Experimental Investigation of Different Fineness and Firing Temperatures on Pellets Properties of Different Iron Ore fines from Indian Mines
Abstract
1. Introduction
2. Experimental Work
2.1. Pellets Making
2.1.1. Fines Sample Preparation
2.1.2. Green Pellets Preparation
2.2. Pellets Heat Hardening
Thermal Treatment Cycle
- ➢
- Drying of pellets occurredin the temperature range of 27–350 °C, within 80 min, and held at 350 °C for 30 min.
- ➢
- Preheating or intensive drying of pellets happened in the temperature range of 350–1000 °C, within 110 min, and held at 1000 °C for 20 min.
- ➢
- The firing of pellets took place at three different temperatures to make 12 sets of pellets.
- A-set firing was done from 1000 to 1250 °C, within 80 min, and soaking time was 20 min.
- B-set firing was done from 1000 to 1300 °C, within 80 min, and soaking time was 20 min.
- C-set firing was done from 1000 to 1350 °C, within 80 min, and soaking time was 20 min.
2.3. Physical and Metallurgical Examination of Pellets
2.4. Reduction DEGRADATION Index (R.D.I.) and Reducibility Index (R.I.)
2.5. Field Emission Scanning Electron Microscopy (F.E.S.E.M.) and Energy Dispersive Spectroscopy (EDS) Analysis
2.6. X-ray Diffraction (XRD)
2.7. Fourier Transformation Infrared Spectrum (FTIR)
3. Result and Discussion
3.1. Green Pellets Characterization
3.2. Fired Pellets Characterization
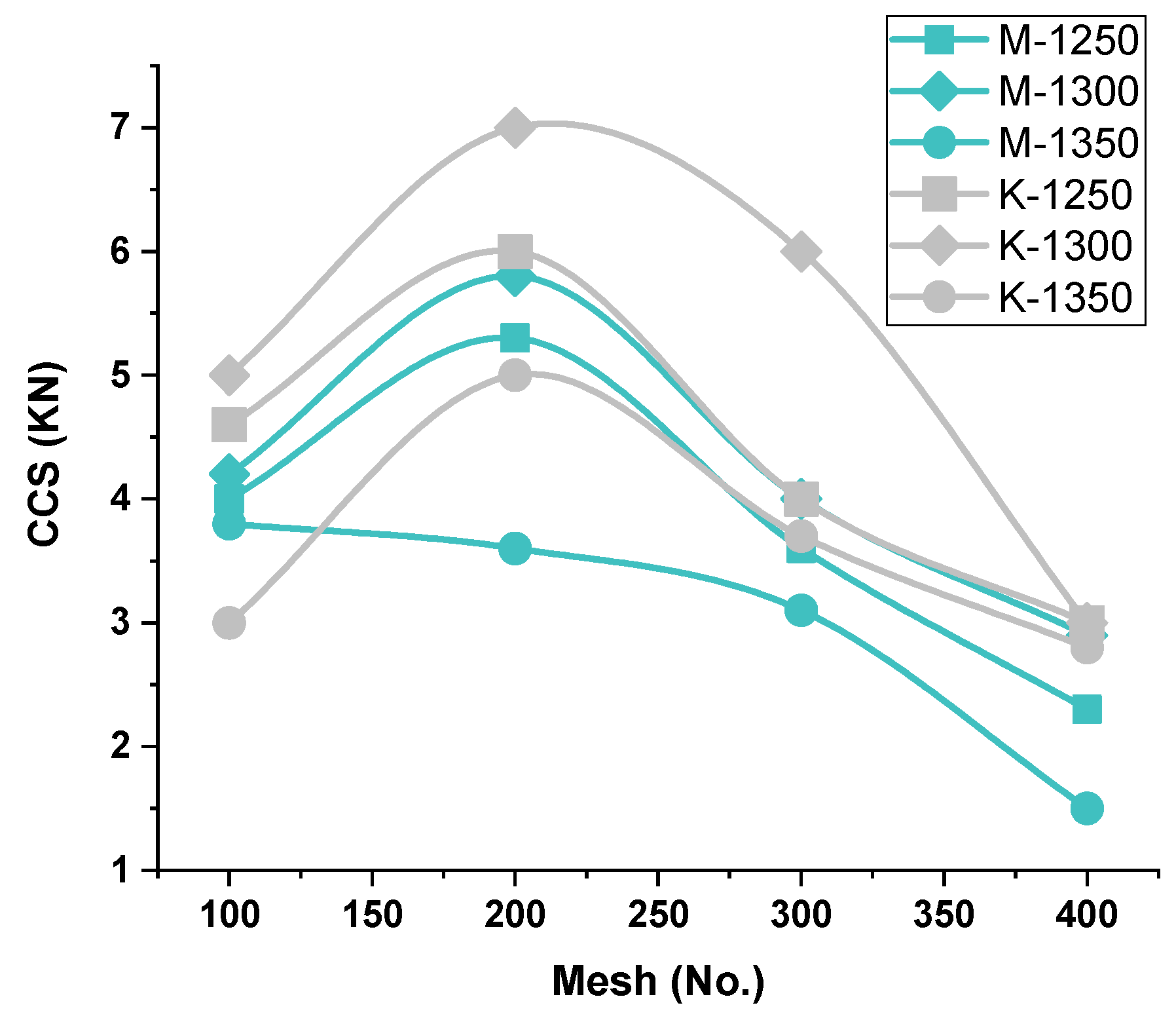
3.2.1. Physical Properties (Cold Compression Strength (C.C.S.) and Apparent Porosity (A.P.)) Examination
C.C.S.-C.C.S.-vs.-Firing Temperature—Iron Ore No.1 and No.2 Pellets
C.C.S.-vs.-Blaine fineness—Iron Ore No.1/No.2 Pellets
C.C.S.-vs.-Ore’s Chemistry—Iron Ore No.1/No.2 Pellets
AP vs. Firing Temperature—Iron Ore No.1/Iron Ore No.2
A.P. vs. Blaine Fineness–Iron Ore No.1/Iron Ore No.2
A.P. vs. Chemistry—Iron Ore No.1/Iron Ore No.2
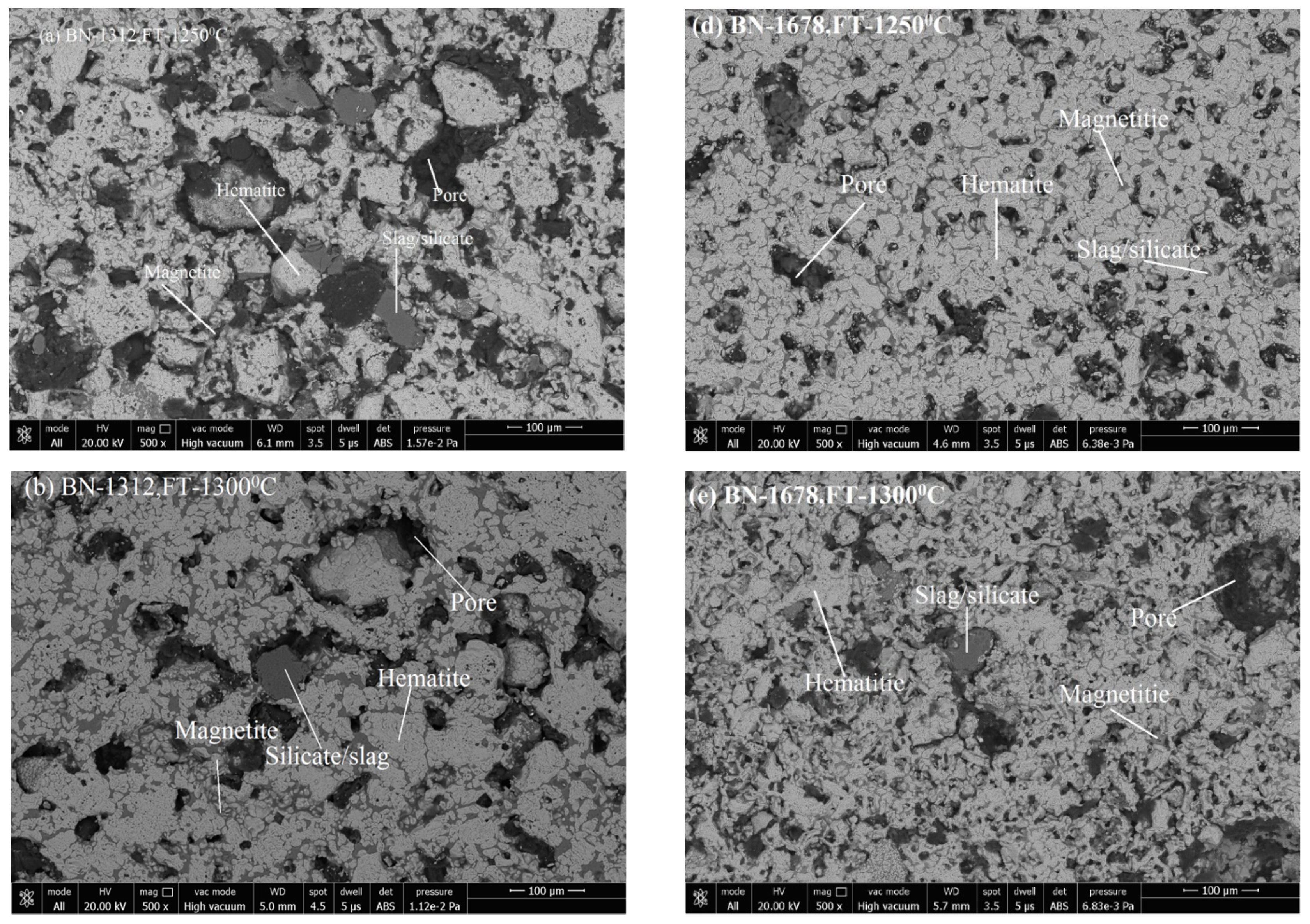
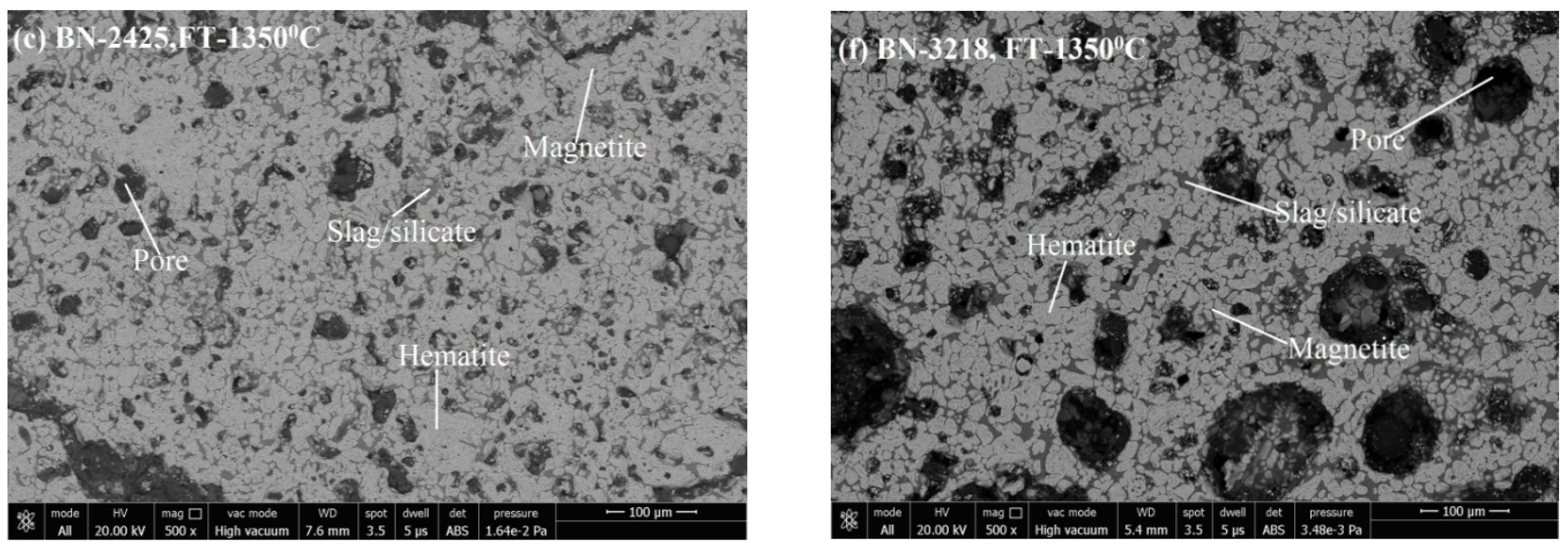
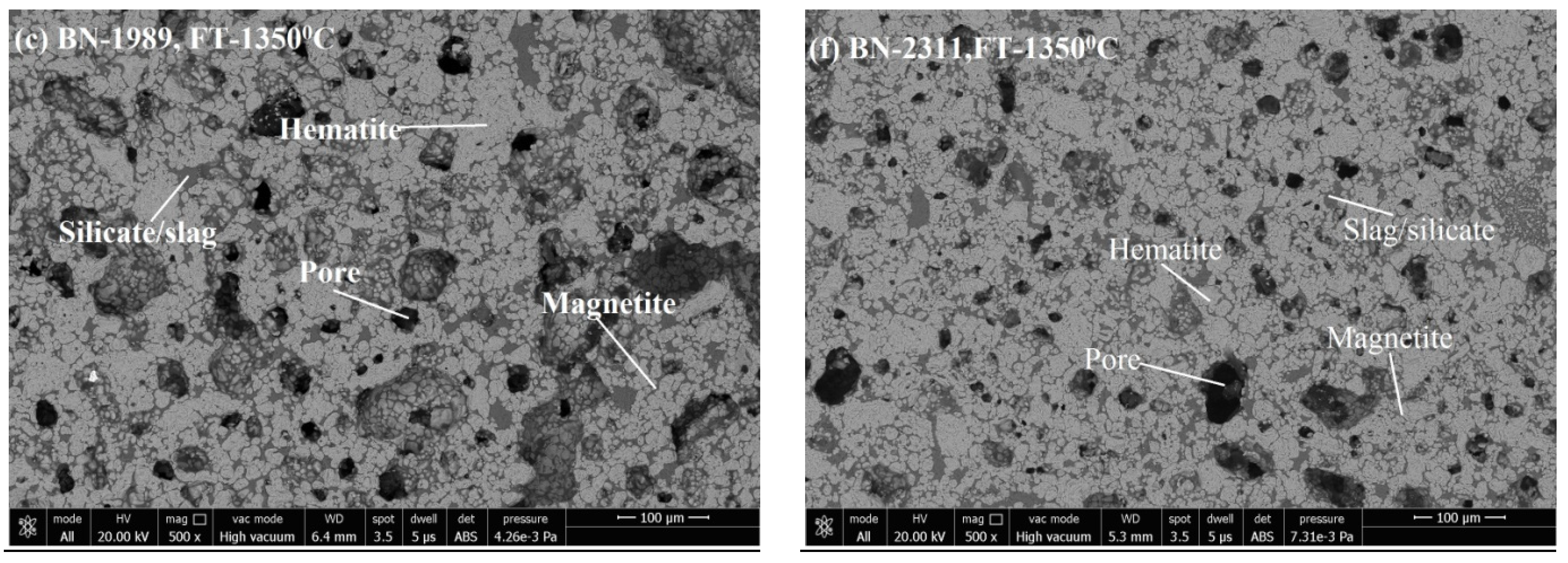
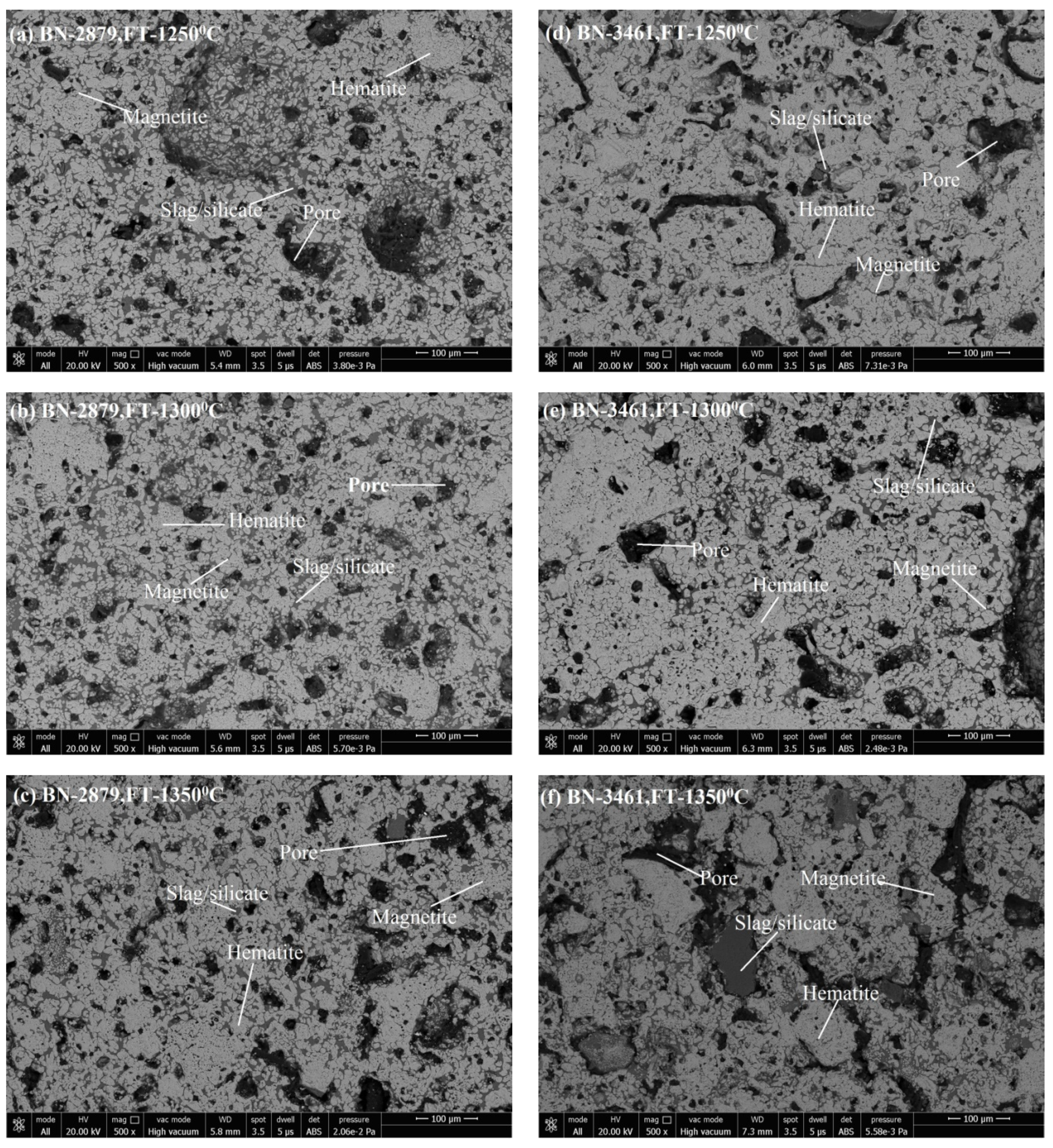
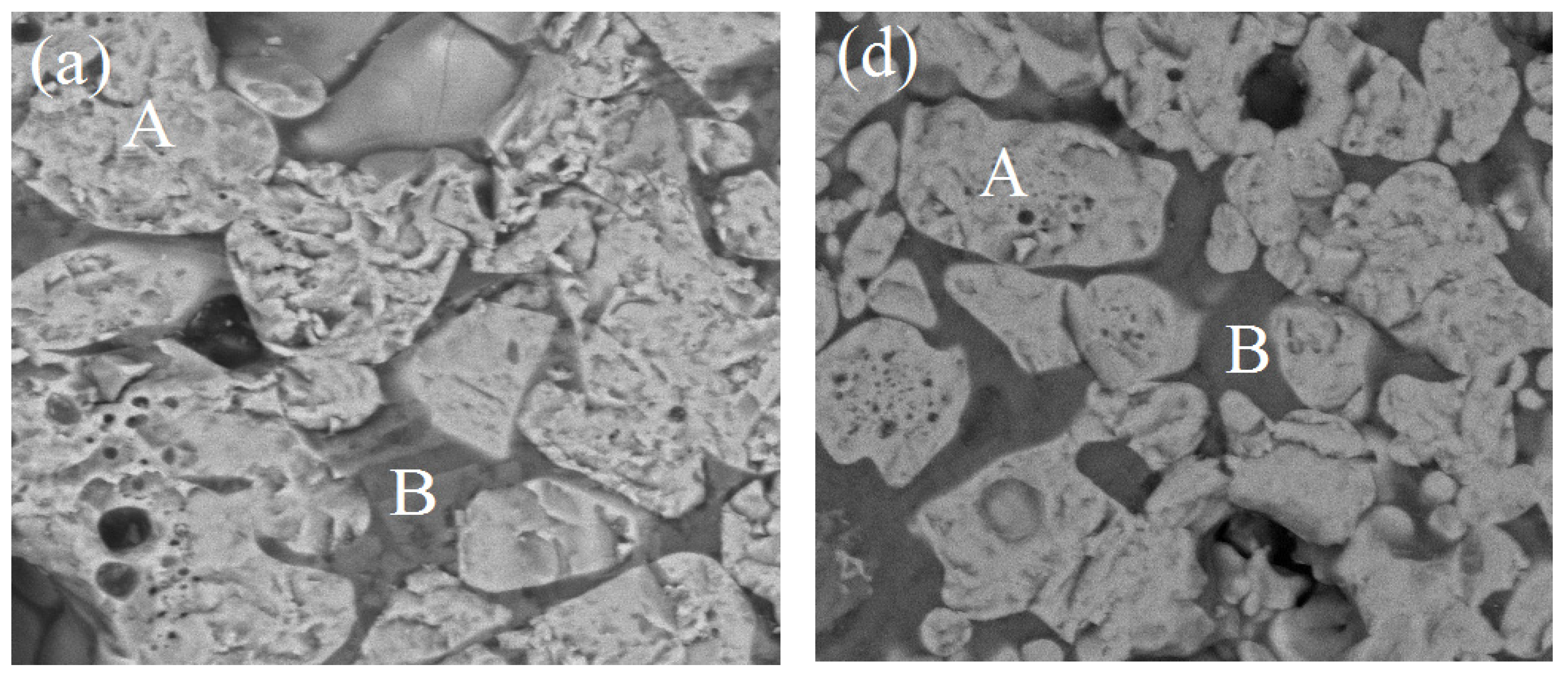
3.3. Microstructure Analysis
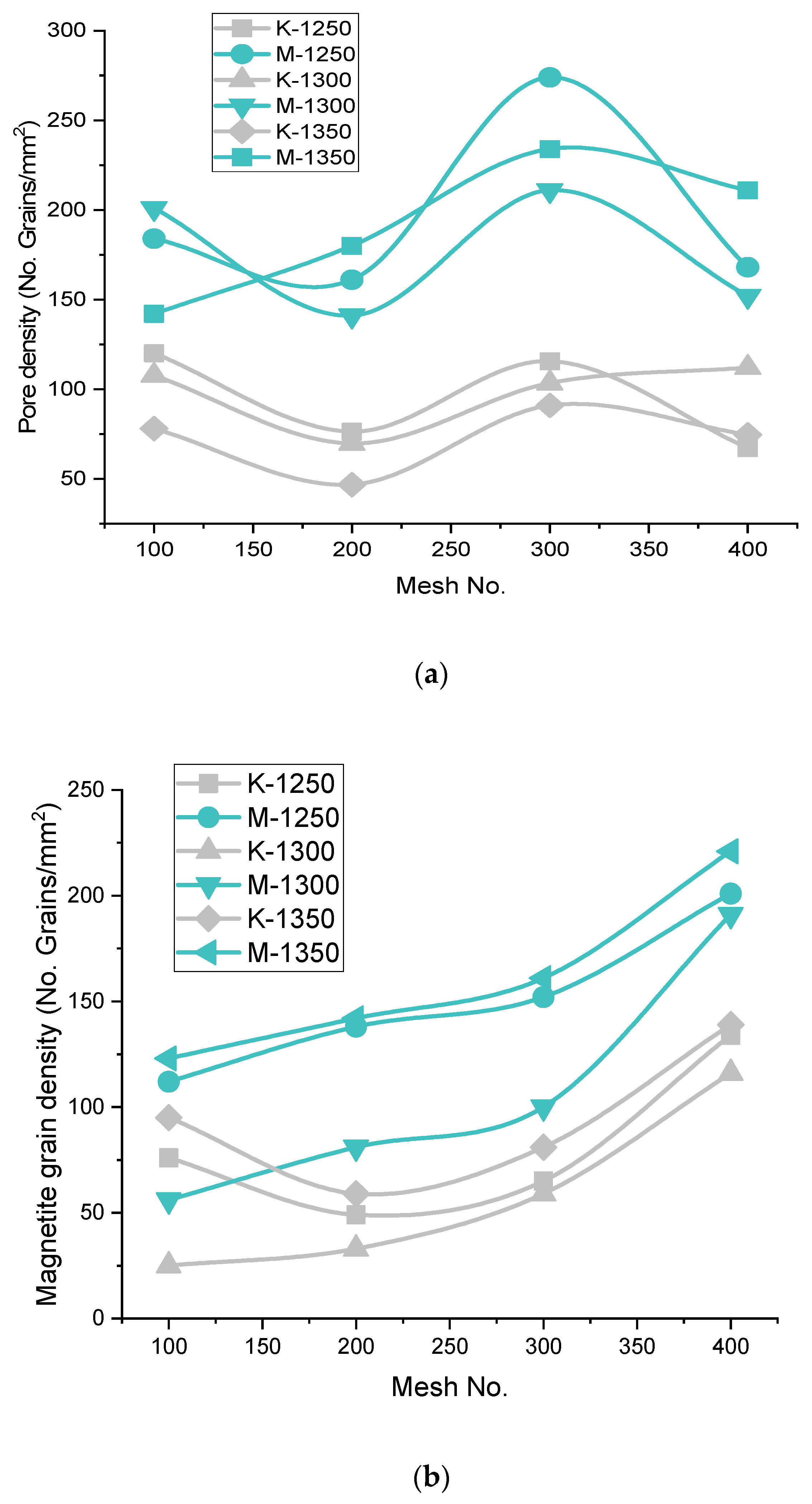
3.3.1. Phase Grains Density Study
Hematite Phase Grain Density (H.P.G.D.)
Silicate/Slag Phase Grain Density (S.P.G.D.)
Pore Phase Grains Density (P.P.G.D.)
Magnetite Phase Grain Density (M.P.G.D.)
3.4. Energy Dispersive Spectroscopy (EDS) Analysis
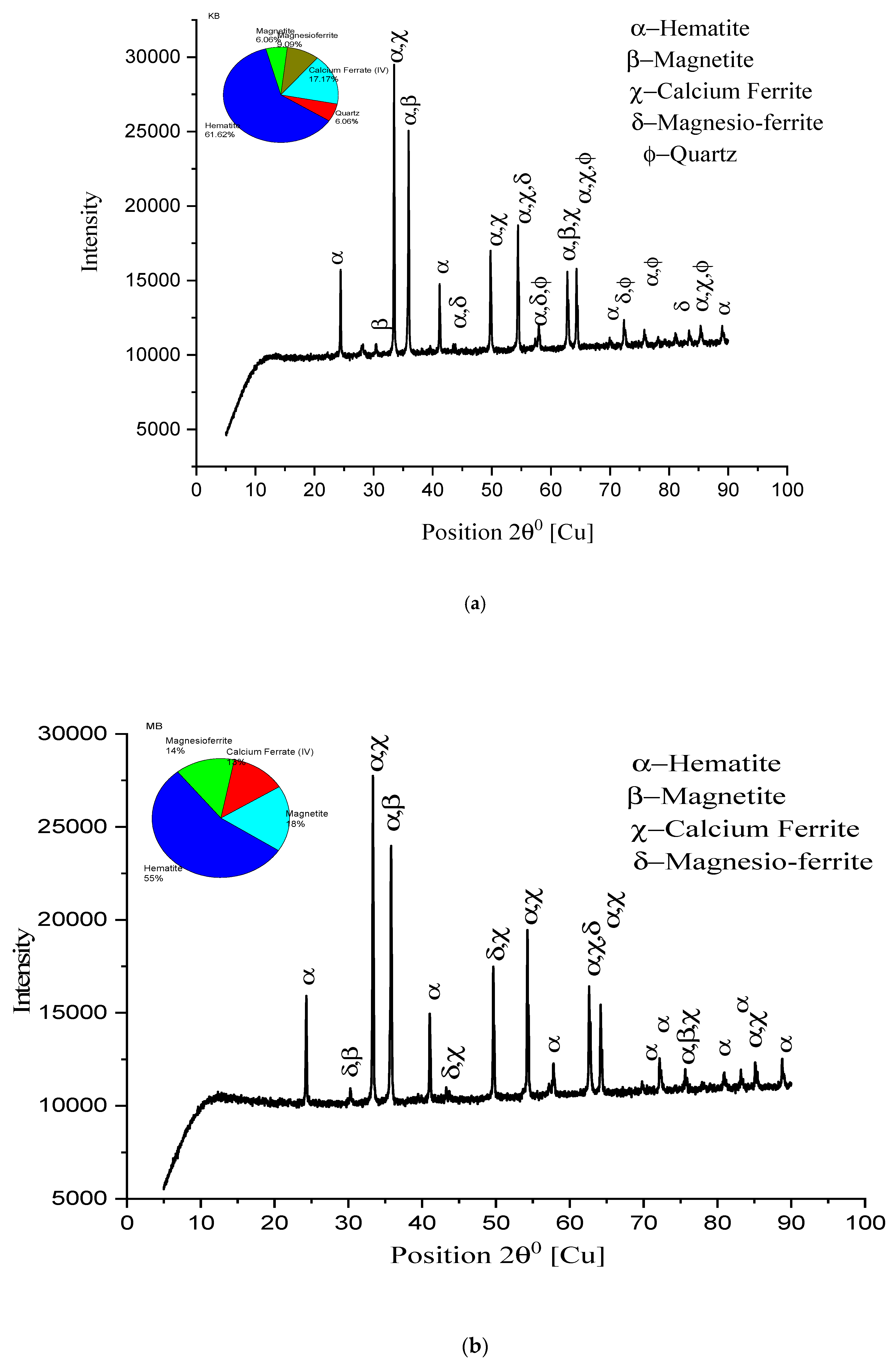
3.5. X-ray Diffraction Study
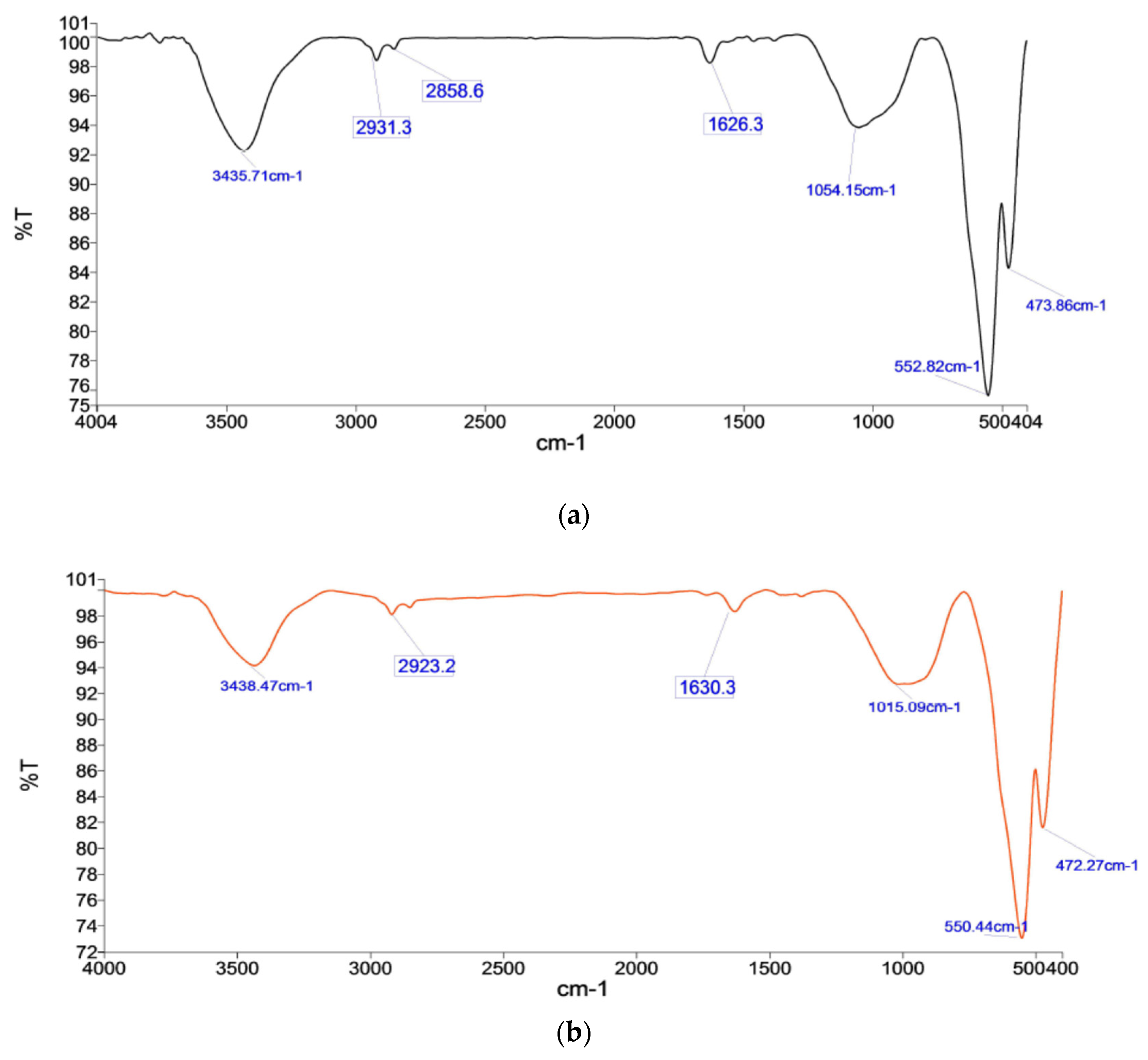
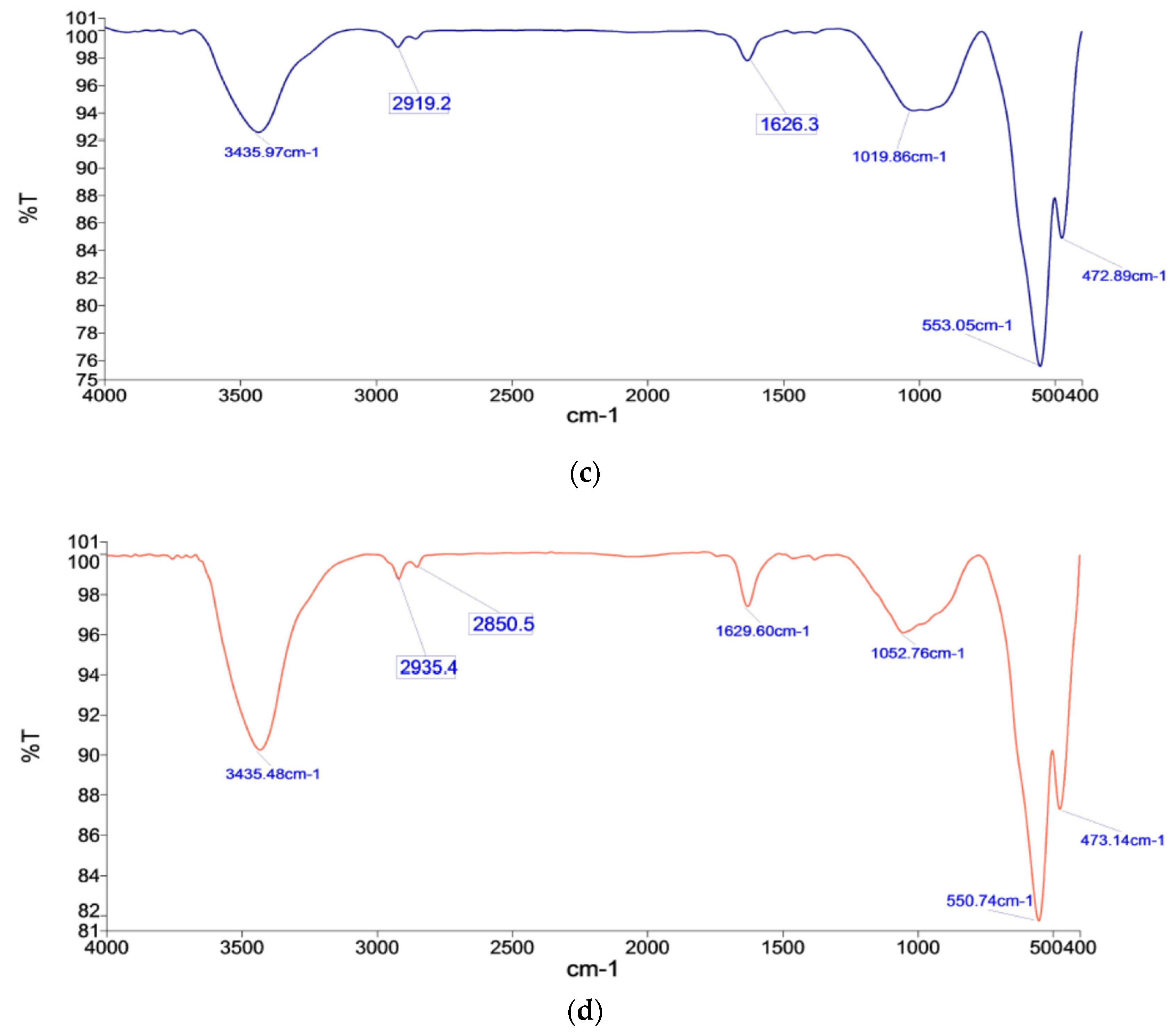
3.6. Fourier Transform Infrared Spectroscopy (FTIR)–Analysis
3.6.1. Highest C.C.S. Pellets
3.6.2. Significant C.C.S. Pellets
3.6.3. Highest Porosity Pellets
3.6.4. Minimal C.C.S. Pellets
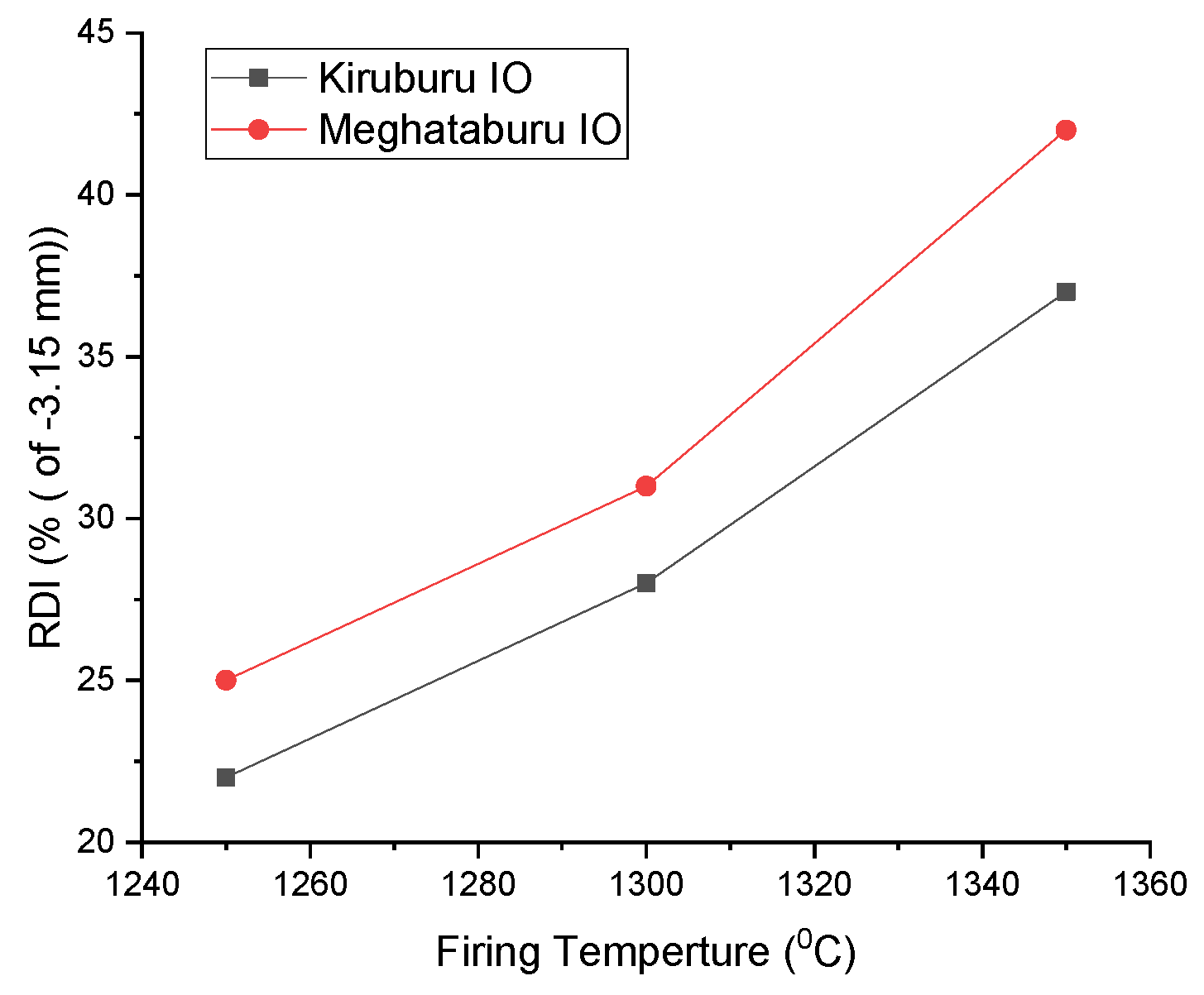
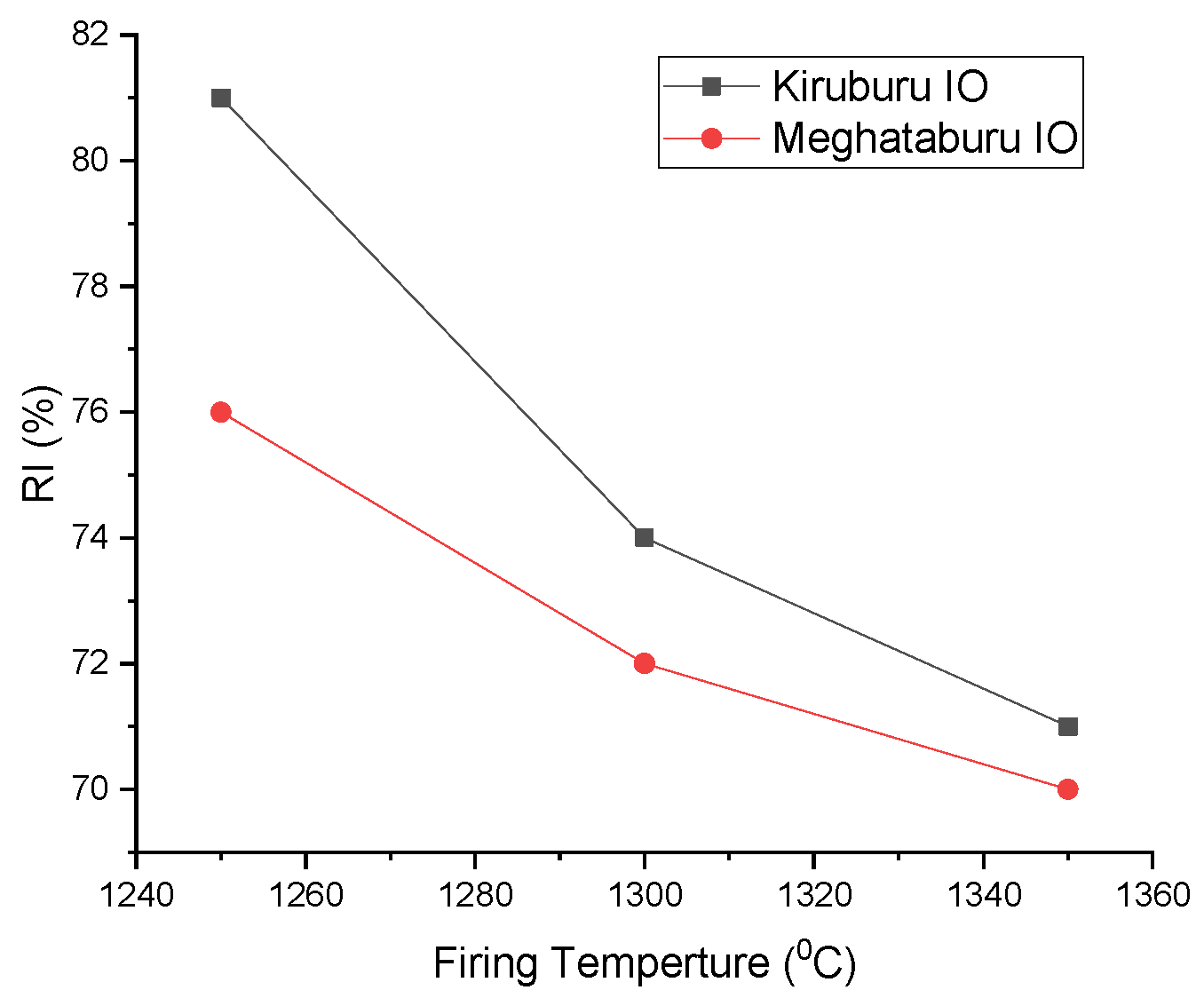
3.7. Reduction Degradation Index (R.D.I.) and Reducibility Index (R.I.) of Pellets
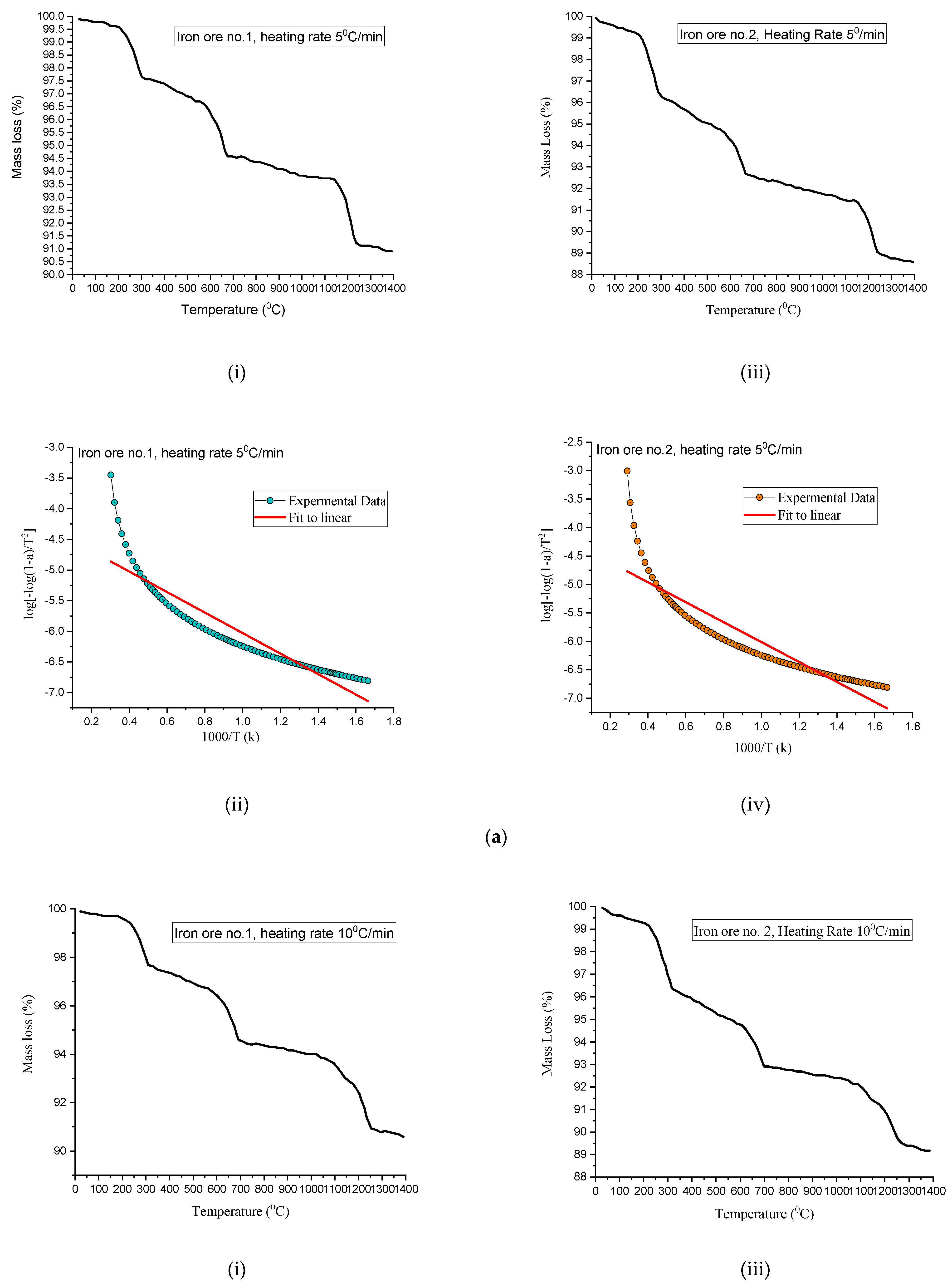
3.8. Thermal Kinetics Analysis of Heating of Pellets
Pellets Heat Treatment Can Be Divided into Three Steps
4. Conclusions
- ➢
- Pellets roasted at 1300 °C exhibit a higher C.C.S. value than the pellets fired at other firing temperatures, of both Iron Ore No.1 and No.2 pellets, of respective Blaine fineness. In the lower Blaine array (1312/1678 cm2/g), Iron Ore No.1 pellets were fired at 1250 °C/1300 °C and had higher C.C.S. than the Iron Ore No.2 pellets (Blaine 1989/2311 cm2/g) due to Ostwald ripening effect. Iron Ore No.2 ore has gangue content compared to Iron Ore No.1 mines, as a high Al2O3/SiO3 ratio influences the sintering of iron oxide grains that make the viscous melt, enhance pore size irregularity, and affect the pellet strength.
- ➢
- H.P.G.D. is high in the microstructure of Iron Ore No.1 and No.2 pellets under 200 mesh, but beyond 200 mesh decreases due to increment of finer particle proportion. At temperate 1300 °C, S.P.G.D. is higher for 200 mesh for both Iron Ore No.1 and No.2 pellets. Blaine increases from 1312–1678 cm2/g for Iron Ore No.1 and 1989–2311 cm2/g for Iron Ore No.2. A decrease in P.P.G.D. shows proper sintering of oxides, which enhance the strength of pellets.
- ➢
- XRD analysis of Iron Ore No.1 and No.2 pellets correspond to 200 mesh for different firings, concluding that iron oxide phases and ferrite phases (calcium and magnesium) directly influence the C.C.S. and AP. As the firing temperature 1250 °C/1300 °C/1350 °C, hematite phase percentage 71.1%, 61.1%, and 57%; magnetite phase 6.1%, 6%, and 3% decreases, while ferrite phases 22.2%, 26%, and 35% increase for Iron Ore No.1 pellet. For Iron Ore No.2, pellet hematite phase percentage varies from 61% to 55% and increases to 67%; magnetite phase varies from 9% to 18% and decreases to 6%, while ferrite phases increase from 21% to 27%, and up to 28.8%.
- ➢
- Firing at 1350 °C has a high H.P.G.D. and shows high R.D.I. compared to pellets firing at 1300 °C/1250 °C with low R.D.I. Pore phase density is high for the Iron Ore No.2 pellet, at 1350 °C rather than 1300 °C. If phase percentage is high, it shows high R.D.I. for pellets fired at 1250 °C. Iron Ore No.1 pellets have coarser fines compared to Iron Ore No.2 pellets, which show good apparent porosity (A.P.) and offer good R.I. The pellet fired at 1250 °C reflects the higher reducibility, since they have a lower magnetite phase and less silicate/slag bond with a higher pore phase. Pellets fired at 1350 °C have more slag bonding with a reduced pore phase, less density, and more magnetite grains, thus showing lower reducibility.
- ➢
- Chemical bonds in phases reflected in IR Spectroscopy show iron oxides phase 471.59 cm−1 and 550.18 cm−1 for K2B, as well as phase peaks 472.27 cm−1 and 550.44 cm−1 for M2B. Here, the broader range of peaks with high intensity reflects the bond affiliation of their dominant presence.
- ➢
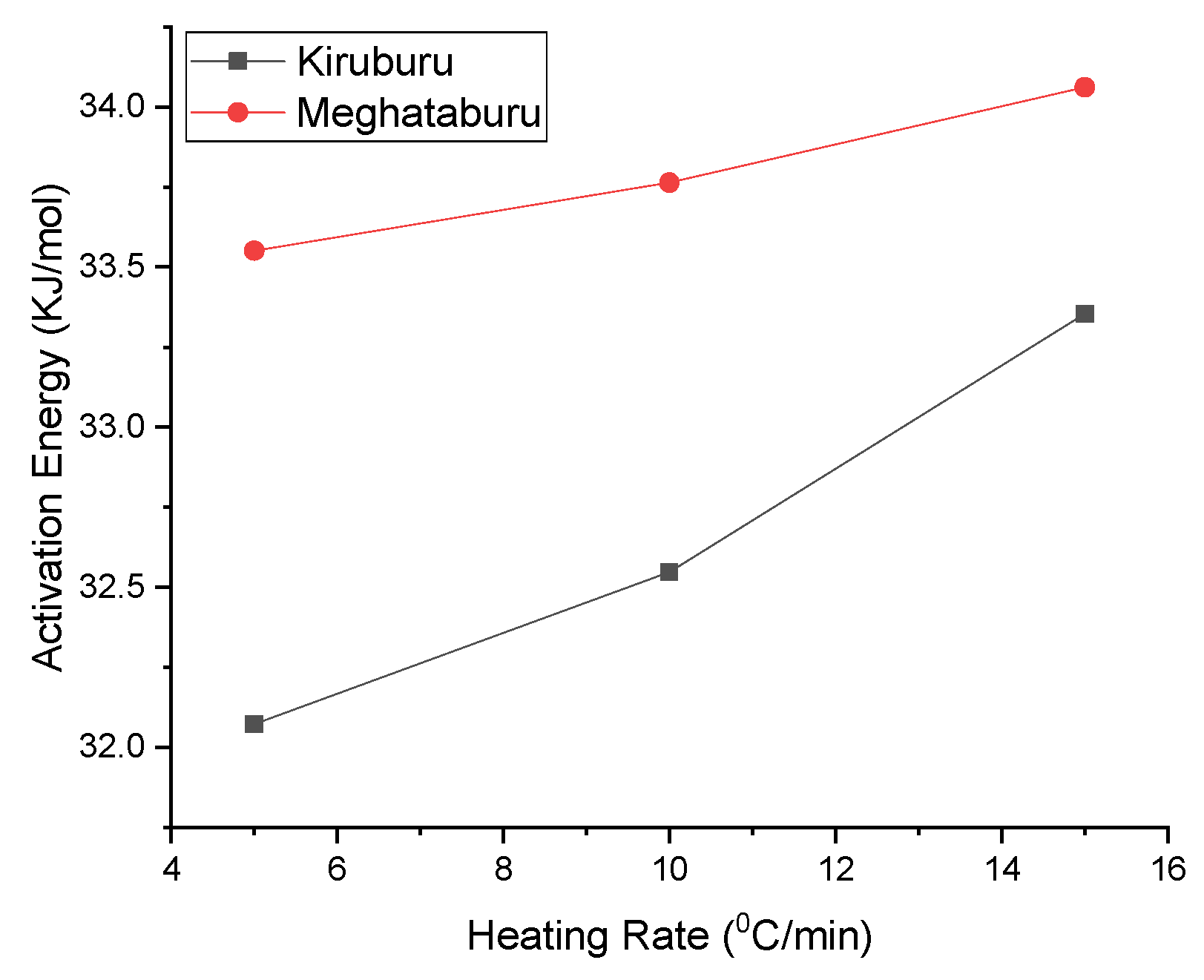
- For high activation energy, hematite dissociation reaction rate dependence at temperature should be high. As compared to Iron Ore No.2, the sample has high activation energy corresponding to the same heating rate of Iron Ore No.1. Due to the fineness of the ore particles and gangue content, it is higher for Iron Ore No.2 fines compared to Iron Ore No.1 fines.
Author Contributions
Funding
Conflicts of Interest
References
- Meyer, K. Pelletizing of Iron Ore; Springer: Berlin/Heidelberg, Germany, 1980; ISBN 0-387-10215-9. [Google Scholar]
- Ball, D. Agglomeration of Iron Ores; American Elsevier Pub. Co.: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Li, G.H.; Li, X.Q.; Zhang, Y.B.; He, G.Q.; Jiang, T. Induration Mechanisms of Oxidised Pellets Prepared from Mixed Magnetite–Haematite Concentrates. Ironmak. Steelmak. 2009, 36, 393–396. [Google Scholar] [CrossRef]
- Umadevi, T.; Lobo, N.F.; Desai, S.; Mahapatra, P.C.; Sah, R.; Prabhu, M. Optimization of Firing Temperature for Hematite Pellets. ISIJ Int. 2013, 53, 1673–1682. [Google Scholar] [CrossRef]
- McCann, G.; Strezov, V.; Lucas, J.A.; Evans, T.; Strezov, L. Iron Ore Characterisation during High Temperature Thermal Processing. Dev. Chem. Eng. Miner. Process. 2010, 12, 369–382. [Google Scholar] [CrossRef]
- Ravisankar, V.; Venugopal, R.; Bhat, H. Transactions of the Institutions of Mining and Metallurgy: Section C Investigation on Beneficiation of Goethite-Rich Iron Ores Using Reduction Roasting Followed by Magnetic Separation. Miner. Process. Extr. Metall. 2017, 128, 175–182. [Google Scholar] [CrossRef]
- Sunil, S.R.; Rayapudi, V.; Dhawan, N. Recovery of Iron Values from Discarded Iron Ore Slimes. Min. Metall. Explor. 2019, 37, 287–295. [Google Scholar] [CrossRef]
- Mining & Mineral Statistics Division-Ibm Statistical Profiles of Minerals; Government of India-Ministry of Mines: Kolkata, India, 2021.
- Meyer, M.; Lagoeiro, L.E.; Graça, L.M.; Silva, C.J. Phase and Microstructural Characterization of Iron Ore Pellet and Their Relation with Cold Crushing Strength Test. Miner. Process. Extr. Metall. Rev. 2016, 37, 295–304. [Google Scholar] [CrossRef]
- Prasad, R.; Venugopal, R.; Kumaraswamidhas, L.A.; Pandey, C.; Pan, S.K. Analysis of the Influence of Blaine Numbers and Firing Temperature on Iron Ore Pellets Properties Using RSM-I-Optimal Design: An Approach Toward Suitability. Min. Metall. Explor. 2020, 37, 1703–1716. [Google Scholar] [CrossRef]
- Kawatra, S.K.; Claremboux, V. Iron Ore Pelletization: Part I. Fundamentals. Miner. Process. Extr. Metall. Rev. 2022, 43, 529–544. [Google Scholar] [CrossRef]
- Dwarapudi, S.; Ghosh, T.K.; Shankar, A.; Tathavadkar, V.; Bhattacharjee, D.; Venugopal, R. Effect of Pellet Basicity and MgO Content on the Quality and Microstructure of Hematite Pellets. Int. J. Miner. Process. 2011, 99, 43–53. [Google Scholar] [CrossRef]
- IS 8625-1986; Determination of Crushing Strength of Iron Ore Pellets. Bureau of Indian Standard: Kolkata, India, 1986.
- IS 1528, Part-VIII-1974; Determination of Apparent Porosity. Bureau of Indian Standard: Kolkata, India, 1974.
- JIS M 8713-2000; Iron Ores—Determination of Reducibility. Japanese Industrial Standard: Tokyo, Japan, 2000.
- JIS M 8720-2001; Iron Ores—Determination of Low-Temperature Reduction-Disintegration. Japanese Industrial Standard: Tokyo, Japan, 2001.
- Ammasi, A. Effect of Heating Rate on Decomposition Temperature of Goethite Ore. Trans. Indian Inst. Met. 2020, 73, 93–98. [Google Scholar] [CrossRef]
- De Villiers, J.P.; Lu, L. Quantitative XRD Analysis and Evaluation of Iron Ore, Sinter, and Pellets. In Iron Ore; Woodhead Publishing: Cambridge, UK, 2022; pp. 109–126. [Google Scholar] [CrossRef]
- Pal, J.; Ghorai, S.; Agarwal, S.; Nandi, B.; Chakraborty, T.; Das, G.; Prakash, S. Effect of Blaine Fineness on the Quality of Hematite Iron Ore Pellets for Blast Furnace. Miner. Process. Extr. Metall. Rev. 2015, 36, 83–91. [Google Scholar] [CrossRef]
- Barik, K.; Prusti, P.; Soren, S.; Meikap, B.C.; Biswal, S.K. Analysis of Iron Ore Pellets Properties Concerning Raw Material Mineralogy for Effective Utilization of Mining Waste. Powder Technol. 2022, 400, 117259. [Google Scholar] [CrossRef]
- Ayyandurai, A.; Pal, J. Reaction Mechanism of In-Situ Carbon in Hematite Ore Pellet during Induration. Miner. Process. Extr. Metall. Rev. 2020, 43, 40–54. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Y.B.; Huang, Z.C.; Li, G.H.; Fan, X.H. Preheating and Roasting Characteristics of Hematite–Magnetite (H–M) Concentrate Pellets. Ironmak. Steelmak. 2008, 35, 21–26. [Google Scholar] [CrossRef]
- Li, G.; Jiang, T.; Zhang, Y.; Tang, Z. Recrystallization of Fe2O3 During the Induration of Iron Ore Oxidation Pellets. Recrystallization 2012, 13, 329–350. [Google Scholar] [CrossRef]
- Ooi, T.C.; Campbell-Hardwick, S.; Zhu, D.; Pan, J. Sintering Performance of Magnetite-Hematite-Goethite and Hematite-Goethite Iron Ore Blends and Microstructure of Products of Sintering. Miner. Process. Extr. Metall. Rev. 2014, 35, 266–281. [Google Scholar] [CrossRef]
- Forsmo, S. Influence of Green Pellet Properties on Pelletizing of Magnetite Iron Ore. Ph.D. Thesis, Luleå Tekniska Universitet, Luleå, Sweden, 2007. [Google Scholar]
- Rajshekar, Y.; Pal, J.; Venugopalan, T. Sintering Characteristics and Kinetics of Acidic Haematite Ore Pellets with and without Mill Scale Addition. Ironmak. Steelmak. 2018, 45, 325–334. [Google Scholar] [CrossRef]
- Pal, J. Innovative Development on Agglomeration of Iron Ore Fines and Iron Oxide Wastes. Miner. Process. Extr. Metall. Rev. 2019, 40, 248–264. [Google Scholar] [CrossRef]
- Umadevi, T.; Deodar, A.V.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of Alumina on Iron Ore Sinter Properties and Productivity in the Conventional and Selective Granulation Sintering Process. Steel Res. Int. 2009, 80, 686–692. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Arol, A.I. The Bonding/Strengthening Mechanism of Colemanite Added Organic Binders in Iron Ore Pelletization. Int. J. Miner. Process. 2012, 110–111, 90–100. [Google Scholar] [CrossRef]
- Zhu, D.; Pan, J.; Lu, L.; Holmes, R.J. Iron Ore Pelletization; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9781782421597. [Google Scholar]
- Pownceby, M.I.; Clout, J.M.F. Importance of Fine Ore Chemical Composition and High Temperature Phase Relations: Applications to Iron Ore Sintering and Pelletising. Miner. Process. Extr. Metall. 2003, 112, 44–51. [Google Scholar] [CrossRef]
- Forsmo, S.P.E.; Forsmo, S.E.; Samskog, P.O.; Björkman, B.M.T. Mechanisms in Oxidation and Sintering of Magnetite Iron Ore Green Pellets. Powder Technol. 2008, 183, 247–259. [Google Scholar] [CrossRef]
- Umadevi, T.; Brahmacharyulu, A.; Roy, A.K.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of Iron Ore Fines Feed Size on Microstructure, Productivity and Quality of Iron Ore Sinter. ISIJ Int. 2011, 51, 922–929. [Google Scholar] [CrossRef]
- Fitton, J.; Goldring, D.J. Constitution of Iron Pellets in Relation to Time and Temperature of Firing. J. Iron Steel Inst. 1955, 452–459. [Google Scholar]
- Firth, A.R.; Garden, J.F.; Douglas, J.D. Phase Equilibria and Slag Formation in the Magnetite Core of Fluxed Iron Ore Pellets. ISIJ Int. 2008, 48, 1485–1492. [Google Scholar] [CrossRef]
- Dwarapudi, S.; Devi, T.U.; Rao, S.M.; Ranjan, M. Influence of Pellet Size on Quality and Microstructure of Iron Ore. ISIJ Int. 2008, 48, 768–776. [Google Scholar] [CrossRef]
- Yang, Y. Fundamental Mechanism of Pore Formation Fundamental Mechanisms in Lron Ore Sinter and Pellets. ISIJ Int. 1991, 31, 468–477. [Google Scholar] [CrossRef][Green Version]
- Prusti, P.; Nayak, B.K.; Biswal, S.K. Study of Temperature Profile in the Induration of Magnetite Iron Ore Pellets. Trans. Indian Inst. Met. 2017, 70, 453–462. [Google Scholar] [CrossRef]
- Prasad, P.S.R.; Shiva Prasad, K.; Krishna Chaitanya, V.; Babu, E.V.S.S.K.; Sreedhar, B.; Ramana Murthy, S. In Situ FTIR Study on the Dehydration of Natural Goethite. J. Asian Earth Sci. 2006, 27, 503–511. [Google Scholar] [CrossRef]
- Umadevi, T.; Sridhara, K.S.; Rameshwar, S. Optimization of Induration Cycle for Magnetite Concentrate and Its Usage in Pellet-Making Process Designed for Hematite Concentrate. Min. Metall. Explor. 2021, 38, 243–255. [Google Scholar] [CrossRef]
- Nguyen, C.-S.; Nguyen, T.-H.; Nguyen, S.-L.; Bui, A.-H. Study on the Reducibility of Iron Ore Pellets at High Temperature. Minist. Sci. Technol. Vietnam 2021, 63, 3–7. [Google Scholar] [CrossRef]
- Umadevi, T.; Kumar, A.; Karthik, P.; Srinidhi, R.; Manjini, S. Characterisation Studies on Swelling Behaviour of Iron Ore Pellets. Ironmak. Steelmak. 2018, 45, 157–165. [Google Scholar] [CrossRef]
- Pimenta, H.P.; Seshadri, V. Influence of Al2O3 and TiO2 on Reduction Degradation Behaviour of Sinter and Hematite at Low Temperatures. Ironmak. Steelmak. 2002, 29, 175–179. [Google Scholar] [CrossRef]
- Rajshekar, Y.; Pal, J.; Venugopalan, T. Mill Scale as a Potential Additive to Improve the Quality of Hematite Ore Pellet. Miner. Process. Extr. Metall. Rev. 2018, 39, 202–210. [Google Scholar] [CrossRef]
- Pal, J.; Ghorai, S.; Venugopalan, T. Effect of High Blaine Iron Ore Fines in Hematite Ore Pelletization for Blast Furnace. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2018, 129, 299–307. [Google Scholar] [CrossRef]
- Sorescu, M.; Xu, T. Particle Size Effects on the Thermal Behavior of Hematite. J. Therm. Anal. Calorim. 2012, 107, 463–469. [Google Scholar] [CrossRef]
- Sah, R.; Dutta, S.K. Kinetic Studies of Iron Ore-Coal Composite Pellet Reduction by TG-DTA. Trans. Indian Inst. Met. 2011, 64, 583–591. [Google Scholar] [CrossRef]
- Ebrahimi-Kahrizsangi, R.; Abbasi, M.H. Evaluation of Reliability of Coats-Redfern Method for Kinetic Analysis of Non-Isothermal TGA. Trans. Nonferr. Met. Soc. China 2008, 18, 217–221. [Google Scholar] [CrossRef]

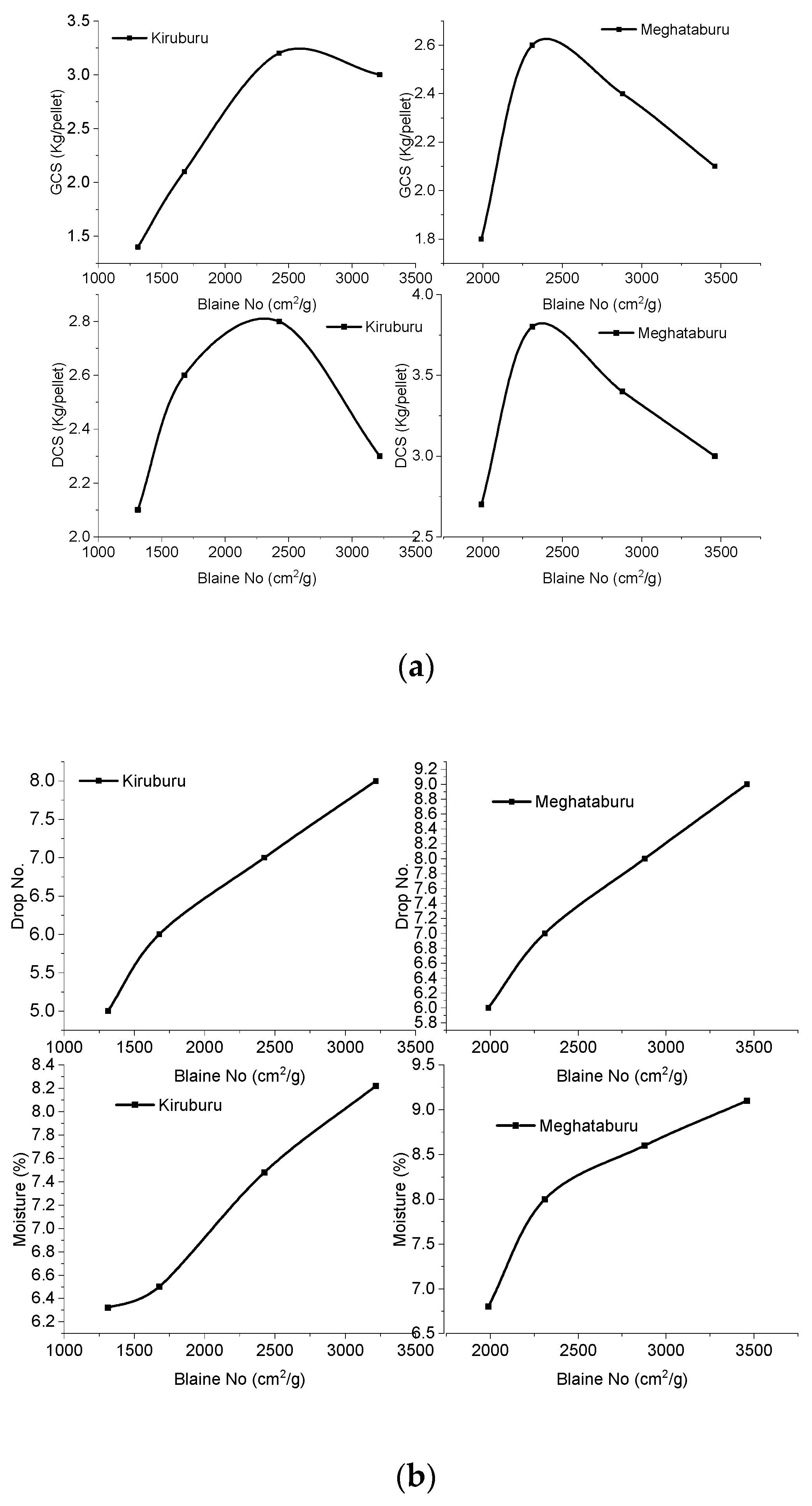












| Blaine No. (cm2/g) | +210 | −210 + 149 | −149 + 105 | −105 + 74 | −74 + 53 | −53 + 44 | −44 |
|---|---|---|---|---|---|---|---|
| 1312 | 31.2 | 12.1 | 7.2 | 5 | 2 | 2.9 | 39.6 |
| 1678 | 23.6 | 11 | 6.4 | 6.5 | 3.1 | 3.4 | 46 |
| 2425 | 11.4 | 9.1 | 7 | 6.2 | 6 | 4.2 | 56.1 |
| 3218 | 6 | 3.1 | 5.7 | 6.1 | 5.8 | 4.3 | 69 |
| Blaine No. (cm2/g) | +210 | −210 + 149 | −149 + 105 | −105 + 74 | −74 + 53 | −53 + 44 | −44 |
|---|---|---|---|---|---|---|---|
| 1989 | 19.2 | 11.7 | 6.1 | 5.6 | 4.1 | 5.1 | 48.2 |
| 2311 | 17.2 | 10.2 | 6.2 | 5.2 | 3.1 | 4.9 | 53.2 |
| 2879 | 9 | 8.1 | 6 | 6.1 | 2.8 | 3 | 65 |
| 3461 | 7 | 3.4 | 3.1 | 2.7 | 2.3 | 3.5 | 78 |
| No. | Mesh No. | Firing Temperature (°C) | Sample-Code IO No.1 | Blain No. IO No.1 | Sample-Code IO No.2 | Blain No. IO No.2 |
|---|---|---|---|---|---|---|
| 1 | 100 | 1250 | K1A | 1312 | M1A | 1989 |
| 2 | 100 | 1300 | K1B | 1312 | M1B | 1989 |
| 3 | 100 | 1350 | K1C | 1312 | M1C | 1989 |
| 4 | 200 | 1250 | K2A | 1678 | M2A | 2311 |
| 5 | 200 | 1300 | K2B | 1678 | M2B | 2311 |
| 6 | 200 | 1350 | K2C | 1678 | M2C | 2311 |
| 7 | 300 | 1250 | K3A | 2425 | M3A | 2879 |
| 8 | 300 | 1300 | K3B | 2425 | M3B | 2879 |
| 9 | 300 | 1350 | K3C | 2425 | M3C | 2879 |
| 10 | 400 | 1250 | K4A | 3218 | M4A | 3461 |
| 11 | 400 | 1300 | K4B | 3218 | M4B | 3461 |
| 12 | 400 | 1350 | K4C | 3218 | M4C | 3461 |
| PHASE | Wt.% | Pellet (a) | Pellet (b) | Pellet (c) | Pellet (d) | Pellet (e) | Pellet (f) |
|---|---|---|---|---|---|---|---|
| K2A | K2B | K2C | M2A | M2B | M2C | ||
| A | Fe | 64.59 | 66.94 | 61.26 | 60.15 | 62.03 | 59.54 |
| O | 28.45 | 32.06 | 29.88 | 30.02 | 29.34 | 35.04 | |
| Al | 1.13 | 1.06 | 1.07 | 0.95 | 2.21 | 2.58 | |
| Si | 1.11 | 0 | 0.27 | 0.61 | 0.8 | 0.74 | |
| B | O | 27.84 | 36.42 | 41.04 | 41.57 | 41.48 | 47.29 |
| Si | 0.39 | 4.35 | 17.35 | 16.79 | 3.96 | 17.14 | |
| Ca | 0.6 | 4.35 | 16.14 | 13.29 | 4.33 | 11.86 | |
| Fe | 6.92 | 17.19 | 14.17 | 7.25 | 19.09 | 11.97 | |
| Al | 0 | 24.2 | 6.01 | 17.42 | 17.25 | 10.96 | |
| Mg | 0 | 3.53 | 3.66 | 1.05 | 5.46 | 1.6 |
| (a) Iron Ore No.1 | |||
| Heating Rate (°C/min) | Slope | R | Activation Energy (kJ/mol) |
| 5 | −1.67504 | 8.314 | 32.07223 |
| 10 | −1.69985 | 8.314 | 32.54727 |
| 15 | −1.74195 | 8.314 | 33.35336 |
| (b) Iron Ore No.2 | |||
| Heating Rate (°C/min) | Slope | R | Activation Energy (kJ/mol) |
| 5 | −1.75224 | 8.314 | 33.55038 |
| 10 | −1.76337 | 8.314 | 33.76349 |
| 15 | −1.77891 | 8.314 | 34.06104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, R.; Soren, S.; Kumaraswamidhas, L.A.; Pandey, C.; Pan, S.K. Experimental Investigation of Different Fineness and Firing Temperatures on Pellets Properties of Different Iron Ore fines from Indian Mines. Materials 2022, 15, 4220. https://doi.org/10.3390/ma15124220
Prasad R, Soren S, Kumaraswamidhas LA, Pandey C, Pan SK. Experimental Investigation of Different Fineness and Firing Temperatures on Pellets Properties of Different Iron Ore fines from Indian Mines. Materials. 2022; 15(12):4220. https://doi.org/10.3390/ma15124220
Chicago/Turabian StylePrasad, Rakesh, Shatrughan Soren, L. A. Kumaraswamidhas, Chandan Pandey, and S. K. Pan. 2022. "Experimental Investigation of Different Fineness and Firing Temperatures on Pellets Properties of Different Iron Ore fines from Indian Mines" Materials 15, no. 12: 4220. https://doi.org/10.3390/ma15124220
APA StylePrasad, R., Soren, S., Kumaraswamidhas, L. A., Pandey, C., & Pan, S. K. (2022). Experimental Investigation of Different Fineness and Firing Temperatures on Pellets Properties of Different Iron Ore fines from Indian Mines. Materials, 15(12), 4220. https://doi.org/10.3390/ma15124220





