Improvements of Flexural Properties and Thermal Performance in Thin Geopolymer Based on Fly Ash and Ladle Furnace Slag Using Borax Decahydrates
Abstract
:1. Introduction
2. Methodology
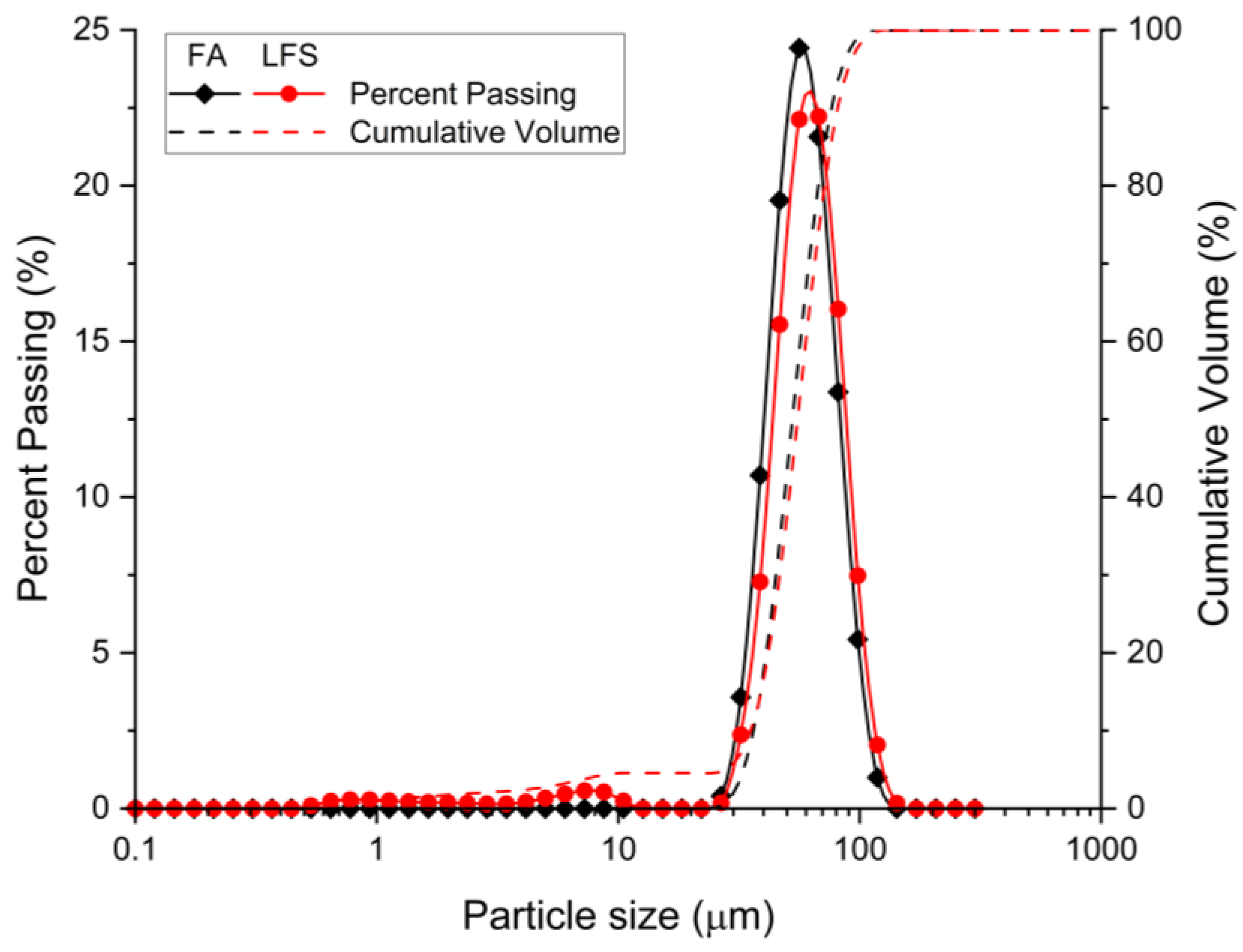
2.1. Materials
2.2. Synthesis of Thin FA/LFS Geopolymers
2.3. Elevated Temperature Exposure
2.4. Testing and Analysis
3. Results and Discussion
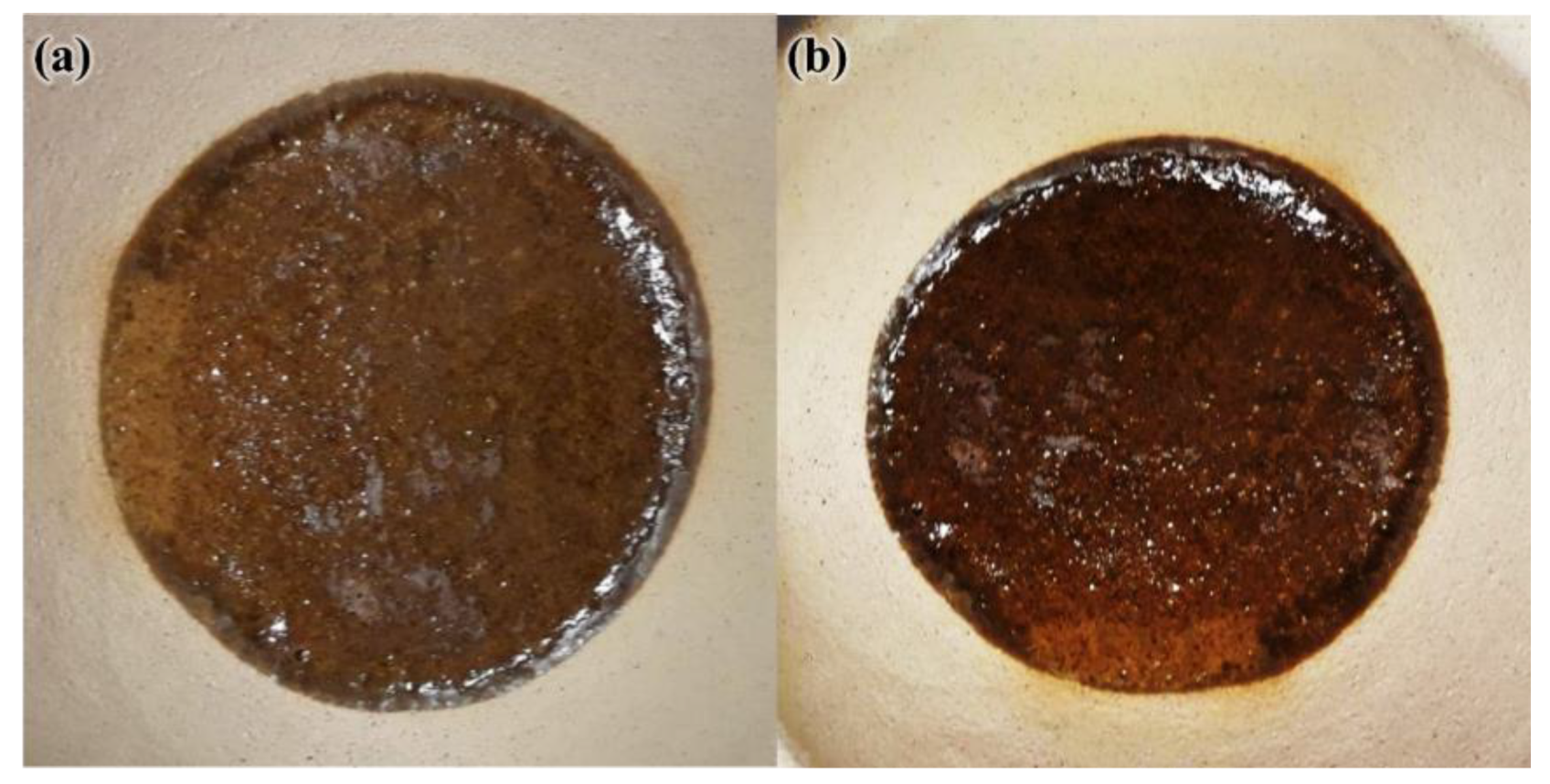
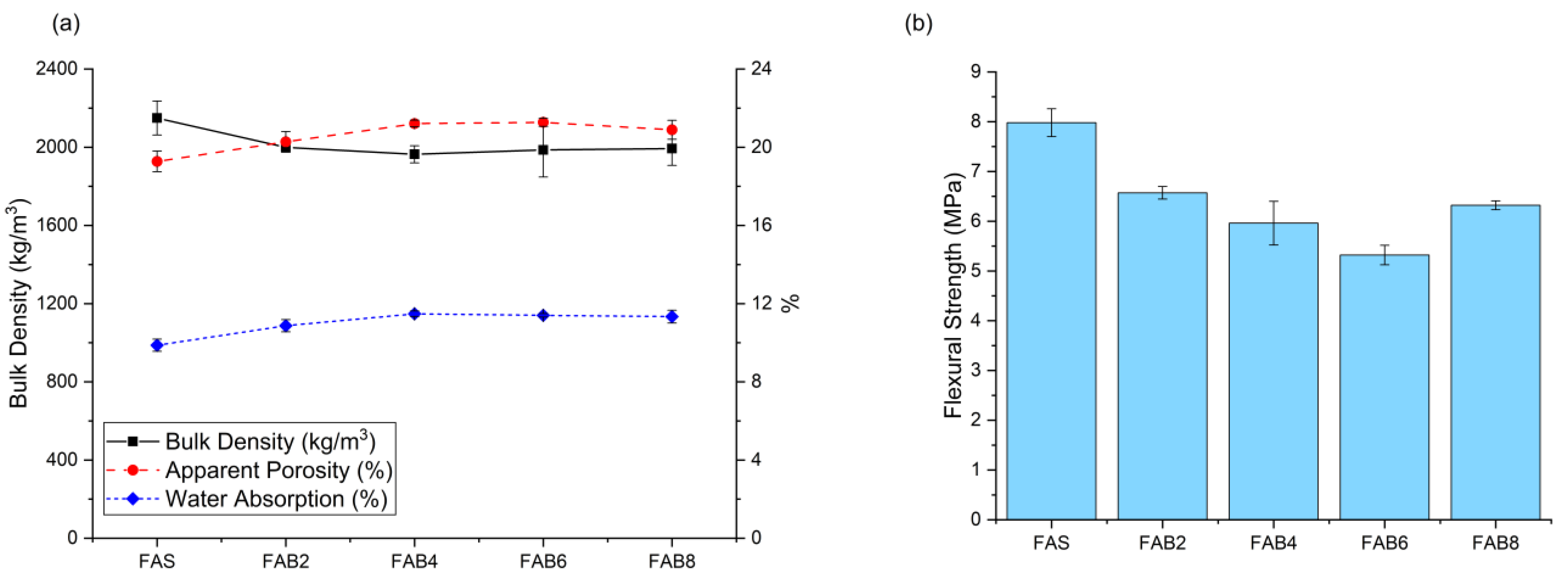
3.1. Properties of Unexposed Thin FAS and FAB Geopolymers
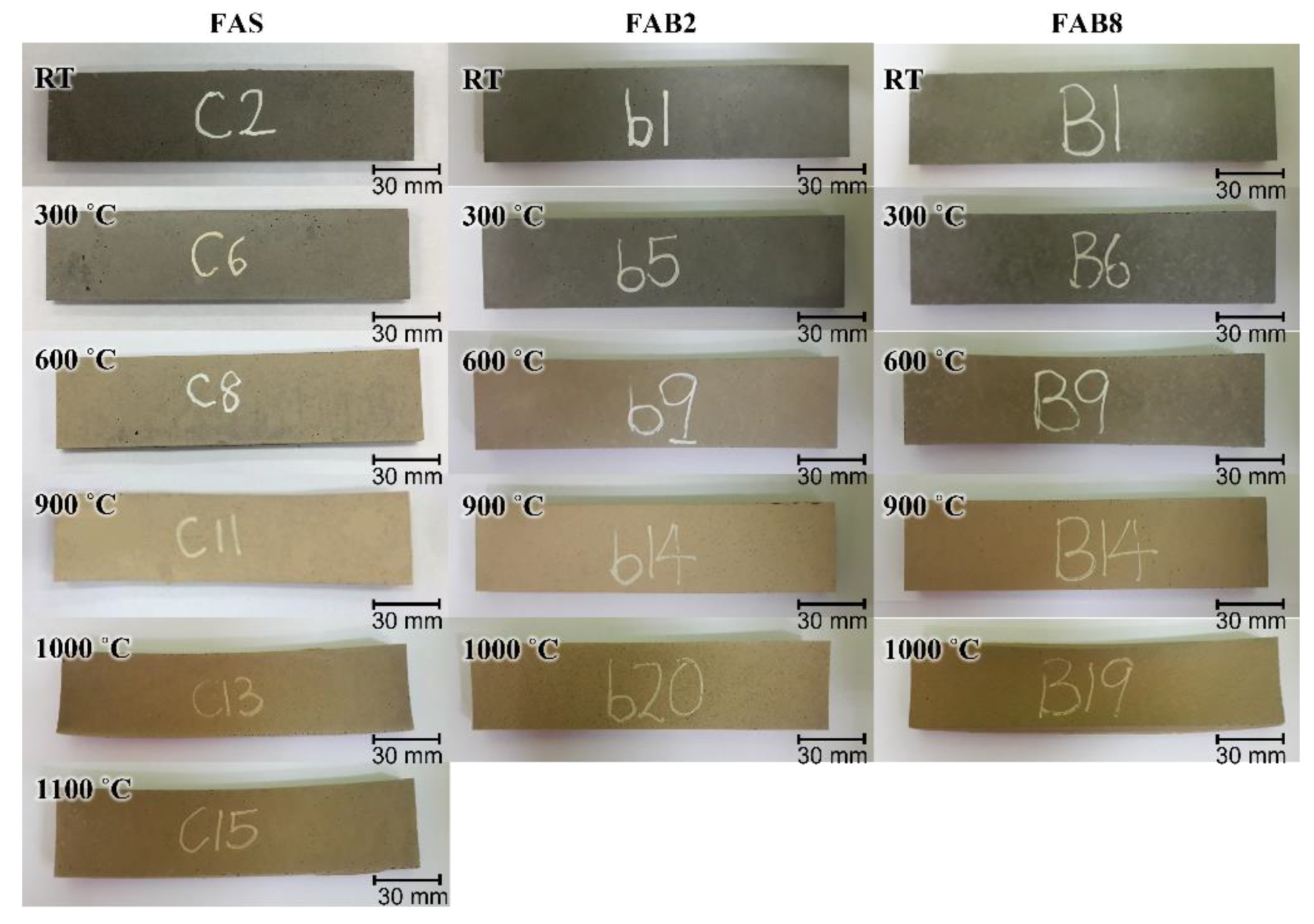
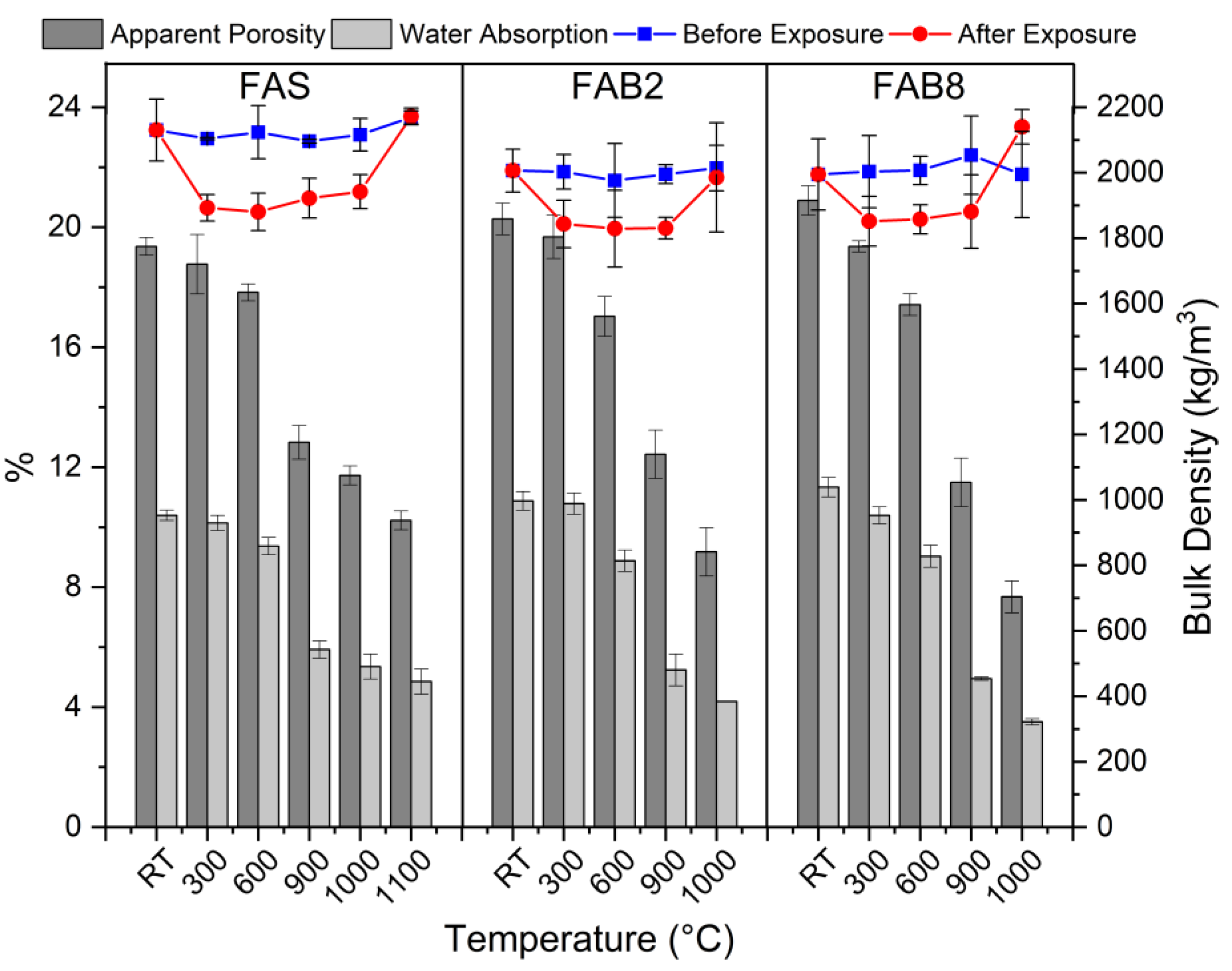
3.2. Elevated Temperature Exposure
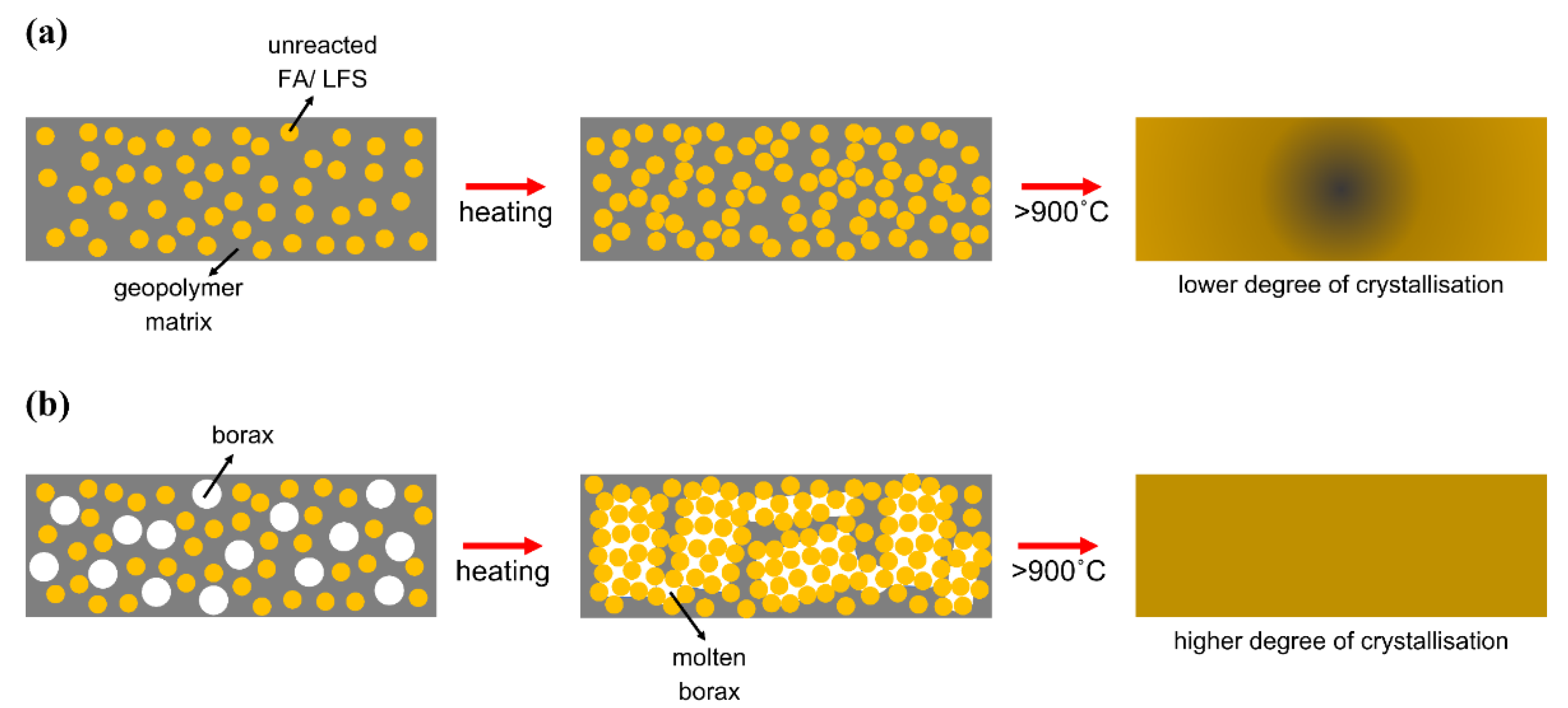
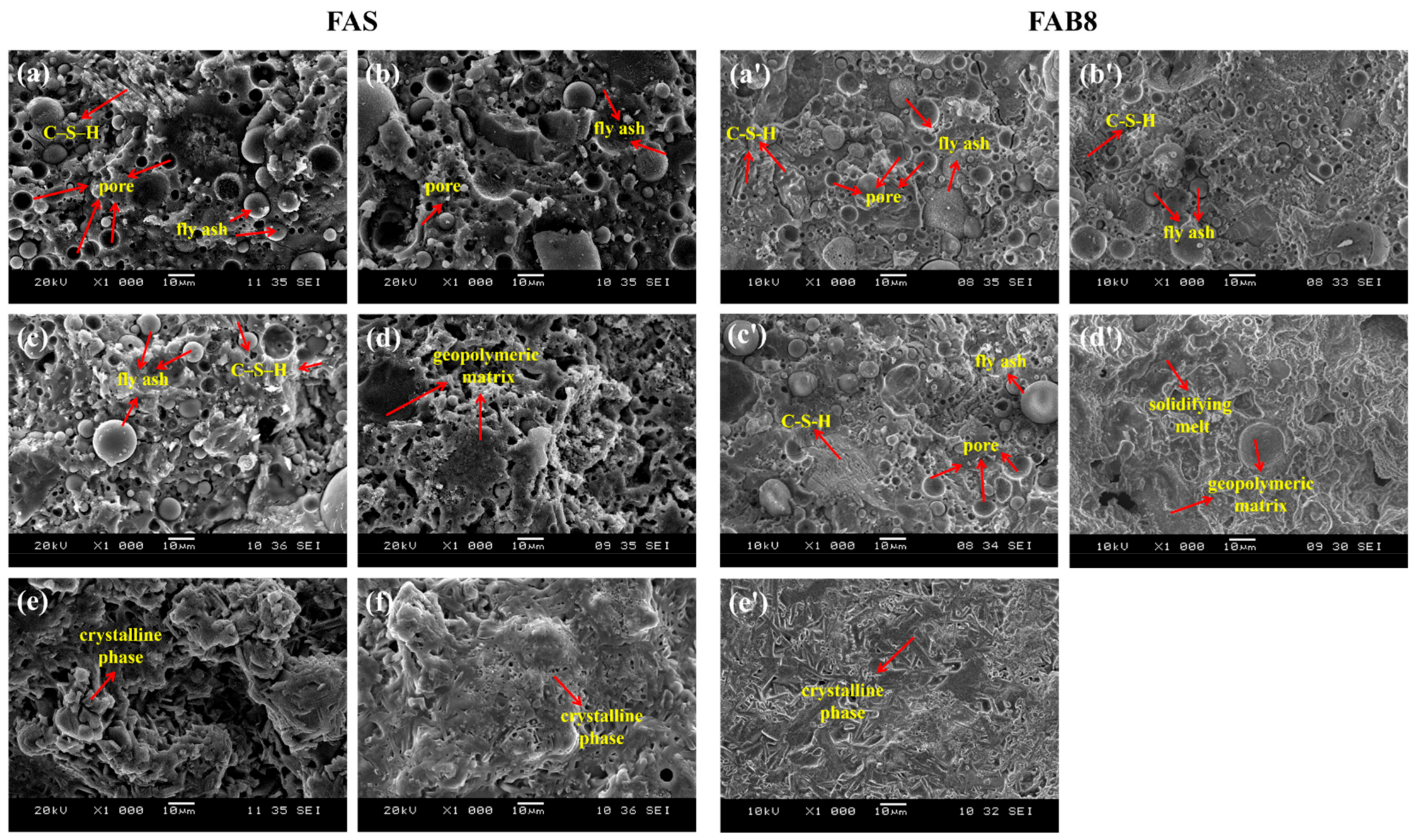
3.3. Microstructural Analysis
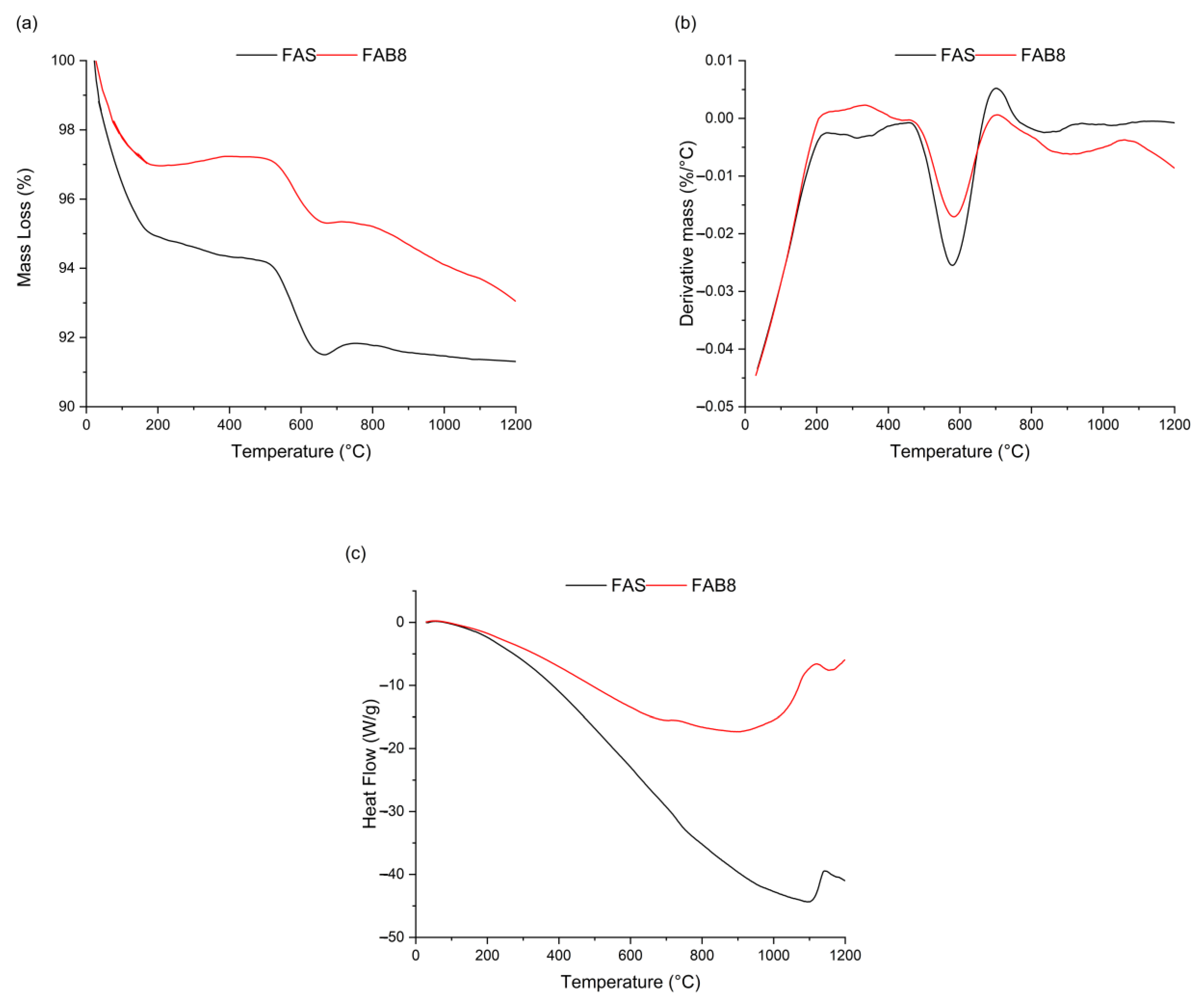
3.4. Thermogravimetric Analysis
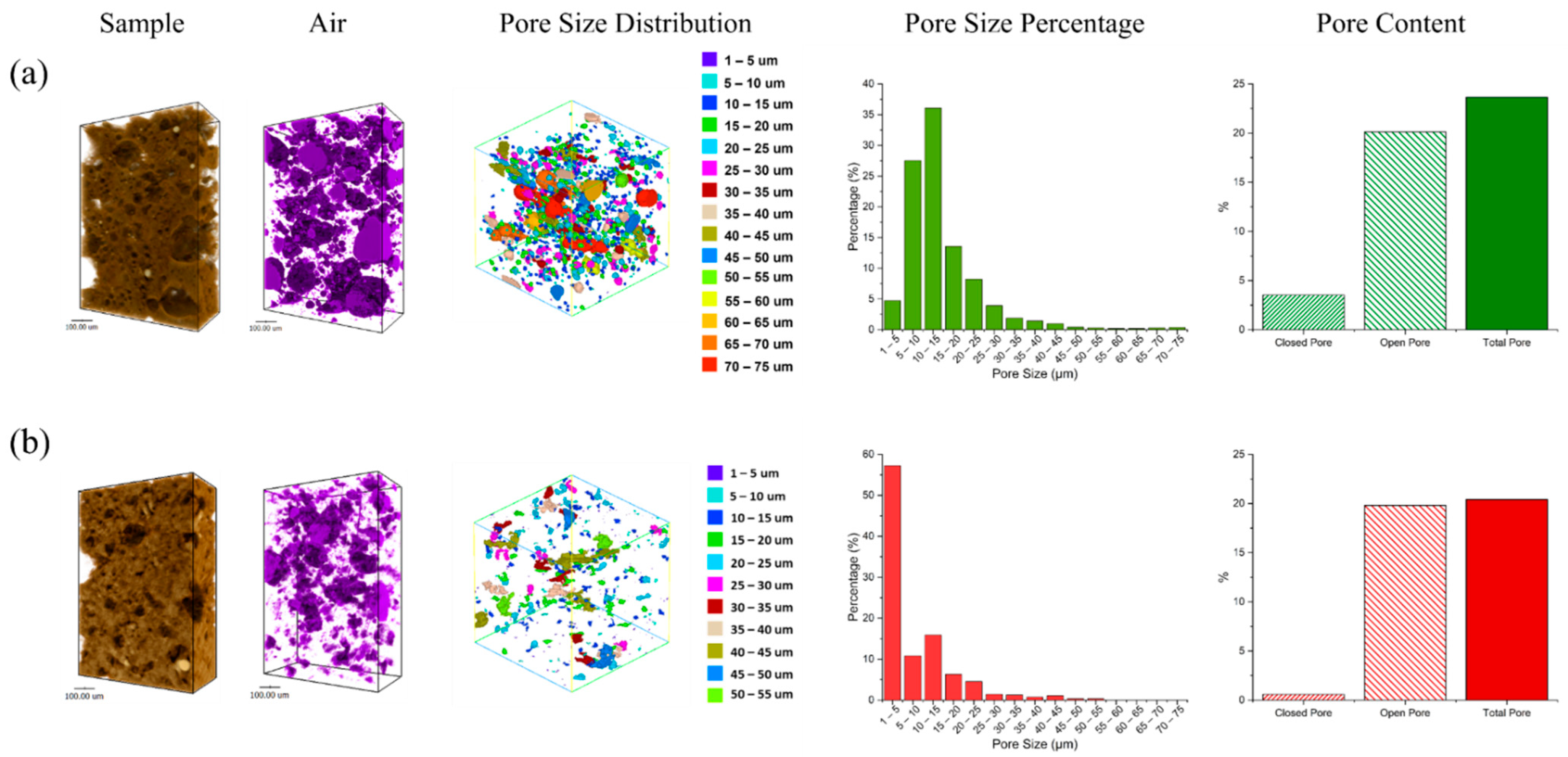
3.5. Pore Distribution Analysis
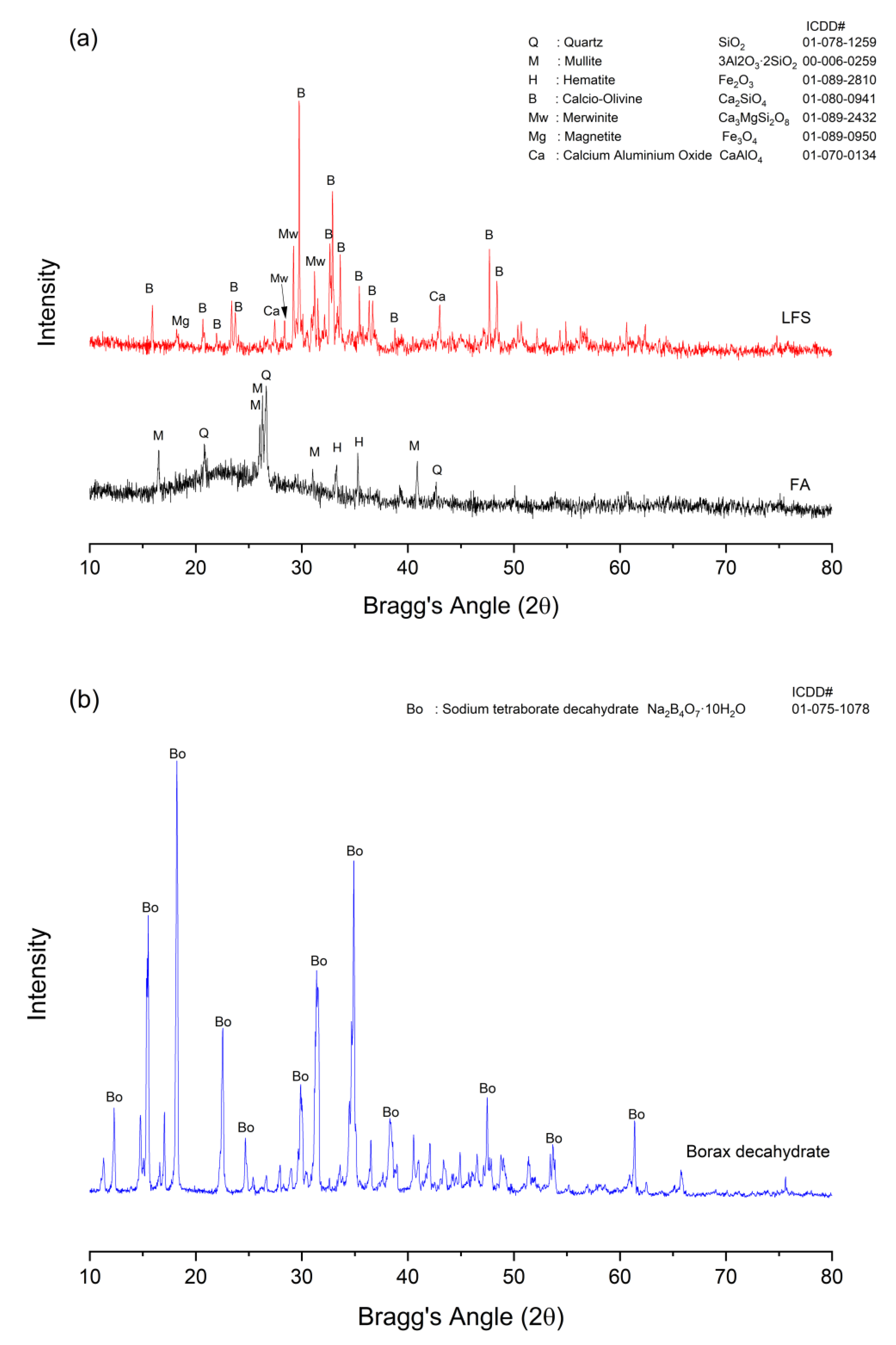
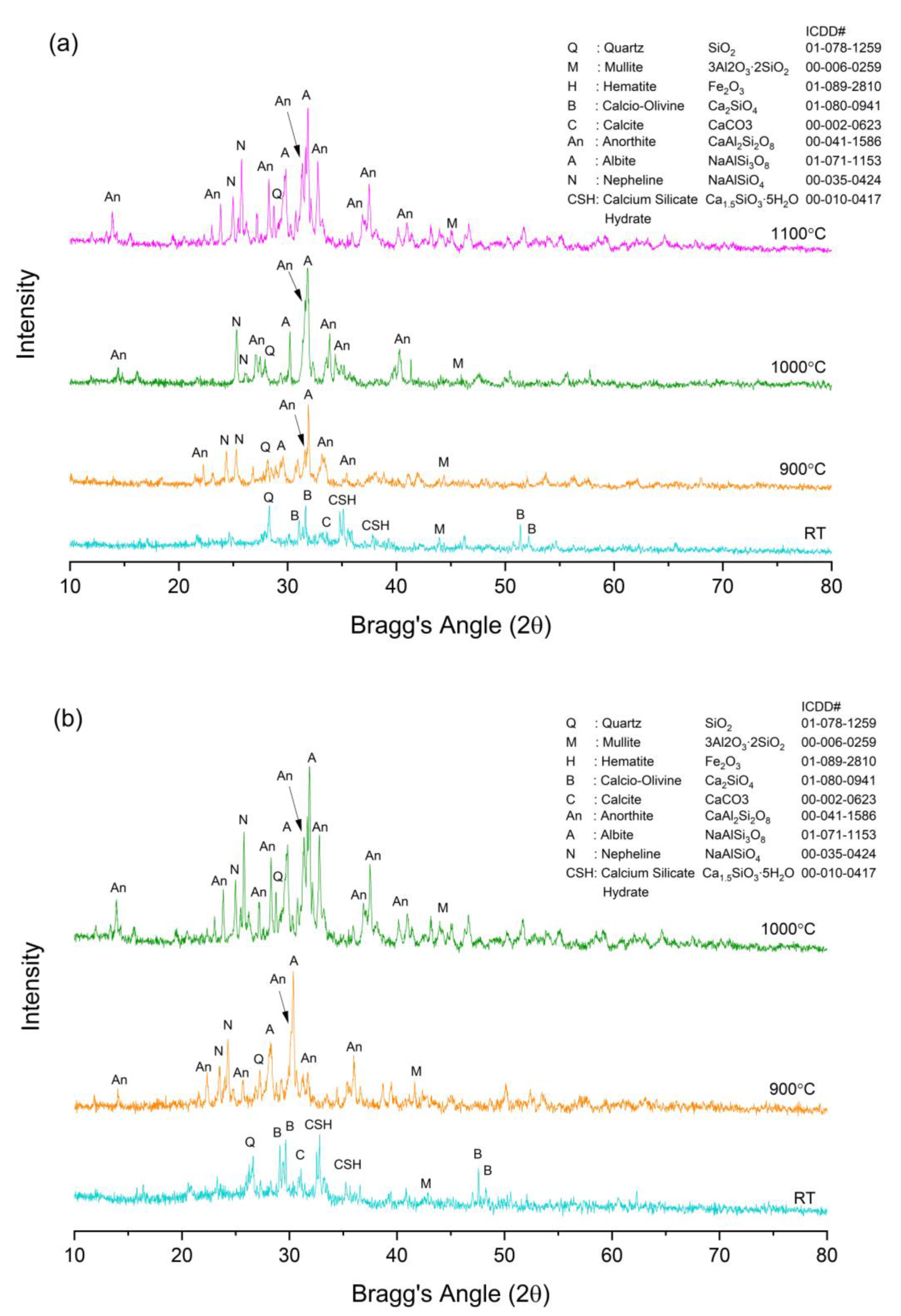
3.6. Phase Analysis
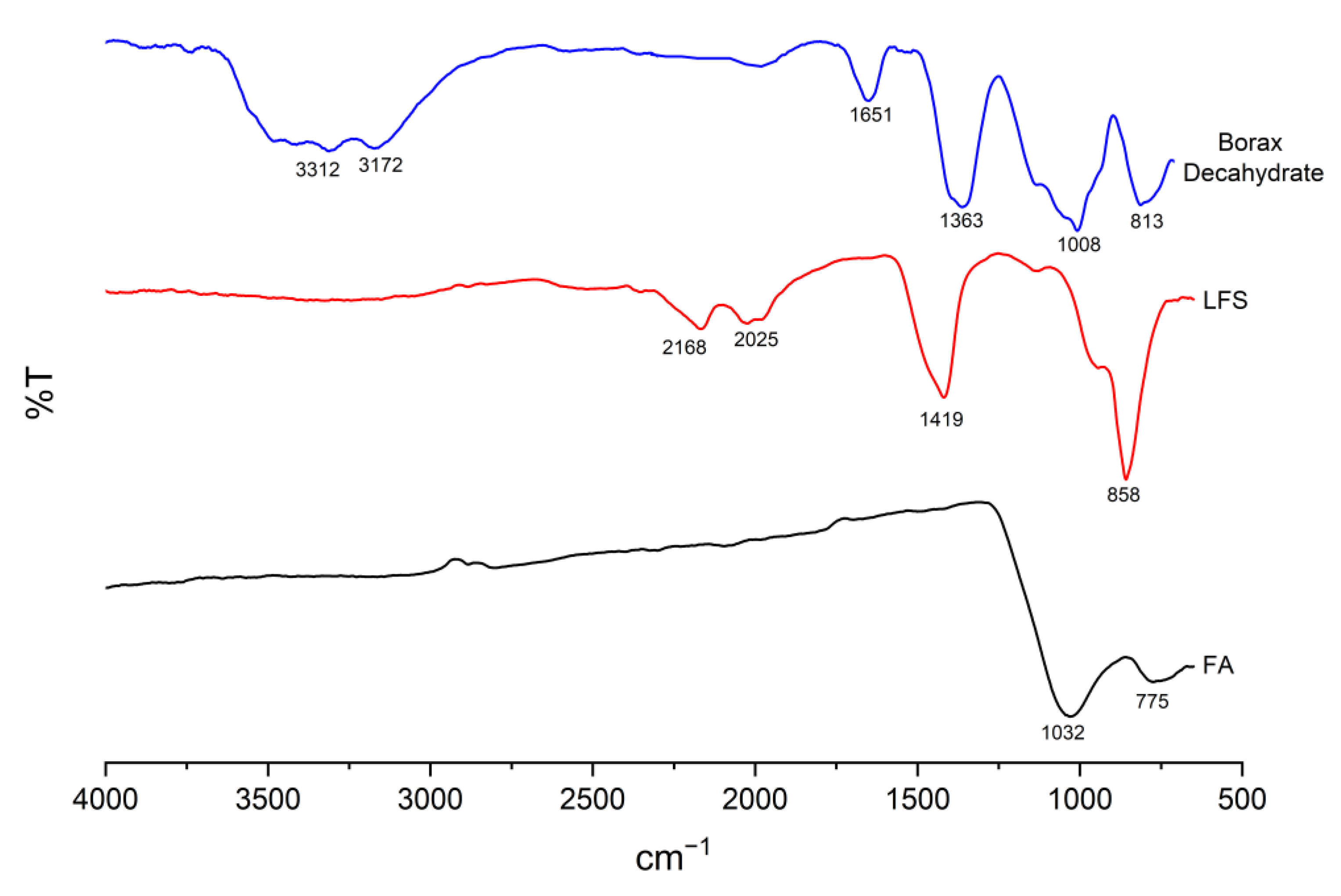
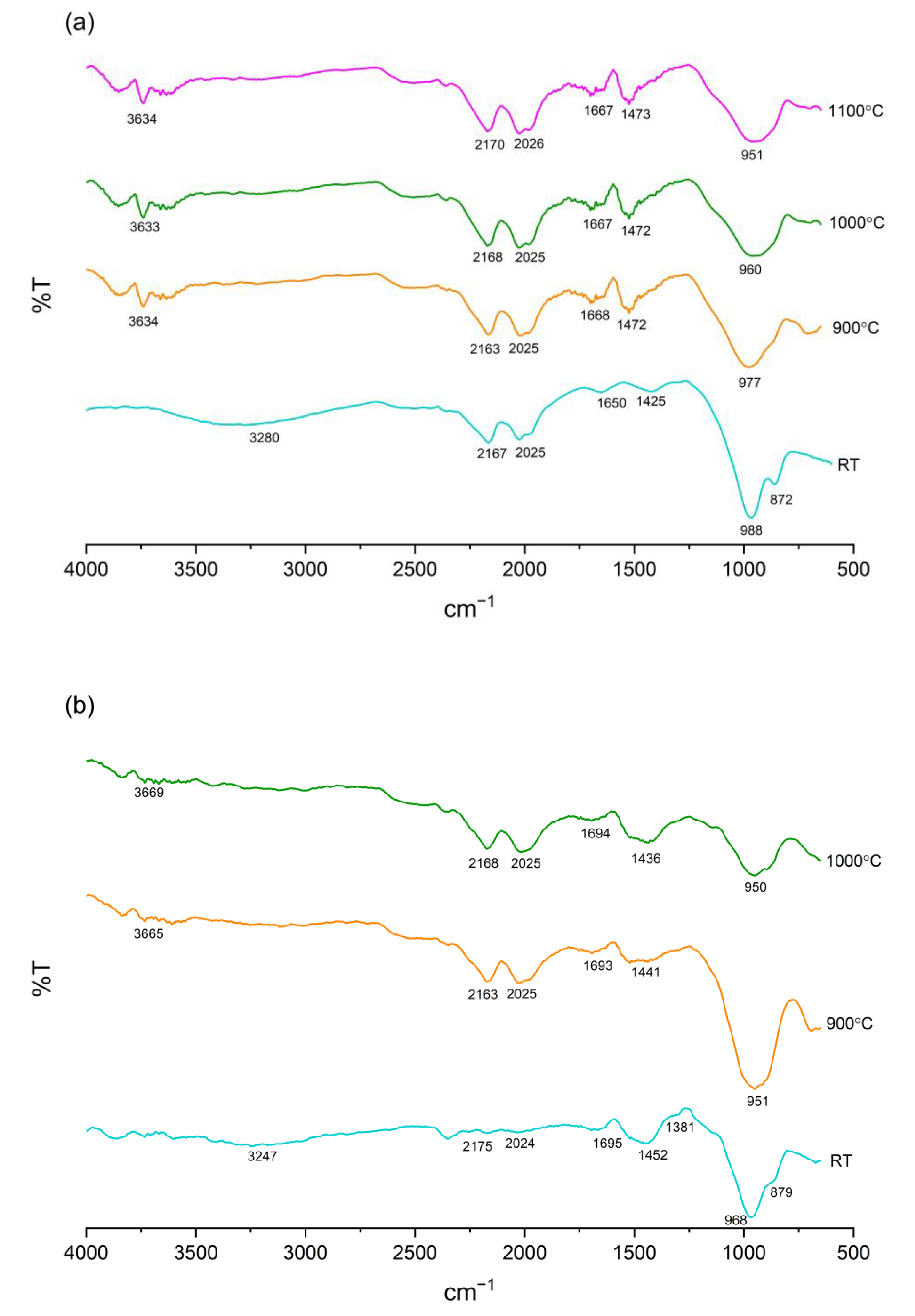
3.7. Functional Group Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar]
- Rakhimova, N.R.; Rakhimov, R.Z. Toward clean cement technologies: A review on alkali-activated fly-ash cements incorporated with supplementary materials. J. Non-Cryst. Solids 2019, 509, 31–41. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, H.J.; Zhou, C.H.; Wang, H. Geopolymer from kaolin in China: An overview. Appl. Clay Sci. 2016, 119, 31–411. [Google Scholar] [CrossRef]
- Albidah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Characteristics of metakaolin-based geopolymer concrete for different mix design parameters. J. Mater. Res. Technol. 2021, 10, 84–98. [Google Scholar] [CrossRef]
- Alomayri, T. Experimental study of the microstructural and mechanical properties of geopolymer paste with nano material (Al2O3). J. Build. Eng. 2019, 25, 100788. [Google Scholar] [CrossRef]
- Awoyera, P.; Adesina, A. A critical review on application of alkali activated slag as a sustainable composite binder. Case Stud. Constr. Mater. 2019, 11, e00268. [Google Scholar] [CrossRef]
- Temuujin, J.; Surenjav, E.; Ruescher, C.H.; Vahlbruch, J. Processing and uses of fly ash addressing radioactivity (critical review). Chemosphere 2019, 216, 866–882. [Google Scholar] [CrossRef]
- Najm, O.; El-Hassan, H.; El-Dieb, A. Ladle slag characteristics and use in mortar and concrete: A comprehensive review. J. Clean. Prod. 2021, 288, 125584. [Google Scholar] [CrossRef]
- Mathew, G.; Joseph, B. Flexural behaviour of geopolymer concrete beams exposed to elevated temperatures. J. Build. Eng. 2018, 15, 311–317. [Google Scholar] [CrossRef]
- Ramagiri, K.K.; Kar, A. Effect of high-temperature on the microstructure of alkali-activated binder. Mater. Today. Proc. 2020, 28, 1123–1129. [Google Scholar] [CrossRef]
- Mucsi, G.; Szenczi, Á.; Nagy, S. Fiber reinforced geopolymer from synergetic utilization of fly ash and waste tire. J. Clean. Prod. 2018, 178, 429–440. [Google Scholar] [CrossRef]
- Xue, G.; Yilmaz, E.; Feng, G.; Cao, S.; Sun, L. Reinforcement effect of polypropylene fiber on dynamic properties of cemented tailings backfill under SHPB impact loading. Constr. Build. Mater. 2021, 279, 122417. [Google Scholar] [CrossRef]
- Shaikh, F.U.A. Effects of slag content on the residual mechanical properties of ambient air-cured geopolymers exposed to elevated temperatures. J. Asian Ceram. Soc. 2018, 6, 342–358. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Tao, Z.; Cao, Y.F.; Wuhrer, R.; Murphy, T. Compressive strength and microstructure of alkali-activated fly ash/slag binders at high temperature. Cem. Concr. Compos. 2018, 86, 9–18. [Google Scholar] [CrossRef]
- Murri, A.N.; Rickard, W.D.A.; Bignozzi, M.C.; van Riessen, A. High temperature behaviour of ambient cured alkali-activated materials based on ladle slag. Cem. Concr. Res. 2013, 43, 51–61. [Google Scholar] [CrossRef]
- Yong-Sing, N.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Pakawanit, P.; Chan, L.W.L.; Hui-Teng, N.; Shee-Ween, O.; Wan-En, O.; Yong-Jie, H. Thin fly ash/ladle furnace slag geopolymer: Effect of elevated temperature exposure on flexural properties and morphological characteristics. Ceram. Int. 2022, 48, 16562–16575. [Google Scholar] [CrossRef]
- Wang, Y.S.; Dai, J.G.; Ding, Z.; Xu, W.T. Phosphate-based geopolymer: Formation mechanism and thermal stability. Mater. Lett. 2017, 190, 209–212. [Google Scholar] [CrossRef]
- Durant, A.T.; MacKenzie, K.J.D. Synthesis of sodium and potassium aluminogermanate inorganic polymers. Mater. Lett. 2011, 65, 2086–2088. [Google Scholar] [CrossRef]
- Williams, R.P.; van Riessen, A. Development of alkali activated borosilicate inorganic polymers (AABSIP). J. Eur. Ceram. Soc. 2011, 31, 1513–1516. [Google Scholar] [CrossRef]
- Nazari, A.; Maghsoudpour, A.; Sanjayan, J.G. Characteristics of boroaluminosilicate geopolymers. Constr. Build. Mater. 2014, 70, 262–268. [Google Scholar] [CrossRef]
- Nazari, A.; Maghsoudpour, A.; Sanjayan, J.G. Flexural strength of plain and fibre-reinforced boroaluminosilicate geopolymer. Constr. Build. Mater. 2015, 76, 207–213. [Google Scholar] [CrossRef]
- Bagheri, A.; Nazari, A.; Sanjayan, J.G.; Rajeev, P.; Duan, W. Fly ash-based boroaluminosilicate geopolymers: Experimental and molecular simulations. Ceram. Int. 2017, 43, 4119–4126. [Google Scholar] [CrossRef]
- Liu, H.; Sanjayan, J.G.; Bu, Y. The application of sodium hydroxide and anhydrous borax as composite activator of class F fly ash for extending setting time. Fuel 2017, 206, 534–540. [Google Scholar] [CrossRef]
- Dupuy, C.; Havette, J.; Gharzouni, A.; Texier-Mandoki, N.; Bourbon, X.; Rossignol, S. Metakaolin-based geopolymer: Formation of new phases influencing the setting time with the use of additives. Constr. Build. Mater. 2019, 200, 272–281. [Google Scholar] [CrossRef]
- Guo, J.; Chen, X.; Zhang, Y. Improving the mechanical and electrical properties of ceramizable silicone rubber/halloysite composites and their ceramic residues by incorporation of different borates. Polymers 2018, 10, 388. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Li, X.; Zhang, F.-J.; Chen, S.; Ren, D. Sintering of monoclinic SrAl2Si2O8 ceramics and their Sr immobilization. Int. J. Miner. Metall. 2021, 28, 1057–1062. [Google Scholar] [CrossRef]
- Imiela, M.; Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Zarzecka-Napierała, M.; Szumera, M. Effect of carbon fibers on thermal properties and mechanical strength of ceramizable composites based on silicone rubber. J. Therm. Anal. Calorim. 2016, 124, 197–203. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Dai, N.; Wang, Z. Thermal and mechanical properties of geopolymers exposed to high temperature: A literature review. Adv. Civ. Eng. 2020, 2020, 7532703. [Google Scholar] [CrossRef] [Green Version]
- Yong-Sing, N.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Chan, L.W.L.; Hui-Teng, N.; Shee-Ween, O.; Wan-En, O.; Yong-Jie, H. Evaluation of flexural properties and characterisation of 10 mm thin geopolymer based on fly ash and ladle furnace slag. J. Mater. Res. Technol. 2021, 15, 163–176. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Mechanical, thermal insulation, thermal resistance and acoustic absorption properties of geopolymer foam concrete. Cem. Concr. Compos. 2015, 62, 97–105. [Google Scholar] [CrossRef]
- Antoni, A.; Purwantoro, A.A.T.; Suyanto, W.S.P.D.; Hardjito, D. Fresh and hardened properties of high calcium fly ash-based geopolymer matrix with high dosage of borax. Iran. J. Sci. Technol. Trans. Civ. Eng. 2020, 44, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Antoni, A.; Wijaya, S.W.; Satria, J.; Sugiarto, A.; Hardjito, D. The use of borax in deterring flash setting of high calcium fly ash based geopolymer. Mater. Sci. Forum 2016, 857, 416–420. [Google Scholar]
- Junru, R.; Huiguo, C.; Ruixi, D.; Tao, S. Behavior of combined fly ash/GBFS-based geopolymer concrete after exposed to elevated temperature. IOP Conf. Ser. Earth Environ. Sci. 2019, 267, 032056. [Google Scholar] [CrossRef]
- Al Saadi, T.H.A.; Badanoiu, A.I.; Nicoara, A.I.; Stoleriu, S.; Voicu, G. Synthesis and properties of alkali activated borosilicate inorganic polymers based on waste glass. Constr. Build. Mater. 2017, 136, 298–306. [Google Scholar] [CrossRef]
- Yuan, J.; He, P.; Liang, X.; Jia, D.; Jia, L.; Cai, D.; Yang, Z.; Duan, X.; Wang, S.; Zhou, Y. Thermal evolution of lithium ion substituted cesium-based geopolymer under high temperature treatment, Part I: Effects of holding temperature. Ceram. Int. 2018, 44, 10047–10054. [Google Scholar] [CrossRef]
- Mandal, K.; Thokchom, S.; Roy, M. Effect of Na2O content on performance of fly ash geopolymers at elevated temperature. Int. J. Civ. Environ. Eng. 2011, 3, 34–40. [Google Scholar]
- Nazari, A.; Bagheri, A.; Sanjayan, J.G.; Dao, M.; Mallawa, C.; Zannis, P.; Zumbo, S. Thermal shock reactions of Ordinary Portland cement and geopolymer concrete: Microstructural and mechanical investigation. Constr. Build. Mater. 2019, 196, 492–498. [Google Scholar] [CrossRef]
- Rickard, W.D.; Temuujin, J.; van Riessen, A. Thermal analysis of geopolymer pastes synthesised from five fly ashes of variable composition. J. Non-Cryst. Solids 2012, 358, 1830–1839. [Google Scholar] [CrossRef]
- Hernández, M.F.; Violini, M.A.; Serra, M.F.; Conconi, M.S.; Suarez, G.; Rendtorff, N.M. Boric acid (H3BO3) as flux agent of clay-based ceramics, B2O3 effect in clay thermal behavior and resultant ceramics properties. J. Therm. Anal. Calorim. 2020, 139, 1717–1729. [Google Scholar] [CrossRef]
- Pan, Z.; Sanjayan, J.G.; Collins, F. Effect of transient creep on compressive strength of geopolymer concrete for elevated temperature exposure. Cem. Concr. Res. 2014, 56 (Suppl. C), 182–189. [Google Scholar] [CrossRef]
- Yang, S.; Lv, G.; Liu, Y.; Wang, Q. Synergism of polysiloxane and zinc borate flame retardant polycarbonate. Polym. Degrad. Stab. 2013, 98, 2795–2800. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Wu, B.; Cao, L.; Wang, F. Thermal behavior and mechanical properties of geopolymer mortar after exposure to elevated temperatures. Constr. Build. Mater. 2016, 109, 17–24. [Google Scholar] [CrossRef]
- Wongsa, A.; Wongkvanklom, A.; Tanangteerapong, D.; Chindaprasirt, P. Comparative study of fire-resistant behaviors of high-calcium fly ash geopolymer mortar containing zeolite and mullite. J. Sustainable Cem.-Based Mater. 2020, 9, 307–321. [Google Scholar] [CrossRef]
- Tanyildizi, H.; Yonar, Y. Mechanical properties of geopolymer concrete containing polyvinyl alcohol fiber exposed to high temperature. Constr. Build. Mater. 2016, 126, 381–387. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Qi, S.L.; Cao, L.; Wu, B. Development of metakaolin–fly ash based geopolymers for fire resistance applications. Constr. Build. Mater. 2014, 55, 38–45. [Google Scholar] [CrossRef]
- Vickers, L.; Pan, Z.; Tao, Z.; van Riessen, A. In situ elevated temperature testing of fly ash based geopolymer composites. Materials 2016, 9, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng-Yong, H.; Yun-Ming, L.; Abdullah, M.M.A.B.; Hussin, K. Thermal resistance variations of fly ash geopolymers: Foaming responses. Sci. Rep. 2017, 7, 45355. [Google Scholar] [CrossRef]
- Mróz, K.; Hager, I.; Korniejenko, K. Material solutions for passive fire protection of buildings and structures and their performances testing. Procedia Eng. 2016, 151, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Aziz, I.H.; Abdullah, M.M.A.B.; Salleh, M.A.A.M.; Yoriya, S.; Chaiprapa, J.; Rojviriya, C.; Li, L.Y. Microstructure and porosity evolution of alkali activated slag at various heating temperatures. J. Mater. Res. Technol. 2020, 9, 15894–15907. [Google Scholar] [CrossRef]
- Azimi, E.A.; Abdullah, M.M.A.B.; Ming, L.Y.; Yong, H.C.; Hussin, K.; Aziz, I.H. Processing and properties of geopolymers as thermal insulating materials: A review. Rev. Adv. Mater. Sci. 2016, 44, 273–285. [Google Scholar]
- Swamy, V.; Jung, I.-H.; Decterov, S.A. Thermodynamic modeling of the Al2O3–B2O3–SiO2 system. J. Non-Cryst. Solids 2009, 355, 1679–1686. [Google Scholar] [CrossRef]
- Zanelli, C.; Raimondo, M.; Guarini, G.; Dondi, M. The vitreous phase of porcelain stoneware: Composition, evolution during sintering and physical properties. J. Non-Cryst. Solids 2011, 357, 3251–3260. [Google Scholar] [CrossRef]
- František, Š.; Rostislav, Š.; Zdeněk, T.; Petr, S.; Vít, Š.; Zuzana, Z.C. Preparation and properties of fly ashbased geopolymer foams. Ceram.-Silik. 2014, 58, 188–197. [Google Scholar]
- He, P.; Jia, D.; Wang, S. Microstructure and integrity of leucite ceramic derived from potassium-based geopolymer precursor. J. Eur. Ceram. Soc. 2013, 33, 689–698. [Google Scholar] [CrossRef]
- Alehyen, S.; Achouri, M.; Taibi, M. Characterization, microstructure and properties of fly ash-based geopolymer. J. Mater. Environ. Sci. 2017, 8, 1783–1796. [Google Scholar]
- Samantasinghar, S.; Singh, S.P. Effect of synthesis parameters on compressive strength of fly ash-slag blended geopolymer. Constr. Build. Mater. 2018, 170, 225–234. [Google Scholar] [CrossRef]
- Guetteche, M.N.; Zergua, A.; Hannachi, S. Investigating the local granulated blast furnace slag. Open J. Civ. Eng. 2012, 2, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Goel, N.; Sinha, N.; Kumar, B. Growth and properties of sodium tetraborate decahydrate single crystals. Mater. Res. Bull. 2013, 48, 1632–1636. [Google Scholar] [CrossRef]
- Criado, M.; Aperador, W.; Sobrados, I. Microstructural and mechanical properties of alkali activated colombian raw materials. Materials 2016, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Lahoti, M.; Wong, K.K.; Tan, K.H.; Yang, E.-H. Effect of alkali cation type on strength endurance of fly ash geopolymers subject to high temperature exposure. Mater. Des. 2018, 154, 8–19. [Google Scholar] [CrossRef]



| Compound | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | TiO2 | K2O | Others | LOI (%) |
|---|---|---|---|---|---|---|---|---|---|
| FA (wt.%) | 56.30 | 28.00 | 3.89 | 6.86 | - | 2.17 | 1.49 | 1.29 | 1.95 |
| LFS (wt.%) | 21.30 | 2.30 | 63.59 | 8.08 | 2.60 | 0.50 | - | 1.63 | 3.74 |
| FAS (%) | FAB2 (%) | FAB8 (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Density Gain/Loss | Mass Gain/Loss | Volume Gain/Loss | Density Gain/Loss | Mass Gain/Loss | Volume Gain/Loss | Density Gain/Loss | Mass Gain/Loss | Volume Gain/Loss | |
| RT | −0.1 | −0.08 | −0.09 | −0.06 | −0.07 | −0.1 | −0.08 | −0.08 | −0.09 |
| 300 °C | −10.0 | −10.2 | −7.8 | −8.0 | −9.9 | −5.6 | −7.6 | −9.7 | −4.2 |
| 600 °C | −11.5 | −12.8 | −8.7 | −7.4 | −12.5 | −6.4 | −7.4 | −11.8 | −3.4 |
| 900 °C | −8.3 | −13.8 | +5.5 | −8.4 | −13.7 | +5.4 | −8.3 | −13.4 | +5.2 |
| 1000 °C | −8.2 | −14.1 | +4.9 | −1.4 | −13.5 | −7.9 | +7.3 | −13.0 | −14.3 |
| 1100 °C | +0.1 | −14.0 | −8.2 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong-Sing, N.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Pakawanit, P.; Vizureanu, P.; Khalid, M.S.; Hui-Teng, N.; Yong-Jie, H.; Nabiałek, M.; et al. Improvements of Flexural Properties and Thermal Performance in Thin Geopolymer Based on Fly Ash and Ladle Furnace Slag Using Borax Decahydrates. Materials 2022, 15, 4178. https://doi.org/10.3390/ma15124178
Yong-Sing N, Yun-Ming L, Cheng-Yong H, Abdullah MMAB, Pakawanit P, Vizureanu P, Khalid MS, Hui-Teng N, Yong-Jie H, Nabiałek M, et al. Improvements of Flexural Properties and Thermal Performance in Thin Geopolymer Based on Fly Ash and Ladle Furnace Slag Using Borax Decahydrates. Materials. 2022; 15(12):4178. https://doi.org/10.3390/ma15124178
Chicago/Turabian StyleYong-Sing, Ng, Liew Yun-Ming, Heah Cheng-Yong, Mohd Mustafa Al Bakri Abdullah, Phakkhananan Pakawanit, Petrica Vizureanu, Mohd Suhaimi Khalid, Ng Hui-Teng, Hang Yong-Jie, Marcin Nabiałek, and et al. 2022. "Improvements of Flexural Properties and Thermal Performance in Thin Geopolymer Based on Fly Ash and Ladle Furnace Slag Using Borax Decahydrates" Materials 15, no. 12: 4178. https://doi.org/10.3390/ma15124178








