Sulfate Freeze–Thaw Resistance of Magnesium Potassium Phosphate Cement Mortar according to Hydration Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
2.3. Experimental Methods
3. Results and Discussion
3.1. Material Analysis and Mix Ratio
3.2. Flexural Strength Development
3.3. Compressive Strength Development
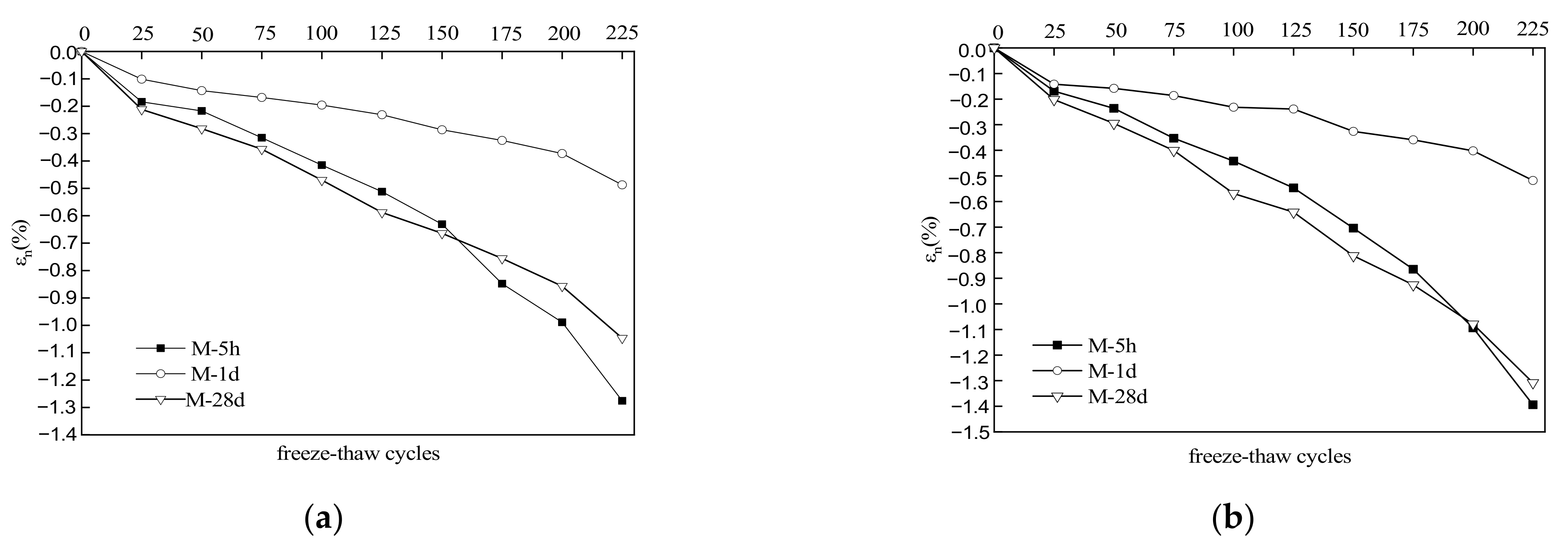
3.4. Volume Deformation
3.5. Water Absorption
3.6. XRD Analysis
3.7. SEM-EDS Analysis
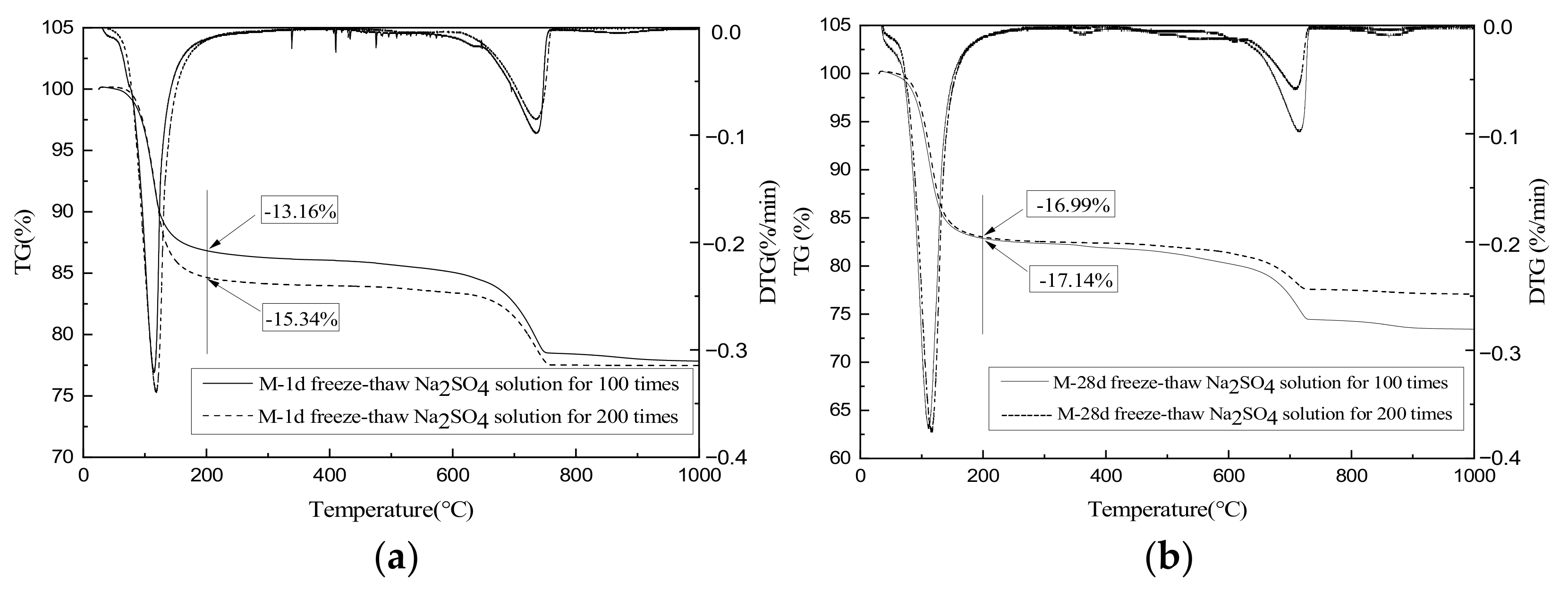
3.8. TG-DTG
4. Conclusions
- (1)
- After 225 freeze–thaw cycles in water, the flexural strengths of specimens M-1d and M-28d were 36.7% and 29.0% of their initial values, respectively, while the compressive strengths were 37.2% and 32.9% of their initial values. After 225 cycles in sulfate solution, the flexural strengths of M-1d and M-28d were 45.1% and 36.0% of their initial values, respectively, and the compressive strengths were 47.4% and 42.1% of their initial values. The results show that, under freeze–thaw conditions, the degree of strength reduction in MKPC mortar with 1 d hydration is similar to that with 28 d hydration. Furthermore, the strength reduction is lower in sulfate solution than in water.
- (2)
- After 225 freeze–thaw cycles, the volume expansion rates of specimens M-1d and M-28d were 0.487% and 1.047% in water and 0.518% and 1.308% in sulfate solution, respectively. Hence, MKPC mortar under freeze–thaw conditions has better deformation resistance with 1 d hydration than with 28 d hydration.
- (3)
- Although the open porosity of specimen M-1d was higher than that of M-28d before freeze–thaw testing, it became lower after 125 freeze–thaw cycles. This indicates that freeze–thaw cycling more strongly degraded the pore structure of M-28d than that of M-1d. The open porosity of MKPC mortar specimens subjected to freeze–thaw cycling was lower in sulfate solution than in water, which proves that salt crystallization and precipitation can improve the pore structure of hardened MKPC bodies to some extent.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Yuan, Y. The mechanism of concrete corrosion damage in sodium sulfate and magnesium sulfate solution. Silicate 2007, 35, 504–508. [Google Scholar]
- Chen, J.; Jiang, M.; Zhu, J. Damage evolution in cement mortar due to erosion of sulphate. Corros. Sci. 2008, 50, 2478–2483. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.H.; Dong, S.H.; Zhu, W.Z.; Li, H.B.; Yin, S.H. Research on the critical freezing strengths of subzero-temperature concrete based on compressive strength. China Concr. Cem. Prod. 2019, 10, 24–26. [Google Scholar]
- Hu, X.; Peng, G.; Niu, D.; Zhao, N. Effect of early frost environment on service performance of concrete. J. Build. Mater. 2020, 23, 1061–1070. [Google Scholar]
- Wu, J.; Lai, Z.; Deng, Q.; Liu, M. Effects of Various Curing Conditions on Volume Stability of Magnesium Phosphate Cement. Adv. Mater. Sci. Eng. 2021, 2021, 6652363. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Paula, G.R.; Morelli, M.R. Effect of boric acid content on the properties of magnesium phosphate cement. Constr. Build. Mater. 2019, 214, 557–564. [Google Scholar] [CrossRef]
- Qiao, F.; Chau, C.K.; Li, Z. Property evaluation of magnesium phosphate cement mortar as patch repair material. Constr. Build. Mater. 2010, 24, 695–700. [Google Scholar] [CrossRef]
- Jia, X.; Luo, J.; Zhang, W.; Qian, J.; Li, J.; Wang, P.; Tang, M. Preparation and Application of Self-Curing Magnesium Phosphate Cement Concrete with High Early Strength in Severe Cold Environments. Materials 2020, 13, 5587. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics: 21st Century Materials with Diverse Applications; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Wagh, A.S. Recent Progress in Chemically Bonded Phosphate Ceramics; Hindawi Publishing Corporation: London, UK, 2013. [Google Scholar]
- Chang, Y.; Shi, C.J.; Yang, N.; Yang, J.M. Research progress on durability of magnesium phosphate cement based materials. J. Chin. Soc. 2014, 42, 486–493. [Google Scholar]
- Jia, X.; Li, J.; Wang, P.; Qian, J.; Tang, M. Preparation and mechanical properties of magnesium phosphate cement for rapid construction repair in ice and snow. Constr. Build. Mater. 2019, 229, 116927. [Google Scholar] [CrossRef]
- Wang, H.T. Study on the High Performance Magnesia-Phosphate Cement Based Composites; Chongqing University: Chongqing, China, 2006. [Google Scholar]
- Lai, Z.Y. Immobilization of Medium and Low Level Radioactive Wastes by Magnesium Phosphate Cement. Ph.D. Thesis, ChongQing University, Chongqing, China, 2012. [Google Scholar]
- Yang, Q.B.; Wu, X. Factors influencing properties of phosphate cement-based binder for rapid repair of concrete. Cem. Concr. Res. 1999, 29, 389–396. [Google Scholar] [CrossRef]
- Tao, Q.; Wang, Y. Mechanical properties and frost resistance of magnesium phosphate cement concrete under negative temperature. J. Glaciol. Geocryol. 2018, 40, 1181–1186. [Google Scholar]
- Jiang, H.; Liang, B.; Zhang, U. Research on MPB ultra-early strength concrete repair materials. J. Build. Mater. 2001, 4, 196–198. [Google Scholar]
- Zhu, D.; Li, Z.; Xing, F. Chemical durability investigation of magnesium phosphosilicate cement. Key Eng. Mater. 2006, 302, 275–281. [Google Scholar]
- Li, D.; Li, P.; Feng, C. Study on water resistance of magnesium phosphate cement. J. Build. Mater. 2009, 12, 505–510. [Google Scholar]
- Zhen, S.; Yang, J.; Zhang, Q. Research on the corrosion resistance of magnesium phosphate cement against chloride ion. J. Build. Mater. 2010, 13, 700–704. [Google Scholar]
- Fan, S.; Chen, B. Experimental research of water stability of magnesium alumina phosphate cements mortar. Constr. Build. Mater. 2015, 94, 164–171. [Google Scholar] [CrossRef]
- Zhu, D. Research of Magnesium Phosphosilicate Cement; Hong Kong University of Science and Technology: Hong Kong, China, 2005. [Google Scholar]
- Yu, H.; Sun, W.; Hua, P.; Yan, L.; Qu, W. Proportion and basic properties of high strength concrete used in salt lakes. J. Build. Mater. 2003, 6940, 410–415. [Google Scholar]
- Feng, C.; Chen, M.; Li, D. Hydration system of magnesium phosphate cement paste. J. Mater. Sci. Eng. 2013, 31, 901–906. [Google Scholar]
- Singh, D.; Wagh, A.S. Phosphate Bonded Structural Product from High Volume Wastes. U.S. Patent 5,846,894, 8 December 1998. [Google Scholar]
- Yang, J.M.; Shi, C.J.; Chang, Y.; Yang, N. Hydration and hardening characteristics of magnesium potassium phosphate cement paste composite retarders. J. Build. Mater. 2013, 16, 43–49. [Google Scholar]
- Yu, C.J. Study on Sulfate Corrosion Behavior and Mechanism of Magnesium Potassium Phosphate Cement (MKPC) Mortar. Ph.D. Thesis, Jiangsu University of Science and Technology, Zhenjiang, China, 2018. [Google Scholar]
- Yang, Q. Mechanisms of deicer-frost scaling of concrete(I)—Capillary-uptake degree of saturation and ice-formation pressure. J. Build. Mater. 2007, 50, 522–527. [Google Scholar]
- Yang, Q. One of mechanisms on the deicer-frost scaling of concret(II): Degree of saturation and ice-formation pressure during freezing-thawing cycles. J. Build. Mater. 2012, 15, 741–746. [Google Scholar]
- Ding, Z.; Xing, F.; Li, Z.J. Repair property of high early strength magnesium phosphosilicate cement. Ind. Constr. 2008, 9, 77–81. [Google Scholar]
- Sotiriadis, K.; Nikolopoulou, E.; Tsivilis, S. Sulfate resistance of limestone cement concrete exposed to combined chloride and sulfate environment at low temperature. Cem. Concr. Compos. 2019, 34, 903–910. [Google Scholar] [CrossRef]
- Chong, L.; Yang, J.; Xu, Z.; Xu, X. Freezing and thawing resistance of MKPC paste under different corrosion solutions. Constr. Build. Mater. 2019, 212, 663–674. [Google Scholar] [CrossRef]
- Tao, T. Study on the Durability of Potassium Magnesium Phosphate Cement (MKPC)-Based Anticorrosive Coating. Ph.D. Thesis, Jiangsu University, Zhenjiang, China, 2020. [Google Scholar]
- Ahmad, M.R.; Chen, B. Effect of silica fume and basalt fiber on the mechanical properties and microstructure of magnesium phosphate cement (MPC) mortar. Constr. Build. Mater. 2018, 190, 466–478. [Google Scholar] [CrossRef]
- Ma, H.; Xu, B.; Li, Z. Magnesium potassium phosphate cement paste: Degree of reaction, porosity and pore structure. Cem. Concr. Res. 2014, 65, 96–104. [Google Scholar] [CrossRef]








| Oxide Species | MgO | SiO2 | CaO | Fe2O3 | Al2O3 | Na2O | TiO2 | Other |
|---|---|---|---|---|---|---|---|---|
| Content (%) | 91.85 | 3.68 | 3.14 | 0.865 | 0.17 | - | - | 0.285 |
| Type | Speciation | Fineness Modulus | Clay Content (%) | Bulk Density (kg/m3) | Particle Gradation |
|---|---|---|---|---|---|
| Ordinary river sand | Medium sand | 2.53 | 0.8 | 1450.00 | Zone II (JGJ52-2006) |
| Limestone sand | Coarse sand | 3.65 | 0.00 | 1460.00 | Zone II (JGJ52-2006) |
| Code | Mass Ratio/% | |||||
|---|---|---|---|---|---|---|
| WMgO/ WKH2PO4 | WCR/ WMKPC | WS/WMKPC | WRS/WS | WLS/ WS | Ww/ WMKPC | |
| M | 150 | 8 | 150 | 33.3 | 66.7 | 17 |
| Element | O | Mg | S | P | Cl | K | Ca | Na | |
|---|---|---|---|---|---|---|---|---|---|
| Atomic percentage (%) | Area A | 68.45 | 12.52 | - | 8.79 | - | 10.24 | - | - |
| Area B | 68.72 | 10.70 | - | 9.47 | - | 9.78 | 1.33 | - | |
| Area C | 70.11 | 10.96 | - | 9.87 | - | 9.06 | - | - | |
| Area D | 72.92 | 8.95 | - | 9.59 | - | 8.54 | - | - | |
| Area E | 73.45 | 8.56 | 2.45 | 7.53 | 0.13 | 5.93 | 1.52 | 0.43 | |
| Area F | 74.33 | 6.41 | 1.99 | 6.12 | - | 6.57 | 3.70 | 0.88 | |
| Area G | 72.3 | 10.36 | - | 8.12 | - | 9.22 | - | - | |
| Area H | 73.52 | 8.43 | - | 6.95 | - | 6.07 | 2.11 | 2.92 | |
| Area I | 66.35 | 7.47 | 2.56 | 8.85 | 0.35 | 9.55 | 2.86 | 2.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, R.-J.; Li, T.; Yang, J.-M.; Xu, J. Sulfate Freeze–Thaw Resistance of Magnesium Potassium Phosphate Cement Mortar according to Hydration Age. Materials 2022, 15, 4192. https://doi.org/10.3390/ma15124192
Ji R-J, Li T, Yang J-M, Xu J. Sulfate Freeze–Thaw Resistance of Magnesium Potassium Phosphate Cement Mortar according to Hydration Age. Materials. 2022; 15(12):4192. https://doi.org/10.3390/ma15124192
Chicago/Turabian StyleJi, Rong-Jian, Tao Li, Jian-Ming Yang, and Jun Xu. 2022. "Sulfate Freeze–Thaw Resistance of Magnesium Potassium Phosphate Cement Mortar according to Hydration Age" Materials 15, no. 12: 4192. https://doi.org/10.3390/ma15124192
APA StyleJi, R.-J., Li, T., Yang, J.-M., & Xu, J. (2022). Sulfate Freeze–Thaw Resistance of Magnesium Potassium Phosphate Cement Mortar according to Hydration Age. Materials, 15(12), 4192. https://doi.org/10.3390/ma15124192







