.1 | 3.3 | - | - | 86.8 | 4.2 | 3.5 | 5.5 | - | - | 93.2 | - | 1.2 | 5.6 | - |
| Structure * | Structure | a, Å | b, Å | c, Å |
|---|---|---|---|---|
| A | Al2TiO5 vasp | 3.572 | 9.404 | 9.679 |
| B | Al2TiO5 Ti1 | 3.596 | 9.479 | 9.678 |
| C | Al2TiO5 Ti2 | 3.609 | 9.568 | 9.698 |
| D | MgTi2O5 vasp | 3.812 | 9.608 | 9.987 |
| E | Al2TiO5 Mg1 | 3.594 | 9.462 | 9.681 |
| F | Al2TiO5 Mg1_1 | 3.631 | 9.495 | 9.665 |
| G | Al2TiO5 Mg2_2 | 3.625 | 9.516 | 9.666 |
| H | Al2TiO5 Mg1Ti1 | 3.605 | 9.514 | 9.721 |
| I | Al2TiO5 Mg1Ti1_1 | 3.630 | 9.483 | 9.690 |
| Structure | a, Å | b, Å | c, Å |
|---|---|---|---|
| Al2TiO5 lit. | 3.557 | 9.436 | 9.648 |
| MgTi2O5 lit. | 3.759 | 9.824 | 10.128 |
| Structure | Structure | a, Å | b, Å | c, Å |
|---|---|---|---|---|
| AT | Al2TiO5 | 3.585 | 9.433 | 9.656 |
| AT 4M | Al2TiO5-MgTi2O5 | 3.609 | 9.481 | 9.696 |
| AT 4M 4S | Al2TiO5-MgTi2O5 | 3.606 | 9.489 | 9.707 |
| AT 9MT | Al2TiO5-MgTi2O5 | 3.597 | 9.460 | 9.673 |
| AT 18MT | Al2TiO5-MgTi2O5 | 3.604 | 9.476 | 9.690 |
| AT 18MT 4S | Al2TiO5-MgTi2O5 | 3.602 | 9.480 | 9.670 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornaus, K.; Czekaj, I.; Sobuś, N.; Lach, R.; Gubernat, A. Remarkable Structural Modifications of Tialite Solid Solutions Obtained by Different Methods. Materials 2022, 15, 3981. https://doi.org/10.3390/ma15113981
Kornaus K, Czekaj I, Sobuś N, Lach R, Gubernat A. Remarkable Structural Modifications of Tialite Solid Solutions Obtained by Different Methods. Materials. 2022; 15(11):3981. https://doi.org/10.3390/ma15113981
Chicago/Turabian StyleKornaus, Kamil, Izabela Czekaj, Natalia Sobuś, Radosław Lach, and Agnieszka Gubernat. 2022. "Remarkable Structural Modifications of Tialite Solid Solutions Obtained by Different Methods" Materials 15, no. 11: 3981. https://doi.org/10.3390/ma15113981
APA StyleKornaus, K., Czekaj, I., Sobuś, N., Lach, R., & Gubernat, A. (2022). Remarkable Structural Modifications of Tialite Solid Solutions Obtained by Different Methods. Materials, 15(11), 3981. https://doi.org/10.3390/ma15113981







