Sr and Zr Co-Doped CaCu3Ti4O12 Ceramics with Improved Dielectric Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Methods
3. Results and Discussion
3.1. Phase Composition
3.2. Microstructure
3.3. Oxidation States of Cations
3.4. Dielectric Properties
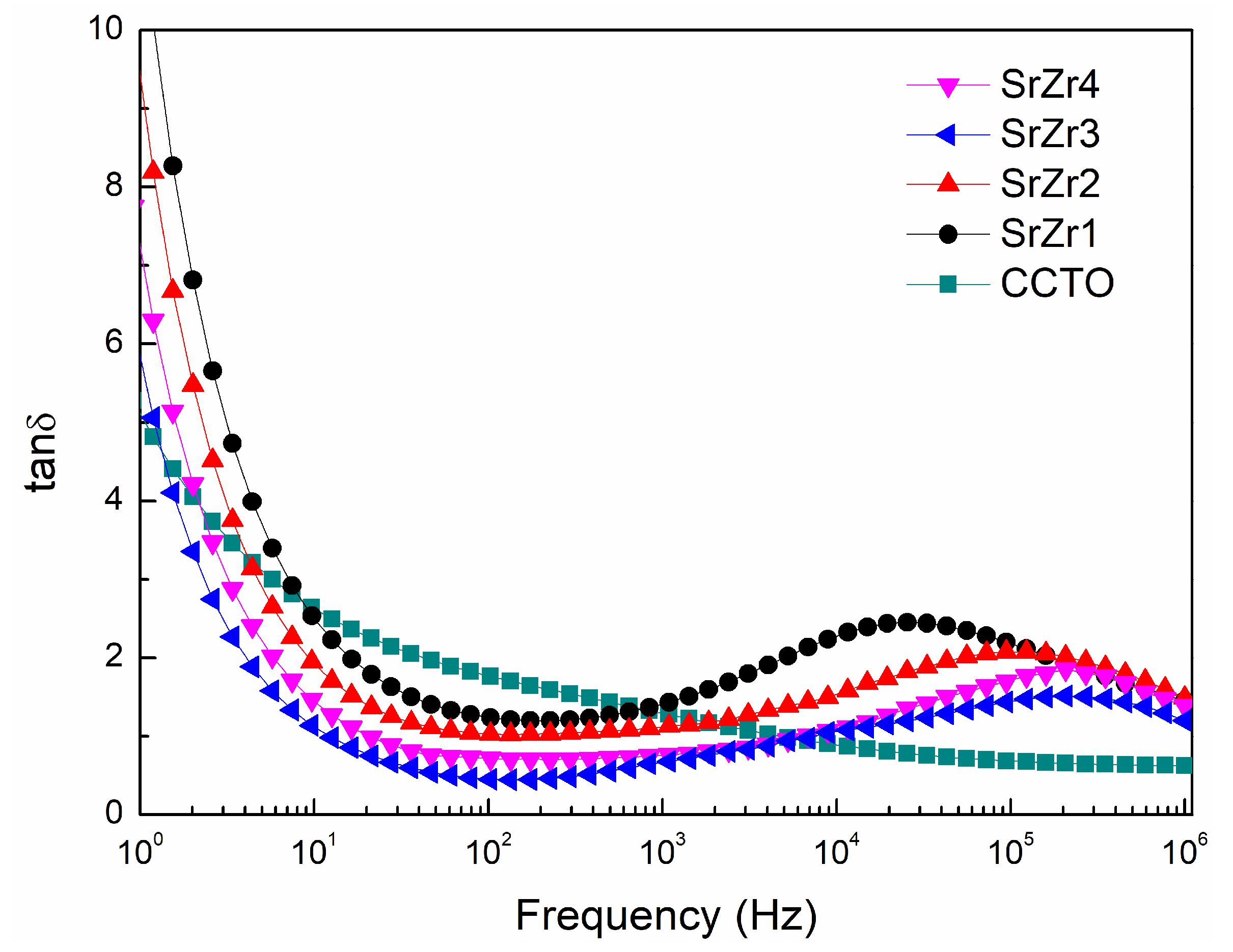
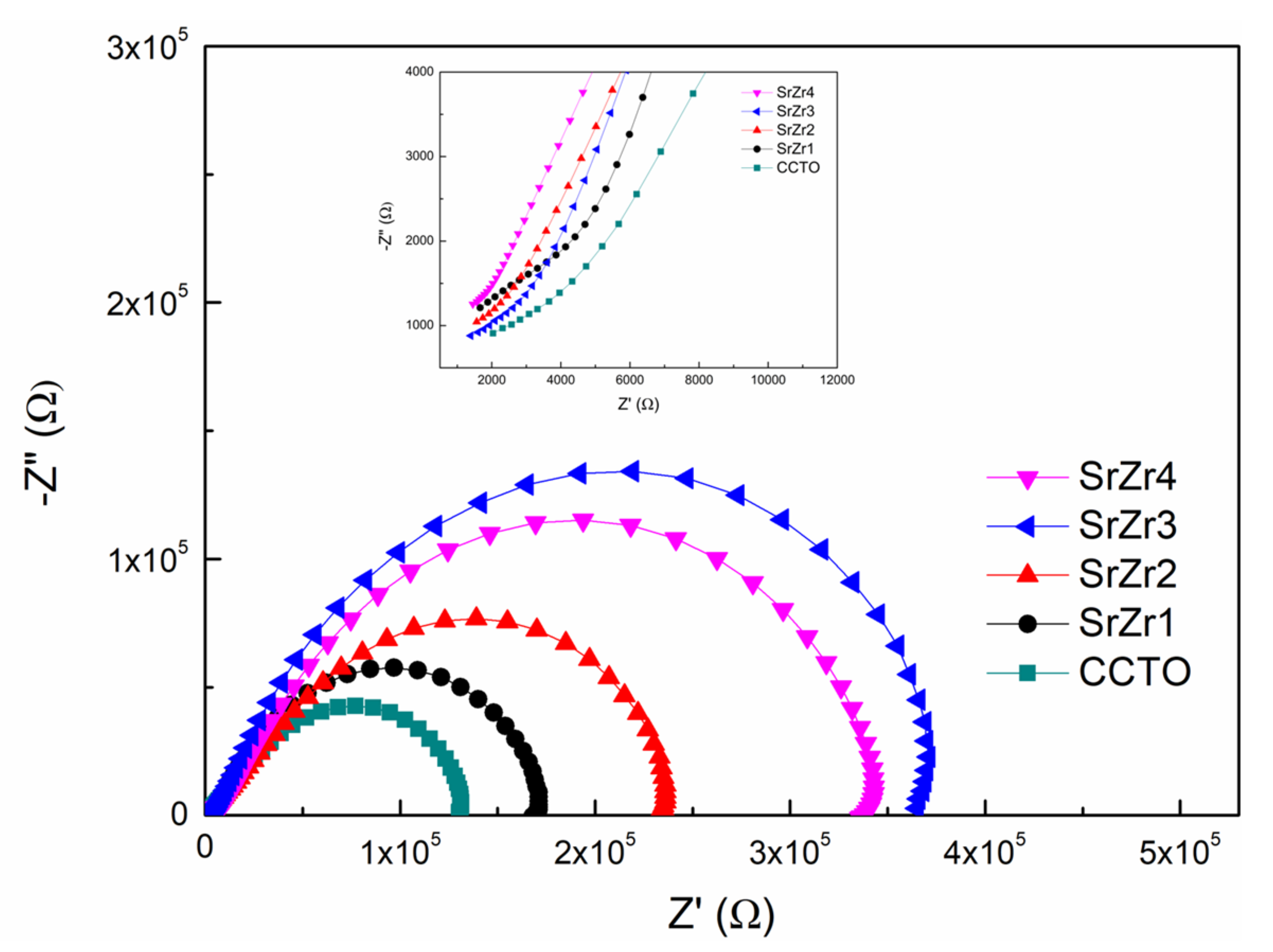
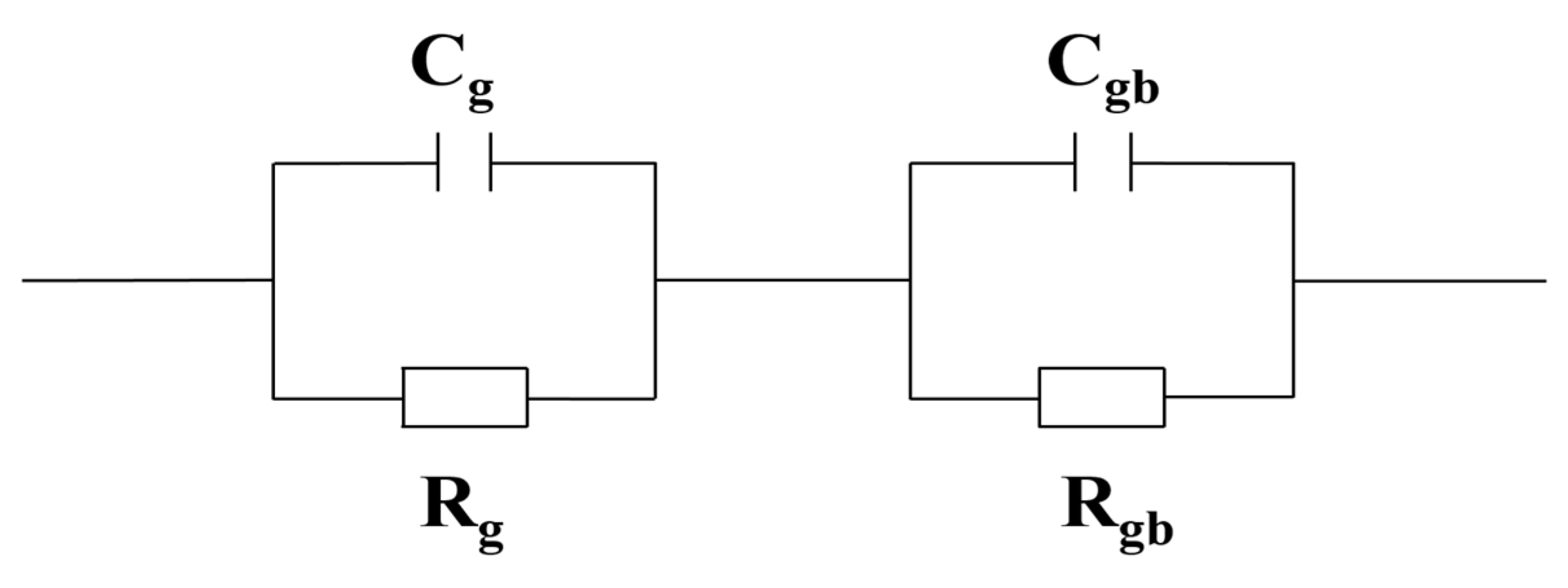
3.5. Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, L.; Guan, Z.; Huang, S.; Liang, K.; Chen, H.; Zhang, J. Enhanced dielectric properties of barium strontium titanate thin films by doping modification. J. Mater. Sci. Mater. Electron. 2019, 30, 12821–12839. [Google Scholar] [CrossRef]
- Norezan, I.; Yahya, A.K.; Talari, M.K. Effect of (Ba0.6Sr0.4)TiO3 (BST) Doping on Dielectric Properties of CaCu3Ti4O12 (CCTO). J. Mater. Sci. Technol. 2012, 28, 1137–1144. [Google Scholar] [CrossRef]
- Kumaria, M.; Baraik, N.; Sarun, P.M. Effect of Nd doping on the structural, optical and dielectric properties of BaTi0.95Sn0.05O3 ceramics. J. Alloys Compd. 2021, 883, 160635. [Google Scholar] [CrossRef]
- Peng, Z.; Li, J.; Liang, P.; Yang, Z.; Chao, X. Improved dielectric properties and grain boundary response of SrTiO3 doped Y2/3Cu3Ti4O12 ceramics fabricated by Sol-gel process for high-energy-density storage applications. J. Eur. Ceram. Soc. 2017, 37, 4637–4644. [Google Scholar] [CrossRef]
- Lee, J.-W.; Koh, J.-H. Enhanced dielectric properties of Ag-doped CCTO ceramics for energy storage devices. Ceram. Int. 2017, 43, 9493–9497. [Google Scholar] [CrossRef]
- Singh, L.; Rai, U.S.; Mandal, K.D.; Singh, N.B. Progress in the growth of CaCu3Ti4O12 and related functional dielectric perovskites. Prog. Cryst. Growth Charact. Mater. 2014, 60, 15–62. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Li, D.; Duan, N.; Reisner, B.A.; Sleight, A.W. High Dielectric Constant in ACu3Ti4O12 and ACu3Ti3FeO12 Phases. J. Solid State Chem. 2000, 151, 323–325. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, L.; Chen, X.M. Effects of Nd-substitution on microstructures and dielectric characteristics of CaCu3Ti4O12 ceramics. J. Mater. Sci. Mater. Electron. 2011, 22, 345–350. [Google Scholar] [CrossRef]
- Senda, S.; Rhouma, S.; Torkani, E.; Megriche, A.; Autret, C. Effect of nickel substitution on electrical and microstructural properties of CaCu3Ti4O12 ceramic. J. Alloys Compd. 2017, 698, 152–158. [Google Scholar] [CrossRef]
- Cheng, P.; Cao, Z.; Zhou, M.; Wang, Q.; Li, S.; Li, J. Dielectric properties of CaCu3Ti4O12 ceramics doped by La3+. Ceram. Int. 2019, 45, 15320–15326. [Google Scholar] [CrossRef]
- Mao, P.; Wang, J.; Liu, S.; Zhang, L.; Zhao, Y.; He, L. Grain size effect on the dielectric and non-ohmic properties of CaCu3Ti4O12 ceramics prepared by the sol-gel process. J. Alloys Compd. 2019, 778, 625–632. [Google Scholar] [CrossRef]
- Pal, K.; Dey, A.; Jana, R.; Ray, P.P.; Bera, P.; Kumar, L.; Mandal, T.K.; Mohanty, P.; Seikh, M.M.; Gayen, A. Citrate combustion synthesized Al-doped CaCu3Ti4O12 quadruple perovskite: Synthesis, characterization and multifunctional properties. Phys. Chem. Chem. Phys. 2020, 22, 3499–3511. [Google Scholar] [CrossRef]
- Lunkenheimer, P.; Fichtl, R.; Ebbinghaus, S.G.; Loidl, A. Non-intrinsic origin of the colossal dielectric constants in CaCu3Ti4O12. Phys. Rev. B 2004, 70, 172102. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, D.C.; Adams, T.B.; Morrison, F.D.; West, A.R. CaCu3Ti4O12: One-step internal barrier layer capacitor. Appl. Phys. Lett. 2002, 80, 2153–2155. [Google Scholar] [CrossRef]
- Adams, T.B.; Sinclair, D.C.; West, A.R. Giant Barrier Layer Capacitance Effects in CaCu3Ti4O12 Ceramics. Adv. Mater. 2002, 14, 1321–1323. [Google Scholar] [CrossRef]
- Adams, T.B.; Sinclair, D.C.; West, A.R. Influence of Processing Conditions on the Electrical Properties of CaCu3Ti4O12 Ceramics. J. Am. Ceram. Soc. 2006, 89, 3129–3135. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Deng, T.; Zhong, C.; Chen, Z. Improved dielectric, nonlinear and magnetic properties of cobalt-doped CaCu3Ti4O12 ceramics. J. Eur. Ceram. Soc. 2018, 38, 3505–3511. [Google Scholar] [CrossRef]
- Wang, X.; Xue, L.; Zhang, Y.; Du, J.; Sun, L.; Shi, Y.; Wang, W.; Liang, Y.; Fu, Y.; Shang, S.; et al. Improved electrical performance of MgO-modified CaCu3Ti4O12 ceramics. Phys. B. 2019, 572, 98–104. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, M.; Srinet, G.; Siqueiros, J.M.; Herrera, O.R. Structural, Raman analysis and exchange bias effects in Mn doped multiferroic Bi0.80La0.10Ca0.10Fe1-xMnxO3 ceramics. Ceram. Int. 2021, 47, 6834–6841. [Google Scholar] [CrossRef]
- Liu, L.; Fan, H.; Fang, P.; Chen, X. Sol-gel derived CaCu3Ti4O12 ceramics: Synthesis, characterization and electrical properties. Mater. Res. Bull. 2008, 43, 1800–1807. [Google Scholar] [CrossRef]
- Sun, D.-L.; Wu, A.-Y.; Yin, S.-T. Structure, Properties, and Impedance Spectroscopy of CaCu3Ti4O12 Ceramics Prepared by Sol–Gel Process. J. Am. Ceram. Soc. 2010, 91, 169–173. [Google Scholar] [CrossRef]
- Kotb, H.M.; Ahmad, M.M.; Aldabal, S.; Alshoaibi, A.; Aljaafari, A. Structural and dielectric behavior of Al-substituted CaCu3Ti4O12 ceramics with giant dielectric constant by spark plasma sintering. J. Mater. Sci. Mater. Electron. 2019, 30, 18259–18267. [Google Scholar] [CrossRef]
- Li, T.; Sun, Y.; Dai, H.Y.; Liu, D.W.; Chen, J.; Xue, R.Z.; Chen, Z.Q. Influence of spark plasma sintering temperature on the microstructures, dielectric and I–V properties of CaCu3Ti4O12 ceramics. J. Alloys Compd. 2020, 829, 154595. [Google Scholar] [CrossRef]
- Ouyang, X.; Cao, P.; Huang, S.F.; Zhang, W.J.; Huang, Z.H.; Gao, W. Microwave-Assisted Synthesis of High Dielectric Constant CaCu3Ti4O12 from Sol–Gel Precursor. J. Electron. Mater. 2015, 44, 2243–2249. [Google Scholar] [CrossRef]
- Gaâbel, F.; Khlifi, M.; Hamdaoui, N.; Beji, L.; Taibi, K.; Dhahri, J. Microstructural, structural and dielectric analysis of Ni-doped CaCu3Ti4O12 ceramic with low dielectric loss. J. Mater. Sci. Mater. Electron. 2019, 30, 14823–14833. [Google Scholar] [CrossRef]
- Jesurani, S.; Kanagesan, S.; Hashim, M.; Ismail, I. Dielectric properties of Zr doped CaCu3Ti4O12 synthesized by sol–gel route. J. Alloys Compd. 2013, 551, 456–462. [Google Scholar] [CrossRef]
- SU, Y.L.; ZHANG, W.Q. Dielectric properties and electrical conductivity of CaCu3Ti4O12 ceramics doped with Zr4+. J. Wuhan Univ. Technol. 2013, 28, 343–346. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, H.; Wei, M.; Lai, Y.; Li, J. Effect of semiconductive grain and microstructure on the dielectric properties of CaCu3Ti4O12 ceramics with Sr2+ doping. J. Alloys Compd. 2017, 708, 1026–1032. [Google Scholar] [CrossRef]
- Ren, L.; Yang, L.; Xu, C.; Zhao, X.; Liao, R. Improvement of breakdown field and dielectric properties of CaCu3Ti4O12 ceramics by Bi and Al co-doping. J. Alloys Compd. 2018, 768, 652–658. [Google Scholar] [CrossRef]
- Xu, Z.; Qiang, H.; Chen, Y.; Chen, Z. Microstructure and enhanced dielectric properties of yttrium and zirconium co-doped CaCu3Ti4O12 ceramics. Mater. Chem. Phys. 2017, 191, 1–5. [Google Scholar] [CrossRef]
- Espinoza-Gonzalez, R.; Hevia, S.; Adrian, A. Effects of strontium/lanthanum co-doping on the dielectric properties of CaCu3Ti4O12 prepared by reactive sintering. Ceram. Int. 2018, 44, 15588–15595. [Google Scholar] [CrossRef]
- Li, J.; Fu, B.; Lu, H.; Huang, C.; Sheng, J.W. Dielectric properties of Sm-doped CaCu3Ti4O12 ceramics. Ceram. Int. 2013, 39, S149–S152. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective Ionic Radii in Oxides and Fluorides. Acta Cryst. 1969, B25, 925–946. [Google Scholar] [CrossRef]
- Saengvong, P.; Boonlakhorn, J.; Chanlek, N.; Putasaeng, B.; Thongbai, P. Giant dielectric permittivity with low loss tangent and excellent non−Ohmic properties of the (Na+,Sr2+,Y3+)Cu3Ti4O12 ceramic system. Ceram. Int. 2020, 46, 9780–9785. [Google Scholar] [CrossRef]
- Mao, P.; Wang, J.; Xiao, P.; Zhang, L.; Kang, F.; Gong, H. Colossal dielectric response and relaxation behavior in novel system of Zr4+ and Nb5+ co-substituted CaCu3Ti4O12 ceramics. Ceram. Int. 2021, 47, 111–120. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Z.; Hao, W.; Cao, E.; Zhang, Y.; Peng, H. Influence of Zirconium doping on microstructure and dielectric properties of CaCu3Ti4O12 synthesized by the sol-gel method. J. Alloys Compd. 2015, 651, 283–289. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Deng, T.; Zhong, C.; Chen, Z. Improved dielectric properties in A’-site nickel-doped CaCu3Ti4O12 ceramics. J. Am. Ceram. Soc. 2017, 100, 4021–4032. [Google Scholar] [CrossRef]
- Neirman, S.; Burn, I. Dielectric properties of polycrystalline CaTiO3 doped with yttrium oxide. J. Mater. Sci. 1984, 19, 737–744. [Google Scholar] [CrossRef]
- Parkash, O.; Kumar, D.; Goyal, A.; Agrawal, A.; Mukherjee, A.; Singh, S.; Singh, P. Electrical behaviour of zirconium doped calcium copper titanium oxide. J. Phys. D Appl. Phys. 2008, 41, 035401. [Google Scholar] [CrossRef]
- Kotb, H.M.; Ahmad, M.M.; Alshoaibi, A.; Yamada, K. Dielectric Response and Structural Analysis of (A3+, Nb5+) Cosubstituted CaCu3Ti4O12 Ceramics (A: Al and Bi). Materials 2020, 13, 5822. [Google Scholar] [CrossRef]
- Jumpatam, J.; Somphan, W.; Boonlakhorn, J.; Putasaeng, B.; Kidkhunthod, P.; Thongbai, P.; Maensiri, S. Non-Ohmic properties and electrical responses of grains and grain boundaries of Na1/2Y1/2Cu3Ti4O12 ceramics. J. Am. Ceram. Soc. 2017, 100, 157–166. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, B.; Ni, L.; Zhou, Y.; Li, M.; Chang, A. New negative temperature coefficient ceramics in Zr-doped CaCu3Ti4O12 system. J. Alloys Compd. 2020, 821, 153476. [Google Scholar] [CrossRef]
- Boonlakhorn, J.; Chanlek, N.; Srepusharawoot, P.; Thongbai, P. Improved dielectric properties of CaCu3−xSnxTi4O12 ceramics with high permittivity and reduced loss tangent. J. Mater. Sci. Mater. Electron. 2020, 31, 15599–15607. [Google Scholar] [CrossRef]
- Chi, Q.G.; Gao, L.; Wang, X.; Lin, J.Q.; Sun, J.; Lei, Q.Q. Effects of Zr doping on the microstructures and dielectric properties of CaCu3Ti4O12 ceramics. J. Alloys Compd. 2013, 559, 45–48. [Google Scholar] [CrossRef]
- Dimitrov, V.; Komatsu, T. An interpretation of optical properties of oxide and oxide glasses in terms of the electronic ion polarizability and average single bond strength. J. Univ. Chem. Technol. Metall. 2010, 45, 219–250. [Google Scholar]
- Lin, L.; Liu, Y.; Zhang, J.; Li, Z.; Lei, Z.; Li, Y.; Tian, M. Enhancement of breakdown electric field and dielectric properties of CaCu3Ti4O12 ceramics by Sr doping. Mater. Chem. Phys. 2020, 224, 122722. [Google Scholar] [CrossRef]
- Li, M.; Sinclair, D.C.; West, A.R. Extrinsic origins of the apparent relaxorlike behavior in CaCu3Ti4O12 ceramics at high temperatures: A cautionary tale. J. Appl. Phys. 2011, 109, 084106. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, R.; Wang, Z.; Cao, E.; Zhang, Y.; Ju, L. Microstructure, dielectric properties and impedance spectroscopy of Ni doped CaCu3Ti4O12 ceramics. RSC Adv. 2016, 6, 55984–55989. [Google Scholar] [CrossRef]
- Rani, S.; Ahlawat, N.; Sangwan, K.M.; Rani, S.; Punia, R.; Malik, J. Structural investigation and giant dielectric response of CaCu3Ti4O12 ceramic by Nd/Zr co-doping for energy storage applications. J. Mater. Sci. Mater. Electron. 2018, 29, 10825–10833. [Google Scholar] [CrossRef]
- Li, T.; Chen, J.; Liu, D.; Zhang, Z.; Chen, Z.; Li, Z.; Cao, X.; Wang, B. Effect of NiO-doping on the microstructure and the dielectric properties of CaCu3Ti4O12 ceramics. Ceram. Int. 2014, 40, 9061–9067. [Google Scholar] [CrossRef]
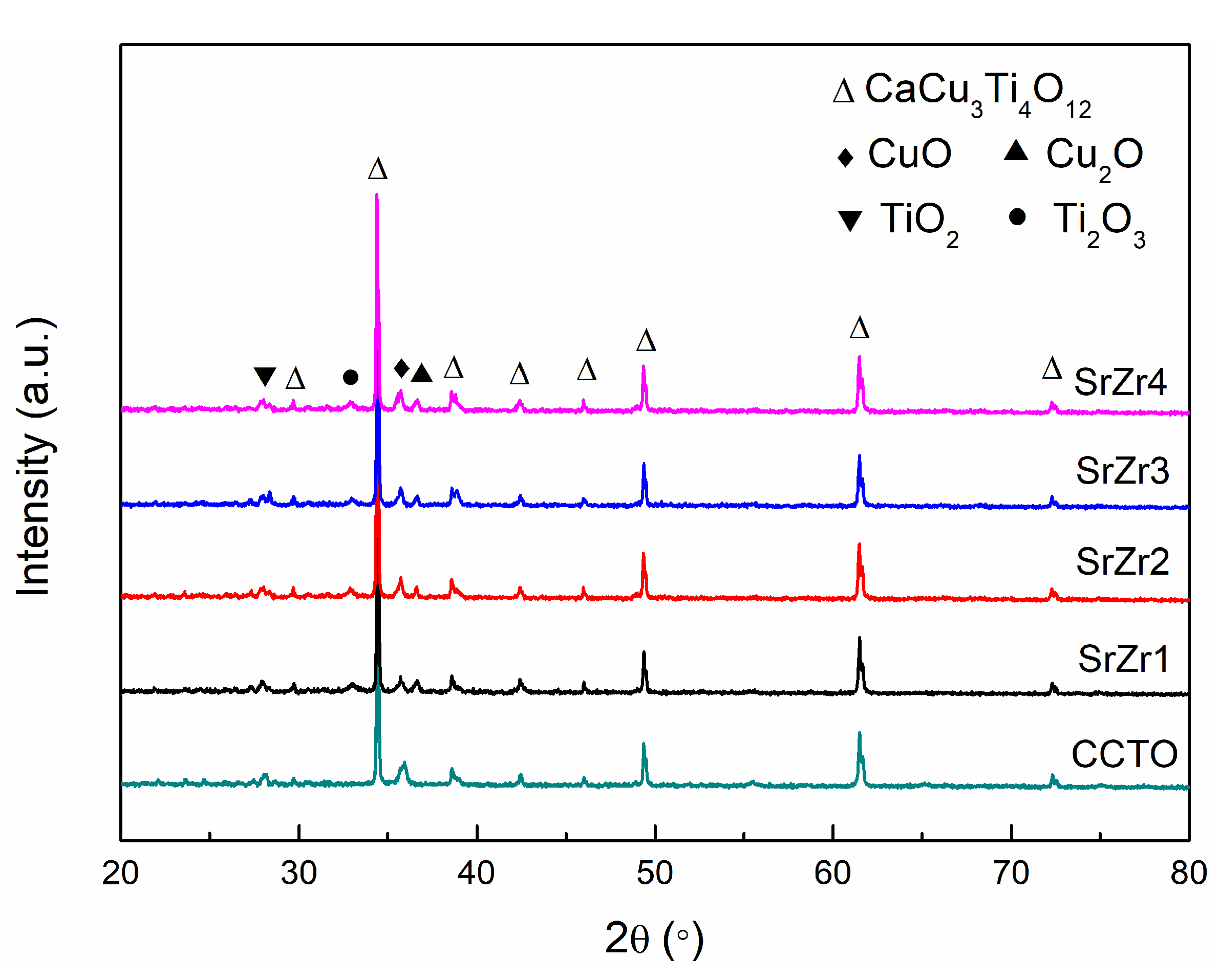
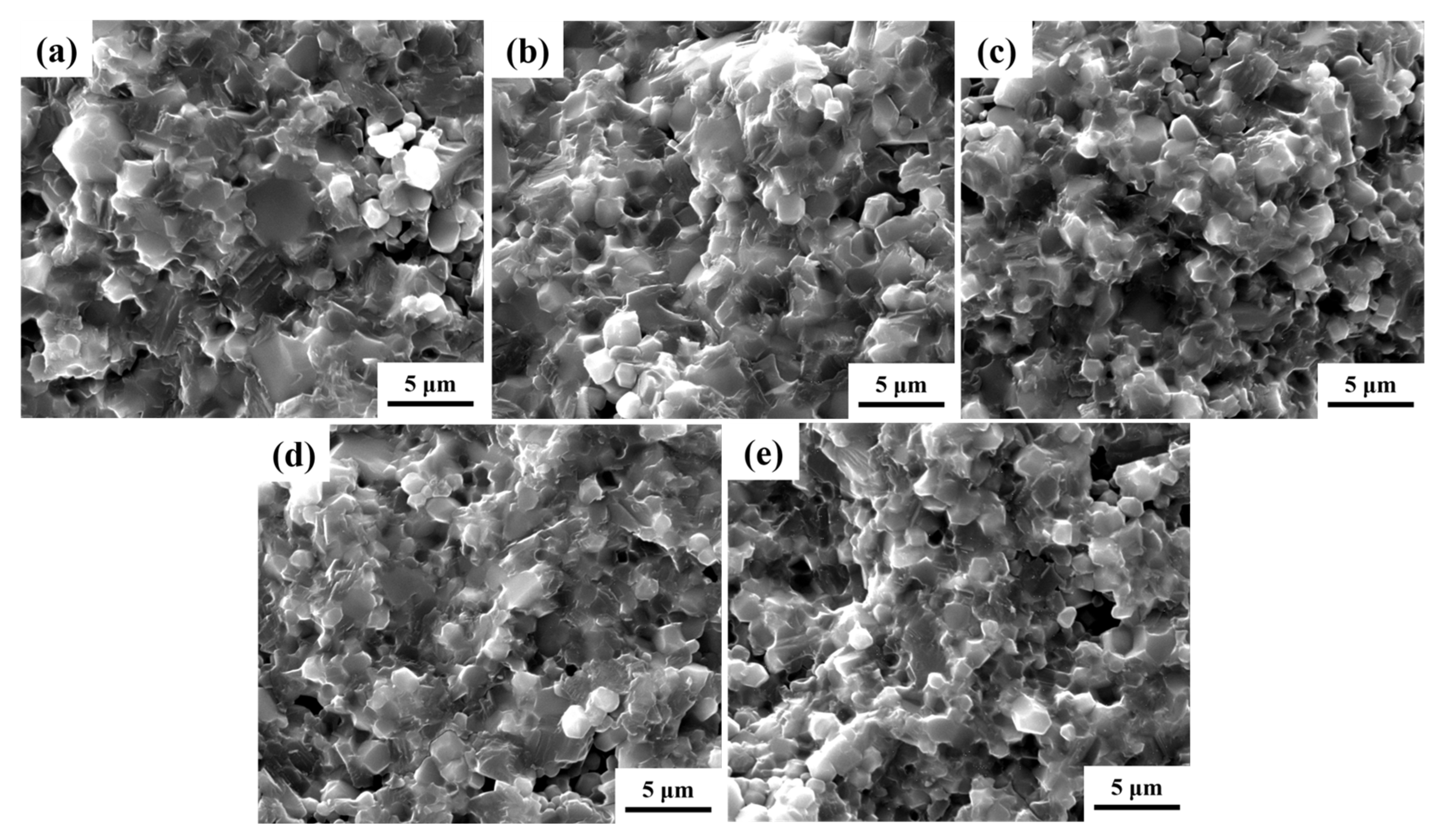
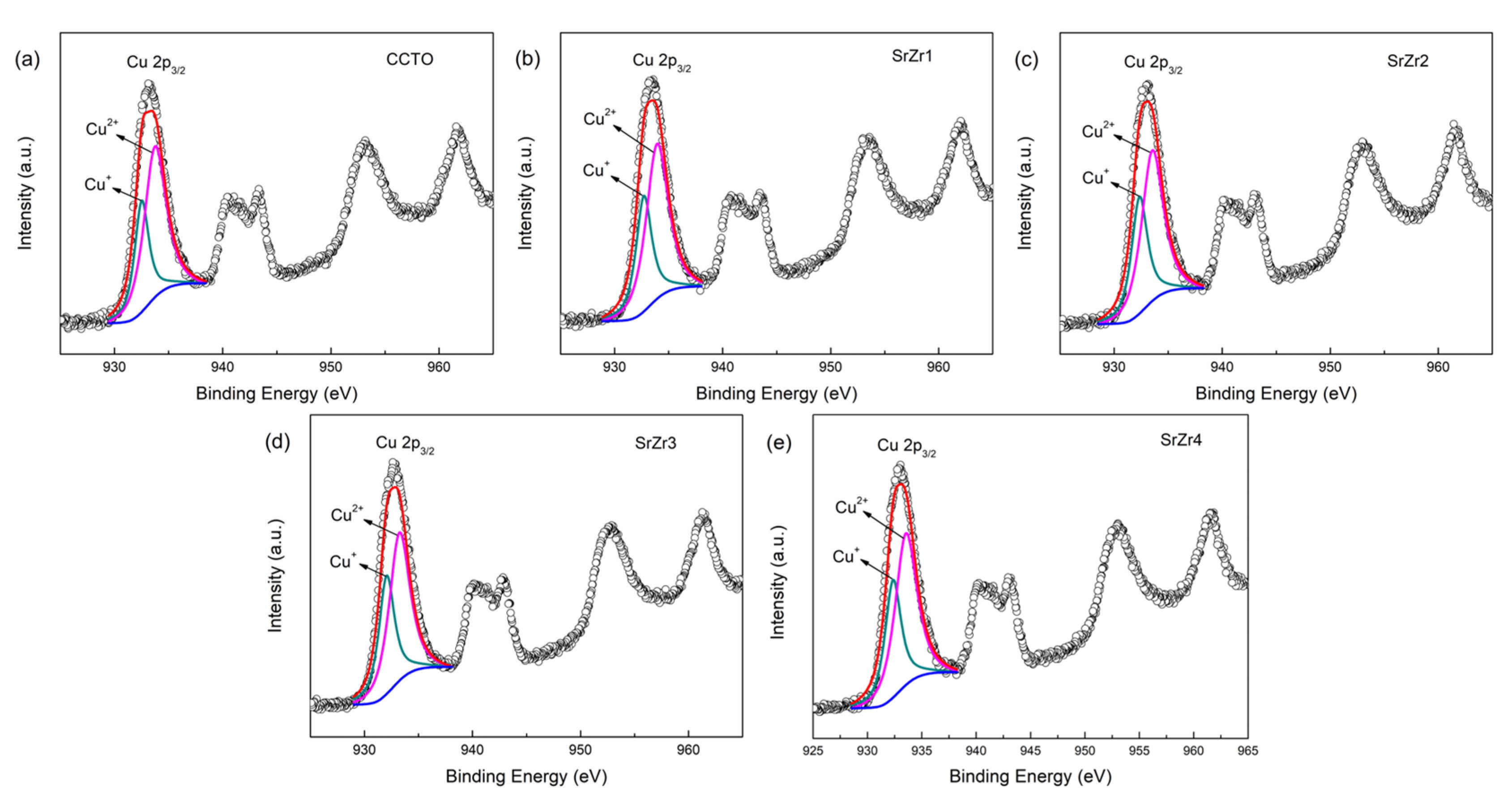
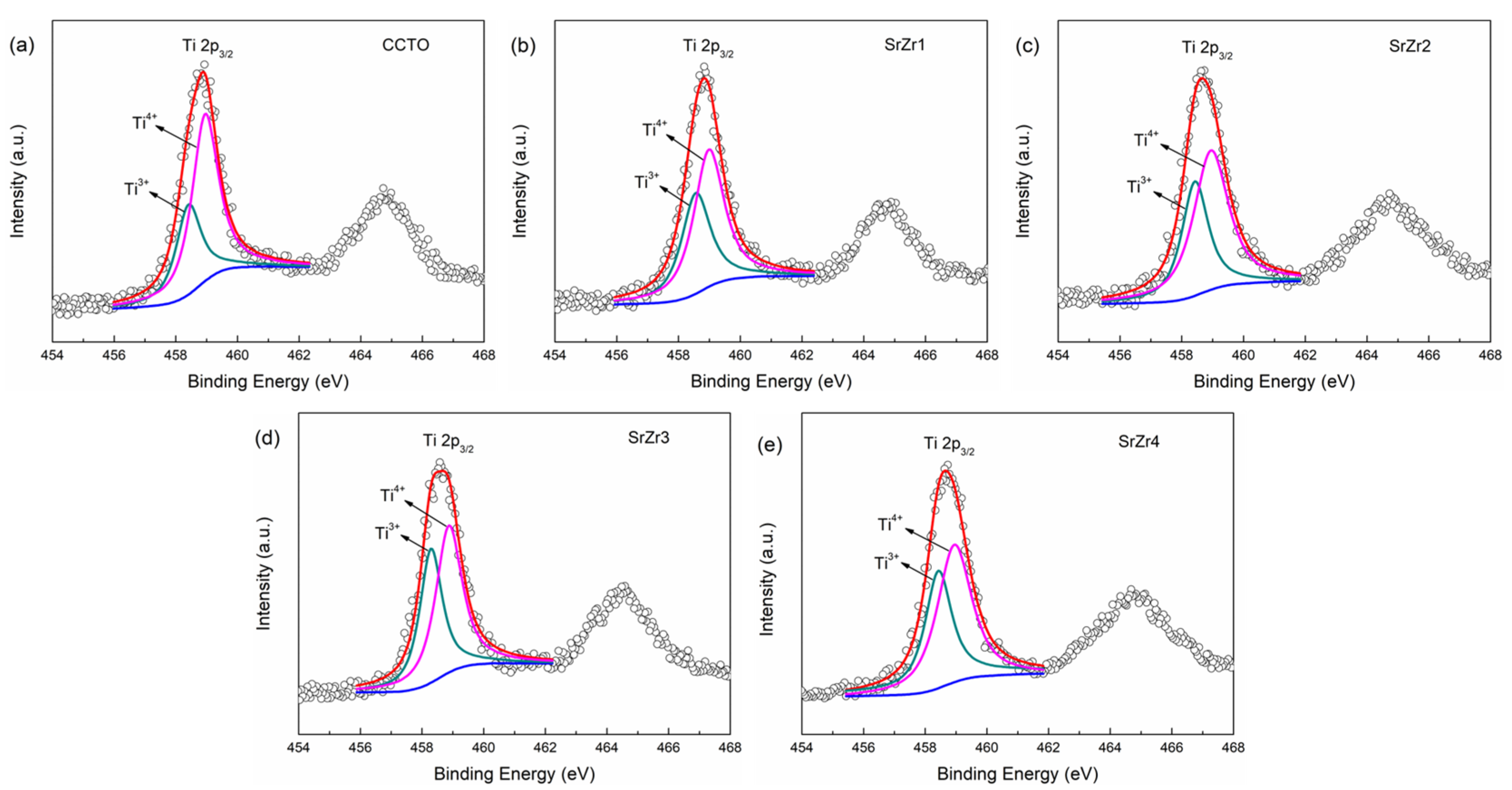

| Samples | CCTO | SrZr1 | SrZr2 | SrZr3 | SrZr4 |
|---|---|---|---|---|---|
| Lattice parameter (Å) | 7.376 | 7.378 | 7.379 | 7.381 | 7.382 |
| Samples | CCTO | SrZr1 | SrZr2 | SrZr3 | SrZr4 |
|---|---|---|---|---|---|
| Cu+/Cu2+ (%) | 31.35 | 34.42 | 35.35 | 37.01 | 36.67 |
| Ti3+/Ti4+ (%) | 34.05 | 38.39 | 41.11 | 43.75 | 42.36 |
| Samples | CCTO | SrZr1 | SrZr2 | SrZr3 | SrZr4 |
|---|---|---|---|---|---|
| Rg (Ω) | 2.0 × 103 | 1.6 × 103 | 1.5 × 103 | 1.3 × 103 | 1.4 × 103 |
| Rgb (Ω) | 1.3 × 105 | 1.7 × 105 | 2.4 × 105 | 3.7 × 105 | 3.3 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, Q.; Li, Y.; Rehman, M.U.; Khan, W.Q. Sr and Zr Co-Doped CaCu3Ti4O12 Ceramics with Improved Dielectric Properties. Materials 2022, 15, 4243. https://doi.org/10.3390/ma15124243
Yu Y, Wang Q, Li Y, Rehman MU, Khan WQ. Sr and Zr Co-Doped CaCu3Ti4O12 Ceramics with Improved Dielectric Properties. Materials. 2022; 15(12):4243. https://doi.org/10.3390/ma15124243
Chicago/Turabian StyleYu, Yunfei, Qun Wang, Yongqing Li, Mehtab Ur Rehman, and Waheed Qamar Khan. 2022. "Sr and Zr Co-Doped CaCu3Ti4O12 Ceramics with Improved Dielectric Properties" Materials 15, no. 12: 4243. https://doi.org/10.3390/ma15124243






