Nonstoichiometric LaO0.65F1.7 Structure and Its Green Luminescence Property Doped with Bi3+ and Tb3+ Ions for Applying White UV LEDs
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, S.; Xiao, F.; Parn, Y.X.; Ma, Y.Y.; Zhang, Q.Y. Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties. Mater. Sci. Eng. R 2010, 71, 1–34. [Google Scholar] [CrossRef]
- Cao, L.; Li, W.; Devakumar, B.; Ma, N.; Huang, X.; Lee, A.F. Full-Spectrum White Light-Emitting Diodes Enabled by an Efficient Broadband Green-Emitting CaY2ZrScAl3O12:Ce3+ Garnet Phosphor. ACS Appl. Mater. Interfaces 2022, 14, 5643–5652. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.N.; Kim, K.D.; Anoop, G.; Kim, G.S.; Yoo, J.S. Design of highly efficient phosphor converted white light-emitting diodes with color rendering indices (R1 − R15) ≥ 95 for artificial lighting. Sci. Rep. 2019, 9, 16848. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.H.; Ni, C.; Zhang, X.; Tsai, Y.T.; Mahlik, S.; Lazarowska, A.; Grinberg, M.; Sheu, H.S.; Lee, J.F.; Cheng, B.M.; et al. Enhance color rendering index via full spectrum employing the important key of cyan phosphor. ACS Appl. Mater. Interfaces 2016, 8, 30677–30682. [Google Scholar] [CrossRef]
- Schubert, E.F.; Kim, J.K. Solid-state light sources getting smart. Science 2005, 308, 1274–1278. [Google Scholar] [CrossRef]
- Yang, S.; Park, S. Bi3+ and Eu3+ Activated Luminescent Behaviors in Non-Stoichiometric LaO0.65F1.7 Structure. Materials 2020, 13, 2326. [Google Scholar] [CrossRef]
- Ning, H.; Tian, L. Enhanced green luminescence in BaZn1.06Al9.94O17:Tb3+ by co-doping with Bi3+ and energy transfer from Bi3+ to Tb3+. Optik 2021, 228, 166218. [Google Scholar] [CrossRef]
- Taikar, D.R. Study of energy transfer from Bi3+ to Tb3+ in Y2O3 phosphor and its application for W-LED. J. Alloys Compd. 2020, 828, 154405. [Google Scholar] [CrossRef]
- Tian, S.; Zhao, L.; Chen, W.; Liu, Z.; Fan, X.; Min, Q.; Yu, H.; Yu, X.; Qiu, J.; Xu, X. Abnormal photo-stimulated luminescence in Ba2Ga2GeO7: Tb3+, Bi3+. J. Lumin. 2018, 202, 414–419. [Google Scholar] [CrossRef]
- Yadav, R.S.; Rai, S.B. Surface analysis and enhanced photoluminescence via Bi3+ doping in a Tb3+ doped Y2O3 nano-phosphor under UV excitation. J. Alloys Compd. 2017, 700, 228–237. [Google Scholar] [CrossRef]
- Noh, W.; Park, S. Synthesis and distinct up-converting behaviors of Er3+, Yb3+ doped LaOF and LaO0.65F1.7 phosphors. Opt. Mater. 2017, 66, 589–594. [Google Scholar] [CrossRef]
- Yang, W.; Park, S. Predominant green emission of Ce3+–Tb3+ activated Y7O6F9 phosphors. RSC Adv. 2016, 6, 12652. [Google Scholar] [CrossRef]
- Laval, J.-P.; Abaouz, A.; Frit, B. High resolution powder neutron diffraction study of the tetragonal anion-excess fluorite-related LaF1.70O0.65 phase. Eur. J. Solid State Inorg. Chem. 1988, 25, 425–434. [Google Scholar] [CrossRef]
- Shin, S.; Yanga, S.; Lee, S.-H.; Shin, T.J.; Park, S. Distinctive occurrences of green-yellow luminescence from orthogermanate-type Ba9Y2(GeO4)6:Ce3+,Na+ phosphors under blue excitation and white-light performance with light-emitting diodes. J. Alloys Compd. 2021, 897, 163213. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J.; Roisnel, T. FullProf.98 and WinPLOTR New Windows Applications for Diffraction. Commission on Powder Diffraction. IUCr, Newsletter 20, May–August, 1998.
- Rodríguez-Carvajal, J. Recent developments of the program FullProf”. Commission on Powder Diffraction. IUCr, Newsletter 26, December, 2001.
- Dexter, D.L.; Schulman, J.H. Theory of concentration quenching in inorganic phosphors. J. Chem. Phys. 1954, 22, 1063–1070. [Google Scholar] [CrossRef]
- Li, K.; Fan, J.; Shang, M.; Lian, H.; Lin, J. Sr2Y8(SiO4)6O2:Bi3+/Eu3+: A single-component white-emitting phosphor via energy transfer for UV w-LEDs. J. Mater. Chem. C 2015, 3, 9989–9998. [Google Scholar] [CrossRef]
- Yang, S.; Kim, H.; Park, S. Color-tunable luminescence in Y7(1-m-n-z)Bi7mDy7nEu7zO6F9 (m = 0.001-0.05, n = 0–0.1, z = 0.005, 0.01) phosphors. Opt. Mater. 2018, 77, 154–160. [Google Scholar] [CrossRef]
- Yun, H.; Kim, S.-H.; Park, S. Bi3+, Eu3+-doped Ba9Y2Si6O24 phosphors based on the site-selected substitution. Opt. Mater. 2017, 72, 571–577. [Google Scholar] [CrossRef]
- Zou, Y.; Min, X.; Liu, Z.; Yu, L.; Liu, B. Photoluminescent properties and energy transfer mechanism of Tb3+-Ce3+ doped CaSi2O2N2 oxynitride phosphors. Mater. Res. Bull. 2020, 124, 110769. [Google Scholar] [CrossRef]
- Xia, M.; Zhao, W.; Zhong, J.; Shi, P.; Liao, Z.; Liu, X.; Song, J.; Luo, L.; Ma, L.; Nie, Z. Tunable luminescence of blue-green emitting NaBaBO3:Ce3+,Tb3+ phosphors for near-UV light emitting diodes. J. Lumin. 2020, 220, 116957. [Google Scholar] [CrossRef]
- Sontakke, A.D.; van Bunningen, A.J.; Rabouw, F.T.; Meijers, S.; Meijerink, A. Unraveling the Eu2+ → Mn2+ Energy Transfer Mechanism in w-LED Phosphors. J. Phys. Chem. C 2020, 124, 13902–13911. [Google Scholar] [CrossRef]
- Yang, S.; Park, S. Ca2+-substitution effects in Ba9−xCaxAl2Si6O24:Ce3+,Na+ phosphors. Opt. Mater. 2020, 99, 109548. [Google Scholar] [CrossRef]
- Yang, S.; Park, S. Luminescent performances of Ba9−pCapAl2Si6O24:Eu2+,Mn2+ orthosilicate phosphors along with Ca2+ contents. Opt. Mater. 2021, 114, 11968. [Google Scholar] [CrossRef]
- Yun, H.; Park, S. Blue-white-orange tunable Ba6Ca3YAlSi6O24:Eu2+, Mn2+ phosphors for NUV-pumped LEDs. Opt. Mater. 2018, 86, 600–605. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, R.S.; Huang, K.W.; Drozd, V. Ca2Al3O6F:Eu2+: A green-emitting oxyfluoride phosphor for white light-emitting diodes. J. Mater. Chem. 2012, 22, 15183–15189. [Google Scholar] [CrossRef]
- Liu, C.; Xia, Z.; Lian, Z.; Zhou, J.; Yan, Q. Structure and luminescence properties of green-emitting NaBaScSi2O7:Eu2+ phosphors for near-UV-pumped light emitting diodes. J. Mater. Chem. C 2013, 1, 7139–7147. [Google Scholar] [CrossRef]

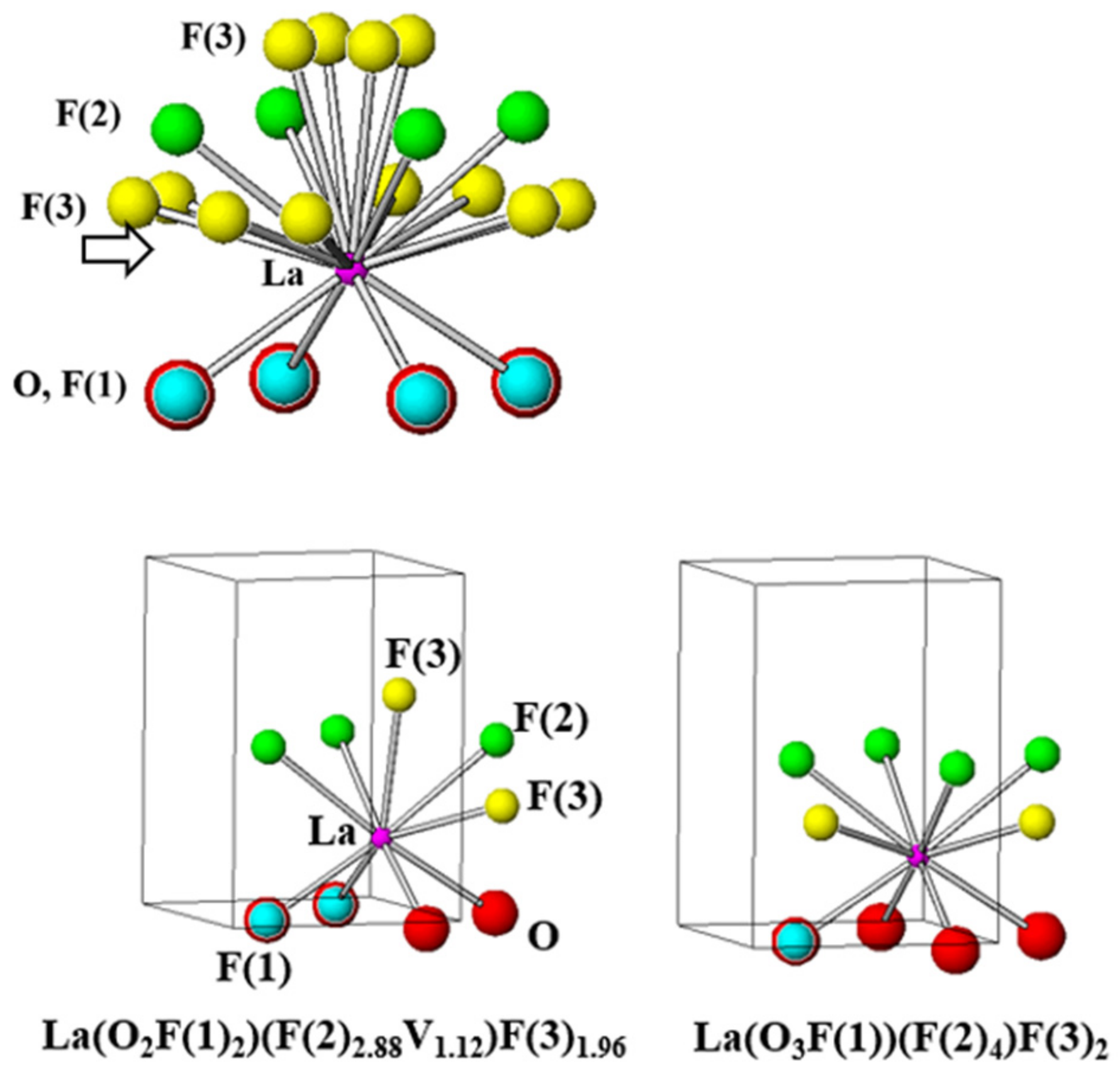
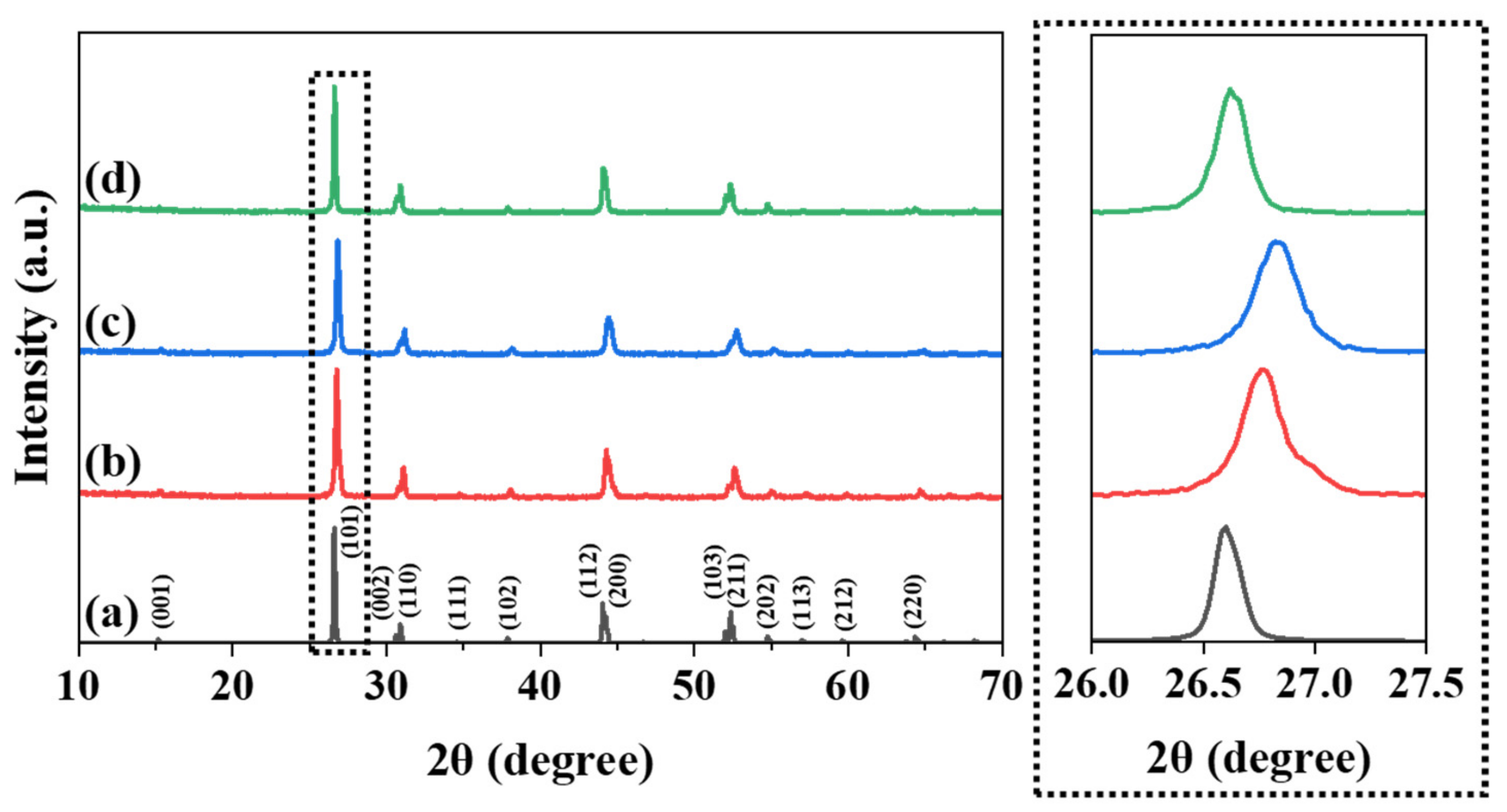
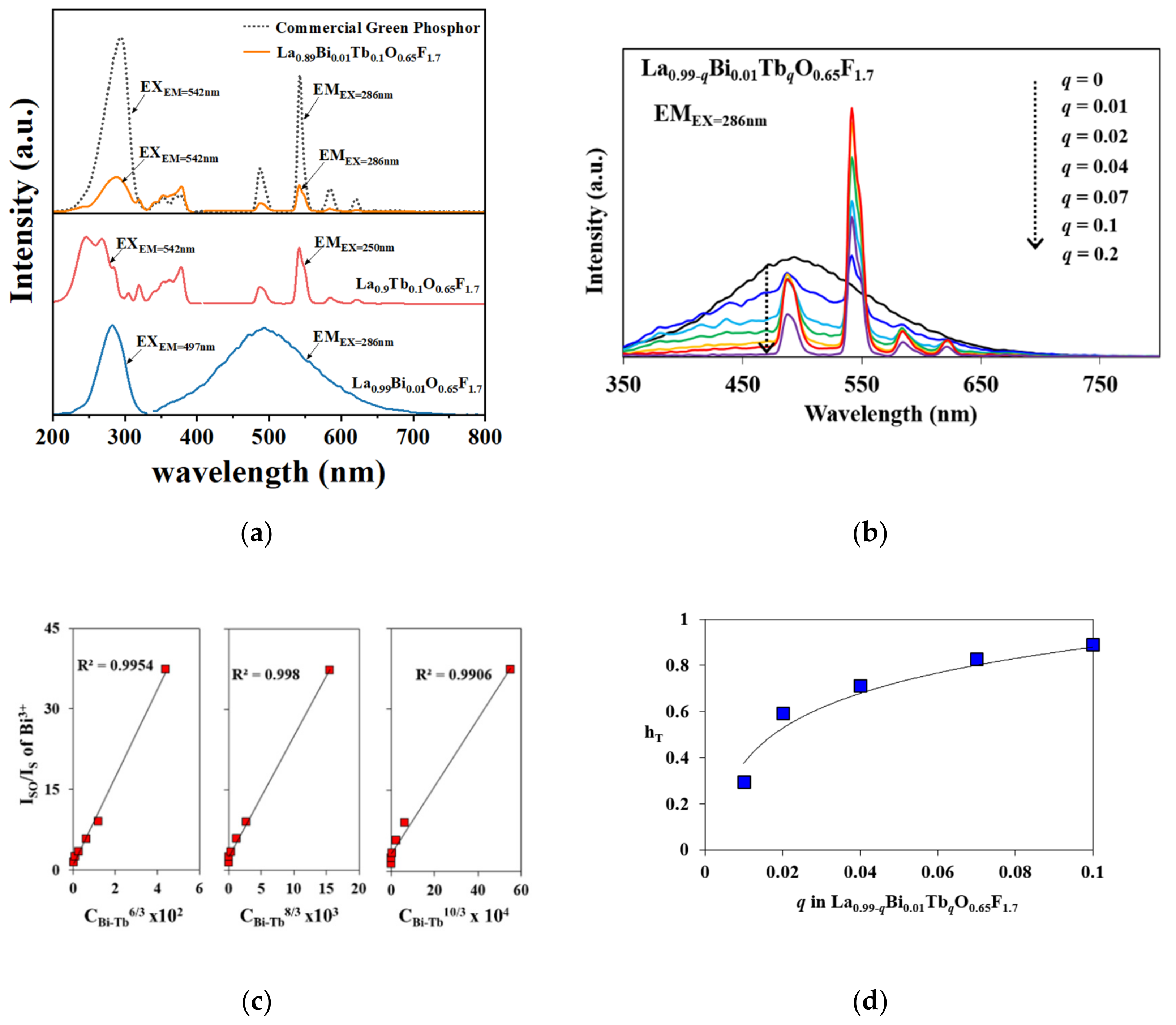

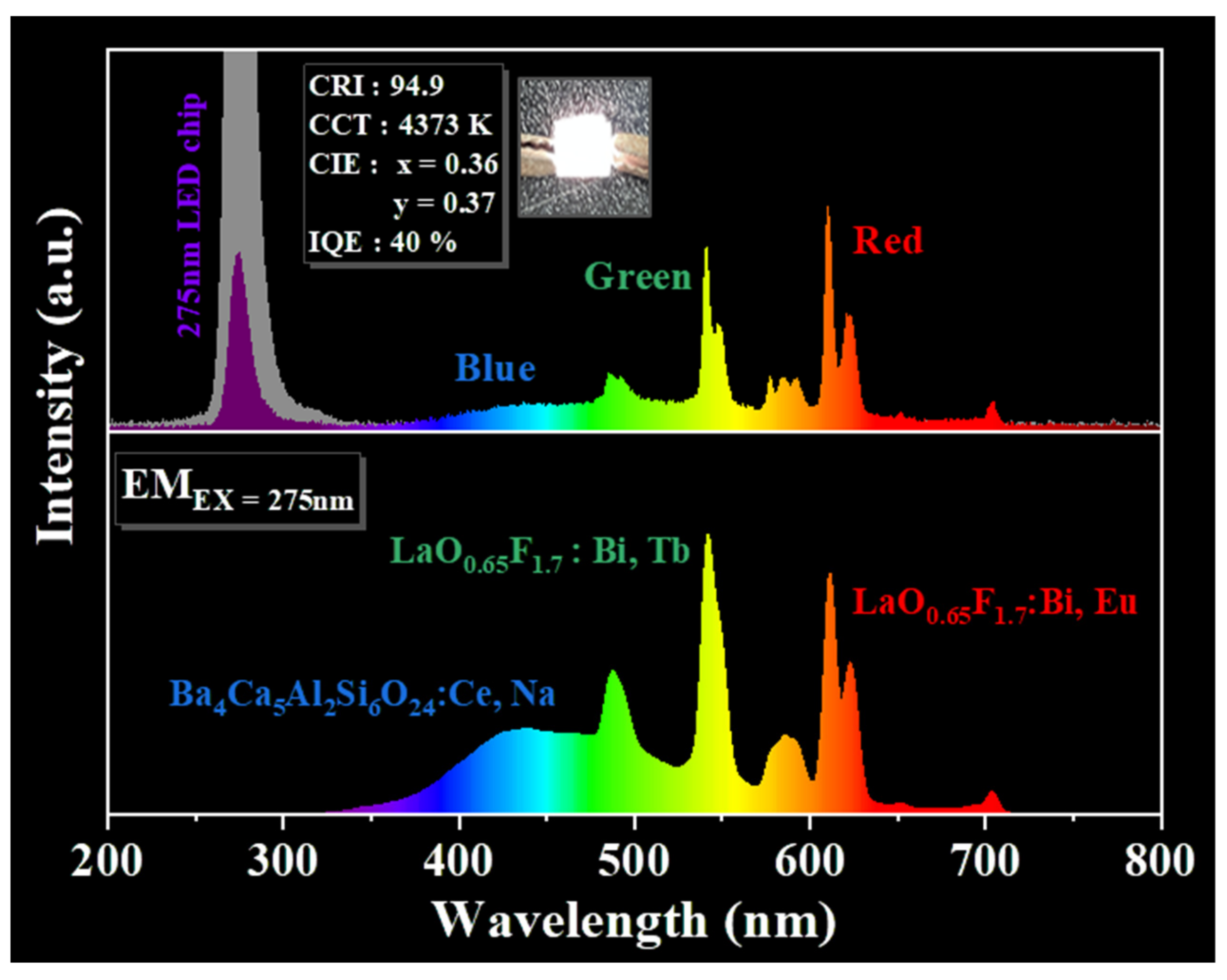
| Chemical Formula | LaO0.65F1.7 |
|---|---|
| Radiation type, λ (Å) | Synchrotron (6D-BM), 0.65303 |
| 2θ range (deg) | 5–35 |
| Crystal system | Tetragonal |
| Space group | P 4/n m m |
| Lattice parameter (Å) | a = 4.10058 (6) |
| c = 5.8468 (1) | |
| Volume (Å3) | V = 98.313 (1) |
| Rp | 6.11 |
| Rwp | 9.06 |
| Rexp | 4.9 |
| S | 1.8 |
| χ2 | 3.42 |
| Atom | Wyckoff Position | x | y | z | Biso | SOF |
|---|---|---|---|---|---|---|
| La | 2c | 0.25 | 0.25 | 0.2268 (3) | 1.02 (6) | 1 |
| O | 2a | 0.75 | 0.25 | 0.00 | 1.1 (5) | 0.65 |
| F(1) | 2a | 0.75 | 0.25 | 0.00 | 1.1 (5) | 0.35 |
| F(2) | 2b | 0.75 | 0.25 | 0.50 | 2.6 (5) | 0.86 |
| F(3) | 8i | 0.25 | 0.077 (15) | 0.652 (10) | 4.8 (25) | 0.1225 |
| Atom | Distance (Å) |
|---|---|
| La-O (x4) | 2.4418 (3) |
| La-F(1) (x4) | 2.4418 (3) |
| La-F(2) (x4) | 2.5990 (4) |
| La-F(3) (x4) | 2.591 (18) |
| La-F(3) (x8) | 2.549 (12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Shin, S.; Ha, H.; Park, S. Nonstoichiometric LaO0.65F1.7 Structure and Its Green Luminescence Property Doped with Bi3+ and Tb3+ Ions for Applying White UV LEDs. Materials 2022, 15, 4222. https://doi.org/10.3390/ma15124222
Yang S, Shin S, Ha H, Park S. Nonstoichiometric LaO0.65F1.7 Structure and Its Green Luminescence Property Doped with Bi3+ and Tb3+ Ions for Applying White UV LEDs. Materials. 2022; 15(12):4222. https://doi.org/10.3390/ma15124222
Chicago/Turabian StyleYang, Sungjun, Seungyong Shin, Heonji Ha, and Sangmoon Park. 2022. "Nonstoichiometric LaO0.65F1.7 Structure and Its Green Luminescence Property Doped with Bi3+ and Tb3+ Ions for Applying White UV LEDs" Materials 15, no. 12: 4222. https://doi.org/10.3390/ma15124222
APA StyleYang, S., Shin, S., Ha, H., & Park, S. (2022). Nonstoichiometric LaO0.65F1.7 Structure and Its Green Luminescence Property Doped with Bi3+ and Tb3+ Ions for Applying White UV LEDs. Materials, 15(12), 4222. https://doi.org/10.3390/ma15124222







