3D Printing of PLLA/Biomineral Composite Bone Tissue Engineering Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Powder
2.2. Preparation and Characterization of Ink
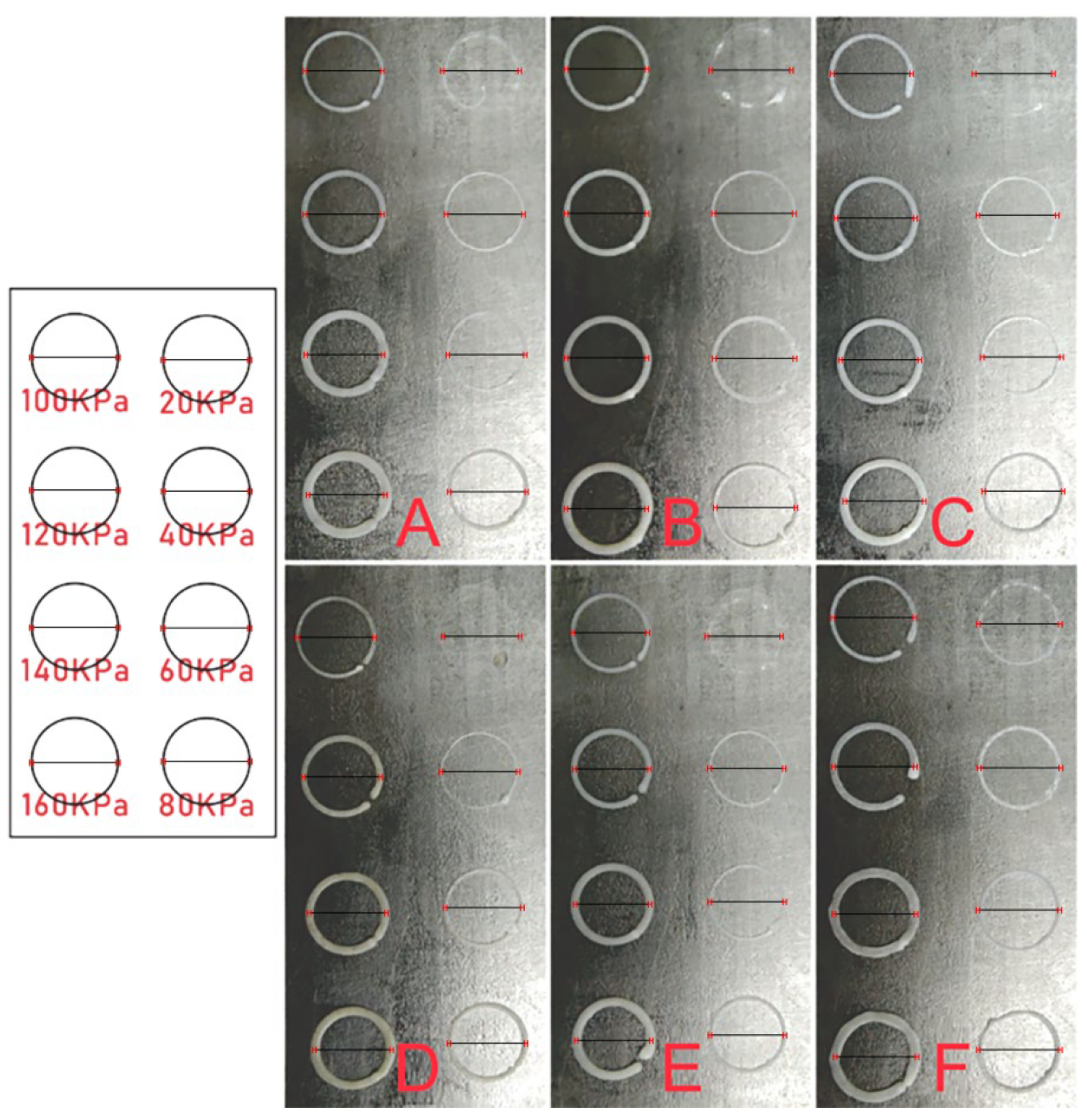
2.3. Preparation of Scaffolds
2.4. SEM Observation
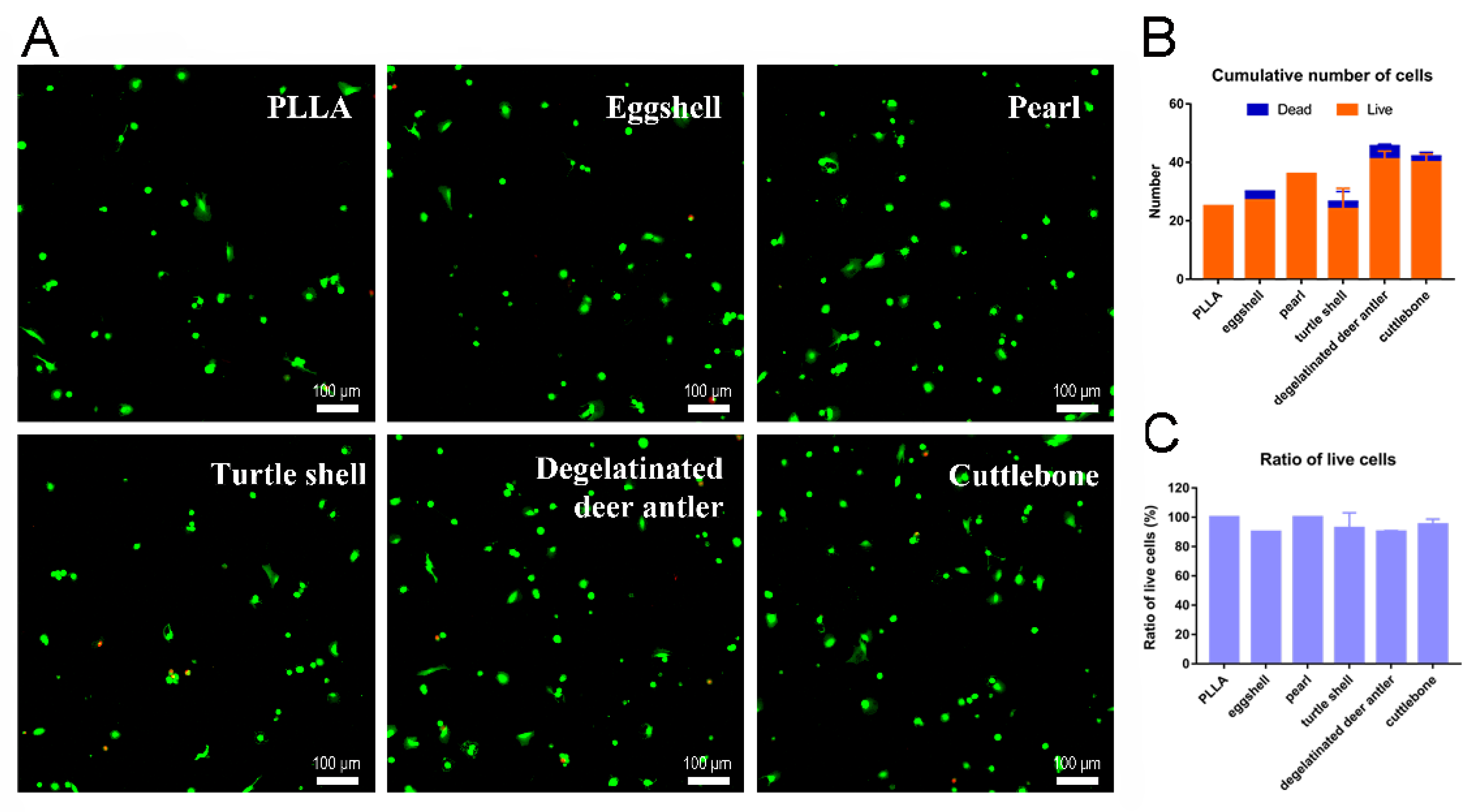
2.5. Cell Live/Dead Staining
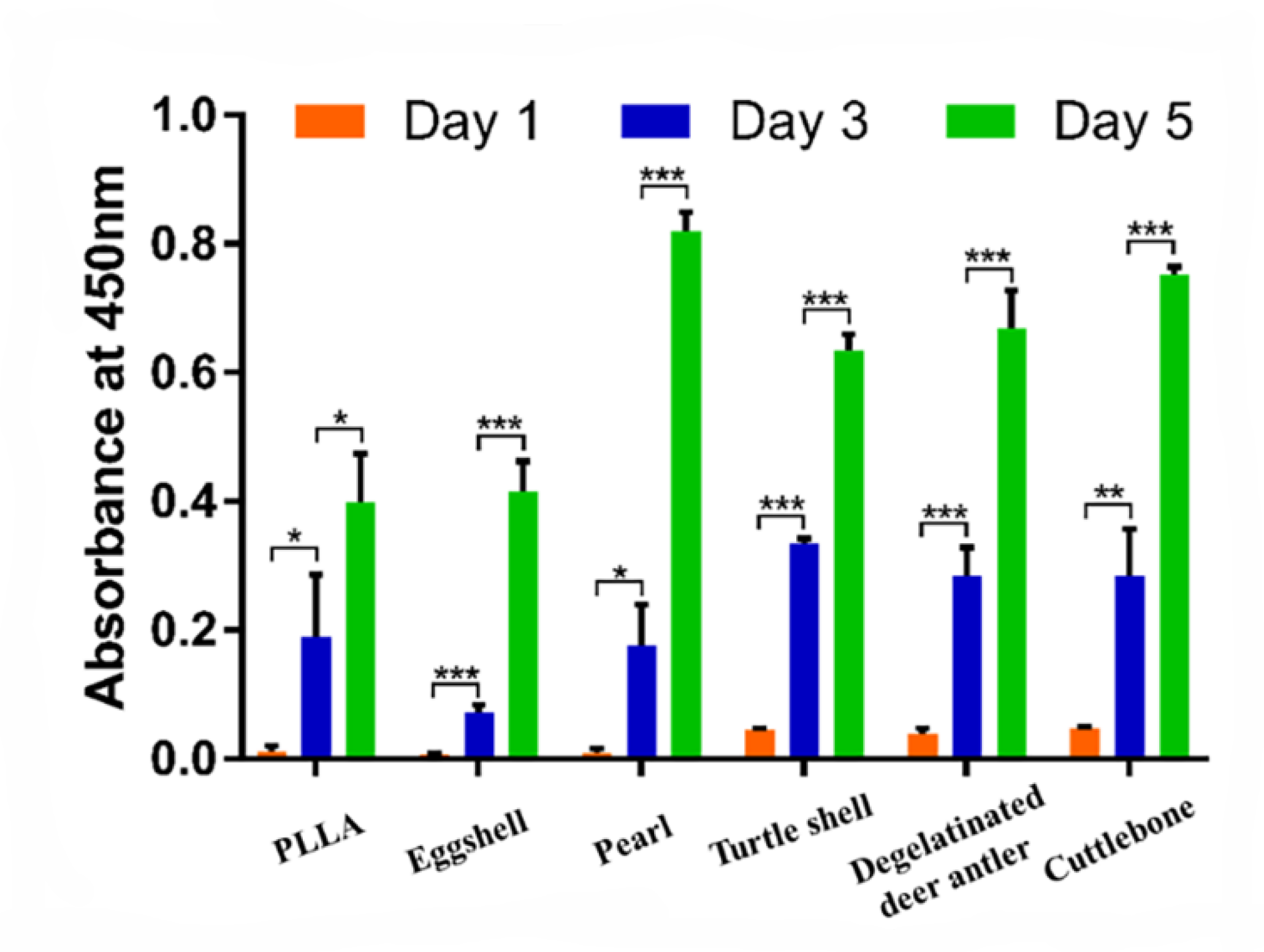
2.6. Cell Proliferation Assay
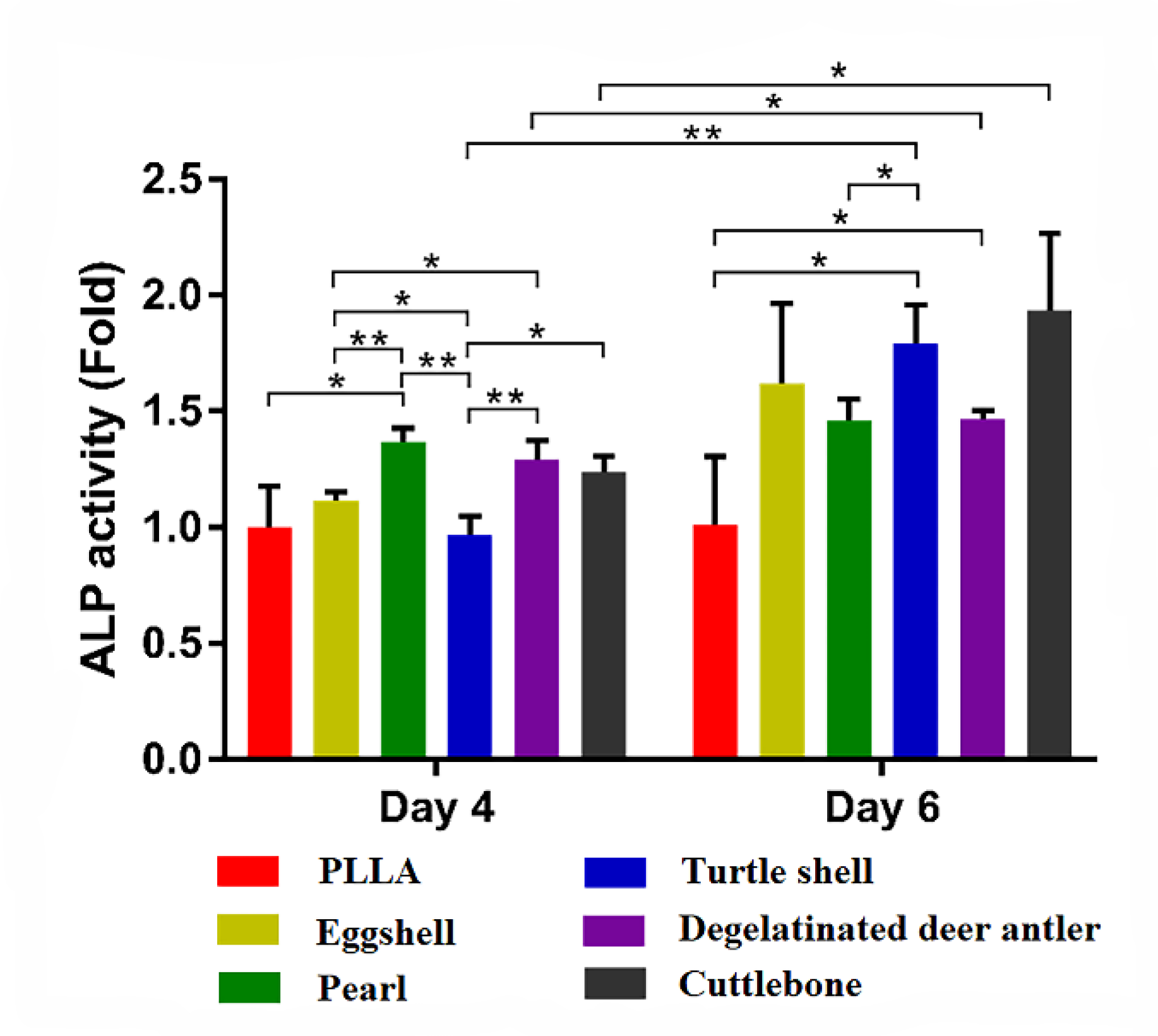
2.7. ALP Detection
3. Results and Discussions
3.1. Powder Test
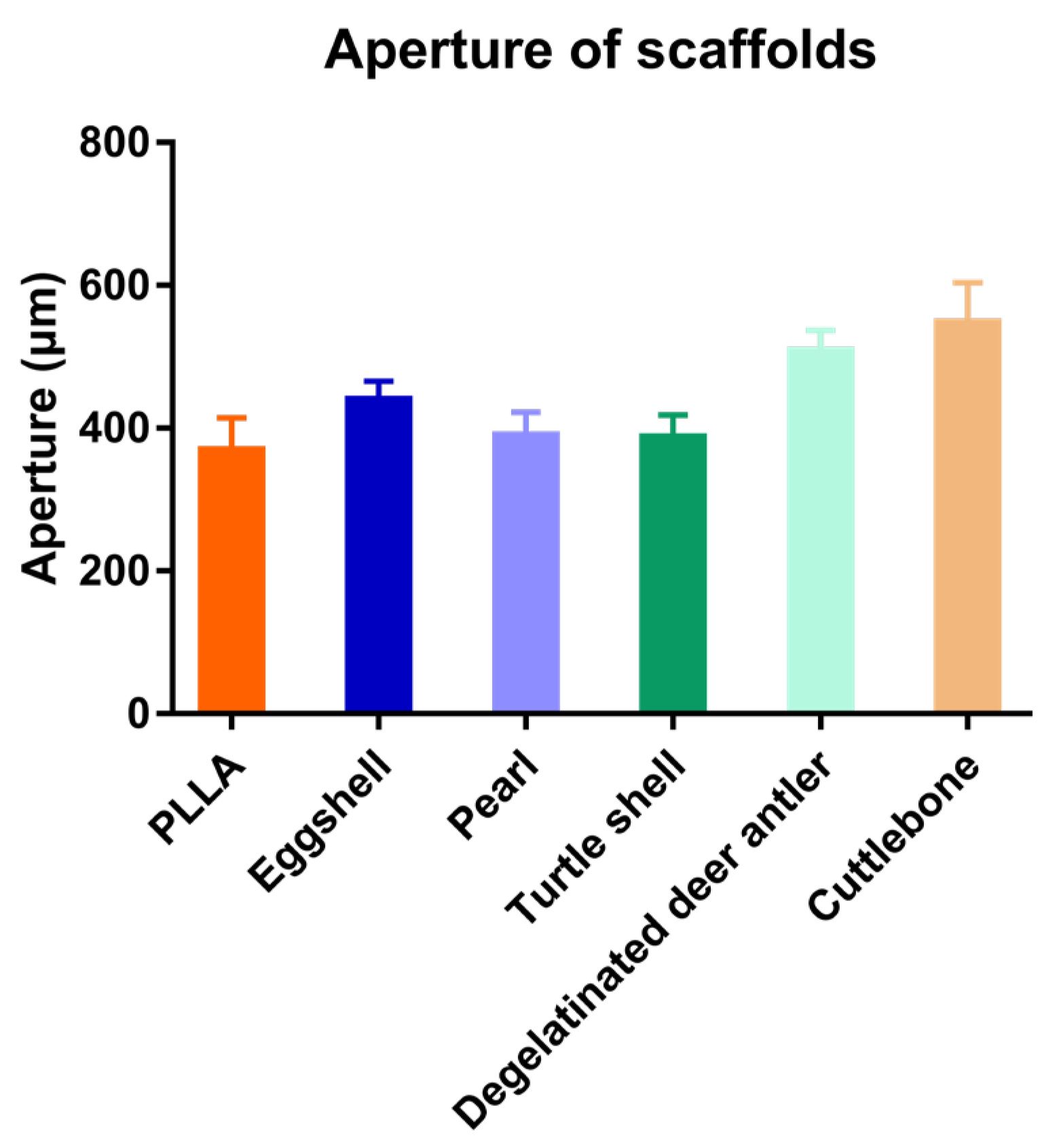
3.2. Ink Test
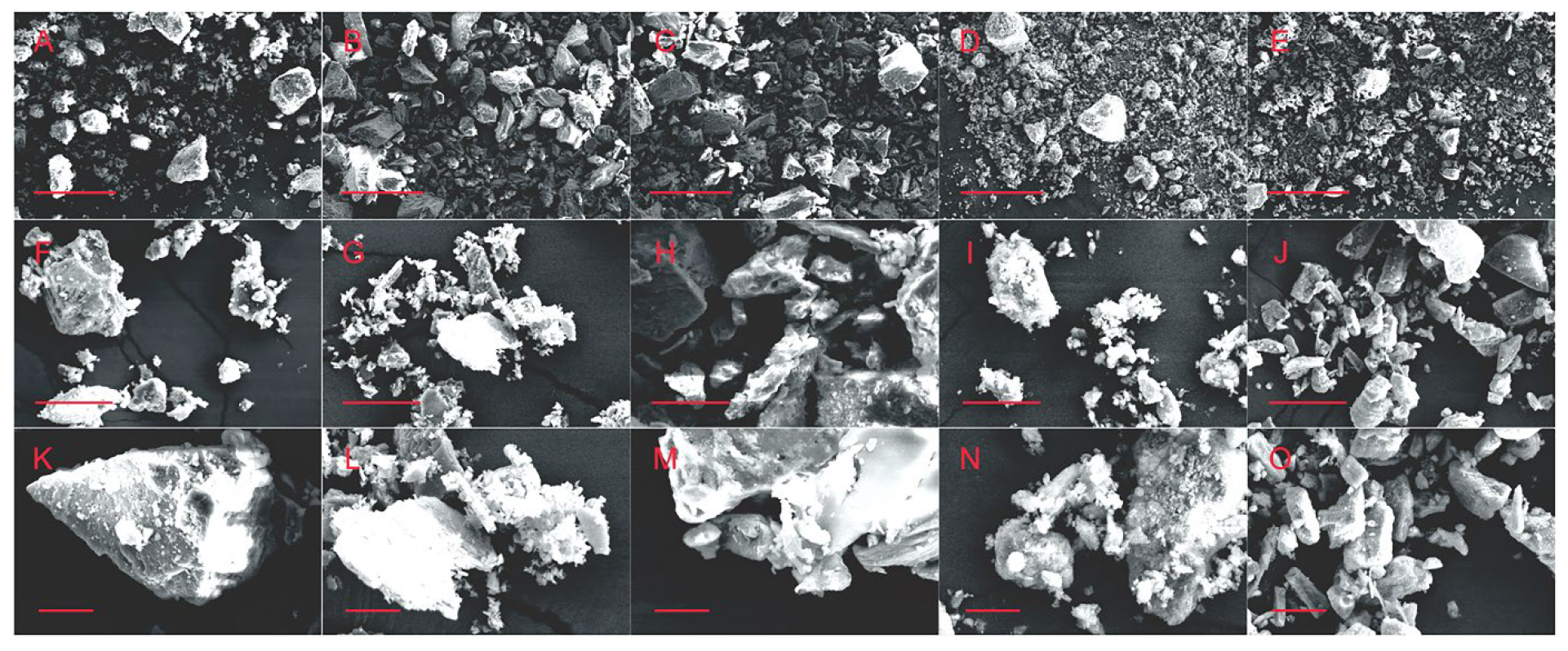
3.3. SEM Observation of the Scaffolds
3.4. Cell Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amini, Z.; Lari, R. A systematic review of decellularized allograft and xenograft–derived scaffolds in bone tissue regeneration. Tissue Cell 2021, 69, 101494. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.; Guedes, M. Morphological and mechanical characterization of 3D printed PLA scaffolds with controlled porosity for trabecular bone tissue replacement. Mat. Sci. Eng. C 2021, 118, 111528. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, T.; Guo, S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen. Ther. 2021, 16, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Khademhosseini, A.; Vacanti, J.P.; Langer, R. Progress in tissue engineering. Sci. Am. 2009, 300, 64–71. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Martino, M.M.; Briquez, P.S.; Ranga, A.; Lutolf, M.P.; Hubbell, J.A. Heparinbinding domain of fibrin (ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. USA 2013, 110, 4563–4568. [Google Scholar] [CrossRef] [Green Version]
- Pakulska, M.M.; Miersch, S.; Shoichet, M.S. Designer protein delivery: From natural to engineered affinity-controlled release systems. Science 2016, 351, aac4750. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S.; et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vegas, A.J.; Veiseh, O.; Doloff, J.C.; Ma, M.; Tam, H.H.; Bratlie, K.; Li, J.; Bader, A.R.; Langan, E.; Olejnik, K.; et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol. 2016, 34, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, R.; Vacanti, J.P.; Vacanti, C.A.; Atala, A.; Freed, L.E.; Vunjak-Novakovic, G. Tissue engineering: Biomedical applications. Tissue Eng. 1995, 1, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354 (Suppl. S1), S32–S34. [Google Scholar] [CrossRef]
- Qi, H.; Ghodousi, M.; Du, Y.; Grun, C.; Bae, H.; Yin, P.; Khademhosseini, A. DNA-directed self-assembly of shape-controlled hydrogels. Nat. Commun. 2013, 4, 2275. [Google Scholar] [CrossRef]
- Todhunter, M.E.; Jee, N.Y.; Hughes, A.J.; Coyle, M.C.; Cerchiari, A.; Farlow, J.; Garbe, J.C.; LaBarge, M.A.; Desai, T.A.; Gartner, Z.J. Programmed synthesis of three-dimensional tissues. Nat. Methods 2015, 12, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Cohen, D.L.; Malone, E.; Lipson, H.; Bonassar, L.J. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006, 12, 1325–1335. [Google Scholar] [CrossRef]
- Khalil, S.; Nam, J.; Sun, W. Multi-nozzle deposition for construction of 3d biopolymer tissue scaffolds. Rapid Prototyp. J. 2005, 11, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Colosi, C.; Shin, S.R.; VManoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Ober, T.J.; Foresti, D.; Lewis, J.A. Active mixing of complex fluids at the microscale. Proc. Natl. Acad. Sci. USA 2015, 112, 12293–12298. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.; Kim, M.; Jeong, Y.; Lee, S. Exosome-coated silk fibroin 3D-scaffold for inducing osteogenic differentiation of bone marrow derived mesenchymal stem cells. Chem. Eng. J. 2021, 406, 127080. [Google Scholar]
- Wang, M.; Li, H.; Yang, Y.; Yuan, K.; Zhou, F.; Liu, H.; Zhou, Q.; Yang, S.; Tang, T. A 3D-bioprinted scaffold with doxycycline-controlled BMP2-expressing cells for inducing bone regeneration and inhibiting bacterial infection. Bioact. Mater. 2021, 6, 1318–1329. [Google Scholar] [CrossRef]
- Ou, M.; Huang, X. Influence of bone formation by composite scaffolds with different proportions of hydroxyapatite and collagen. Dent. Mater. 2021, 37, 231–244. [Google Scholar] [CrossRef]
- Li, N.; Guo, X.; Tang, X.; Xing, Y.; Pang, H. Three-dimensional Co2V2O7·nH2O superstructures assembled by nanosheets for electrochemical energy storage. Chin. Chem. Lett. 2022, 33, 462–465. [Google Scholar] [CrossRef]
- Su, X.; Xian, C.; Gao, M.; Liu, G.; Wu, J. Edible materials in tissue regeneration. Macromol. Biosci. 2021, 21, 2100114. [Google Scholar] [CrossRef]
- Huang, K.; Liu, G.; Gu, Z.; Wu, J. Tofu as excellent scaffolds for potential bone regeneration. Chin. Chem. Lett. 2020, 31, 3190–3194. [Google Scholar] [CrossRef]
- Huang, K.; Gu, Z.; Wu, J. Tofu-Incorporated Hydrogels for Potential Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Q.; Feng, Q. 3D scaffold of PLLA/pearl and PLLA/nacre powder for bone regeneration. Biomed. Mater. 2013, 8, 065001. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhu, J.; Zhang, H.; Zhao, F. In vitro osteogenetic activity of pearl. Biomaterials 2006, 27, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yu, B.; Pei, P.; Ding, H.; Yu, B.; Zhu, Y. 3D printing of pearl/CaSO4 composite scaffolds for bone regeneration. J. Mater. Chem. B 2018, 6, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, Y.; Ouyang, Z.; Feng, Q. Comparing the regeneration potential between PLLA/Aragonite and PLLA/Vaterite pearl composite scaffolds in rabbit radius segmental bone defects. Bioact. Mater. 2020, 5, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K. Role of calcium bio-minerals in regenerative medicine and tissue engineering. J. Stem Cell Res. Ther. 2017, 2, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Jiang, L.; Wang, Y.; Gang, F.; Xu, N.; Li, T.; Liu, Z.; Chi, Y.; Wang, X.; Zhao, L. 3D printing of conductive tissue engineering scaffolds containing polypyrrole nanoparticles with different morphologies and concentrations. Materials 2019, 12, 2491. [Google Scholar] [CrossRef] [Green Version]
- Shuai, C.; Li, Y.; Feng, P.; Guo, W.; Yang, W.; Peng, S. Positive feedback effects of Mg on the hydrolysis of poly-l-lactic acid (PLLA): Promoted degradation of PLLA scaffolds. Polym. Test. 2018, 68, 27–33. [Google Scholar] [CrossRef]
- Yu, B.; Meng, L.; Fu, S.; Zhao, Z.; Liu, Y.; Wang, K.; Fu, Q. Morphology and internal structure control over PLA microspheres by compounding PLLA and PDLA and effects on drug release behavior. Colloid. Surf. B 2018, 172, 105–112. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Q.; Du, B.; Cao, L.; Lin, H.; Fan, Z.; Dong, J. Enhanced bone regeneration composite scaffolds of PLLA/β-TCP matrix grafted with gelatin and Hap. Mat. Sci. Eng. C 2018, 87, 60. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wu, M.; Yan, F.; Xie, Y.; Liu, Z.; Huang, H.; Yang, Z.; Yao, S.; Cai, L. A radial 3D polycaprolactone nanofiber scaffold modified by biomineralization and silk fibroin coating promote bone regeneration in vivo. Int. J. Biol. Macromol. 2021, 172, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dai, X.; Peng, Y.; Liu, M.; Lu, F.; Yang, X.; Gou, Z.; Ye, J. Digital light processing strength-strong ultra-thin bioceramic scaffolds for challengeable orbital bone regeneration and repair in Situ. Appl. Mater. Today 2021, 22, 100889. [Google Scholar] [CrossRef]
- Eghtesad, S.; Nurminskaya, M.V. Binding of pro-migratory serum factors to electrospun PLLA nano-fibers. J. Biomater. Sci. Polym. Ed. 2013, 24, 2006–2017. [Google Scholar] [CrossRef] [Green Version]
- Piran, M.; Shiri, M.; Soufi Zomorrod, M.; Esmaeili, E.; Zomorrod, M.S.; Shiran, N.V.; Mahboudi, H.; Daneshpazhouh, H.; Dehghani, N.; Hosseinzadeh, S. Electrospun triple-layered PLLA/gelatin. PRGF/PLLA scaffold induces fibroblast migration. J. Cell. Biochem. 2019, 120, 11441–11453. [Google Scholar] [CrossRef]
- Zhepao, Y.; Huihua, Y.; Bingcheng, Y.; Xianliu, W.; Zhaowenbin, Z.; Yanzhong, Z. Effects of Electrospun Fiber-Stiffness on Adhesion and Migration of iPS-MSCs. Chem. J. Chin. Univ.-Chin. 2018, 39, 807–816. [Google Scholar]
- Hu, C.; Liu, S.; Zhang, Y.; Li, B.; Yang, H.; Fan, C.; Cui, W. Long-term drug release from electrospun fibers for in vivo inflammation prevention in the prevention of peritendinous adhesions. Acta Biomater. 2013, 9, 7381–7388. [Google Scholar] [CrossRef]
- Dong, Q.N.; Kanno, T.; Bai, Y.; Sha, J.; Hideshima, K. Bone regeneration potential of uncalcined and unsintered hydroxyapatite/poly l-lactide bioactive/osteoconductive sheet used for maxillofacial reconstructive surgery: An in vivo study. Materials 2019, 12, 2931. [Google Scholar] [CrossRef] [Green Version]
- Kanno, T.; Sukegawa, S.; Karino, M.; Furuki, Y. Navigation-assisted orbital trauma reconstruction using a bioactive osteoconductive/bioresorbable u-HA/PLLA system. J. Maxillofac. Oral Surg. 2019, 18, 329–338. [Google Scholar] [CrossRef]
- Wei, H.; Yan, S.; Menary, G. modelling stretch blow moulding of poly (l-lactic acid) for the manufacture of bioresorbable vascular scaffold. Polymers 2021, 13, 967. [Google Scholar] [CrossRef]
- Sadeghi-Avalshahr, A.; Khorsand-Ghayeni, M.; Nokhasteh, S.; Shahri, M.M.; Molavi, A.M.; Sadeghi-Avalshahr, M. Effects of hydroxyapatite (HA) particles on the PLLA polymeric matrix for fabrication of absorbable interference screws. Polym. Bull. 2018, 75, 2559–2574. [Google Scholar] [CrossRef]
- Ide, A.; Sakane, M.; Chen, G.; Shimojo, H.; Ushida, T.; Tateishi, T.; Wadano, Y.; Miyanag, Y. Collagen hybridization with poly (L-lactic acid) braid promotes ligament cell migration. Mater. Sci. Eng. C 2001, 17, 95–99. [Google Scholar] [CrossRef]
- He, Z.; Xiong, L. Evaluation of in-vitro cytotoxicity of composite materials composed of poly-L-lactic acid and β-tricalcium phosphate. Polym.-Plast. Technol. Eng. 2010, 49, 381–386. [Google Scholar] [CrossRef]
- Abazari, M.F.; Nasiri, N.; Nejati, F.; Kohandani, M.; Hajati-Birgani, N.; Sadeghi, S.; Piri, P.; Soleimanifar, F.; Rezaei-Tavirani, M.; Mansouri, V. Acceleration of osteogenic differentiation by sustained release of BMP2 in PLLA/graphene oxide nanofibrous scaffold. Polym. Adv. Technol. 2021, 32, 272–281. [Google Scholar] [CrossRef]
- Bai, J.; Dai, J.; Li, G. Electrospun composites of PHBV/pearl powder for bone repairing. Prog. Nat. Sci. Mater. Int. 2015, 25, 327–333. [Google Scholar] [CrossRef]
- Widyowati, R.; Suciati, S.; Haryadi, D.M.; Chang, H.-I.; Suryawan, I.N.; Tarigan, N. The effect of deer antler from East Kalimantan to increase trabecular bone density and calcium levels in serum on osteoporotic mice. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 1145–1150. [Google Scholar] [CrossRef]
- Palaveniene, A.; Tamburaci, S.; Kimna, C.; Glambaite, K.; Baniukaitiene, O.; Tihminlioğlu, F.; Liesiene, J. Osteoconductive 3D porous composite scaffold from regenerated cellulose and cuttlebone-derived hydroxyapatite. J. Biomater. Appl. 2019, 33, 876–890. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, Z.; Li, W.; Zhu, M. Understanding hydration effects on mechanical and impacting properties of turtle shell. J. Mech. Behav. Biomed. Mater. 2018, 78, 116–123. [Google Scholar] [CrossRef]
- Chen, M.; Hu, N.; Zhou, C.; Lin, X.; Xie, H.; He, Q. The hierarchical structure and mechanical performance of a natural nanocomposite material: The turtle shell. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 97–104. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Wang, Y.; Zhang, L.; Zhang, J.; Shi, J.; Si, J.; Yuan, Y.; Liu, C. Direct three-dimensional printing of a highly customized freestanding hyperelastic bioscaffold for complex craniomaxillofacial reconstruction. Chem. Eng. J. 2021, 411, 128541. [Google Scholar] [CrossRef]
- Wysokowski, M.; Jesionowski, T.; Ehrlich, H. Biosilica as a source for inspiration in biological materials science. Am. Mineral. J. Earth Planet. Mater. 2018, 103, 665–691. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Q.; Pu, X.; Hou, Z.; Zhang, Q. Biphasic calcium phosphate macroporous scaffolds derived from oyster shells for bone tissue engineering. Chem. Eng. J. 2011, 173, 837–845. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, S.; Lan, Y.; Huang, C.; Wang, C.; Lai, X.; Chen, H.; Ao, J. 3D printed porous polycaprolactone/oyster shell powder (PCL/OSP) scaffolds for bone tissue engineering. Mat. Res. Exp. 2018, 5, 045403. [Google Scholar] [CrossRef]
- Wang, Z.; Han, L.; Sun, T.; Wang, W.; Wu, B. Construction of tissue-engineered bone with differentiated osteoblasts from adipose-derived stem cell and coral scaffolds at an ectopic site. Br. J. Oral Maxillofac. Surg. 2021, 59, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Granito, R.N.; Custódio, M.R.; Rennó, A.C.M. Natural marine sponges for bone tissue engineering: The state of art and future perspectives. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1717–1727. [Google Scholar] [CrossRef]
- Gabbai-Armelin, P.R.; Kido, H.W.; Cruz, M.A.; Prado, J.P.S.; Avanzi, I.R.; Custódio, M.R.; Renno, A.C.M.; Granito, R.N. Characterization and cytotoxicity evaluation of a marine sponge biosilica. Mar. Biotechnol. 2019, 21, 65–75. [Google Scholar] [CrossRef] [PubMed]

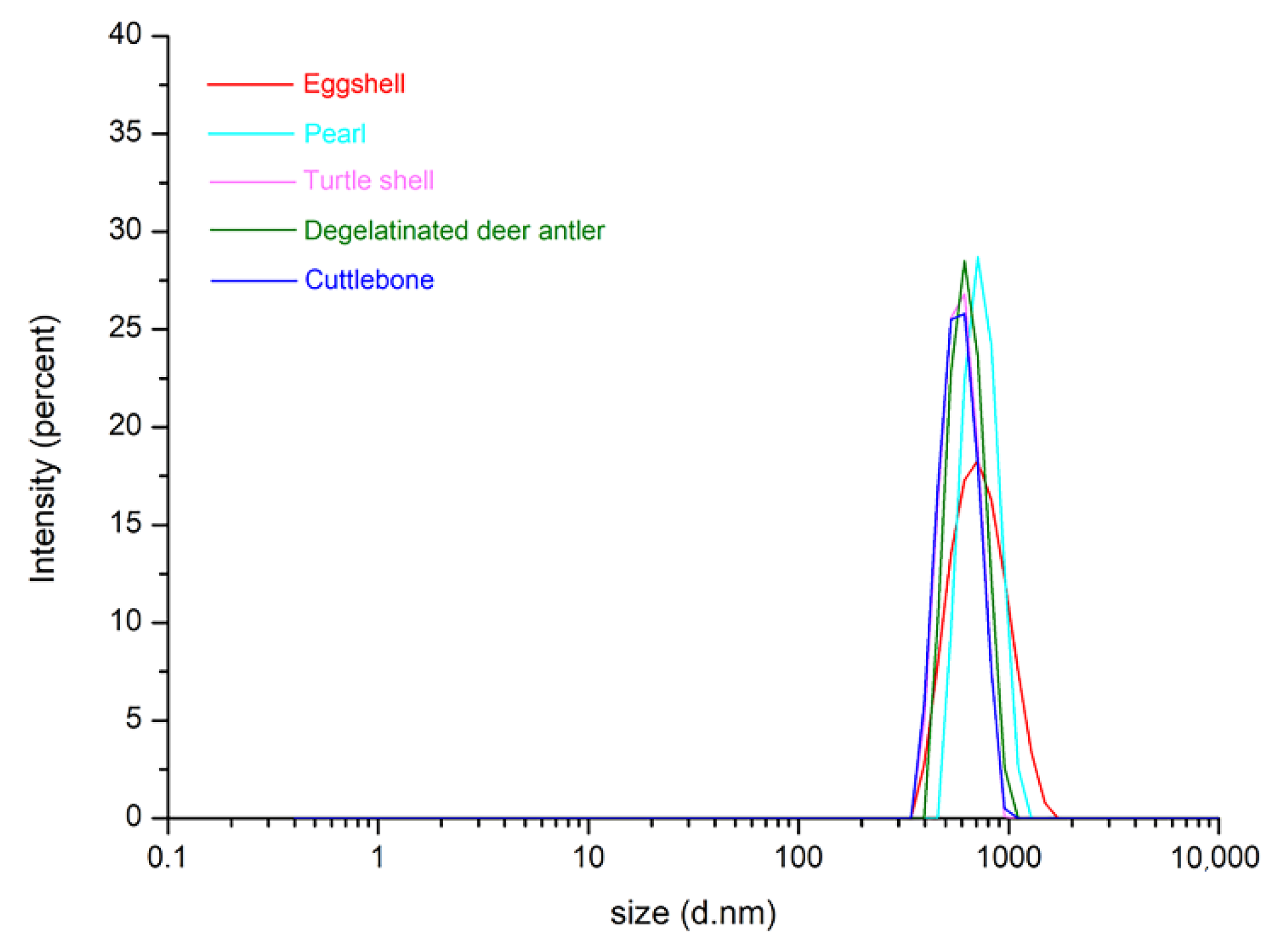




| Biomineral | Chemical Composition | Particle Size |
|---|---|---|
| Eggshell | CaCO3 | 400–1200 nm |
| Pearl | CaCO3 | 350–1100 nm |
| Turtle shell | Ca5(PO4)3(OH) | 260–610 nm |
| Degelatinated deer antler | Ca5(PO4)3(OH) | 180–6500 nm |
| Cuttle bone | CaCO3 | 300–610 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gang, F.; Ye, W.; Ma, C.; Wang, W.; Xiao, Y.; Liu, C.; Sun, X. 3D Printing of PLLA/Biomineral Composite Bone Tissue Engineering Scaffolds. Materials 2022, 15, 4280. https://doi.org/10.3390/ma15124280
Gang F, Ye W, Ma C, Wang W, Xiao Y, Liu C, Sun X. 3D Printing of PLLA/Biomineral Composite Bone Tissue Engineering Scaffolds. Materials. 2022; 15(12):4280. https://doi.org/10.3390/ma15124280
Chicago/Turabian StyleGang, Fangli, Weilong Ye, Chunyang Ma, Wenting Wang, Yi Xiao, Chang Liu, and Xiaodan Sun. 2022. "3D Printing of PLLA/Biomineral Composite Bone Tissue Engineering Scaffolds" Materials 15, no. 12: 4280. https://doi.org/10.3390/ma15124280
APA StyleGang, F., Ye, W., Ma, C., Wang, W., Xiao, Y., Liu, C., & Sun, X. (2022). 3D Printing of PLLA/Biomineral Composite Bone Tissue Engineering Scaffolds. Materials, 15(12), 4280. https://doi.org/10.3390/ma15124280







