Synthesis, Performance Measurement of Bi2SmSbO7/ZnBiYO4 Heterojunction Photocatalyst and Photocatalytic Degradation of Direct Orange within Dye Wastewater under Visible Light Irradiation
Abstract
:1. Introduction
2. Result and Discussion
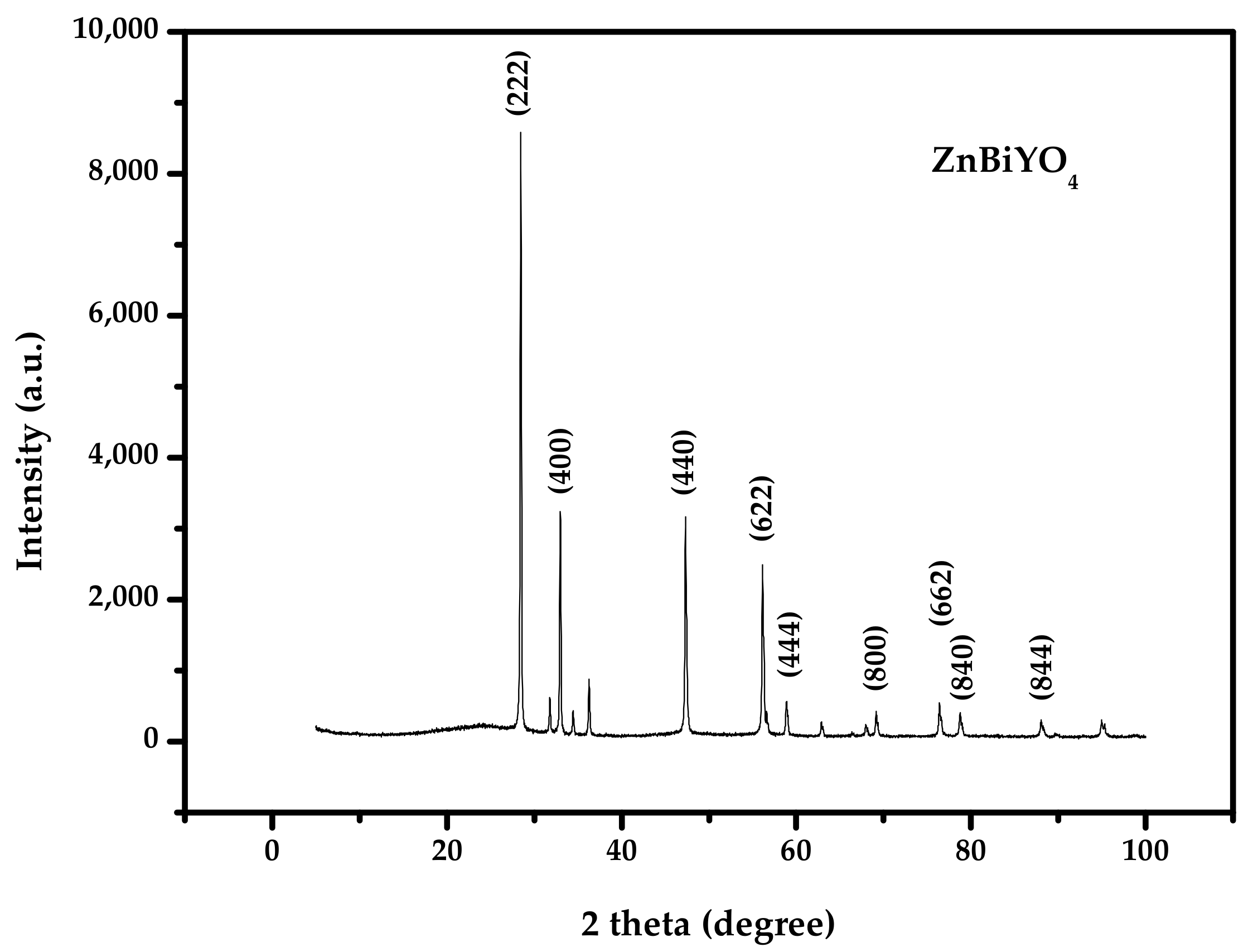
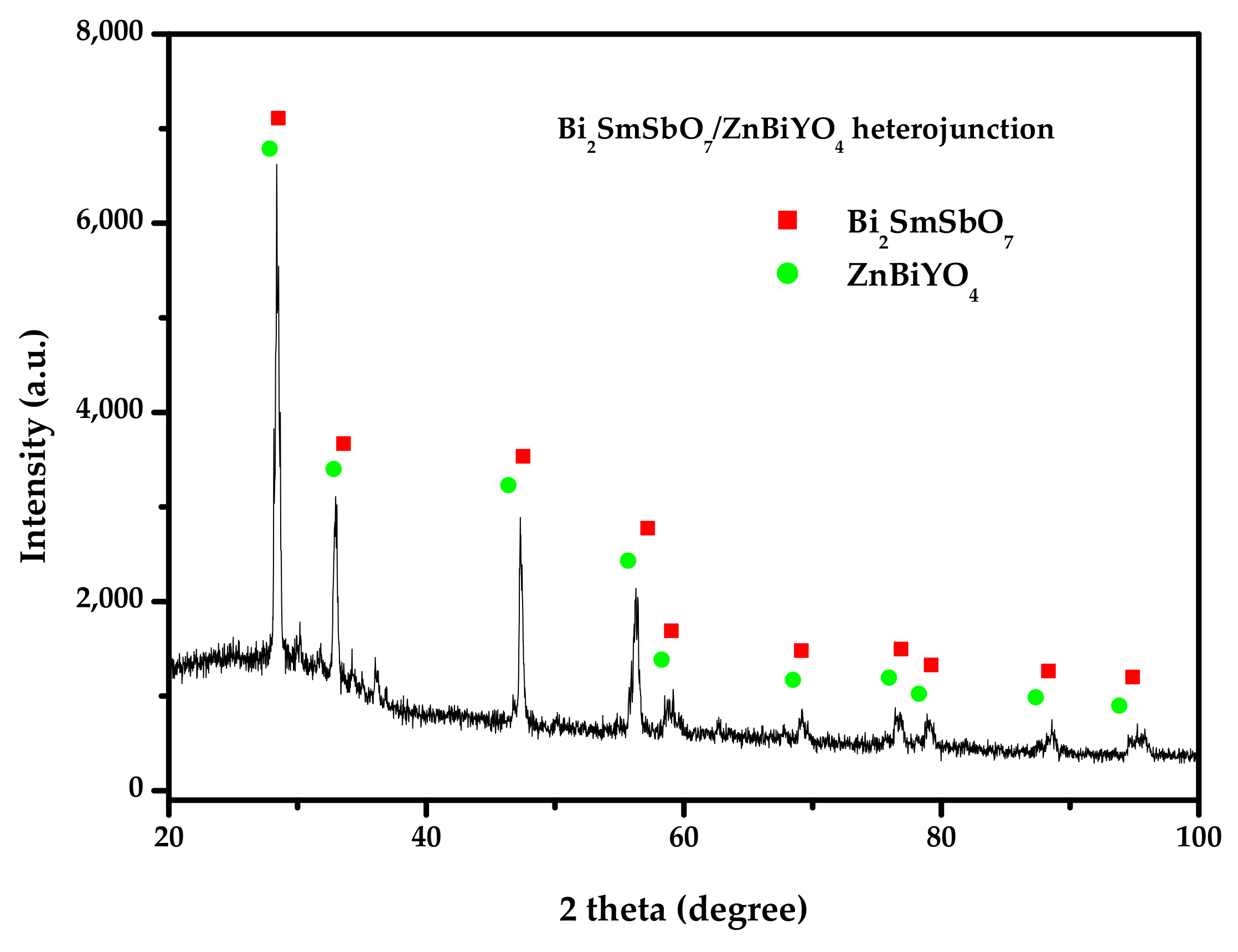
2.1. XRD Analysis
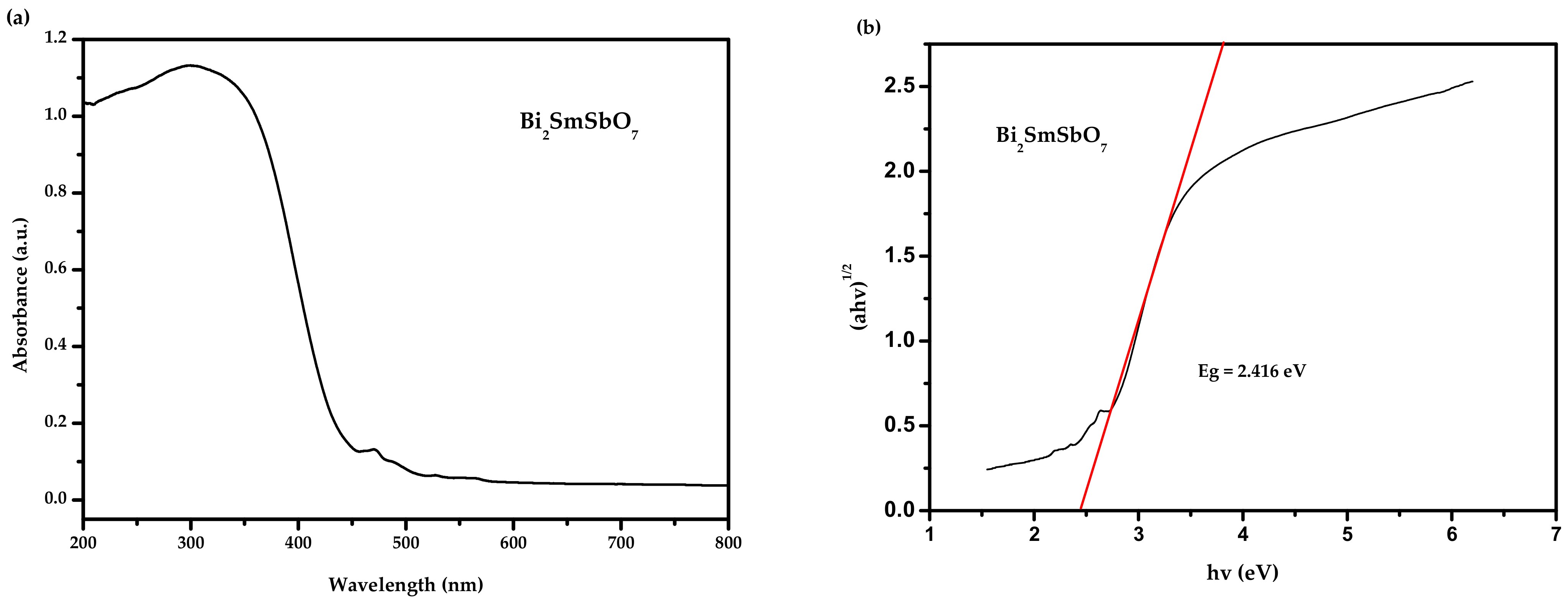
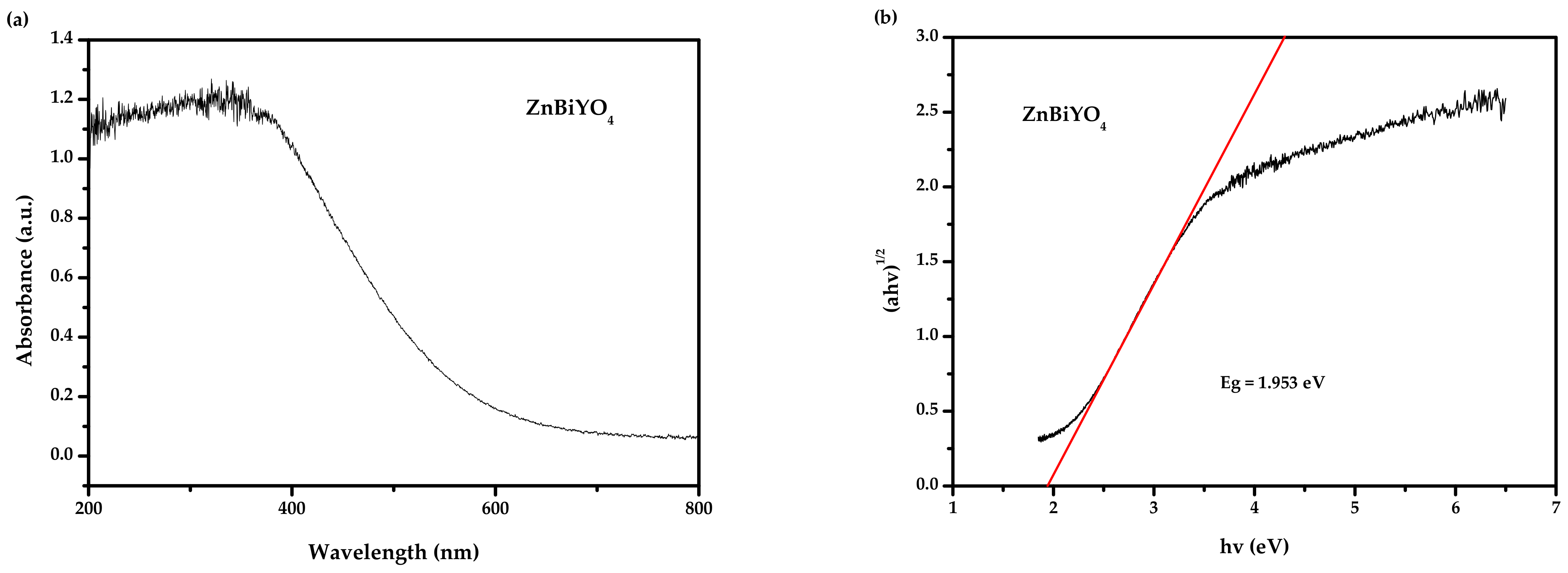
2.2. Diffused Reflection Spectrum
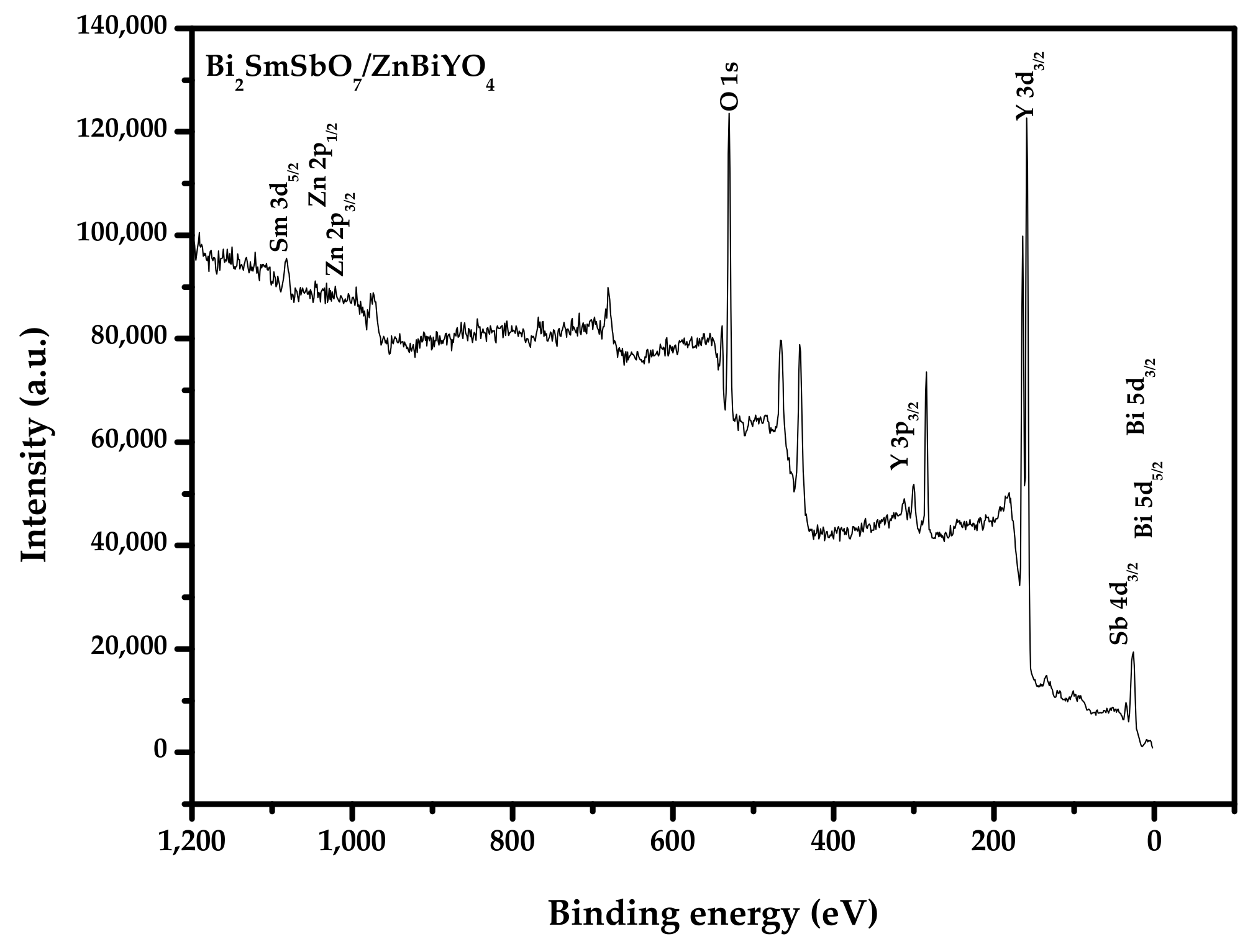
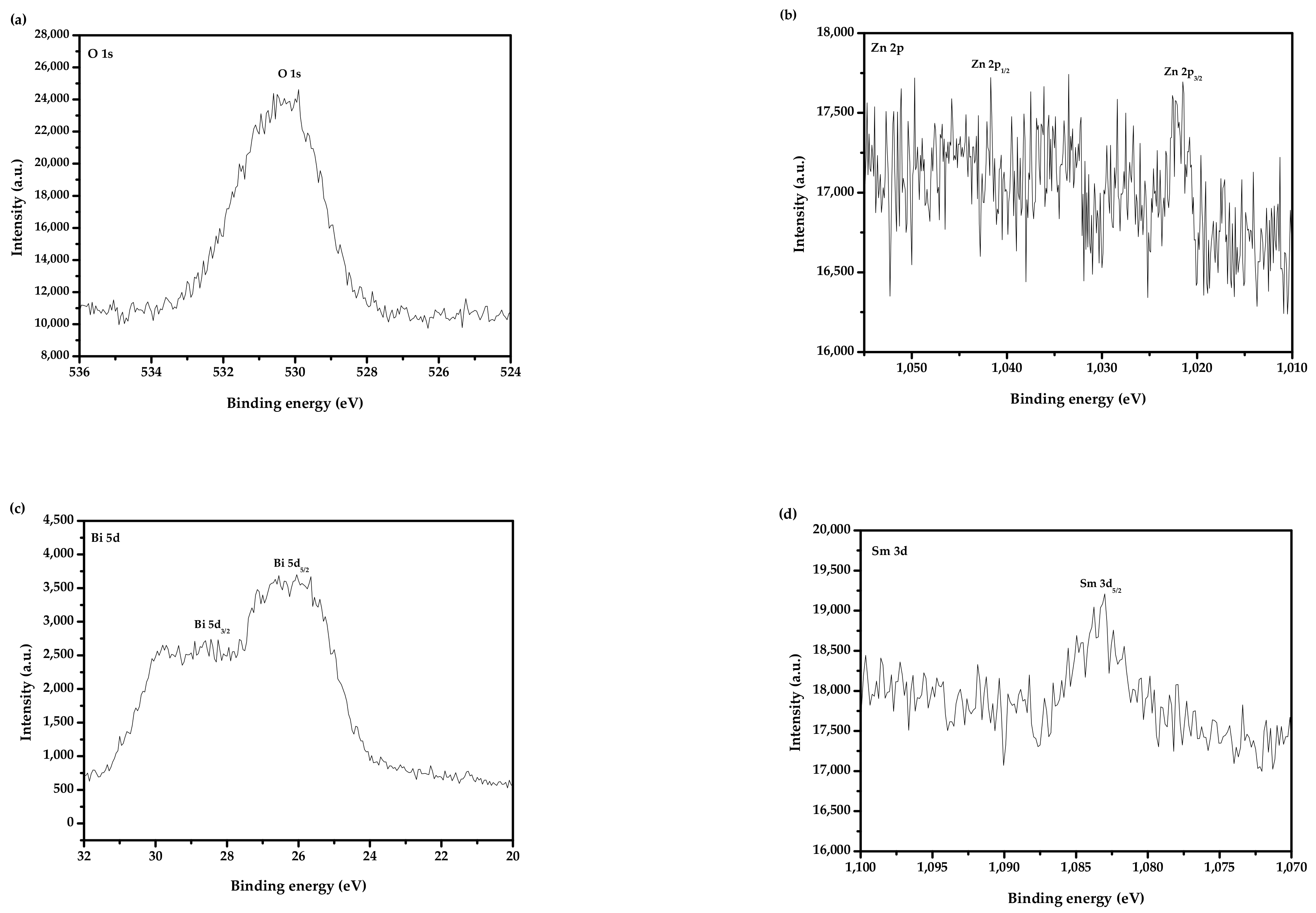
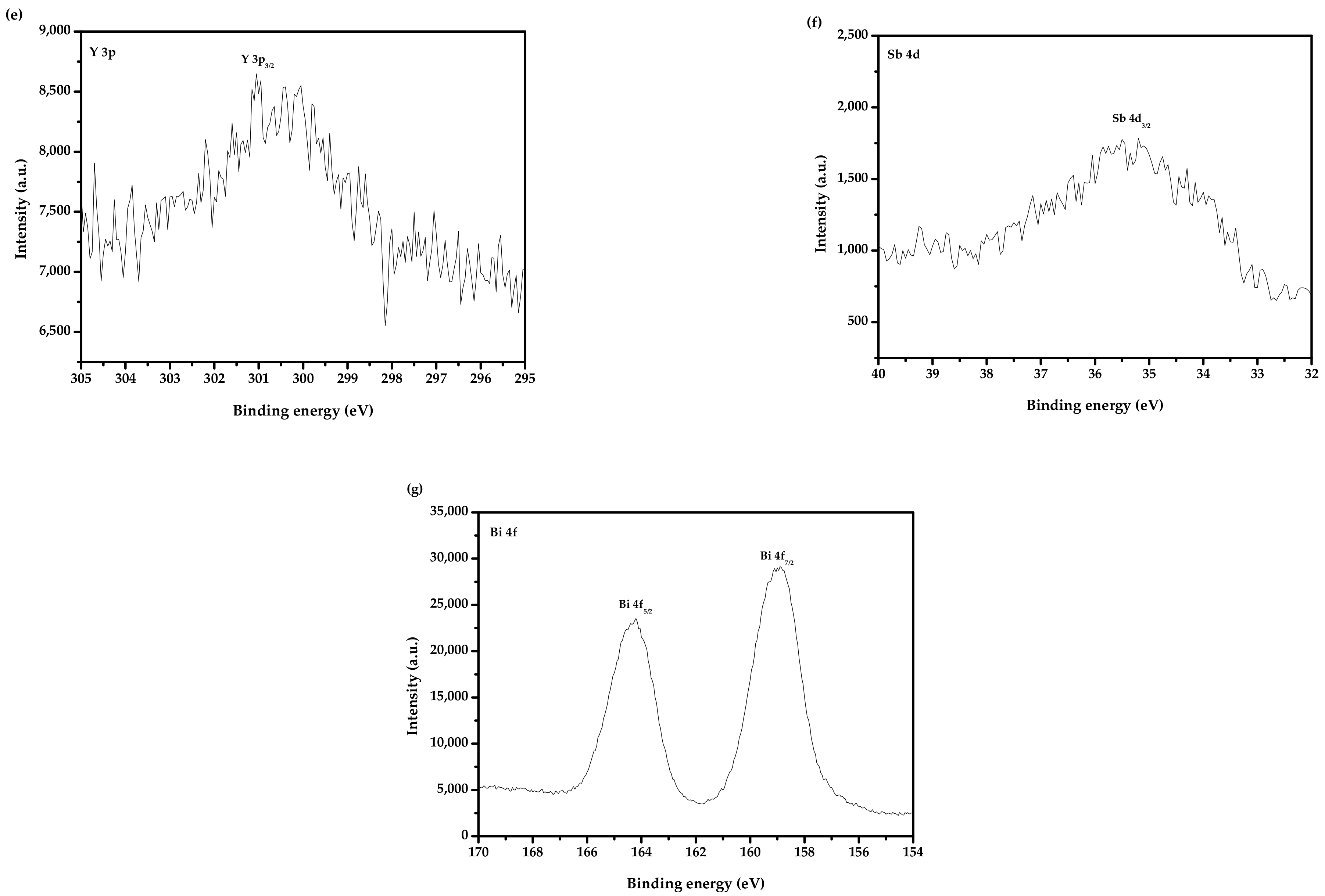
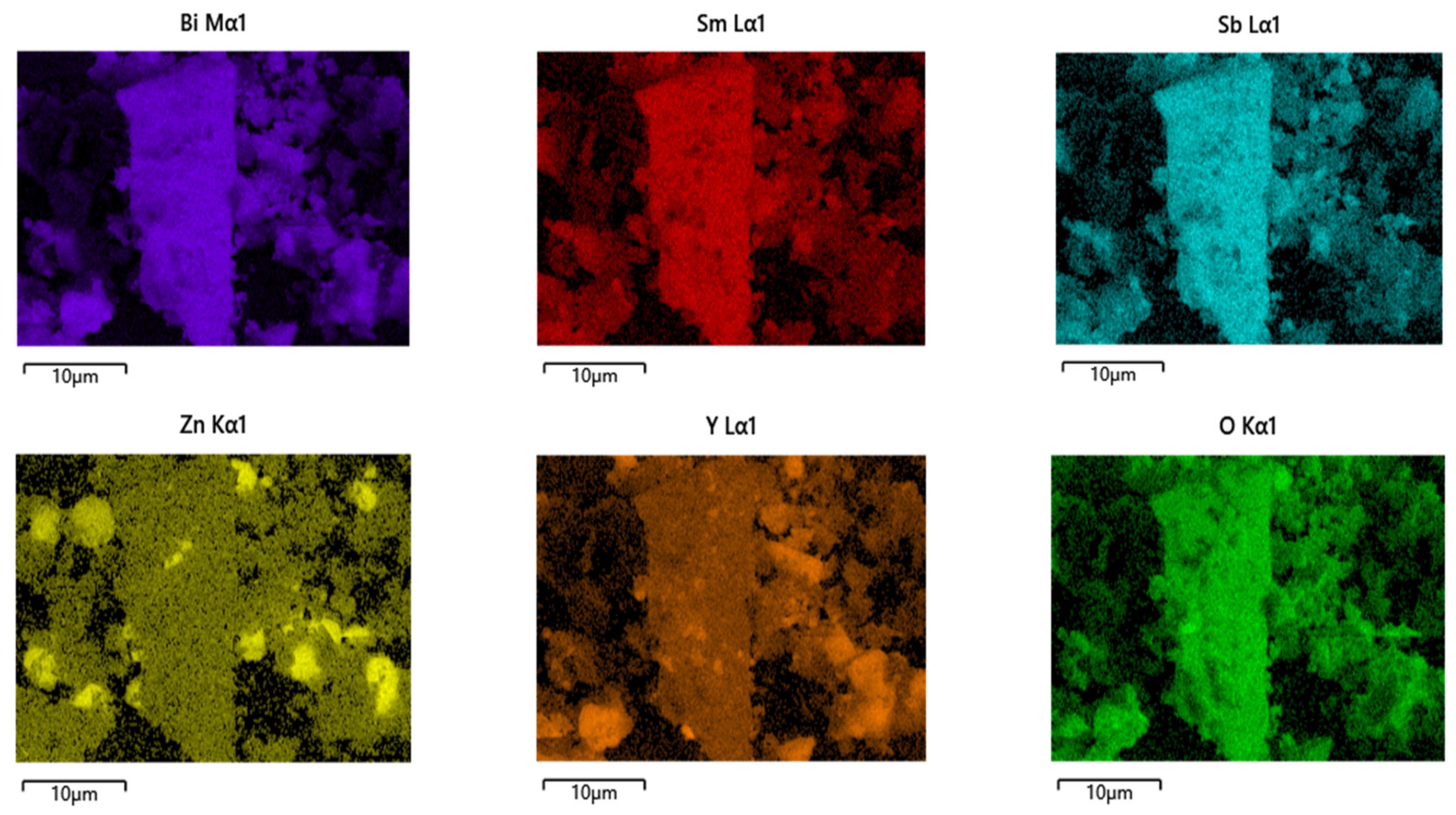
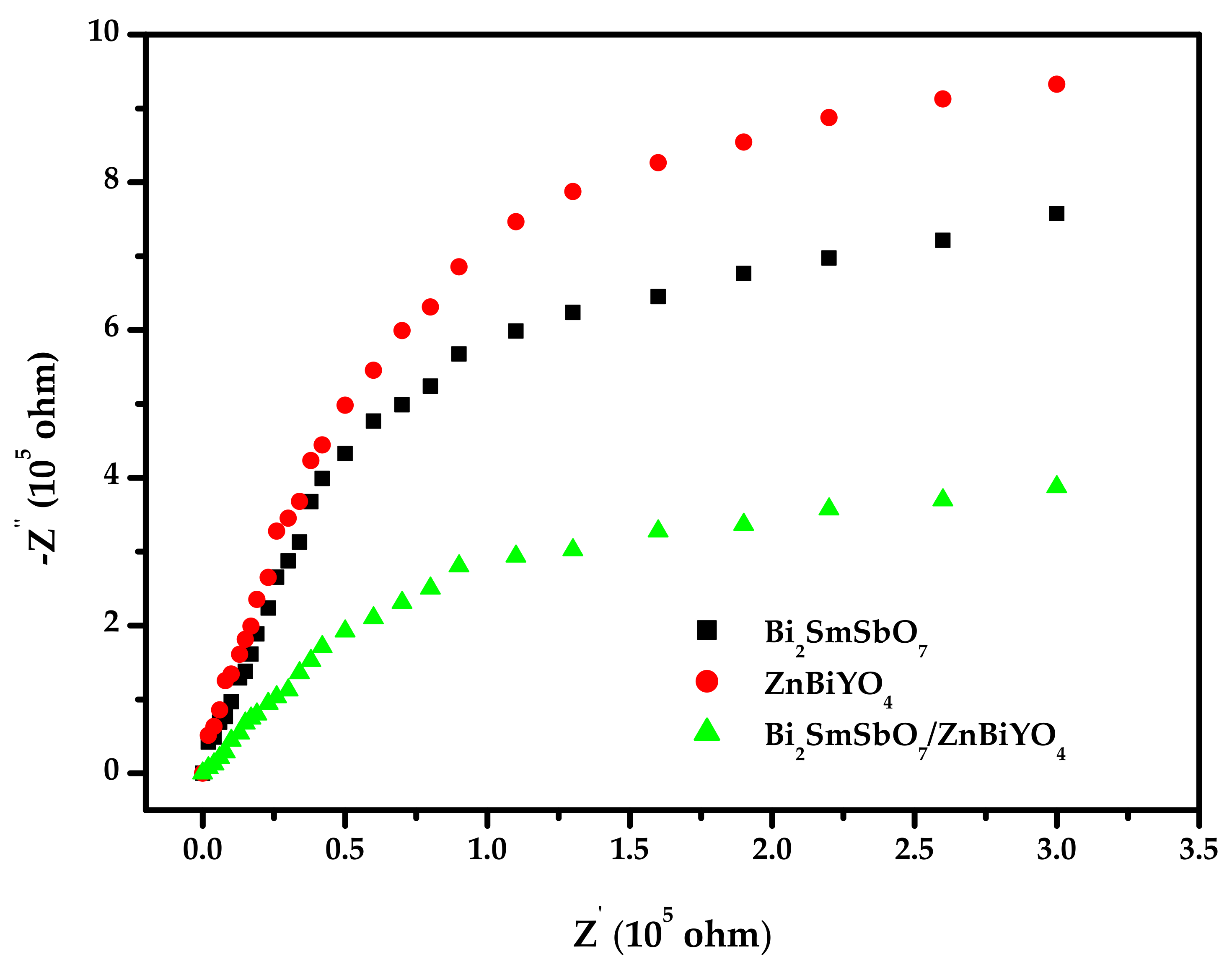
2.3. Performance Representation of Bi2SmSbO7/ZnBiYO4 Heterojunction Photocatalyst
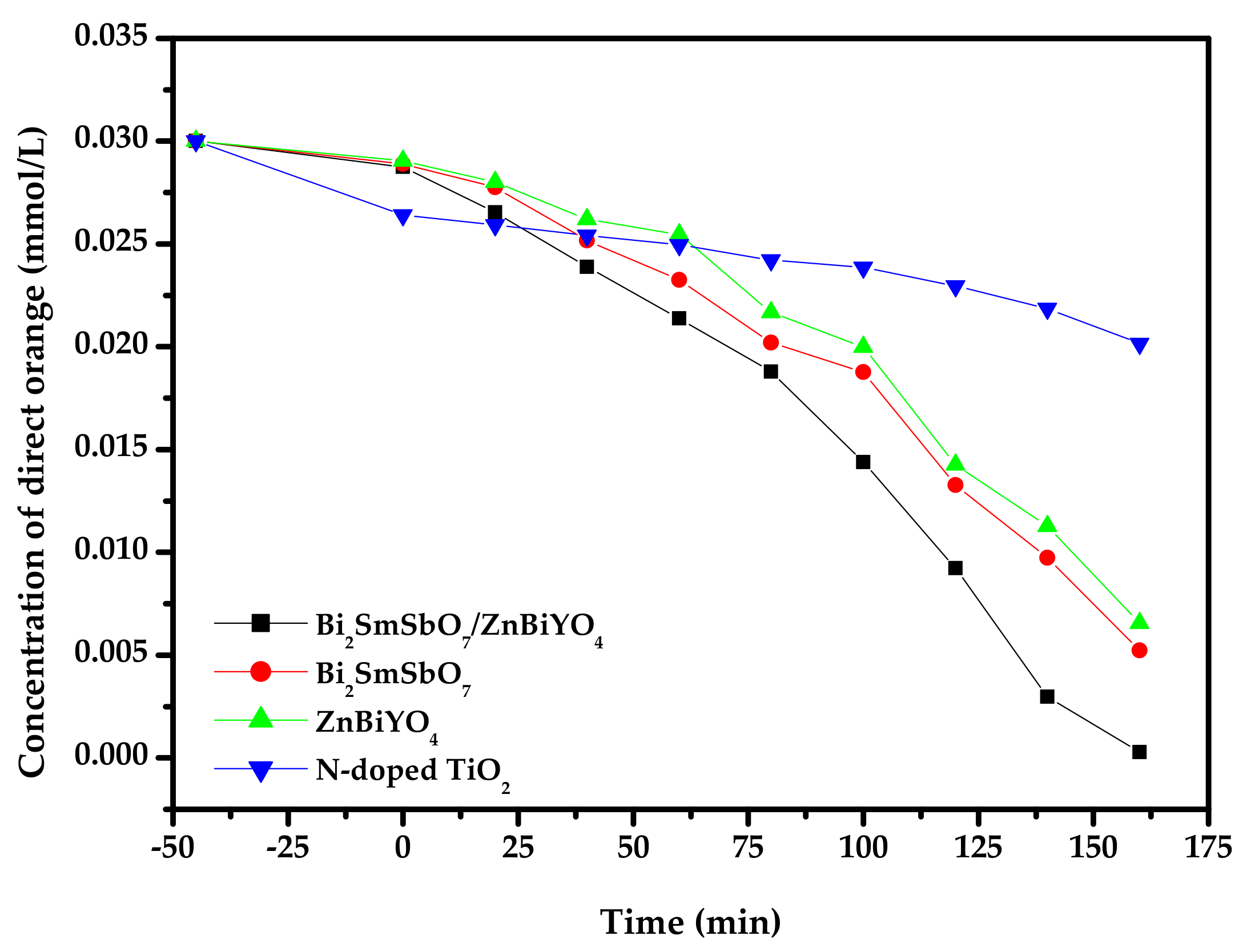
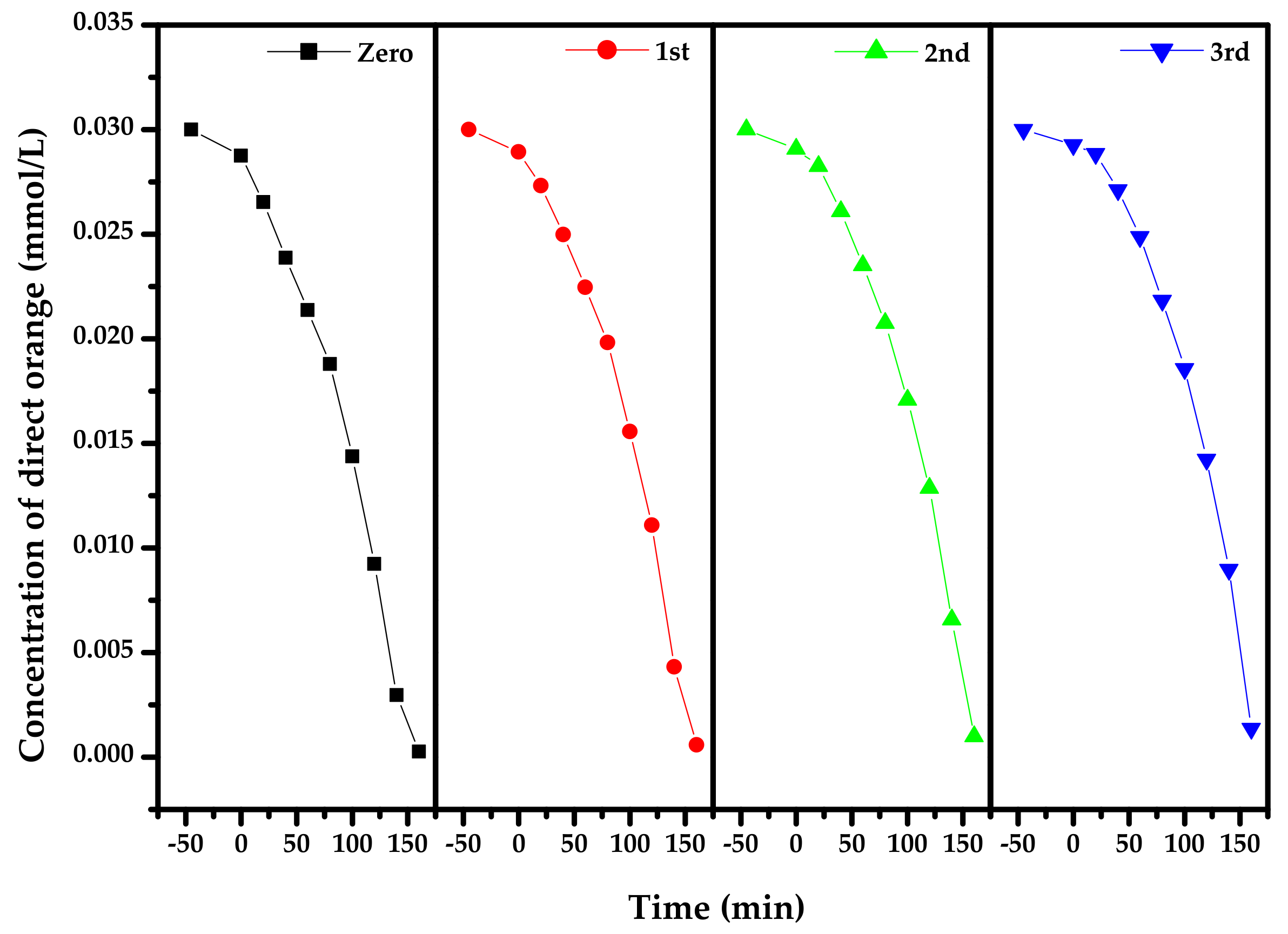
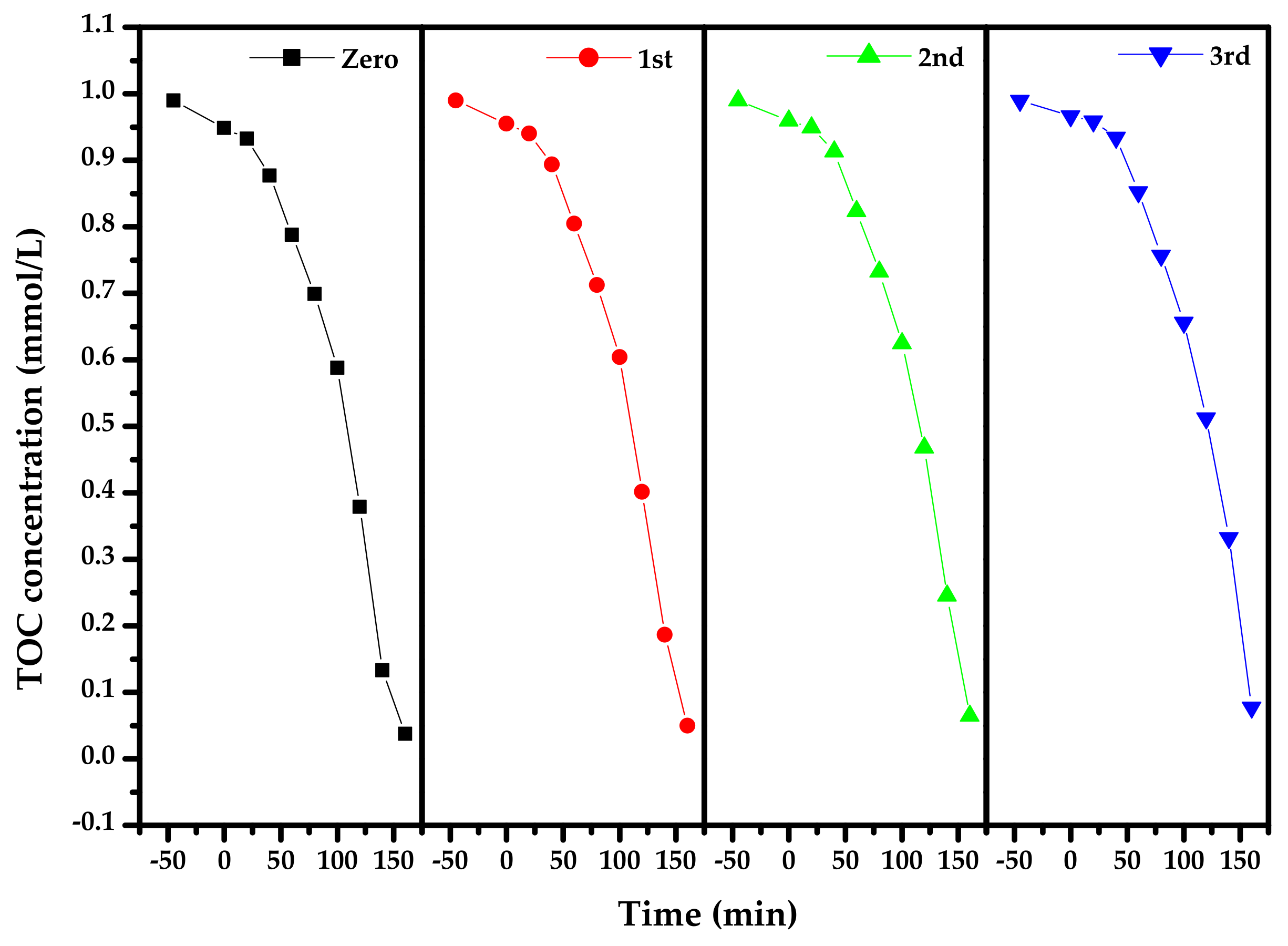
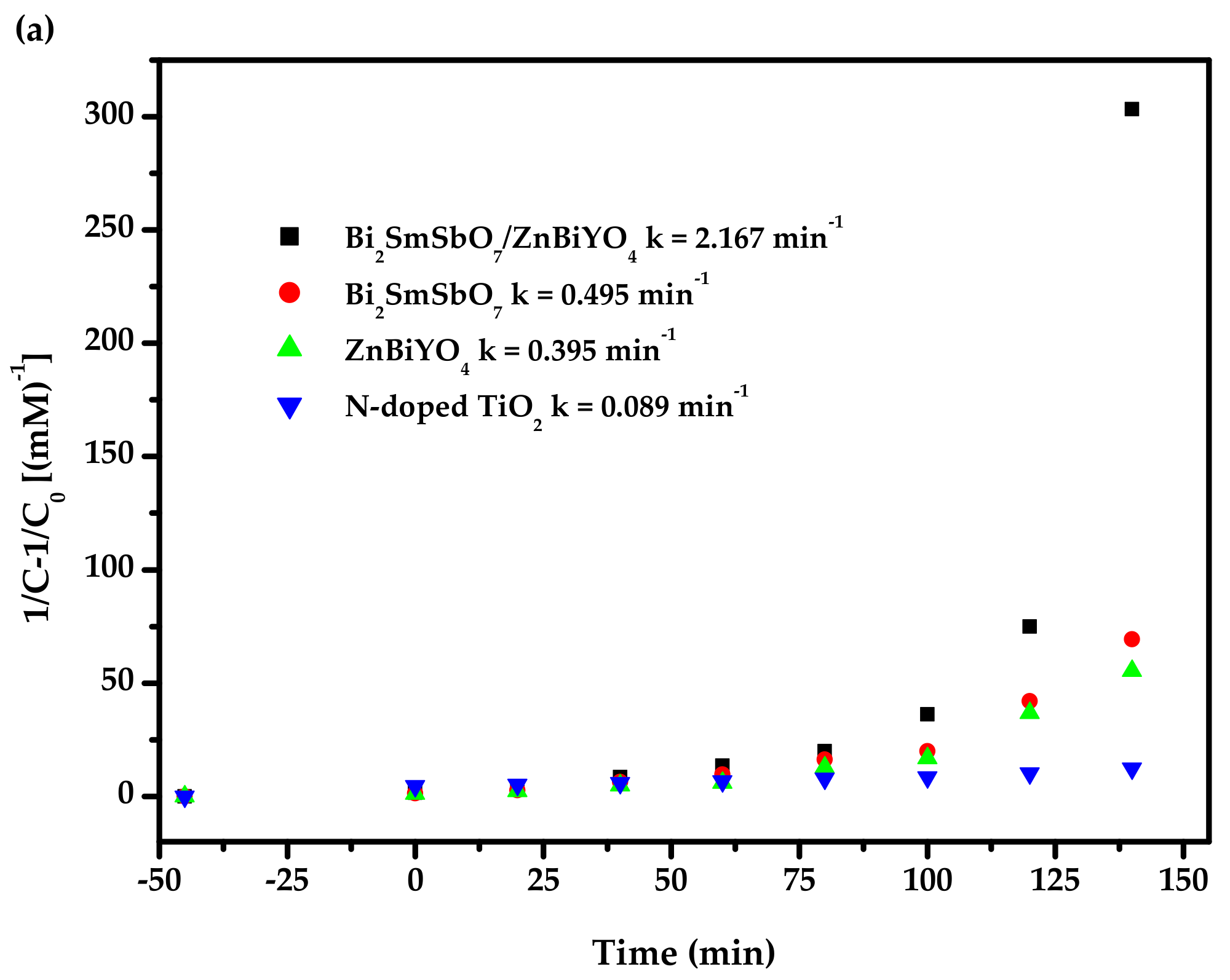
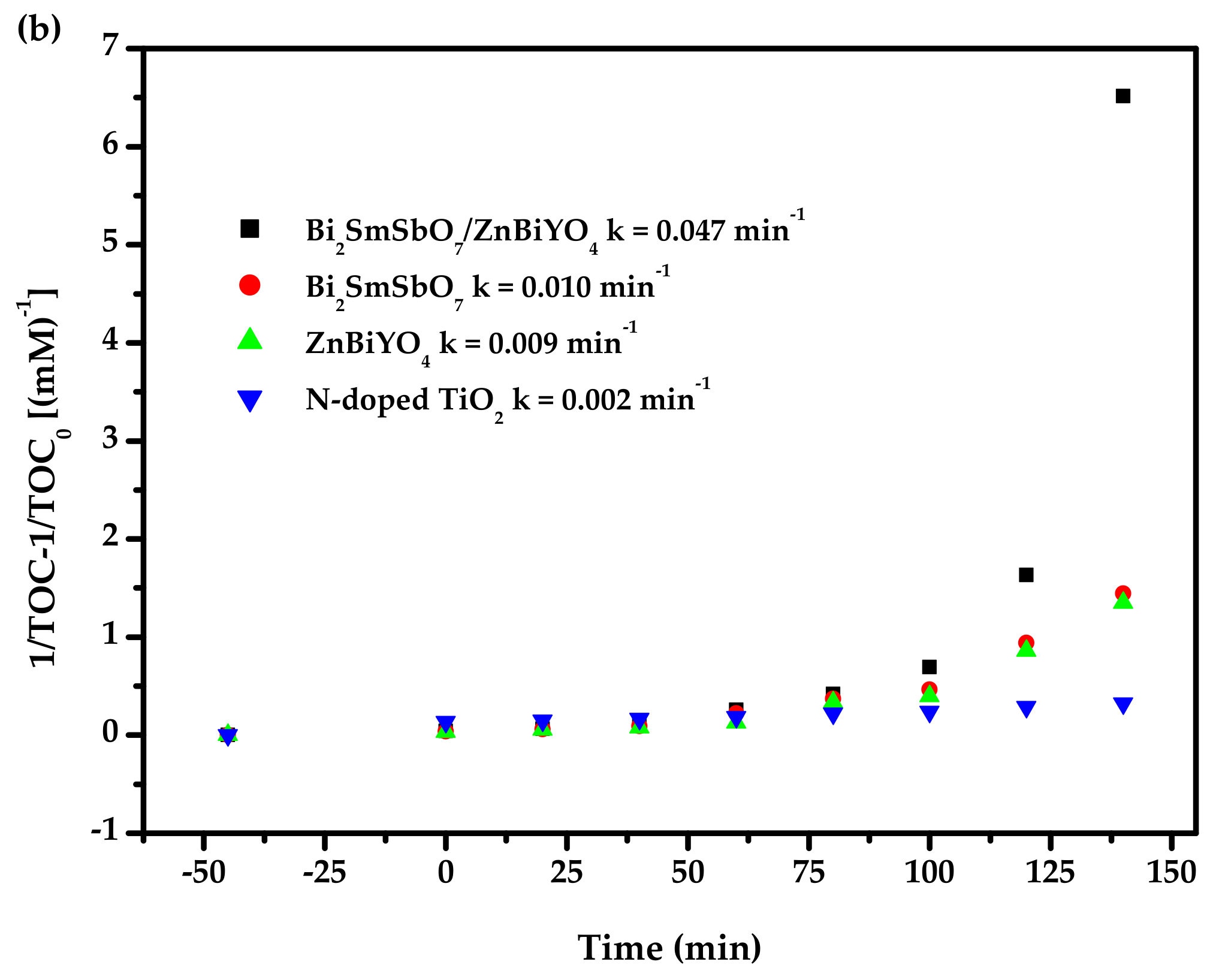
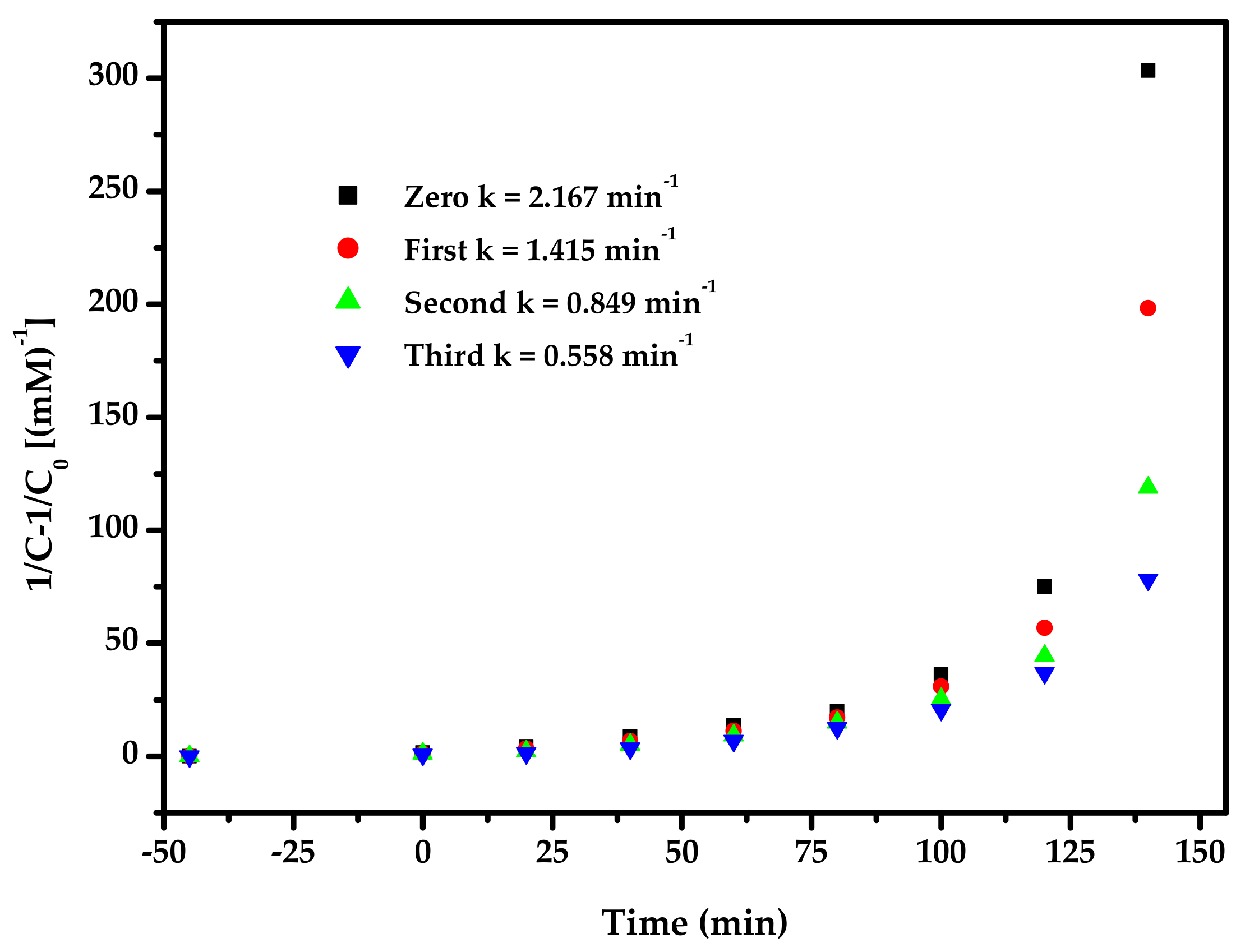
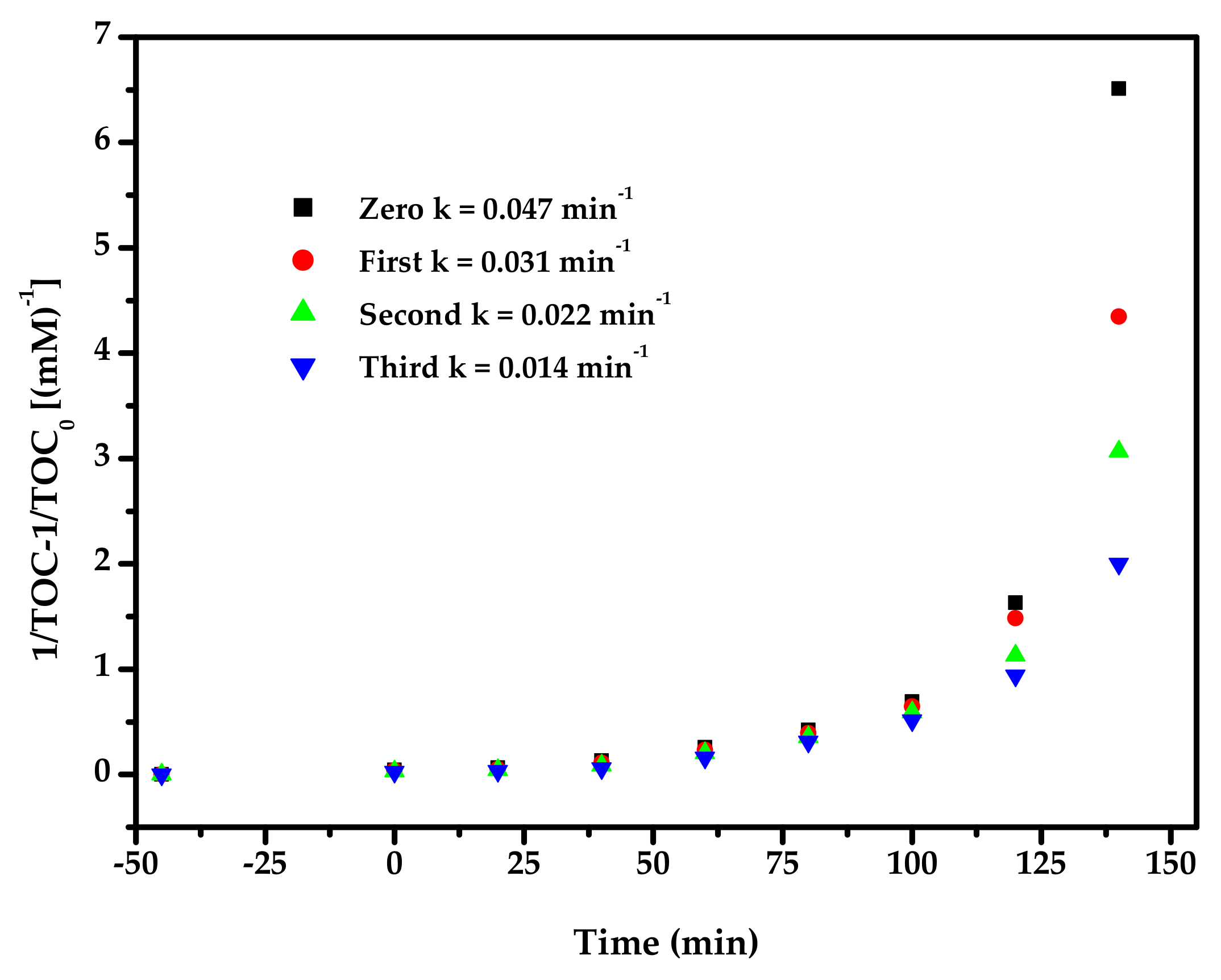
2.4. Photocatalytic Activity
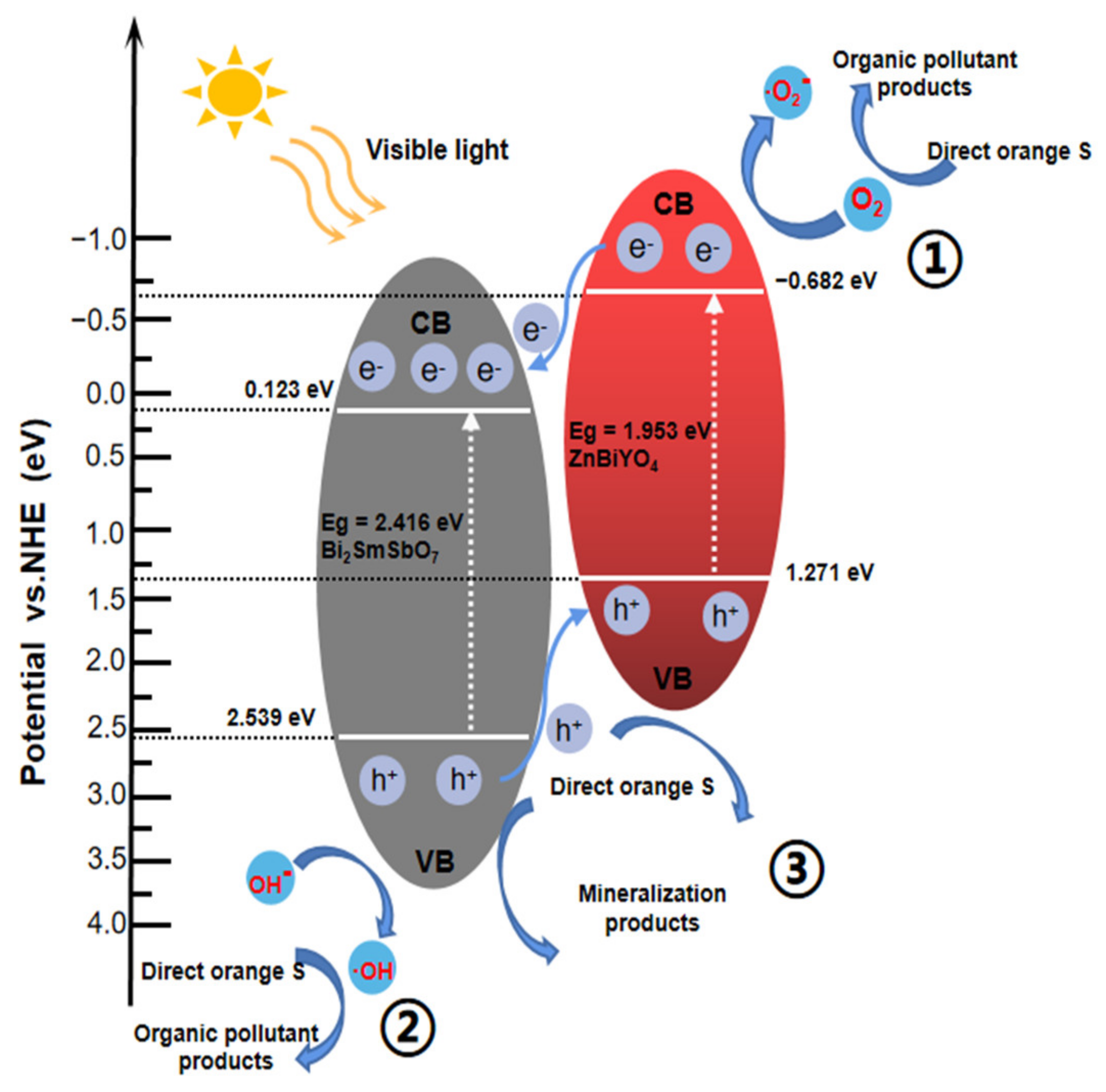
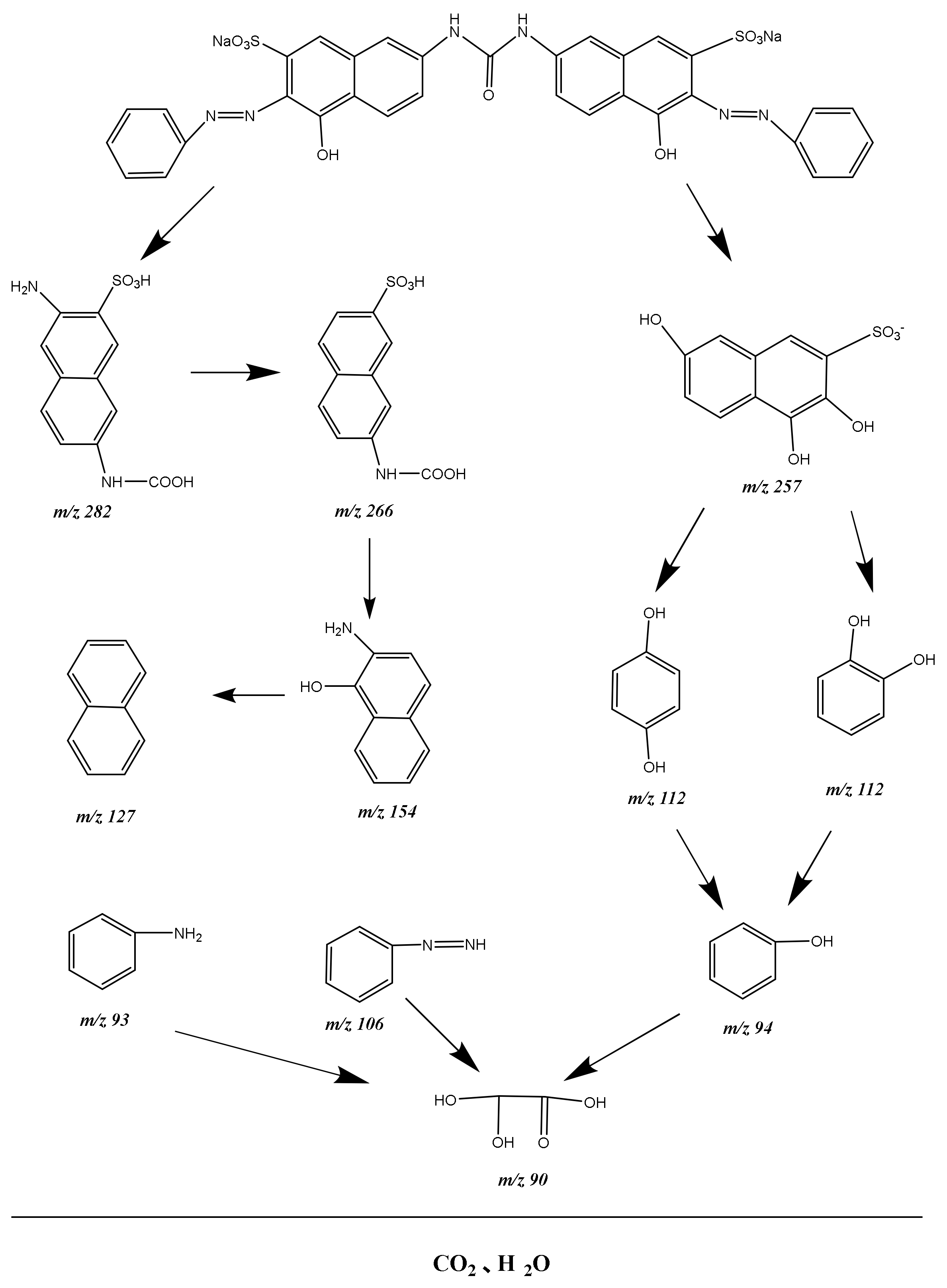
2.5. Probable Degradation Mechanism Analysis
3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation Method of Bi2SmSbO7
3.3. Preparation Method of ZnBiYO4
3.4. Synthesis of N-Doped TiO2
3.5. Synthesis of Bi2SmSbO7/ZnBiYO4 Heterojunction Photocatalyst
3.6. Characterizations
3.7. Photoelectrochemical Experiments
3.8. Experimental Setup and Procedure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meng, Z.L.; Zhang, Y.H.; Zhang, Z.L.; Zhang, Q.; Chu, P.K.; Komarneni, S.; Lv, F.Z. Anomalous but massive removal of two organic dye pollutants simultaneously. J. Hazard. Mater. 2016, 318, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Vo, D.V.N. Critical review on hazardous pollutants in water environment: Occurrence, monitoring, fate, removal technologies and risk assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.Z.; Shen, H.Z. Ultrasound enhancement of the reduction of the Basic Green dye in wastewater by cast iron. J. Environ. Sci. 2006, 18, 1–3. [Google Scholar]
- Qamar, M.; Saquib, M.; Muneer, M. Photocatalytic degradation of two selected dye derivatives, chromotrope 2B and amido black 10B, in aqueous suspensions of titanium dioxide. Dye. Pigment. 2005, 65, 1–9. [Google Scholar] [CrossRef]
- Jeevanantham, S.; Saravanan, A.; Hemavathy, R.V.; Kumar, P.S.; Yaashikaa, P.R.; Yuvaraj, D. Removal of toxic pollutants from water environment by phytoremediation: A survey on application and future prospects. Environ. Technol. Inno. 2019, 13, 264–276. [Google Scholar] [CrossRef]
- Parvathi, V.P.; Parimaladevi, R.; Sathe, V.; Mahalingam, U. Graphene boosted silver nanoparticles as surface enhanced Raman spectroscopic sensors and photocatalysts for removal of standard and industrial dye contaminants. Sensor. Actuat. B-Chem. 2019, 281, 679–688. [Google Scholar]
- Kasten, F.H. Cytochemical studies with acridine orange and the influence of dye contaminants in the staining of nucleic acids. Int. Rev. Cytol. 1967, 21, 141–202. [Google Scholar]
- Zhao, S.G.; Yang, F.; Kong, F.; Li, B.F.; Xue, Z.L.; Wang, T. Decolorization of azo-type dye Direct Orange S catalyzed by laccase /mediator system. Chin. J. Environ. Eng. 2016, 10, 3912–3918. [Google Scholar]
- He, G.L.; Zhang, Y.T. BPNN simulating photocatalytic degradation of direct orange S. Comput. Appl. Chem. 2008, 25, 1359–1364. [Google Scholar]
- Zhong, J.B.; Li, J.Z.; Zeng, F.C. Photocatalytic Decolorization of Direct Orange S Solution by Phospho-tungstic Acid. J. Sichuan Nor. Univ. 2013, 36, 618–621. [Google Scholar]
- Yao, P.; Xing, T.L.; Chen, G.Q. Biosynthesis of Eucommia ulmoides silver nanoparticles and application thereof in reductive catalytic degradation of Direct Orange 26. J. Text. Res. 2018, 39, 104–110. [Google Scholar]
- Safa, Y.; Bhatti, H.N.; Bhatti, I.A.; Asgher, M. Removal of direct red-31 and direct orange-26 by low cost rice husk: Influence of immobilisation and pretreatments. Can. J. Chem. Eng. 2011, 89, 1554–1565. [Google Scholar] [CrossRef]
- Karthikeyeni, S.; Vijayakumar, T.S.; Vasanth, S.; Ganesh, A.; Vignesh, V.; Akalya, J.; Thirumurugan, R.; Subramanian, P. Decolourisation of Direct Orange S dye by ultra sonication using iron oxide nanoparticles. J. Exp. Nanosci. 2015, 10, 199–208. [Google Scholar] [CrossRef]
- Jadhav, J.P.; Phugare, S.S.; Dhanve, R.S.; Jadhav, S.B. Rapid biodegradation and decolorization of Direct Orange 39 (Orange TGLL) by an isolated bacterium Pseudomonas aeruginosa strain BCH. Biodegradation 2010, 21, 453–463. [Google Scholar] [CrossRef]
- Wolf, J.H.; Korf, J. 4-bromomethyl-7-methoxycoumarin and analogs as derivatization agents for high-performance liquid-chromatography determinations—A Review. J. Pharmaceut. Biomed. 1992, 10, 99–107. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F.; Bilal, M.; Iqbal, H.M.N. Biogenic synthesis and characterization of cobalt oxide nanoparticles for catalytic reduction of direct yellow-142 and methyl orange dyes. Biocaral. Agr. Biotechnol. 2019, 19, 101154. [Google Scholar] [CrossRef]
- Vasudevan, S.; Oturan, M.A. Electrochemistry: As cause and cure in water pollution-an overview. Environ. Chem. Lett. 2014, 12, 97–108. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Gao, G.D.; Liu, H. Electrochemical Carbon Nanotube Filter for Adsorption, Desorption, and Oxidation of Aqueous Dyes and Anions. J. Phys. Chem. 2011, 115, 3621–3629. [Google Scholar] [CrossRef]
- Bao, N.; Li, Y.; Yu, X.H.; Niu, J.J.; Wu, G.L.; Xu, X.H. Removal of anionic azo dye from aqueous solution via an adsorption-photosensitized regeneration process on a TiO2 surface. Environ. Sci. Pollut. Res. 2013, 20, 897–906. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Huang, S.S.; Dong, M.Z. Asphaltene deposition during CO2 flooding. Spe Prod. Facil. 1999, 14, 235–245. [Google Scholar] [CrossRef]
- Abejon, A.; Garea, A.; Irabien, A. Arsenic removal from drinking water by reverse osmosis: Minimization of costs and energy consumption. Sep. Purif. Technol. 2015, 144, 46–53. [Google Scholar] [CrossRef]
- Bofill-Mas, S.; Rusinol, M. Recent trends on methods for the concentration of viruses from water samples. Curr. Opin. Environ. Sci. Health 2020, 16, 7–13. [Google Scholar] [CrossRef]
- Luan, J.F.; Shen, Y.; Li, Y.Y.; Paz, Y. The Structural, Photocatalytic Property Characterization and Enhanced Photocatalytic Activities of Novel Photocatalysts Bi2GaSbO7 and Bi2InSbO7 during Visible Light Irradiation. Materials 2016, 9, 801. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- He, J.Y.; Jia, Q.M.; Wu, S.S.; Lv, H.T.; Shan, S.Y. Application of photocatalysis in wastewater treatment. New Chem. Mater. 2014, 42, 230–232. [Google Scholar]
- Ren, G.M.; Han, H.T.; Wang, Y.X.; Liu, S.T.; Zhao, J.Y.; Meng, X.C.; Li, Z.Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Bora, T.; Dutta, J. Applications of Nanotechnology in Wastewater Treatment-A Review. J. Nanosci. Nanotechno. 2014, 14, 613–626. [Google Scholar] [CrossRef]
- Xu, B.T.; Ahmed, M.B.; Zhou, J.L.; Altaee, A.; Wu, M.H.; Xu, G. Photocatalytic removal of perfluoroalkyl substances from water and wastewater: Mechanism, kinetics and controlling factors. Chemosphere 2017, 189, 717–729. [Google Scholar] [CrossRef]
- Akpotu, S.O.; Oseghe, E.O.; Ayanda, O.S.; Skelton, A.A.; Msagati, T.A.M.; Ofomaja, A.E. Photocatalysis and biodegradation of pharmaceuticals in wastewater: Effect of abiotic and biotic factors. Clean Technol. Environ. 2019, 21, 1701–1721. [Google Scholar] [CrossRef]
- Xu, J.; Wan, Y.P.; Huang, Y.L.; Wang, Y.R.; Qin, L.; Seo, H.J. Layered oxide semiconductor In2Fe2CuO7: Optical properties and visible-light responsive photocatalytic abilities. Mater. Lett. 2016, 179, 175–178. [Google Scholar] [CrossRef]
- Bu, Y.Y.; Chen, Z.Y.; Sun, C.J. Highly effificient Z-Scheme Ag3PO4/Ag/WO3 -x photocatalyst for its enhanced photocatalytic performance. Appl. Catal. B 2015, 179, 363–371. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, M.; Lin, L.; Zeng, Q.Q.; He, D.N. Synthesis of parallel squared nanosheet-assembled Bi2WO6 microstructures under alkalescent hydrothermal treatment. Ceram. Int. 2014, 40, 5831–5835. [Google Scholar] [CrossRef]
- Alemi, A.A.; Kashfifi, R.; Shabani, B. Preparation and characterization of novel Ln (Gd3+, Ho3+ and Yb3+)-doped Bi2MoO6 with Aurivillius layered structures and photocatalytic activities under visible light irradiation. J. Mol. Catal. A Chem. 2014, 392, 290–298. [Google Scholar] [CrossRef]
- Nazim, S.; Kousar, T.; Shahid, M.; Khan, M.A.; Nasar, G.; Sher, M.; Warsi, M.F. New graphene-CoxZn1 -xFe2O4 nano-heterostructures: Magnetically separable visible lightphotocatalytic materials. Ceram. Int. 2016, 42, 7647–7654. [Google Scholar] [CrossRef]
- Ghaffar, I.; Warsi, M.F.; Shahid, M.; Shakir, I. Unprecedented photocatalytic activity of carbon coated/MoO3 core-shell nanoheterostructurs under visible light irradiation. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 79, 1–7. [Google Scholar] [CrossRef]
- Kiransan, M.; Khataee, A.; Karaca, S.; Sheydaei, M. Artifificial neural network modeling of photocatalytic removal of a disperse dye using synthesized of ZnO nanoparticles on montmorillonite. Spectrochim. Acta A 2015, 140, 465–473. [Google Scholar] [CrossRef]
- Khataee, A.; Karimi, A.; Arefifi-Oskoui, S.; Soltani, R.D.C.; Hanifehpour, Y.; Soltani, B.; Joo, S.W. Sonochemical synthesis of Pr-doped ZnO nanoparticles for sonocatalytic degradation of Acid Red 17. Ultrason. Sonochem. 2015, 22, 371–381. [Google Scholar] [CrossRef]
- Yi, X.; Li, J.L. Synthesis and optical property of NaTaO3 nanofifibers prepared by electrospinning. J. Sol-Gel Sci. Technol. 2010, 53, 480–484. [Google Scholar] [CrossRef]
- Yang, J.X.; Akbarzadeh, J.; Maurer, C.; Peterlik, H.; Schubert, U. Sol-gel synthesis of ZnTiO3 using a single-source precursor based on p-carboxybenzaldehyde oxime as a linker. J. Mater. Chem. 2012, 22, 24034–24041. [Google Scholar] [CrossRef]
- Suresh, R.; Giribabu, K.; Manigandan, R.; Munusamy, S.; Kumar, S.P.; Muthamizh, S.; Stephen, A.; Narayanan, V. Doping of Co into V2O5 nanoparticles enhances of methylene blue. J. Alloy. Compd. 2014, 598, 151–160. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, X.H. Synthesis of novel visible light driven BiVO4 photocatalysts via microemulsion process and its photocatalytic performance. J. Inorg. Mater. 2009, 24, 453–456. [Google Scholar] [CrossRef]
- Han, Q.F.; Chen, L.; Wang, M.J.; Yang, X.J.; Lu, L.D.; Wang, X. Low-temperature synthesis of uniform Sb2S3 nanorods and its visible-light-driven photocatalytic activities. Mater. Sci. Eng. B 2010, 166, 118–121. [Google Scholar] [CrossRef]
- Yi, X.F.; Zheng, J.S.; Zhao, Y.B. Hydrothermal synthesis of CdS nanorods in NaOH solution. Chem. J. Chin. Univ. 2012, 33, 2597–2603. [Google Scholar]
- Yang, S.X.; Yue, Q.; Wu, F.M.; Huo, N.J.; Chen, Z.H.; Yang, J.H.; Li, J.B. Synthesis of the nanostructured Cd4GeS6 photocatalysts and their visible-light-driven photocatalytic degradation property. J. Alloy. Compd. 2014, 597, 91–94. [Google Scholar] [CrossRef]
- Wu, W.M.; Lin, R.; Shen, L.J.; Liang, R.W.; Yuan, R.S.; Wu, L. Visible-light-induced photocatalytic hydrogenation of 4-nitroaniline over In2S3 photocatalyst in water. Catal. Commun. 2013, 40, 1–4. [Google Scholar] [CrossRef]
- Anandan, S.; Rao, T.N.; Gopalan, R.; Ikuma, Y. Fabrication of visible-light-driven N-doped ordered mesoporous TiO2 photocatalysts and their photocatalytic applications. J. Nanosci. Nanotechnol. 2014, 14, 3181–3186. [Google Scholar] [CrossRef]
- Zou, Z.G.; Ye, J.H.; Arakawa, H. Photocatalytic behavior of a new series of In0.8M0.2TaO4 (M = Ni, Cu, Fe) photocatalysts in aqueous solutions. Catal. Lett. 2001, 75, 209–213. [Google Scholar] [CrossRef]
- Luan, J.F.; Li, M.; Ma, K.; Li, Y.M.; Zou, Z.G. Photocatalytic activity of novel Y2InSbO7 and Y2GdSbO7 nanocatalysts for degradation of environmental pollutant rhodamine B under visible light irradiation. Chem. Eng. J. 2011, 167, 162–171. [Google Scholar] [CrossRef]
- Luan, J.F.; Ma, K.; Pan, B.C.; Li, Y.M.; Wu, X.S.; Zou, Z.G. Synthesis and catalytic activity of new Gd2BiSbO7 and Gd2YSbO7 nanocatalysts. J. Mol. Catal. A-Chem. 2010, 321, 1–9. [Google Scholar] [CrossRef]
- Eder, D.; Motta, M.; Windle, A.H. Iron-doped Pt-TiO2 nanotubes for photo-catalytic water splitting. Nanotechnology 2009, 20, 055602. [Google Scholar] [CrossRef]
- Biswas, S.K.; Baeg, J.O. Enhanced photoactivity of visible light responsive W incorporated FeVO4 photoanode for solar water splitting. Int. J. Hydrog. Energy 2013, 38, 14451–14457. [Google Scholar] [CrossRef]
- Xu, X.L.; Song, W. Synthesis and photocatalytic activity of heterojunction ZnFe2O4-BiVO4. Mater. Technol. 2017, 32, 472–479. [Google Scholar] [CrossRef]
- Zong, X.; Yan, H.J.; Wu, G.P.; Ma, G.J.; Wen, F.Y.; Wang, L.; Li, C. Enhancement of photocatalytic H2 evolution on CdS by loading MOS2 as cocatalyst under visible light irradiation. J. Am. Chem. Soc. 2008, 130, 7176–7177. [Google Scholar] [CrossRef]
- Zong, X.; Han, J.F.; Ma, G.J.; Yan, H.J.; Wu, G.P.; Li, C. Photocatalytic H2 evolution on CdS loaded with WS2 as cocatalyst under visible light irradiation. J. Phys. Chem. C 2011, 115, 12202–12208. [Google Scholar] [CrossRef]
- Chen, X.F.; Zhang, J.; Huo, Y.N.; Li, H.X. Preparation and visible light catalytic activity of three-dimensional ordered macroporous CdS/TiO2 fifilms. Chin. J. Catal. 2013, 34, 949–955. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A. Photosensitization of ZnO by AgBr and Ag2CO3: Nanocomposites with tandem n-n heterojunctions and highly enhanced visible-light photocatalytic activity. J. Colloid Interface Sci. 2016, 474, 103–113. [Google Scholar] [CrossRef]
- Cao, J.; Xu, B.Y.; Lin, H.L.; Luo, B.D.; Chen, S.F. Novel Bi2S3 -sensitized BiOCl with highly visible light photocatalytic activity for the removal of rhodamine B. Catal. Commun. 2012, 26, 204–208. [Google Scholar] [CrossRef]
- Lu, H.J.; Xu, L.L.; Wei, B.; Zhang, M.Y.; Gao, H.; Sun, W.J. Enhanced photosensitization process induced by the p-n junction of Bi2O2CO3/BiOCl heterojunctions on the degradation of rhodamine B. Appl. Surf. Sci. 2014, 303, 360–366. [Google Scholar] [CrossRef]
- Biswas, S.; Sundstrom, V.; De, S. Facile synthesis of luminescent TiO2 nanorods using an anionic surfactant: Their photosensitization and photocatalytic effificiency. Mater. Chem. Phys. 2014, 147, 761–771. [Google Scholar] [CrossRef]
- Han, C.C.; Ge, L.; Chen, C.F.; Li, Y.J.; Xiao, X.L.; Zhang, Y.N.; Guo, L.L. Novel visible light induced Co3O4 -g-C3N4 heterojunction photocatalysts for effificient degradation of methyl orange. Appl. Catal. B Environ. 2014, 147, 546–553. [Google Scholar] [CrossRef]
- Xie, T.P.; Liu, C.L.; Xu, L.J.; Yang, J.; Zhou, W. Novel heterojunction Bi2O3/SrFe12O19 magnetic photocatalyst with highly enhanced photocatalytic activity. J. Phys. Chem. C 2013, 117, 24601–24610. [Google Scholar] [CrossRef]
- Dai, K.; Lv, J.L.; Lu, L.H.; Liang, C.H.; Geng, L.; Zhu, G.P. A facile fabrication of plasmonic g-C3N4/Ag2WO4/Ag ternary heterojunction visible-light photocatalyst. Mater. Chem. Phys. 2016, 177, 529–537. [Google Scholar] [CrossRef]
- Wang, X.F.; Hu, H.M.; Chen, S.H.; Zhang, K.H.; Zhang, J.; Zou, W.S.; Wang, R.X. One-step fabrication of BiOCl/CuS heterojunction photocatalysts with enhanced visible-light responsive activity. Mater. Chem. Phys. 2015, 158, 67–73. [Google Scholar] [CrossRef]
- Luan, J.F.; Wang, S.; Ma, K.; Li, Y.M.; Pan, B.C. Structural Property and Catalytic Activity of New In2YbSbO7 and Gd2YbSbO7 Nanocatalysts under Visible Light Irradiation. J. Phys. Chem. C. 2010, 114, 9398–9407. [Google Scholar] [CrossRef]
- Sun, S.F.; Wu, Y.F.; Zhang, X.; Zhang, Z.J.; Yan, Y.; Guan, W.S. Enhanced visible-light-driven photocatalytic degradation performance of cip on BiVO4-Bi2WO6 nano-heterojunction photocatalysts. Nona 2014, 9, 1450015. [Google Scholar] [CrossRef]
- Yang, B.Y.; Li, H.; Shang, N.Z.; Feng, C.; Gao, S.T.; Wang, C. Visible-Light Responsive Photocatalyst g-C3N4@BiOCl with Hollow Flower-like Structure: Preparation and Photocatalytic Performance. Chin. J. Inorg. Chem. 2017, 33, 396–404. [Google Scholar]
- Nguyen, T.B.; Doong, R.A. Heterostructured ZnFe2O4/TiO2 nanocomposites with a highly recyclable visible-light-response for bisphenol A degradation. RSC Adv. 2017, 7, 50006–50016. [Google Scholar] [CrossRef] [Green Version]
- Simsek, E.B.; Kilic, B.; Asgin, M.; Akan, A. Graphene oxide based heterojunction TiO2-ZnO catalysts with outstanding photocatalytic performance for bisphenol-A, ibuprofen and flurbiprofen. J. Ind. Eng. Chem. 2018, 59, 115–126. [Google Scholar] [CrossRef]
- Zhang, C.J.; Li, N.J.; Chen, D.Y.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. The ultrasonic-induced-piezoelectric enhanced photocatalytic performance of ZnO/CdS nanofibers for degradation of bisphenol A. J. Alloy. Compd. 2021, 885, 160987. [Google Scholar] [CrossRef]
- Luo, Y.D.; Wei, X.Q.; Gao, B.; Zou, W.X.; Zheng, Y.L.; Yang, Y.C.; Zhang, Y.; Tong, Q.; Dong, L. Synergistic adsorption-photocatalysis processes of graphitic carbon nitrate (g-C3N4) for contaminant removal: Kinetics, models, and mechanisms. Chem. Eng. J. 2019, 375, 122019. [Google Scholar] [CrossRef]
- Vo, T.S.; Vo, T.T.B.C.; Suk, J.W.; Kim, K. Recycling performance of graphene oxide-chitosan hybrid hydrogels for removal of cationic and anionic dyes. Nano Converg. 2020, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Zyoud, A.H.; Saleh, F.; Helal, M.H.; Shawahna, R.; Hilal, H.S. Anthocyanin-Sensitized TiO2 Nanoparticles for Phenazopyridine Photodegradation under Solar Simulated Light. J. Nanomater. 2018, 2018, 2789616. [Google Scholar] [CrossRef] [Green Version]
- Kohantorabi, M.; Moussavi, G.; Oulego, P.; Giannakis, S. Heterogeneous catalytic ozonation and peroxone-mediated removal of Acetaminophen using natural and modified hematite-rich soil, as efficient and environmentally friendly catalysts. Appl. Catal. B-Environ. 2022, 301, 120786. [Google Scholar] [CrossRef]
- Rostami, R.; Moussavi, G.; Darbari, S.; Jafari, A.J. Non-thermal plasma by positive corona glow discharge using nano-structured Cu/CuO coated electrodes for benzene removal from air flow; removal enhancement and energy efficiency improvement. Sep. Purif. Technol. 2021, 275, 119156. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Mohammadi, S.; Oulego, P.; Giannakis, S. Photocatalytic activation of peroxymonosulfate (PMS) by novel mesoporous Ag/ZnO@NiFe2O4 nanorods, inducing radical-mediated acetaminophen degradation under UVA irradiation. Chemosphere 2021, 277, 130271. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Oulego, P.; Giannakis, S. Radical-based degradation of sulfamethoxazole via UVA/PMS-assisted photocatalysis, driven by magnetically separable Fe3O4@CeO2@BiOI nanospheres. Sep. Purif. Technol. 2021, 267, 118665. [Google Scholar] [CrossRef]
- Khavar, A.H.C.; Moussavi, G.; Mahjoub, A.R.; Satari, M.; Abdolmaleki, P. Synthesis and visible-light photocatalytic activity of In, S-TiO2@rGO nanocomposite for degradation and detoxification of pesticide atrazine in water. Chem. Eng. J. 2018, 345, 300–311. [Google Scholar] [CrossRef]
- Namini, A.S.; Delbari, S.A.; Mousavi, M.; Ghasemi, J.B. Synthesis and characterization of novel ZnO/NiCr2O4 nanocomposite for water purification by degradation of tetracycline and phenol under visible light irradiation. Mater. Res. Bull. 2021, 139, 111247. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Mousavi, M.; Ghasemi, J.B.; Delbari, S.A.; Le, Q.V.; Asl, M.S.; Shokouhimehr, M.; Mohammadi, M.; Azizian-Kalandaragh, Y.; Namini, A.S. Synthesis, characterization, and photocatalytic performance of Ag/AgFeO2 decorated on g-C3N4-nanosheet under the visible light irradiation. J. Taiwan Inst. Chem. Eng. 2020, 115, 279–292. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Mousavi, M.; Ghasemi, J.B.; Delbari, S.A.; Le, Q.V.; Namini, A.S.; Asl, M.S.; Shokouhimehr, M.; Azizian-Kalandaragh, Y.; Mohammadi, M. Z-scheme g-C3N4 nanosheet/MgBi2O6 systems with the visible light response for impressive photocatalytic organic contaminants degradation. J. Photoch. Photobio. A. 2021, 406, 113023. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Mousavi, M.; Ghasemi, J.B.; Le, Q.V.; Delbari, S.A.; Asl, M.S.; Shokouhimehr, M.; Mohammadi, M.; Azizian-Kalandaragh, Y.; Namini, A.S. In situ preparation of g-C3N4 nanosheet/FeOCl: Achievement and promoted photocatalytic nitrogen fixation activity. J. Colloid. Interf. Sci. 2021, 587, 538–549. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Mousavi, M.; Ghasemi, J.B.; Le, Q.V.; Delbari, S.A.; Namini, A.S.; Asl, M.S.; Shokouhimehr, M.; Jang, H.W.; Mohammadi, M. 8g-C3N4 nanosheet adorned with Ag3BiO3 as a perovskite: An effective photocatalyst for efficient visible-light photocatalytic processes. Mater. Sci. Semicon. Proc. 2021, 125, 105651. [Google Scholar] [CrossRef]
- Wang, J.H.; Zou, Z.G.; Ye, J.H. Synthesis, Structure and Photocatalytic Property of a New Hydrogen Evolving Photocatalyst Bi2InTaO7. Mater. Sci. Forum. 2003, 423–425, 485–490. [Google Scholar] [CrossRef]
- Kohno, M.; Ogura, S.; Sato, K.; Inoue, Y. Properties of photocatalysts with tunnel structures: Formation of a surface lattice O− radical by the UV irradiation of BaTi4O9 with a pentagonal-prism tunnel structure. Chem. Phys. Lett. 1997, 267, 72–76. [Google Scholar] [CrossRef]
- Kudo, A.; Kato, H.; Nakagawa, S. Water Splitting into H2 and O2 on New Sr2M2O7 (M = Nb and Ta) Photocatalysts with Layered Perovskite Structures: Factors Affecting the Photocatalytic Activity. J. Phys. Chem. B 2000, 104, 571–575. [Google Scholar] [CrossRef]
- Nowak, M.; Kauch, B.; Szperlich, P. Determination of energy band gap of nanocrystalline SbSI. Rev. Sci. Instrum. 2009, 80, 046107. [Google Scholar] [CrossRef]
- Zhou, F.; Kang, K.; Maxisch, T.; Ceder, G.; Morgan, D. The electronic structure and band gap of LiFePO4 and LiMnPO4. Solid State Commun. 2004, 132, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Tauc, J.; Grigorov, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis with YFeO3 electrodes. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Cui, B.Y.; Cui, H.T.; Li, Z.R.; Dong, H.Y.; Li, X.; Zhao, L.F.; Wang, J.W. Novel Bi3O5I2 hollow microsphere and its enhanced photocatalytic activity. Catalysts 2019, 9, 709. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, W.; Cantillo, A.; Salazar, B.; Diaz-Uribe, C.; Ramos, W.; Romero, E.; Hurtado, M. Comparative study of ZnO thin films doped with transition metals (Cu and Co) for methylene blue photodegradation under visible irradiation. Catalysts 2020, 10, 528. [Google Scholar] [CrossRef]
- Deonikar, V.G.; Patil, S.S.; Tamboli, M.S.; Ambekar, J.D.; Kulkarni, M.V.; Panmand, R.P.; Umarji, G.G.; Shinde, M.D.; Rane, S.B.; Munirathnam, N.R.; et al. Growth study of hierarchical Ag3PO4/LaCO3OH heterostructures. Phys. Chem. Chem. Phys. 2017, 19, 20541–20550. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Tamboli, M.S.; Deonikar, V.G.; Umarji, G.G.; Ambekar, J.D.; Kulkarni, M.V.; Kolekar, S.S.; Kale, B.B.; Patil, D.R. Magnetically separable Ag3PO4/NiFe2O4 composites with enhanced photocatalytic activity. Dalton Trans. 2015, 44, 20426–20434. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.B.; Yuan, X.Z.; Zeng, G.M.; Liang, J.; Chen, X.H.; Yu, H.B.; Wang, H.; Wu, Z.B.; Zhang, J.; Xiong, T. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant. Appl. Catal. B 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Cao, W.; Jiang, C.Y.; Chen, C.; Zhou, H.F.; Wang, Y.P. A novel Z-scheme CdS/Bi4O5Br2 heterostructure with mechanism analysis: Enhanced photocatalytic performance. J. Alloys Compd. 2021, 861, 158554. [Google Scholar] [CrossRef]






| Atomy | x | y | z | Occupation Factor |
|---|---|---|---|---|
| Bi | 0 | 0 | 0 | 1 |
| Sm | 0.5 | 0.5 | 0.5 | 0.5 |
| Sb | 0.5 | 0.5 | 0.5 | 0.5 |
| O(1) | −0.185 | 0.125 | 0.125 | 1 |
| O(2) | 0.125 | 0.125 | 0.125 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, J.; Ma, B.; Yao, Y.; Liu, W.; Niu, B.; Yang, G.; Wei, Z. Synthesis, Performance Measurement of Bi2SmSbO7/ZnBiYO4 Heterojunction Photocatalyst and Photocatalytic Degradation of Direct Orange within Dye Wastewater under Visible Light Irradiation. Materials 2022, 15, 3986. https://doi.org/10.3390/ma15113986
Luan J, Ma B, Yao Y, Liu W, Niu B, Yang G, Wei Z. Synthesis, Performance Measurement of Bi2SmSbO7/ZnBiYO4 Heterojunction Photocatalyst and Photocatalytic Degradation of Direct Orange within Dye Wastewater under Visible Light Irradiation. Materials. 2022; 15(11):3986. https://doi.org/10.3390/ma15113986
Chicago/Turabian StyleLuan, Jingfei, Bingbing Ma, Ye Yao, Wenlu Liu, Bowen Niu, Guangmin Yang, and Zhijie Wei. 2022. "Synthesis, Performance Measurement of Bi2SmSbO7/ZnBiYO4 Heterojunction Photocatalyst and Photocatalytic Degradation of Direct Orange within Dye Wastewater under Visible Light Irradiation" Materials 15, no. 11: 3986. https://doi.org/10.3390/ma15113986







