Wettability and Surface Roughness of Parylene C on Three-Dimensional-Printed Photopolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Sample Characterization
3. Results
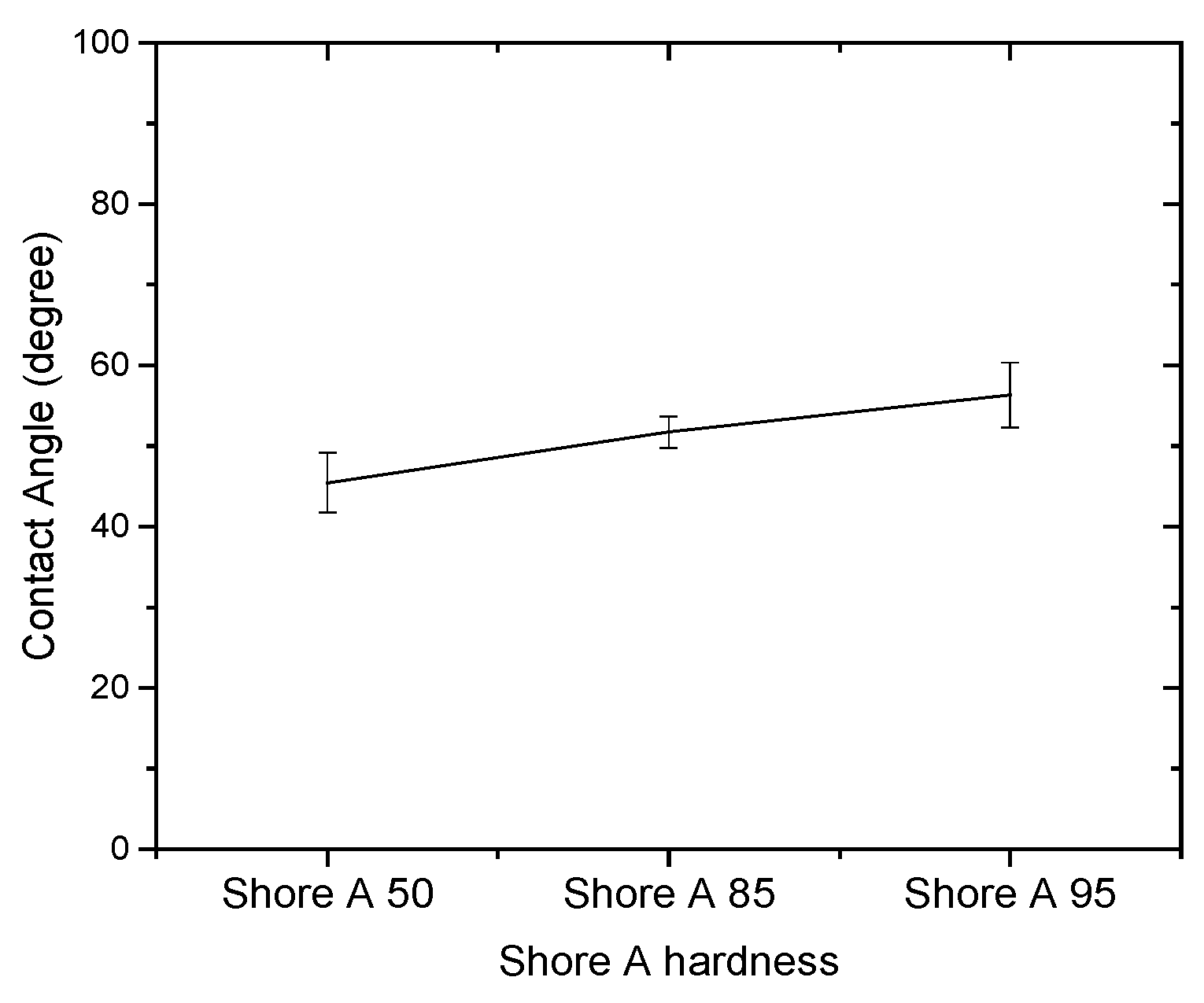
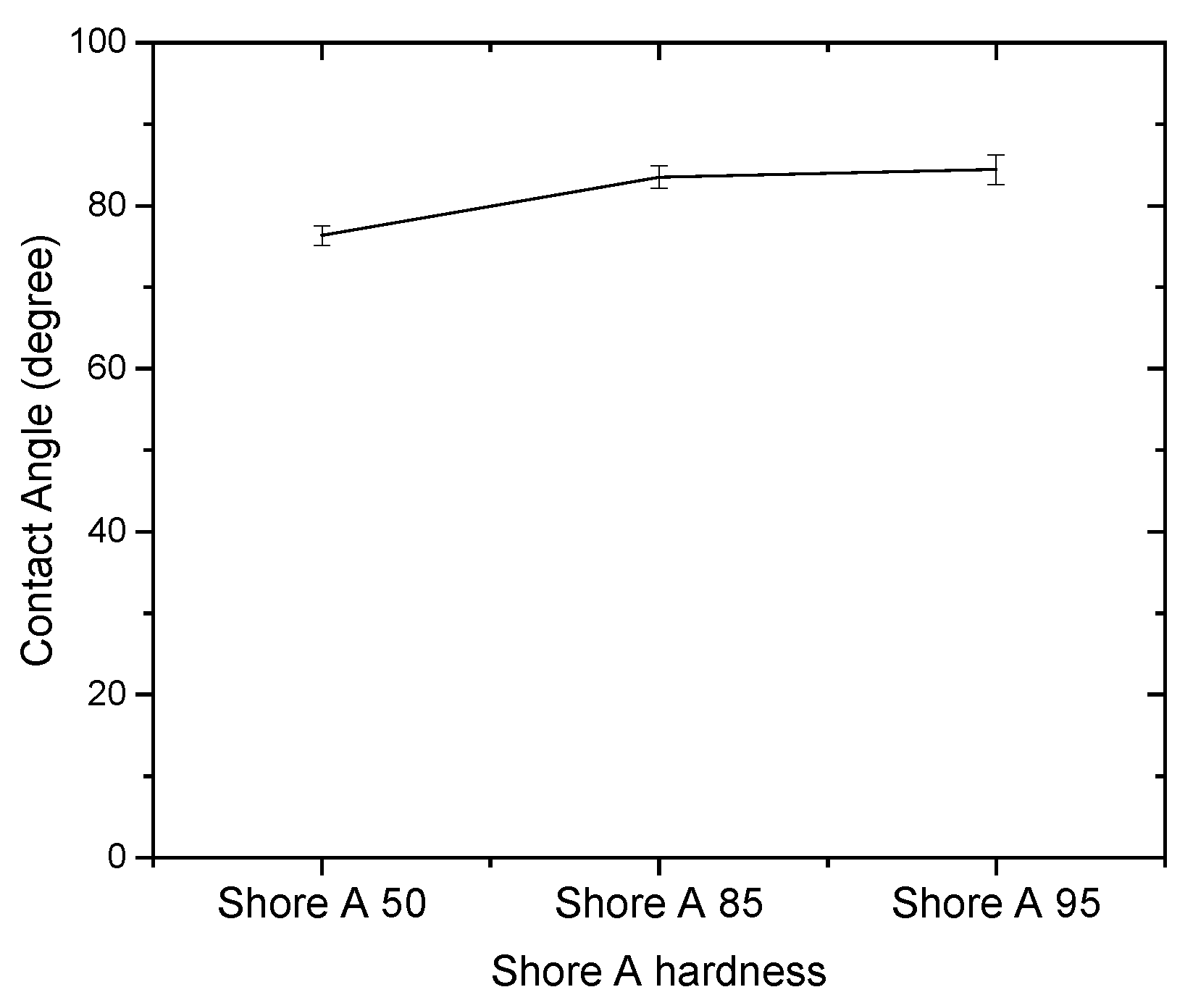
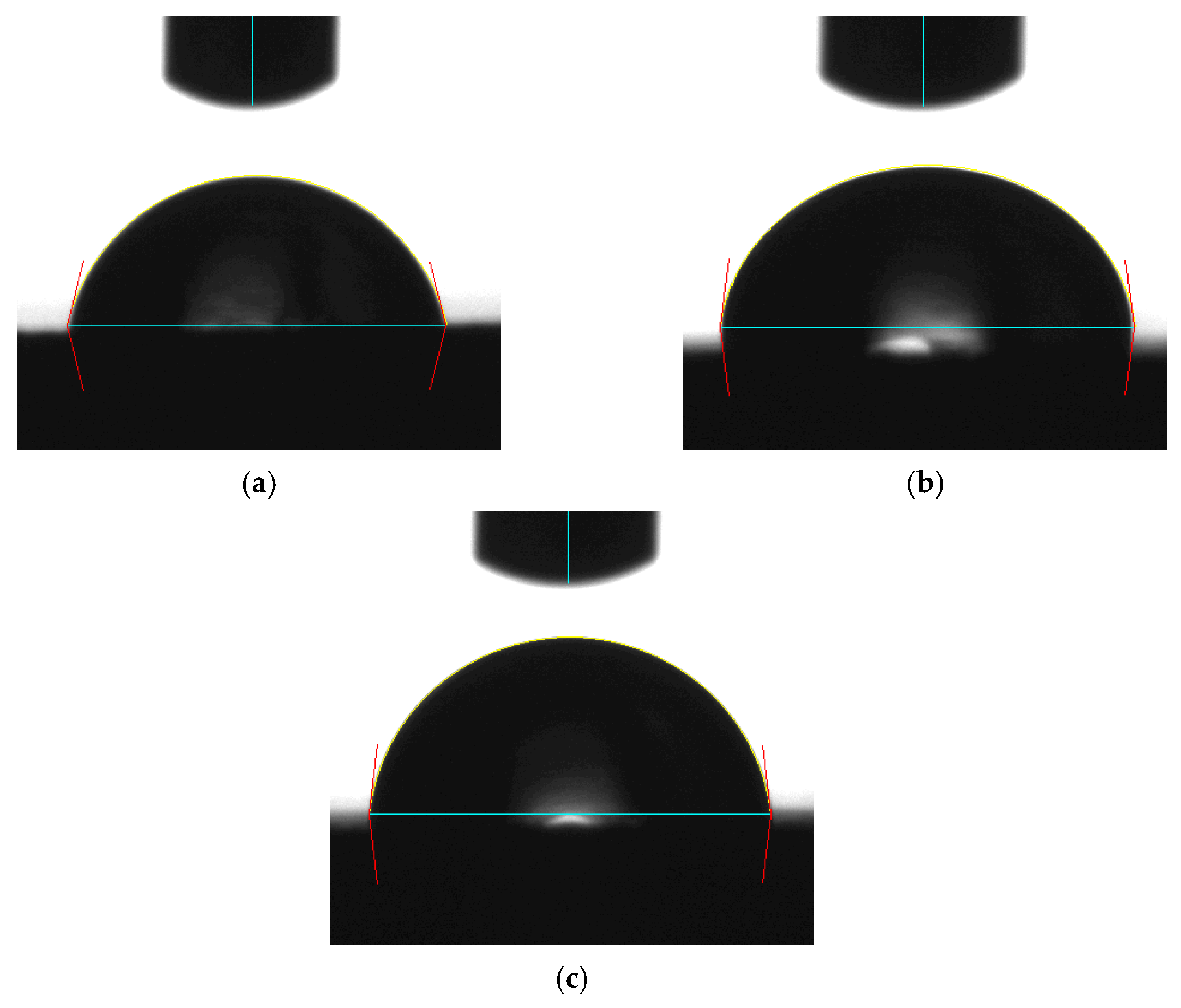
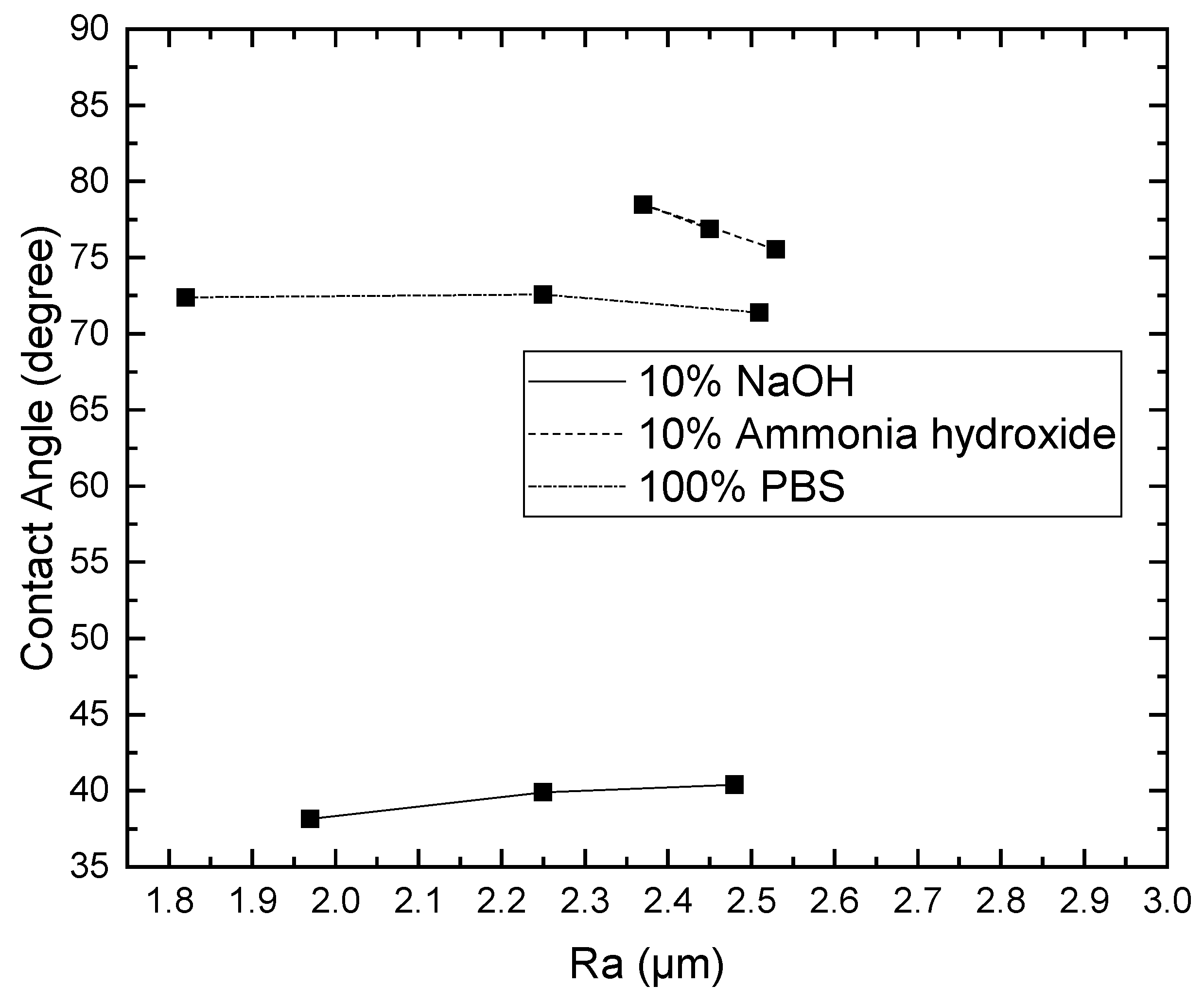
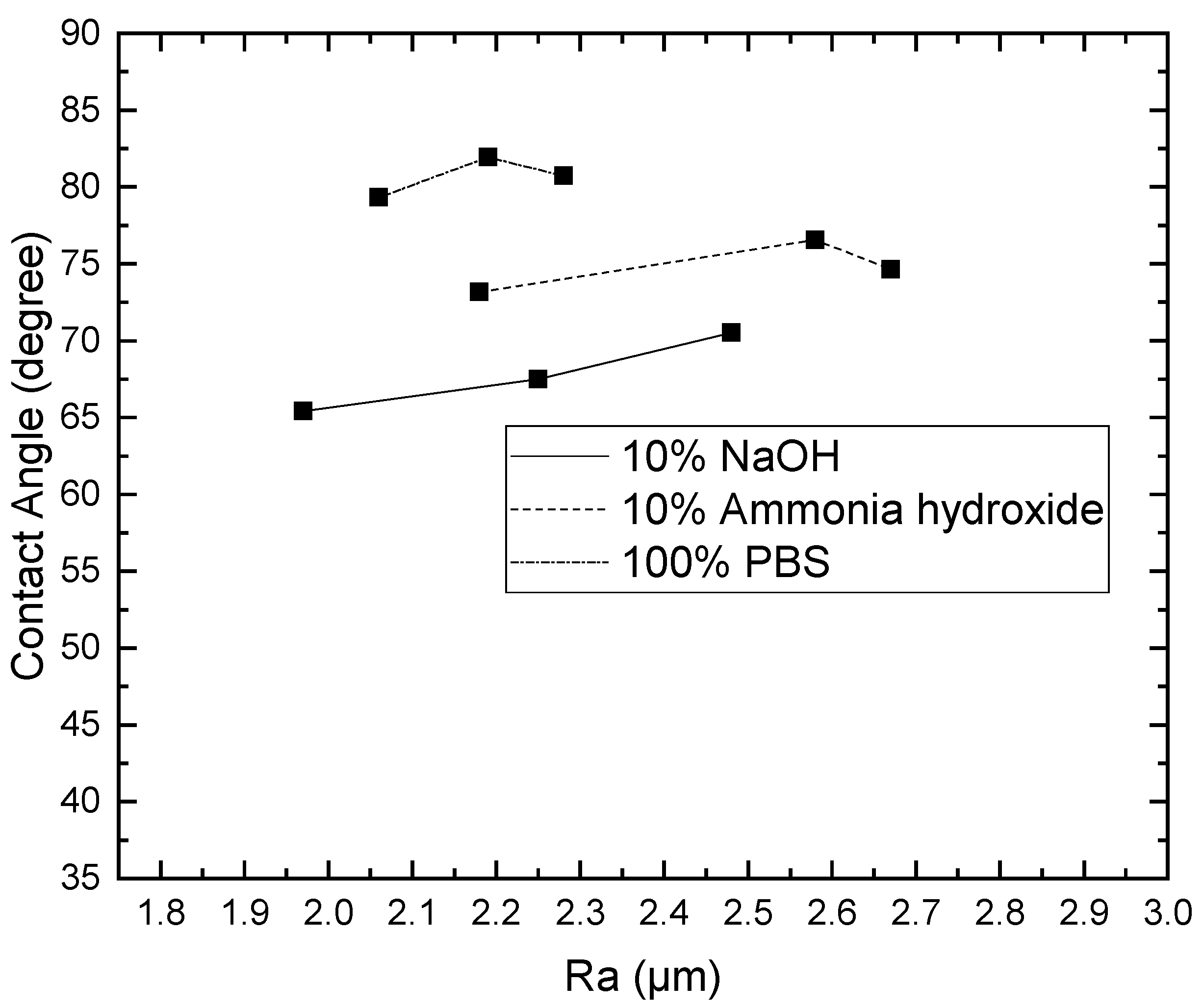
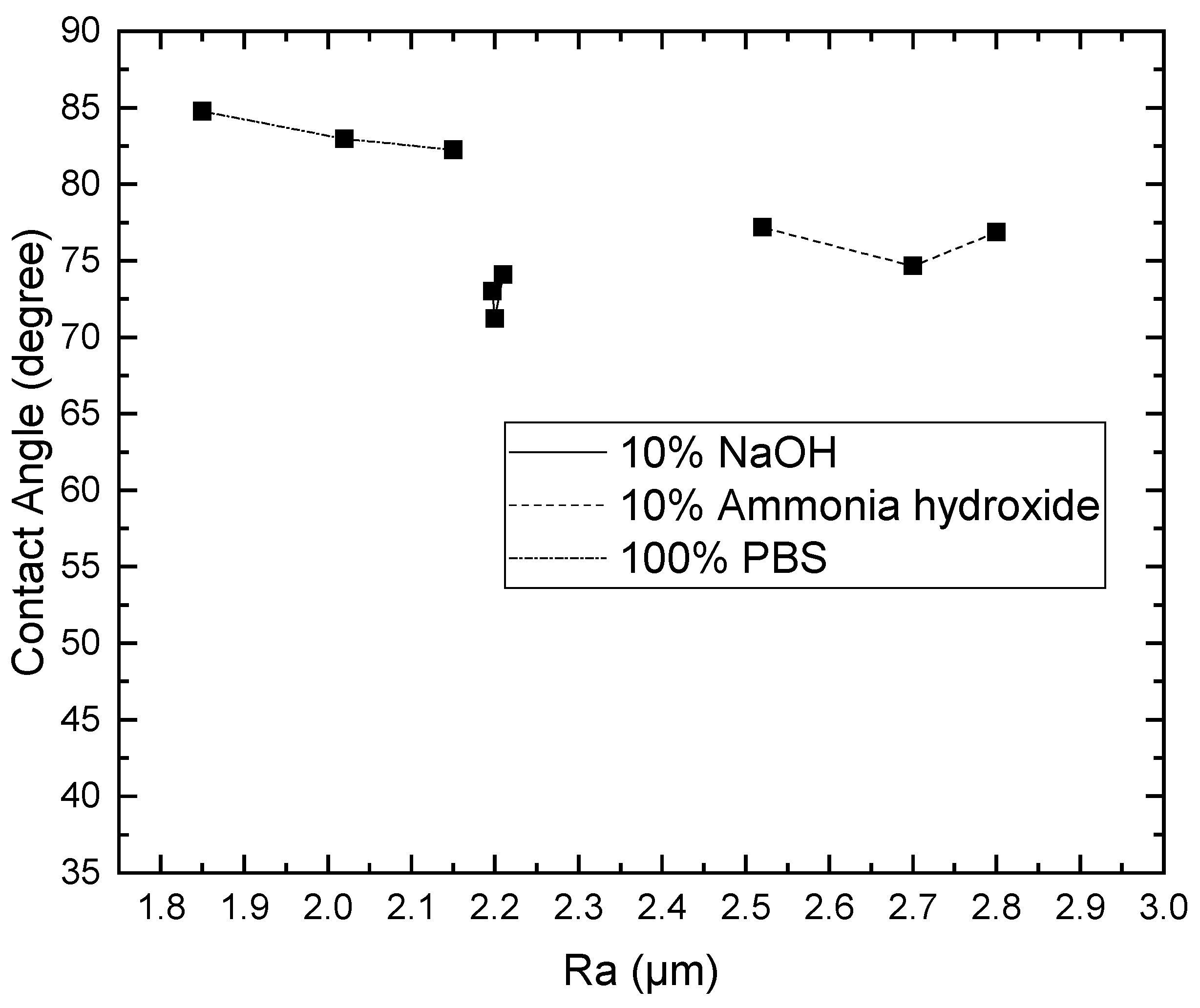
3.1. Effect of Solutions on the Contact Angle of Parylene C
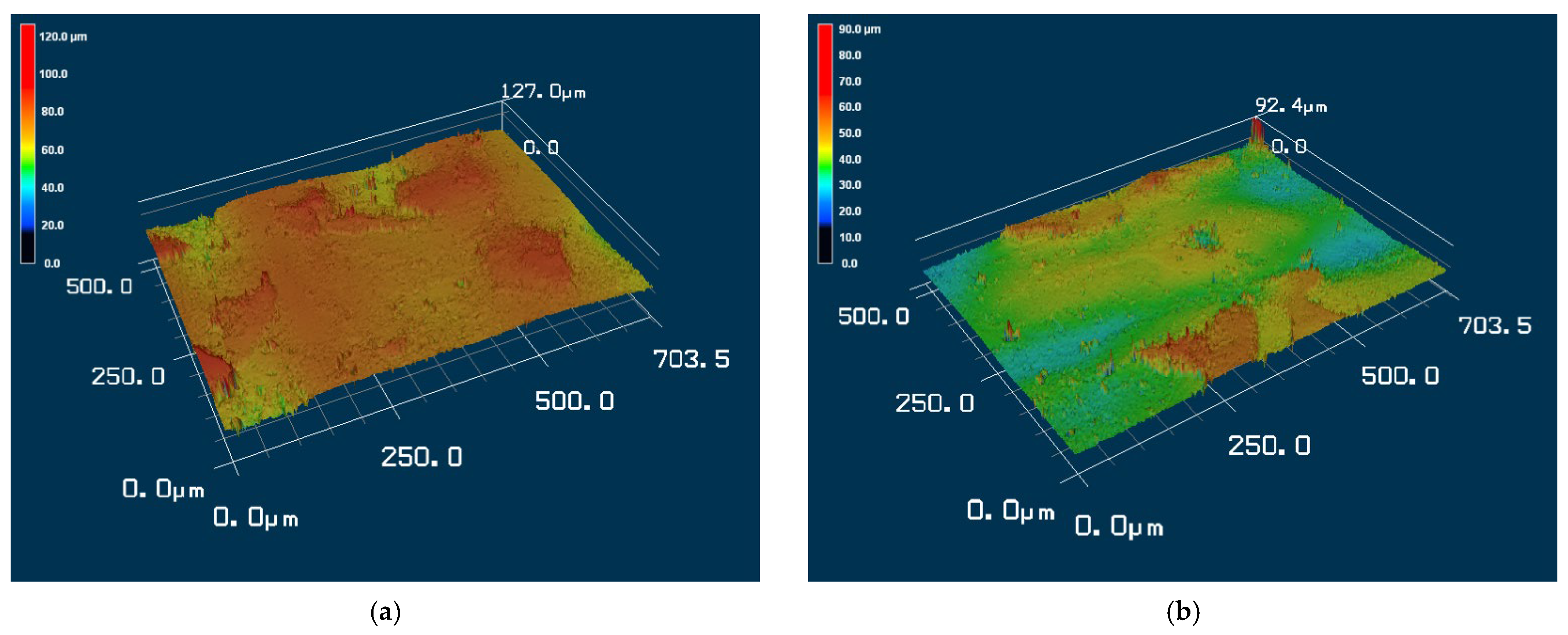
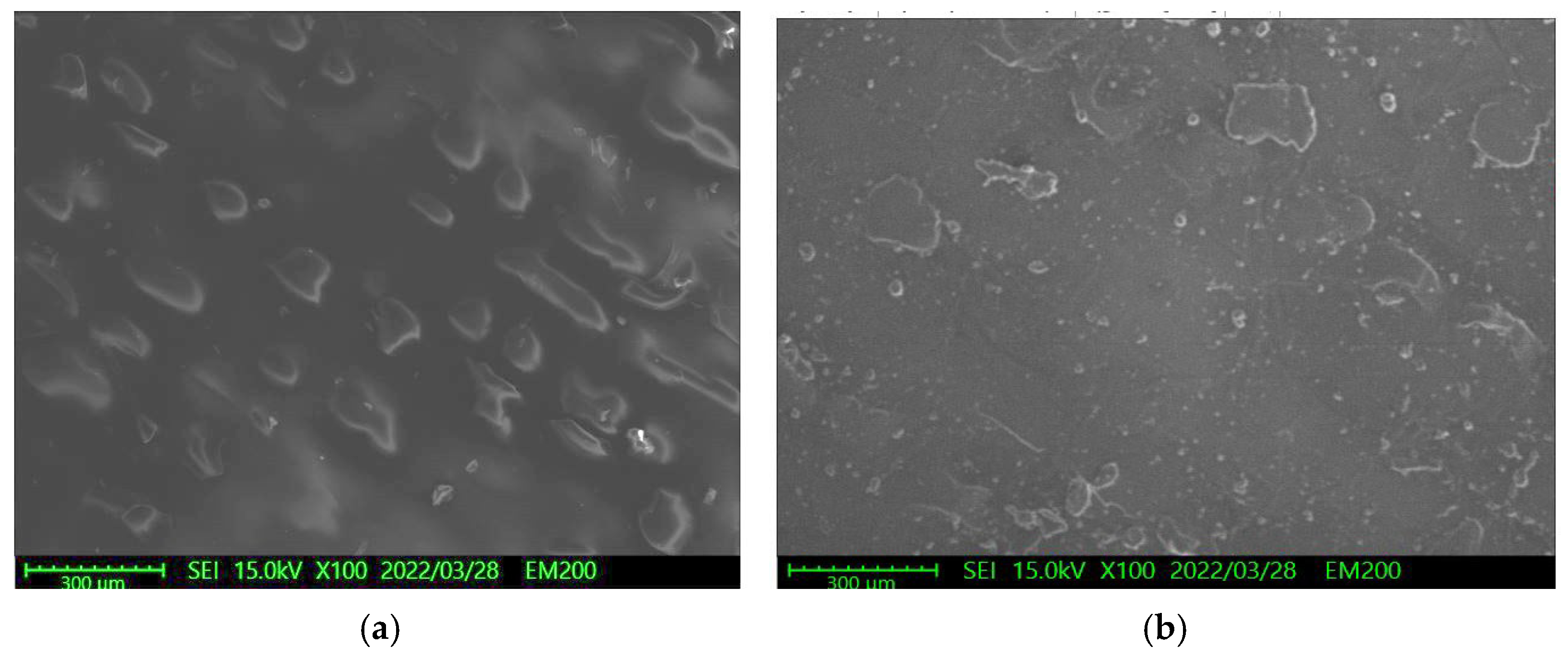
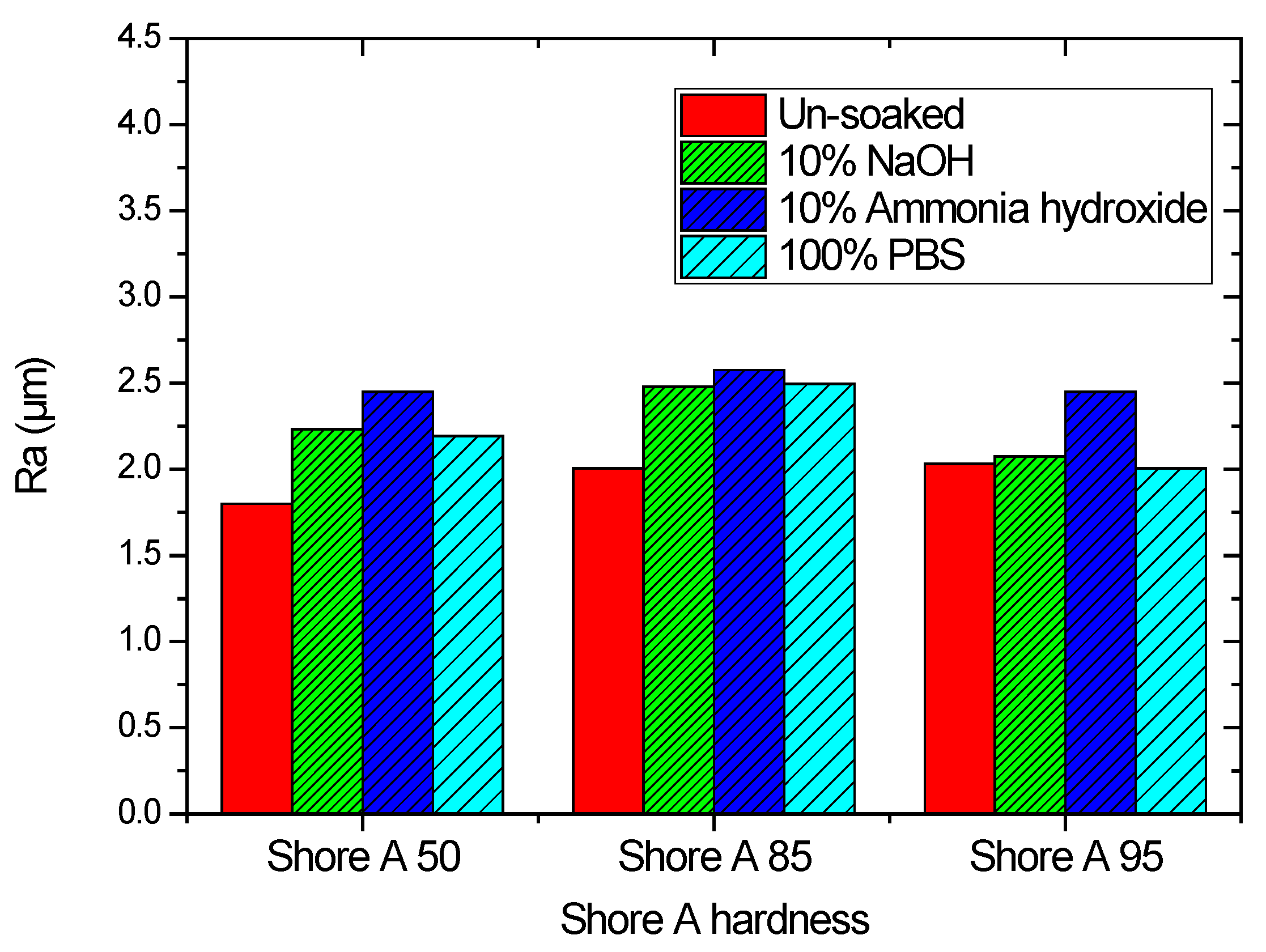
3.2. Effect of Solutions on the Surface Roughness of Parylene C
- (a)
- For the substrate with Shore A hardness 50 that was coated with Parylene C and soaked in 10% sodium hydroxide
- (b)
- For the substrate with Shore A hardness 50 that was coated with Parylene C and soaked in 10% ammonium hydroxide
- (c)
- For the substrate with Shore A hardness 50 that was coated with Parylene C and soaked in 100% PBS
- (d)
- For the substrate with Shore A hardness 85 that was coated with Parylene C and soaked in 10% sodium hydroxide
- (e)
- For the substrate with Shore A hardness 85 that was coated with Parylene C and soaked in 10% ammonium hydroxide
- (f)
- For the substrate with Shore A hardness 85 that was coated with Parylene C and soaked in 100% PBS
- (g)
- For the substrate with Shore A hardness 95 that was coated with Parylene C and soaked in 10% sodium hydroxide
- (h)
- For the substrate with Shore A hardness 95 that was coated with Parylene C and soaked in 10% ammonium hydroxide
- (i)
- For the substrate with Shore A hardness 95 that was coated with Parylene C and soaked in 100% PBS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, Z.; Yoshida, K.; Kim, J.W. Fast packaging by a partially-crosslinked SU-8 adhesive tape for microfluidic sensors and actuators. Sens. Actuators A Phys. 2019, 289, 77–86. [Google Scholar] [CrossRef]
- Borók, A.; Laboda, K.; Bonyár, A. PDMS Bonding Technologies for Microfluidic Applications: A Review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Constantin, C.P.; Aflori, M.; Damian, R.F.; Rusu, R.D. Biocompatibility of Polyimides: A Mini-Review. Materials 2019, 12, 3166. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Meng, E. Micromachining of Parylene C for bioMEMS. Polym. Adv. Technol. 2016, 27, 564–576. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 45,753,301, 11 March 1986. [Google Scholar]
- Vijayan, S.; Parthiban, P.; Hashimoto, M. Evaluation of Lateral and Vertical Dimensions of Micromolds Fabricated by a PolyJet™ Printer. Micromachines 2021, 12, 302. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Mott, E.J.; Busso, M.; Luo, X.; Dolder, C.; Wang, M.O.; Fisher, J.P.; Dean, D. Digital micromirror device (DMD)-based 3D printing of poly(propylene fumarate) scaffolds. Mater. Sci. Eng. C 2016, 61, 301–311. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet Printing for Materials and Devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Chung, M.; Radacsi, N.; Robert, C.; McCarthy, E.D.; Callanan, A.; Conlisk, N.; Hoskins, P.R.; Koutsos, V. On the optimization of low-cost FDM 3D printers for accurate replication of patient-specific abdominal aortic aneurysm geometry. 3D Print. Med. 2018, 4, 2. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Cesarano, J. A Review of Robocasting Technology. MRS Online Proc. Libr. 1998, 542, 133–139. [Google Scholar] [CrossRef]
- Luong, D.X.; Subramanian, A.K.; Silva, G.A.L.; Yoon, J.; Cofer, S.; Yang, K.; Owuor, P.S.; Wang, T.; Wang, Z.; Lou, J.; et al. Laminated Object Manufacturing of 3D-Printed Laser-Induced Graphene Foams. Adv. Mater. 2018, 30, 1707416. [Google Scholar] [CrossRef] [PubMed]
- Gayer, C.; Ritter, J.; Bullemer, M.; Grom, S.; Jauer, L.; Meiners, W.; Pfister, A.; Reinauer, F.; Vučak, M.; Wissenbach, K.; et al. Development of a solvent-free polylactide/calcium carbonate composite for selective laser sintering of bone tissue engineering scaffolds. Mater. Sci. Eng. C 2019, 101, 660–673. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Attar, H. Selective Laser Melting of Titanium Alloys and Titanium Matrix Composites for Biomedical Applications: A Review. Adv. Eng. Mater. 2016, 18, 463–475. [Google Scholar] [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M.C. Additive manufacturing: Technology, applications and research needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Zhai, Y.; Lados, D.A.; Brown, E.J.; Vigilante, G.N. Fatigue crack growth behavior and microstructural mechanisms in Ti-6Al-4V manufactured by laser engineered net shaping. Int. J. Fatigue 2016, 93, 51–63. [Google Scholar] [CrossRef]
- Brandhoff, L.; van den Driesche, S.; Lucklum, F.; Vellekoop, M.J. Creation of hydrophilic microfluidic devices for biomedical application through stereolithography. In Proceedings of the Bio-MEMS and Medical Microdevices II, Barcelona, Spain, 5–6 May 2015; Volume 9518, p. 95180D. [Google Scholar]
- Zhang, J.M.; Ji, Q.; Duan, H. Three-Dimensional Printed Devices in Droplet Microfluidics. Micromachines 2019, 10, 754. [Google Scholar] [CrossRef]
- Macdonald, N.P.; Cabot, J.M.; Smejkal, P.; Guijt, R.M.; Paull, B.; Breadmore, M.C. Comparing microfluidic performance of three-dimensional (3D) printing platforms. Anal. Chem. 2017, 89, 3858–3866. [Google Scholar] [CrossRef]
- Li, F.; Macdonald, N.P.; Guijt, R.M.; Breadmore, M.C. Using printing orientation for tuning fluidic behavior in microfluidic chips made by fused deposition modeling 3D printing. Anal. Chem. 2017, 89, 12805–12811. [Google Scholar] [CrossRef]
- Rehmani, M.A.A.; Jaywant, S.A.; Arif, K.M. Study of Microchannels Fabricated Using Desktop Fused Deposition Modeling Systems. Micromachines 2021, 12, 14. [Google Scholar] [CrossRef]
- Ortigoza-Diaz, J.; Scholten, K.; Larson, C.; Cobo, A.; Hudson, T.; Yoo, J.; Baldwin, A.; Weltman Hirschberg, A.; Meng, E. Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems. Micromachines 2018, 9, 422. [Google Scholar] [CrossRef]
- Moss, T.; Greiner, A. Functionalization of Poly(para-xylylene)s—Opportunities and Challenges as Coating Material. Adv. Mater. Interfaces 2020, 7, 1901858. [Google Scholar] [CrossRef]
- Peng, H.-L.; Liu, J.-Q.; Dong, Y.-Z.; Yang, B.; Chen, X.; Yang, C.-S. Parylene-based flexible dry electrode for bioptential recording. Sens. Actuators B Chem. 2016, 231, 1–11. [Google Scholar] [CrossRef]
- Baldwin, A.; Yu, L.; Pratt, M.; Scholten, K.; Meng, E. Passive wireless transduction of electrochemical impedance across thin-film microfabricated coils using reflected impedance. Biomed. Microdevices 2017, 19, 87. [Google Scholar] [CrossRef]
- Mueller, M.; de la Oliva, N.; del Valle, J.; Delgado-Martínez, I.; Navarro, X.; Stieglitz, T. Rapid prototyping of flexible intrafascicular electrode arrays by picosecond laser structuring. J. Neural Eng. 2017, 14, 066016. [Google Scholar] [CrossRef]
- Hara, S.A.; Kim, B.J.; Kuo, J.T.W.; Lee, C.D.; Meng, E.; Pikov, V. Long-term stability of intracortical recordings using perforated and arrayed Parylene sheath electrodes. J. Neural Eng. 2016, 13, 066020. [Google Scholar] [CrossRef]
- Reddy, J.W.; Lassiter, M.; Chamanzar, M. Parylene photonics: A flexible, broadband optical waveguide platform with integrated micromirrors for biointerfaces. Microsyst. Nanoeng. 2020, 20206, 85. [Google Scholar] [CrossRef]
- Shin, G. Studies of parylene/silicone-coated soft bio-implantable optoelectronic device. Coatings 2020, 10, 404. [Google Scholar] [CrossRef]
- Forouzandeh, F.; Ahamed, N.N.; Hsu, M.-C.; Walton, J.P.; Frisina, R.D.; Borkholder, D.A. A 3D-printed modular microreservoir for drug delivery. Micromachines 2020, 11, 648. [Google Scholar] [CrossRef]
- Cobo, A.M.; Larson, C.E.; Scholten, K.; Miranda, J.A.; Elyahoodayan, S.; Song, D.; Pikov, V.; Meng, E. Parylene-Based Cuff Electrode with Integrated Microfluidics for Peripheral Nerve Recording, Stimulation, and Drug Delivery. J. Microelectromechanical Syst. 2019, 28, 36–49. [Google Scholar] [CrossRef]
- Rodger, D.C.; Fong, A.J.; Li, W.; Ameri, H.; Ahuja, A.K.; Gutierrez, C.; Lavrov, I.; Zhong, H.; Menon, P.R.; Meng, E. Flexible Parylene-based multielectrode array technology for high-density neural stimulation and recording. Sens. Actuators B Chem. 2008, 132, 449–460. [Google Scholar] [CrossRef]
- Nandra, M.S.; Lavrov, I.A.; Edgerton, V.R.; Tai, Y.-C. A Parylene-based microelectrode array implant for spinal cord stimulation in rats. In Proceedings of the 2011 IEEE 24th International Conference on Micro Electro Mechanical Systems (MEMS), Cancun, Mexico, 23–27 January 2011; pp. 1007–1010. [Google Scholar]
- Ortigoza-Diaz, J.; Scholten, K.; Meng, E. Characterization and modification of adhesion in dry and wet environments in thin-film Parylene systems. J. Microelectromech. Syst. 2018, 27, 874–885. [Google Scholar] [CrossRef]
- Bae, J.; Lee, I.J. Wettability, interface structure, and chemistry in functionalized poly(chloro-para-xylylene) films. Appl. Surf. Sci. 2014, 303, 344–349. [Google Scholar] [CrossRef]
- Chindam, C.; Lakhtakia, A.; Awadelkarim, O.O. Surface energy of Parylene C. Mater. Lett. 2015, 153, 18–19. [Google Scholar] [CrossRef]
- Van den Driesche, S.; Lucklum, F.; Bunge, F.; Vellekoop, M.J. 3D Printing Solutions for Microfluidic Chip-To-World Connections. Micromachines 2018, 9, 71. [Google Scholar] [CrossRef]
- Kreß, S.; Schaller-Ammann, R.; Feiel, J.; Priedl, J.; Kasper, C.; Egger, D. 3D Printing of Cell Culture Devices: Assessment and Prevention of the Cytotoxicity of Photopolymers for Stereolithography. Materials 2020, 13, 3011. [Google Scholar] [CrossRef]
- Golda-Cepa, M.; Kulig, W.; Cwiklik, L.; Kotarba, A. Molecular Dynamics Insights into Water–Parylene C Interface: Relevance of Oxygen Plasma Treatment for Biocompatibility. ACS Appl. Mater. Interfaces 2017, 9, 16685–16693. [Google Scholar] [CrossRef]
- Jordan, H.J.; Wegner, M.; Tiziani, H. Highly accurate non-contact characterization of engineering surfaces using confocal microscopy. Meas. Sci. Technol. 1998, 9, 1142–1151. [Google Scholar] [CrossRef]
- Lam, C.N.C.; Wu, R.; Li, D.; Hair, M.L.; Neumann, A.W. Study of the advancing and receding contact angles: Liquid sorption as a cause of contact angle hysteresis. Adv. Colloid Interface Sci. 2002, 96, 169–191. [Google Scholar] [CrossRef]
- Tan, C.P.; Craighead, H.G. Surface Engineering and Patterning Using Parylene for Biological Applications. Materials 2010, 3, 1803–1832. [Google Scholar] [CrossRef]
- Bi, X.; Crum, B.P.; Li, W. Super Hydrophobic Parylene-C Produced by Consecutive Plasma Treatment. J. Microelectromechanical Syst. 2014, 23, 628–635. [Google Scholar] [CrossRef]
- Brancato, L.; Decrop, D.; Lammertyn, J.; Puers, R. Surface Nanostructuring of Parylene-C Coatings for Blood Contacting Implants. Materials 2018, 11, 1109. [Google Scholar] [CrossRef]
- Verwolf, A.; White, G.; Poling, C. Effects of substrate composition and roughness on mechanical properties and conformality of parylene C coatings. J. Appl. Polym. Sci. 2013, 127, 2969–2976. [Google Scholar] [CrossRef]
- Hubbell, W.H., Jr.; Brandt, H.; Munir, Z.A. Transient and steady-state water vapor permeation through polymer films. J. Polym. Sci. Polym. Phys. Ed. 1975, 13, 493–507. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.M. Film properties. In Chemical Vapor Deposition Polymerization—The Growth and Properties of Parylene Thin Films; Kluwer Academic Publishers: Norwell, MA, USA, 2004; p. 58. [Google Scholar]

| Substrate Material | Mean Receding Contact Angle (Degree) | Mean Advancing Contact Angle (Degree) | Mean Contact Angle Hysteresis (Degree) |
|---|---|---|---|
| Shore A hardness 50 | 43.79 | 47.09 | 3.30 |
| Shore A hardness 85 | 50.20 | 53.26 | 3.06 |
| Shore A hardness 95 | 56.14 | 62.52 | 6.38 |
| Substrate Material | Ra (µm) | |
|---|---|---|
| Uncoated Parylene C | Coated Parylene C | |
| Shore A hardness 50 | 2.48 | 1.8 |
| Shore A hardness 85 | 2.91 | 2.01 |
| Shore A hardness 95 | 3.06 | 2.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, F.-C.; Huang, C.-Y.; Lu, Y.-P. Wettability and Surface Roughness of Parylene C on Three-Dimensional-Printed Photopolymers. Materials 2022, 15, 4159. https://doi.org/10.3390/ma15124159
Hsieh F-C, Huang C-Y, Lu Y-P. Wettability and Surface Roughness of Parylene C on Three-Dimensional-Printed Photopolymers. Materials. 2022; 15(12):4159. https://doi.org/10.3390/ma15124159
Chicago/Turabian StyleHsieh, Fan-Chun, Chien-Yao Huang, and Yen-Pei Lu. 2022. "Wettability and Surface Roughness of Parylene C on Three-Dimensional-Printed Photopolymers" Materials 15, no. 12: 4159. https://doi.org/10.3390/ma15124159
APA StyleHsieh, F.-C., Huang, C.-Y., & Lu, Y.-P. (2022). Wettability and Surface Roughness of Parylene C on Three-Dimensional-Printed Photopolymers. Materials, 15(12), 4159. https://doi.org/10.3390/ma15124159






