Influence of Fe and Cu Co-Doping on Structural, Magnetic and Electrochemical Properties of CeO2 Nanoparticles
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. XRD Analysis
3.2. Morphological Analysis
3.3. UV-Visible Spectroscopy
3.4. Raman Spectroscopy
3.5. Magnetic Properties
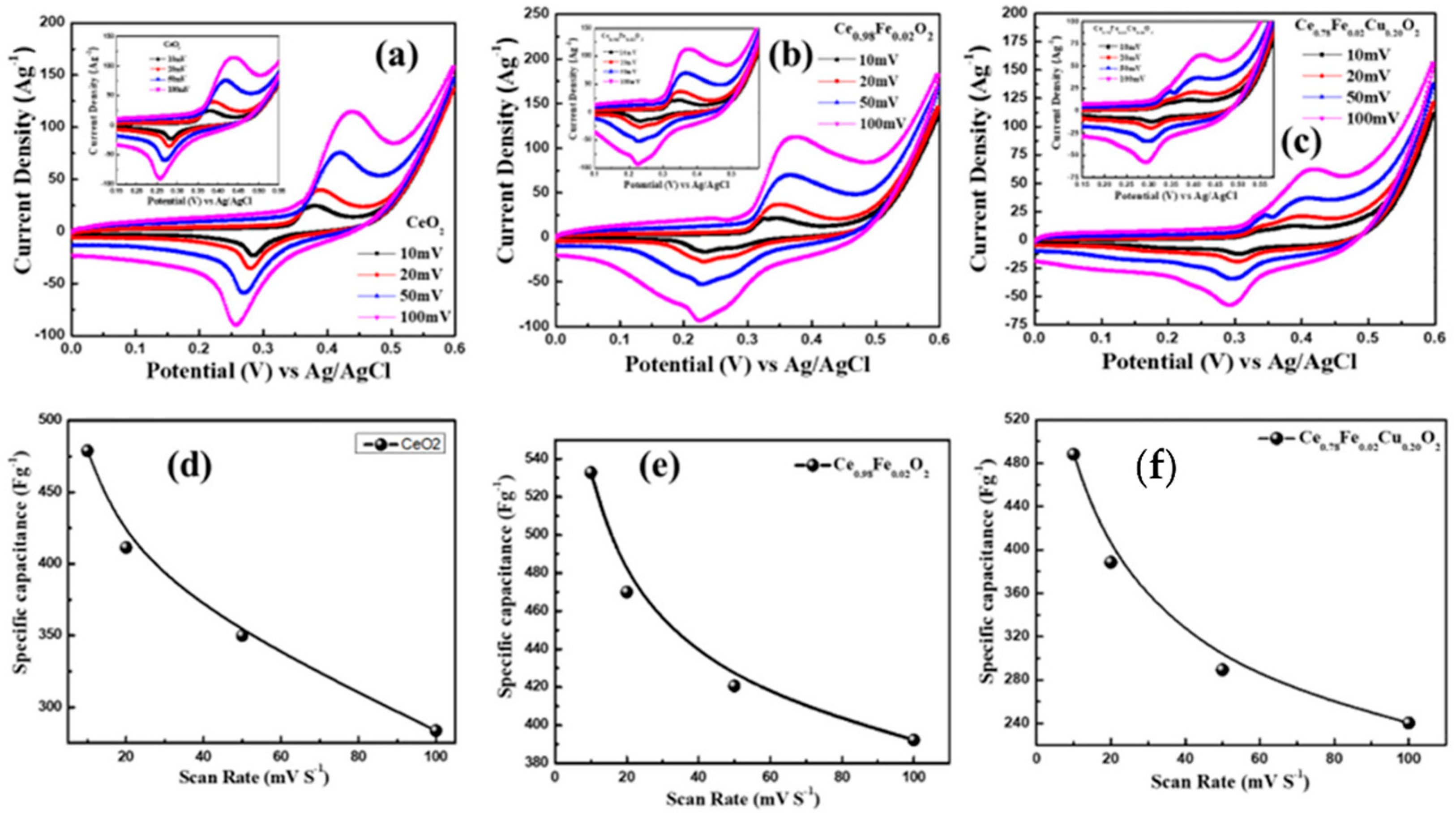
3.6. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, M.A.; Catto, A.C.; Longo, E.; Nossol, E.; Lima, R.C. Characterization and electrochemical performance of CeO2 and Eu-doped CeO2 films as a manganese redox flow battery component. J. Rare Earths 2018, 36, 1074–1083. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, Q.A.; Li, M.G.; Huang, X.J.; Fang, B.; Wang, L. CeO2 nanoparticles decorated multi-walled carbon nanotubes for electrochemical determination of guanine and adenine. Electrochim. Acta 2011, 56, 8571–8575. [Google Scholar] [CrossRef]
- Jeyaranjan, A.; Sakthivel, T.S.; Molinari, M.; Sayle, D.C.; Seal, S. Morphology and Crystal Planes Effects on Supercapacitance of CeO2 Nanostructures: Electrochemical and Molecular Dynamics Studies. Part. Part. Syst. Charact. 2018, 35, 1800176. [Google Scholar] [CrossRef]
- Rajendran, S.; Khan, M.M.; Gracia, F.; Qin, J.; Gupta, V.K.; Arumainathan, S. Ce3+-ion-induced visible-light photocatalytic degradation and electrochemical activity of ZnO/CeO2 nanocomposite. Sci. Rep. 2016, 6, 31641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.-Q.; Zhang, H.-W.; Wen, Q.-Y.; Peng, L.; Xiao, J.Q. Direct evidence of oxygen vacancy mediated ferromagnetism of Co doped CeO 2 thin films on Al2O3 (0001) substrates. J. Phys. Condens. Matter 2008, 20, 255210. [Google Scholar] [CrossRef]
- Coey, M.; Ackland, K.; Venkatesan, M.; Sen, S. Collective magnetic response of CeO2 nanoparticles. Nat. Phys. 2016, 12, 694–699. [Google Scholar] [CrossRef]
- Anwar, M.S.; Kumar, S.; Arshi, N.; Ahmed, F.; Seo, Y.J.; Koo, B.H. Structural and optical study of samarium doped cerium oxide thin films prepared by electron beam evaporation. J. Alloys Compd. 2018, 752, 520–531. [Google Scholar] [CrossRef]
- Skorodumova, N.V.; Simak, S.I.; Lundqvist, B.I.; Abrikosov, I.A.; Johansson, B. Quantum Origin of the Oxygen Storage Capability of Ceria. Phys. Rev. Lett. 2002, 89, 166601. [Google Scholar] [CrossRef]
- Maqbool, Q.; Nazar, M.; Naz, S.; Hussain, T.; Jabeen, N.; Kausar, R.; Anwaar, S.; Abbas, F.; Jan, T.; Maqbool, Q.; et al. Antimicrobial potential of green synthesized CeO2 nanoparticles from Olea europaea leaf extract. Int. J. Nanomed. 2016, 11, 5015–5025. [Google Scholar] [CrossRef] [Green Version]
- Chueh, W.C.; Haile, S.M. Electrochemical studies of capacitance in cerium oxide thin films and its relationship to anionic and electronic defect densities. Phys. Chem. Chem. Phys. 2009, 11, 8144–8148. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; He, H.; Zhang, Y.; Qin, X.; Wang, B. Oxygen vacancy clusters essential for the catalytic activity of CeO2 nanocubes for o-xylene oxidation. Sci. Rep. 2017, 7, 12845. [Google Scholar] [CrossRef] [PubMed]
- Tsunekawa, S.; Fukuda, T.; Kasuya, A. Blue shift in ultraviolet absorption spectra of monodisperse CeO2-xnanoparticles. J. Appl. Phys. 2000, 87, 1318–1321. [Google Scholar] [CrossRef]
- Sharma, S.K.; Thakur, P.; Kumar, S.; Shukla, D.K.; Brookes, N.B.; Lee, C.G.; Pirota, K.R.; Koo, B.H.; Knobel, M. Room temperature ferromagnetism in Fe-doped CeO2 thin films grown on LaAlO3 (001). Thin Solid Film. 2010, 519, 410–413. [Google Scholar] [CrossRef]
- Soni, S.; Chouhan, N.; Meena, R.K.; Kumar, S.; Dalela, B.; Mishra, M.; Meena, R.S.; Gupta, G.; Kumar, S.; Alvi, P.A.; et al. Electronic Structure and Room Temperature Ferromagnetism in Gd-doped Cerium Oxide Nanoparticles for Hydrogen Generation via Photocatalytic Water Splitting. Glob. Chall. 2019, 3, 1800090. [Google Scholar] [CrossRef]
- Bhatti, S.; Sbiaa, R.; Hirohata, A.; Ohno, H.; Fukami, S.; Piramanayagam, S.N. Spintronics based random access memory: A review. Mater. Today 2017, 20, 530–548. [Google Scholar] [CrossRef]
- Dietl, T.; Ohno, H.; Matsukura, F.; Cibert, J.; Ferrand, D. Zener Model Description of Ferromagnetism in Zinc-Blende Magnetic Semiconductors. Science 2000, 287, 1019–1022. [Google Scholar] [CrossRef] [Green Version]
- Ohno, H. Properties of ferromagnetic III–V semiconductors. J. Magn. Magn. Mater. 1999, 200, 110–129. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, M.; Aljawfi, R.N.; Chae, K.H.; Kumar, R.; Dalela, S.; Alshoaibi, A.; Ahmed, F.; Alvi, P.A. Tailoring the structural, electronic structure and optical properties of Fe: SnO2 nanoparticles. J. Electron Spectros. Relat. Phenom. 2020, 240, 146934. [Google Scholar] [CrossRef]
- Deng, D.; Chen, N.; Xiao, X.; Du, S.; Wang, Y. Electrochemical performance of CeO2 nanoparticle-decorated graphene oxide as an electrode material for supercapacitor. Ionics 2017, 23, 121–129. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Chiba, K.; Egashira, M.; Favier, F.; Long, J.; Miller, J.R.; Morita, M.; Naoi, K.; Simon, P.; et al. Materials for electrochemical capacitors. In Springer Handbook of Electrochemical Energy; Springer: Berlin, Germany, 2017; pp. 495–561. [Google Scholar] [CrossRef]
- Maheswari, N.; Muralidharan, G. Supercapacitor Behavior of Cerium Oxide Nanoparticles in Neutral Aqueous Electrolytes. Energy Fuels 2015, 29, 8246–8253. [Google Scholar] [CrossRef]
- Prasanna, K.; Santhoshkumar, P.; Jo, Y.N.; Sivagami, I.N.; Kang, S.H.; Joe, Y.C.; Lee, C.W. Highly porous CeO2 nanostructures prepared via combustion synthesis for supercapacitor applications. Appl. Surf. Sci. 2018, 449, 454–460. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, S.; Dalela, B.; Kumar, S.; Alvi, P.A.; Dalela, S. Defects and oxygen vacancies tailored structural and optical properties in CeO2 nanoparticles doped with Sm3+ cation. J. Alloys Compd. 2018, 752, 520–531. [Google Scholar] [CrossRef]
- Ahmed, F.; Kumar, S.; Arshi, N.; Anwar, M.S.; Koo, B.H.; Lee, C.G. Defect induced room temperature ferromagnetism in well-aligned ZnO nanorods grown on Si (100) substrate. Thin Solid Film. 2011, 519, 8199–8202. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, Y.J.; Koo, B.H.; Gautam, S.; Chae, K.H.; Kumar, R.; Lee, C.G. Room temperature ferromagnetism in chemically synthesized ZnO rods. Mater. Lett. 2009, 63, 194–196. [Google Scholar] [CrossRef] [Green Version]
- Kumari, K.; Naji, R.; Katharria, Y.S.; Dwivedi, S.; Chae, K.H.; Kumar, R.; Alshoaibi, A.; Alvi, P.A.; Dalela, S.; Kumar, S. Study the contribution of surface defects on the structural, electronic structural, magnetic and photocatalyst properties of Fe: CeO2 nanoparticles. J. Electron Spectros. Relat. Phenom. 2019, 235, 29–39. [Google Scholar] [CrossRef]
- Kumari, K.; Aljawfi, R.N.; Chawla, A.K.; Kumar, R.; Alvi, P.A.; Alshoaibi, A.; Vij, A.; Ahmed, F.; Abu-samak, M.; Kumar, S. Engineering the optical properties of Cu doped CeO2 NCs for application in white LED. Ceram. Int. 2020, 46, 7482–7488. [Google Scholar] [CrossRef]
- Swatsitang, E.; Phokha, S.; Hunpratub, S.; Maensiri, S. Characterization of Sm-doped CeO2 nanoparticles and their magnetic properties. Phys. B Condens. Matter 2016, 485, 14–20. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Douvalis, A.P.; Fitzgerald, C.B.; Venkatesan, M. Ferromagnetism in Fe-doped SnO2 thin films. Appl. Phys. Lett. 2004, 84, 1332–1334. [Google Scholar] [CrossRef] [Green Version]
- Ohno, H.; Sen, A.; Matsukura, F.; Oiwa, A.; Endo, A.; Katsumoto, S.; Lye, Y. (Ga, Mn) As: A new diluted magnetic semiconductor based on GaAs. Appl. Phys. Lett. 1996, 69, 363–365. [Google Scholar] [CrossRef]
- Ahmed, F.; Almutairi, G.; Alotaibi, B.; Kumar, S.; Arshi, N. Binder-Free Electrode Based on ZnO Nanorods Directly Grown on Aluminum Substrate for High-Performance Supercapacitors. Nanomaterials 2020, 10, 1979. [Google Scholar] [CrossRef]
- Padmanathan, N.; Selladurai, S. Shape controlled synthesis of CeO2 nanostructures for high performance supercapacitor electrodes. RSC Adv. 2014, 4, 6527–6534. [Google Scholar] [CrossRef]
- Aravinda, L.S.; Bhat, K.U.; Bhat, B.R. Nano CeO2/activated carbon based composite electrodes for high performance supercapacitor. Mater. Lett. 2013, 112, 158–161. [Google Scholar] [CrossRef]

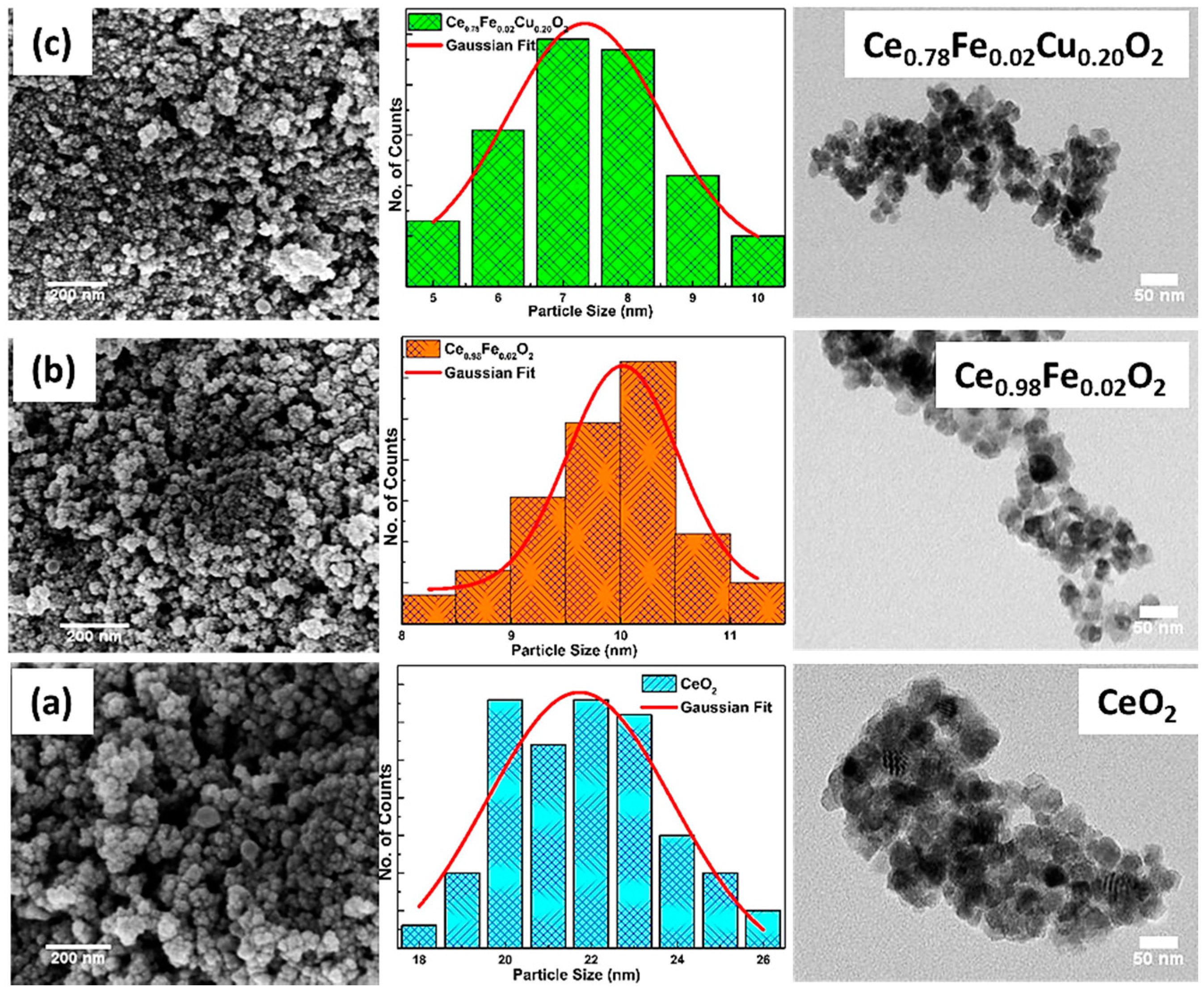
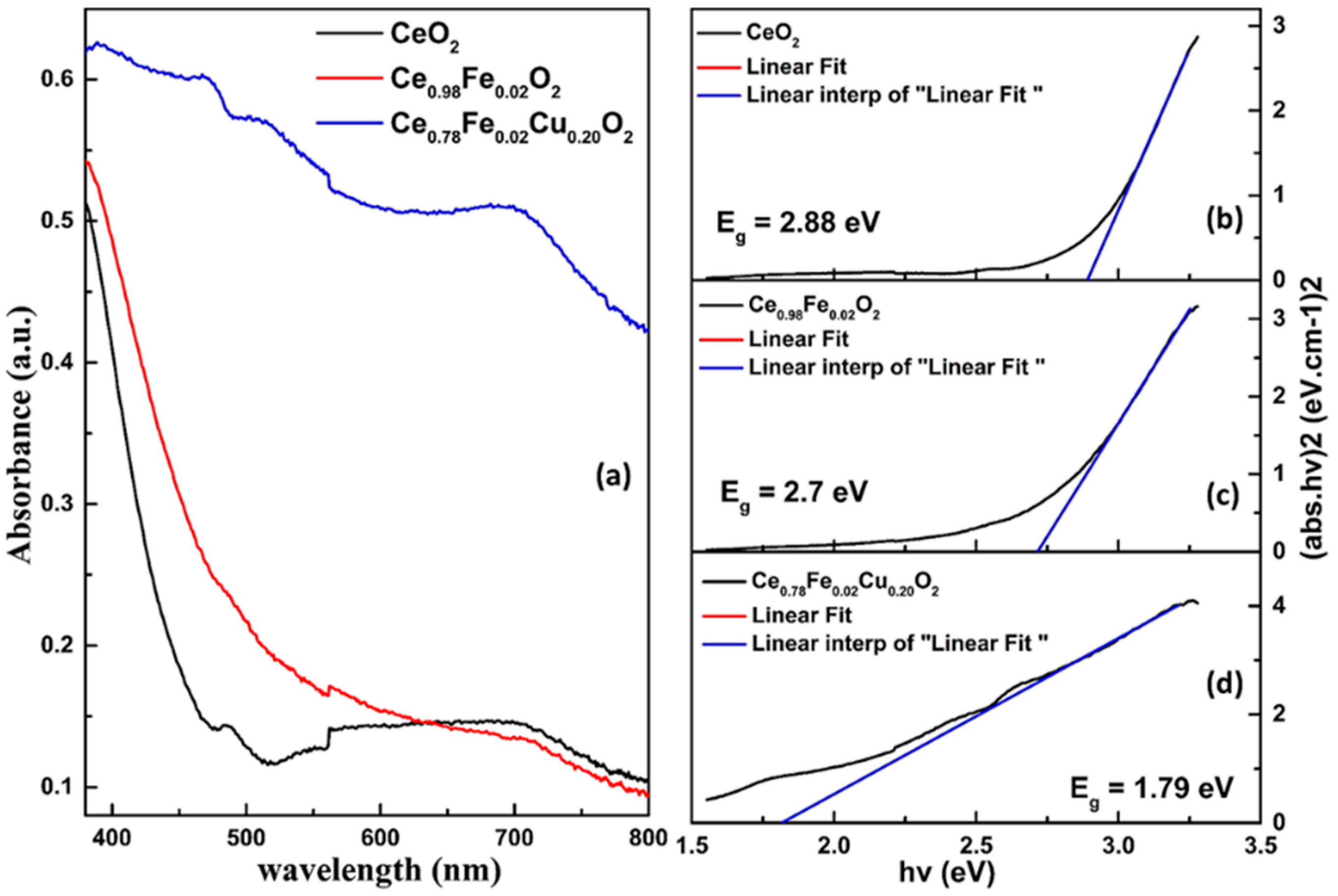
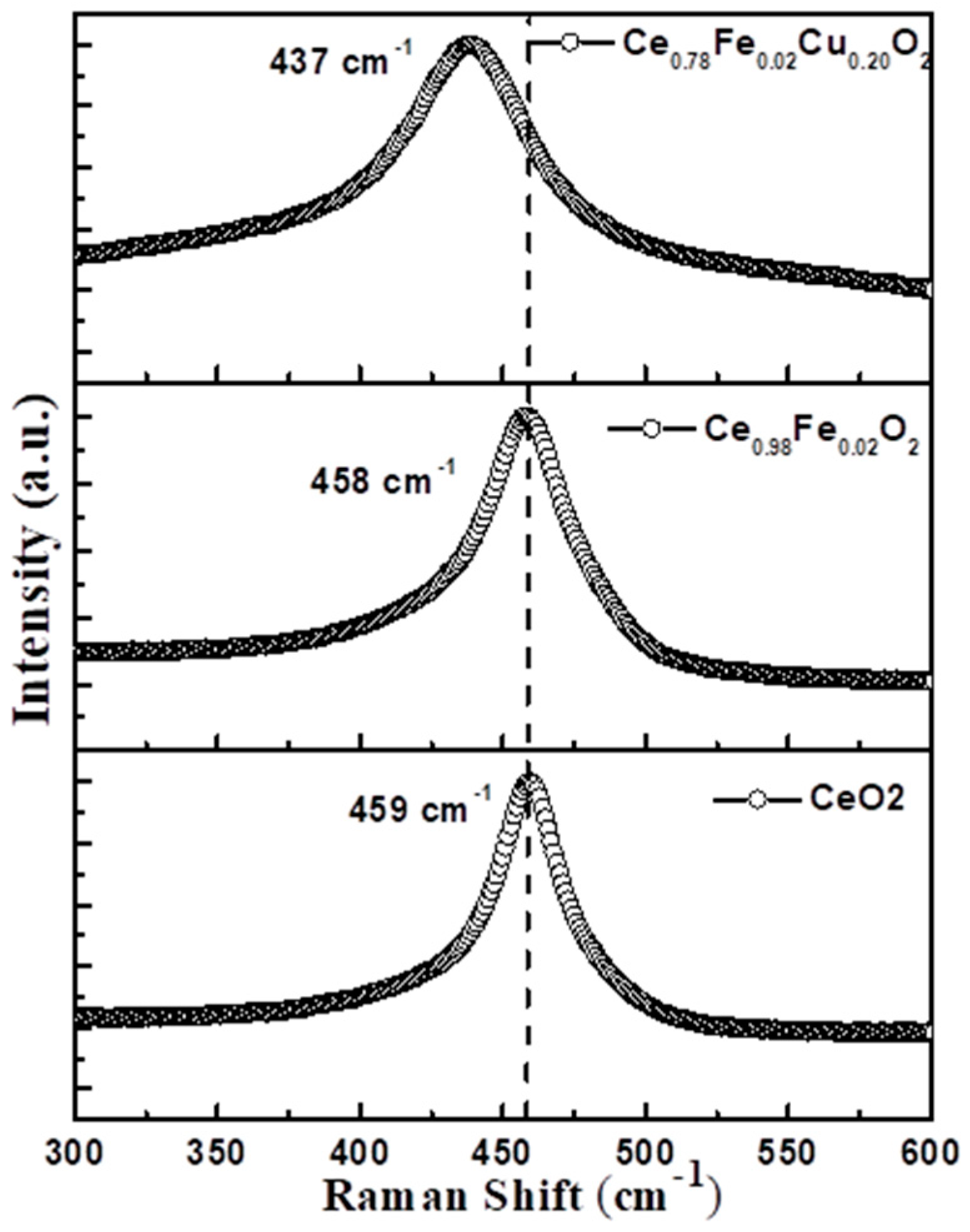
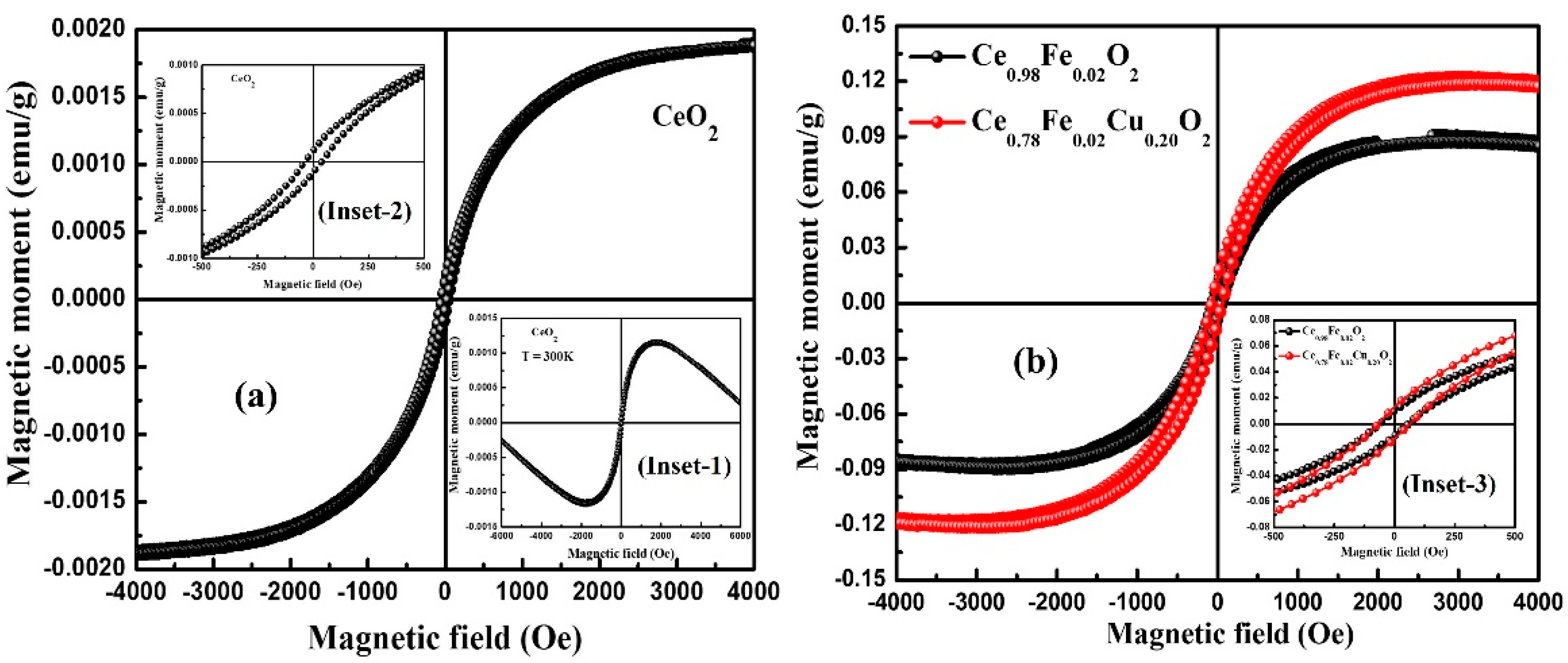

| Sample | D (nm) | d(111) (nm) | a (Å) | Strain ×10−2 | Eg (eV) |
|---|---|---|---|---|---|
| CeO2 | 10.25 | 0.3142 | 5.442 | 1.02 | 2.88 |
| Ce0.98Fe0.02O2 | 8.57 | 0.3145 | 5.447 | 1.21 | 2.7 |
| Ce0.78Fe0.02Cu0.20O2 | 5.59 | 0.3137 | 5.434 | 2.22 | 1.79 |
| Sample | MR (emu/g) | HC (Oe) | MS (emu/g) |
|---|---|---|---|
| CeO2 | 1.1 × 10−4 | 38.0 | 1.9 × 10−3 |
| Ce0.98Fe0.02O2 | 9.7 × 10−3 | 60.0 | 9.0 × 10−2 |
| Ce0.78Fe0.02Cu0.20O2 | 13 × 10−3 | 68.0 | 12.2 × 10−2 |
| Electrode Materials | Specific Capacitance (F g−1) | Ref. |
|---|---|---|
| CeO2 | 81.18 | [19] |
| CeO2/GO (1:1) | 45.29 | |
| CeO2/GO (1:2) | 82.35 | |
| CeO2/GO (1:4) | 382.94 | |
| CeO2/GO (1:5) | 18.37 | |
| CeO2 nanorods | 162.47 | [3] |
| CeO2 nanocubes | 149.03 | |
| CeO2 nanoparticle | 87.73 | |
| CeO2 | 400 | [32] |
| Nano CeO2/activated carbon | 162 | [33] |
| CeO2 | 487 | This work |
| Ce0.98Fe0.02O2 | 532 | |
| Ce0.78Fe0.02Cu0.20O2 | 488 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Ahmed, F.; Ahmad, N.; Shaalan, N.M.; Kumar, R.; Alshoaibi, A.; Arshi, N.; Dalela, S.; Alvi, P.A.; Kumari, K. Influence of Fe and Cu Co-Doping on Structural, Magnetic and Electrochemical Properties of CeO2 Nanoparticles. Materials 2022, 15, 4119. https://doi.org/10.3390/ma15124119
Kumar S, Ahmed F, Ahmad N, Shaalan NM, Kumar R, Alshoaibi A, Arshi N, Dalela S, Alvi PA, Kumari K. Influence of Fe and Cu Co-Doping on Structural, Magnetic and Electrochemical Properties of CeO2 Nanoparticles. Materials. 2022; 15(12):4119. https://doi.org/10.3390/ma15124119
Chicago/Turabian StyleKumar, Shalendra, Faheem Ahmed, Naushad Ahmad, Nagih M. Shaalan, Rajesh Kumar, Adil Alshoaibi, Nishat Arshi, Saurabh Dalela, Parvez Ahmad Alvi, and Kavita Kumari. 2022. "Influence of Fe and Cu Co-Doping on Structural, Magnetic and Electrochemical Properties of CeO2 Nanoparticles" Materials 15, no. 12: 4119. https://doi.org/10.3390/ma15124119










