Evaluation of Shear Bond Strength between Resin Composites and Conventional Glass Ionomer Cement in Class II Restorative Technique—An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Samples Preparation
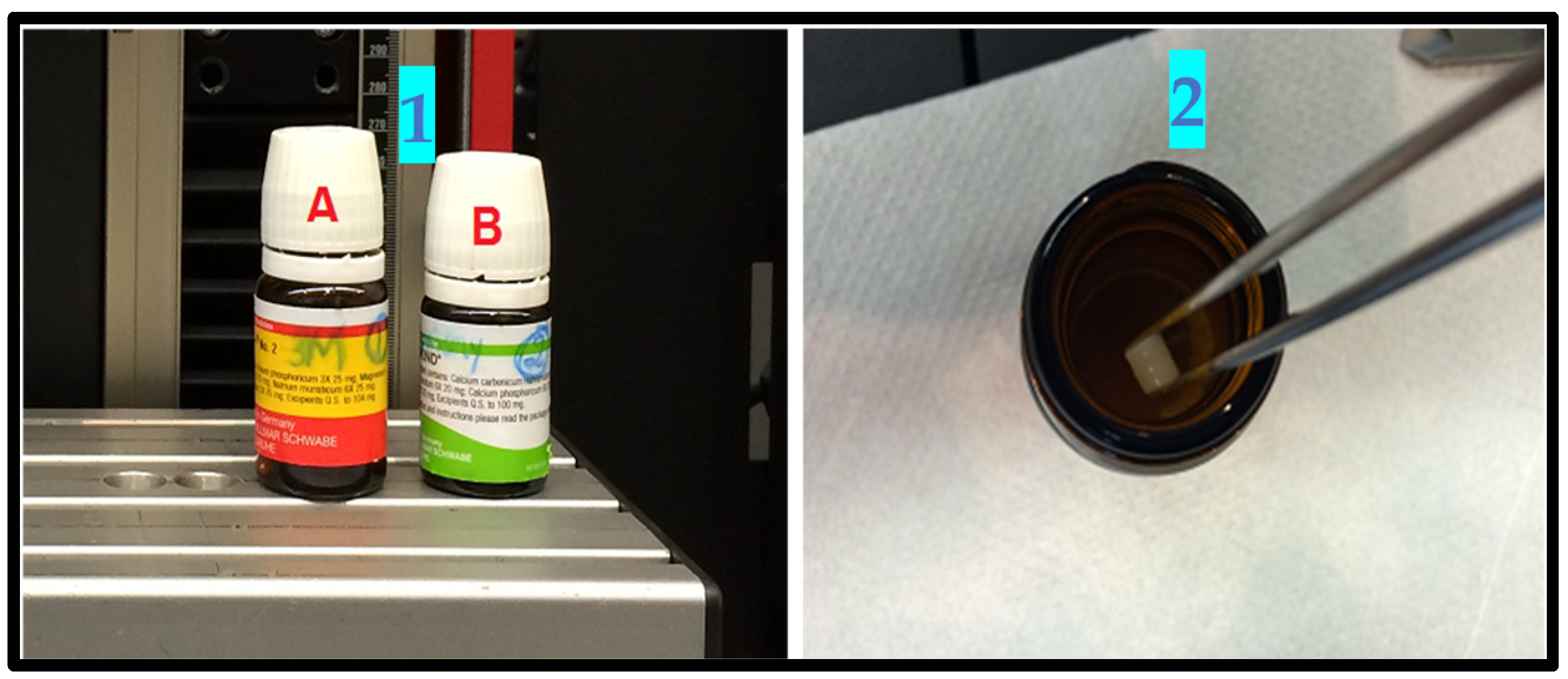
2.3. Materials Included in the Study
- Methacrylate Z350™ (3M ESPE);
- Ceram X™ (DENTSPLY, SIRONA);
- GIC (Ketac Molar™, 3M ESPE);
- Microhybrid composite (Spectrum™ (DENSTPLY, SIRONA);
- Phosphoric acid gel (3M ESPE & DENTSPLY).
2.4. Sample Size Calculation and Grouping
- Group A: 08 samples (GIC + composite A, Z350™ (3M ESPE, St. Paul, MN, USA).
- Group B: 08 samples (GIC + composite B, Ceram X™ (DENTSPLY SIRONA, Charlotte, NC, USA).
- Control group C: 08 samples (GIC + Microhybrid composite C, Spectrum (DENTSPLY SIRONA, Charlotte, NC, USA).
2.5. Sampling Technique and Criteria
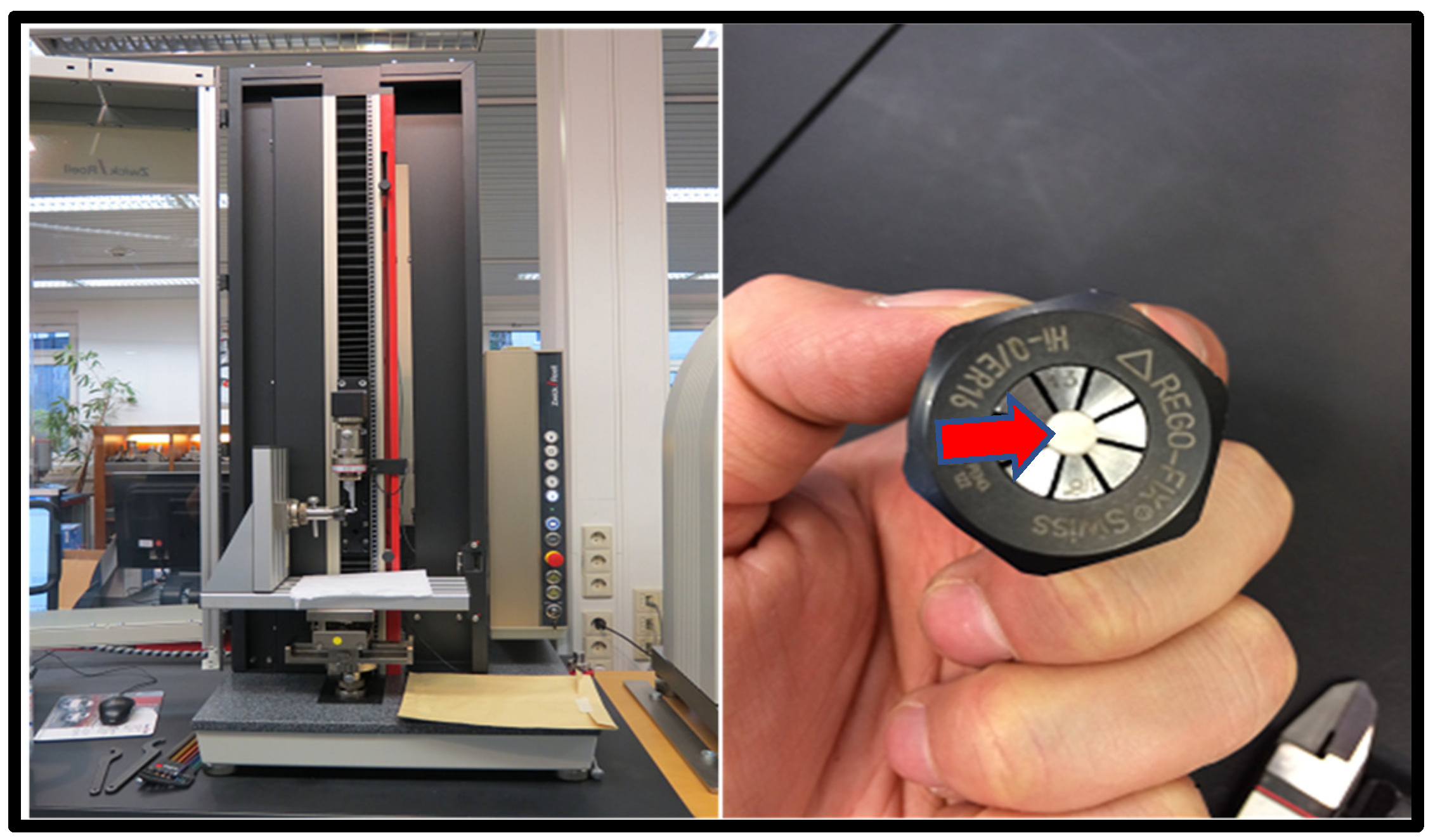
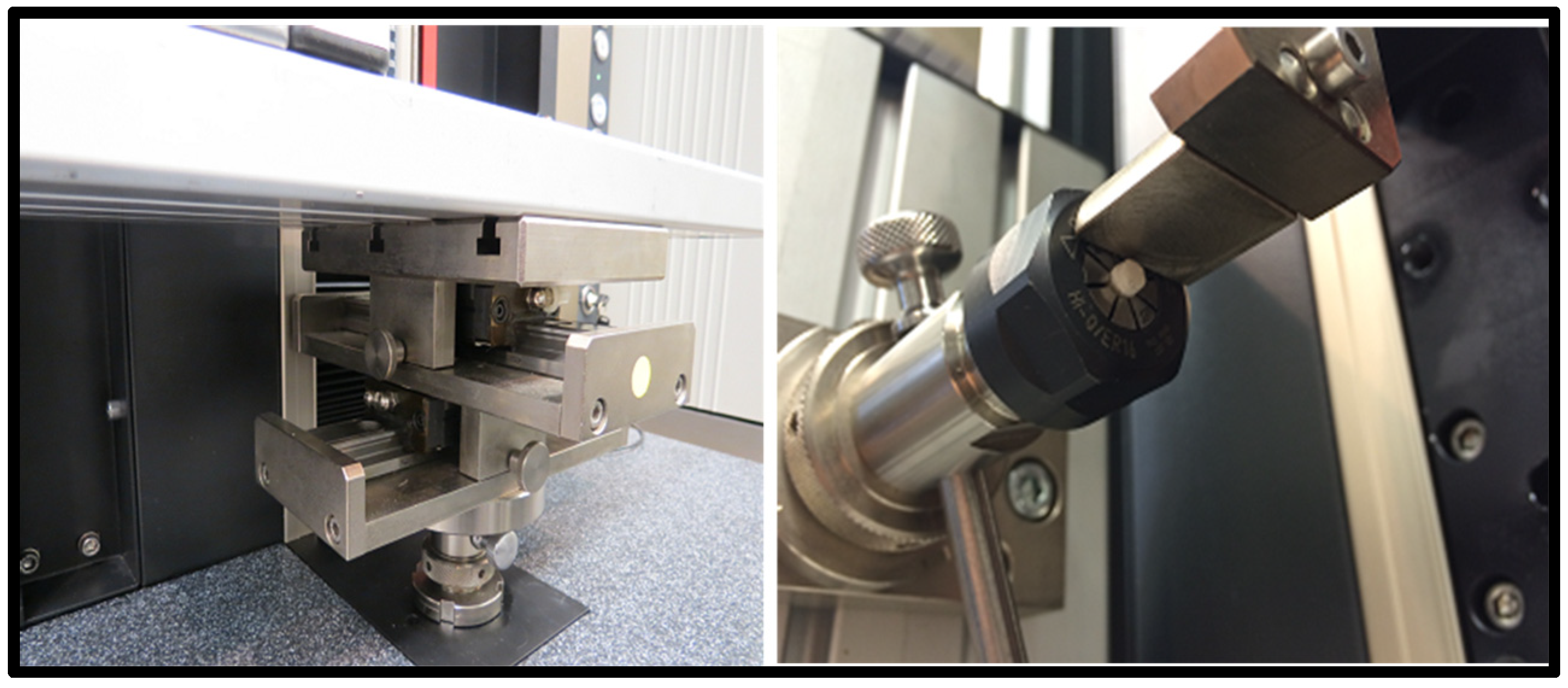
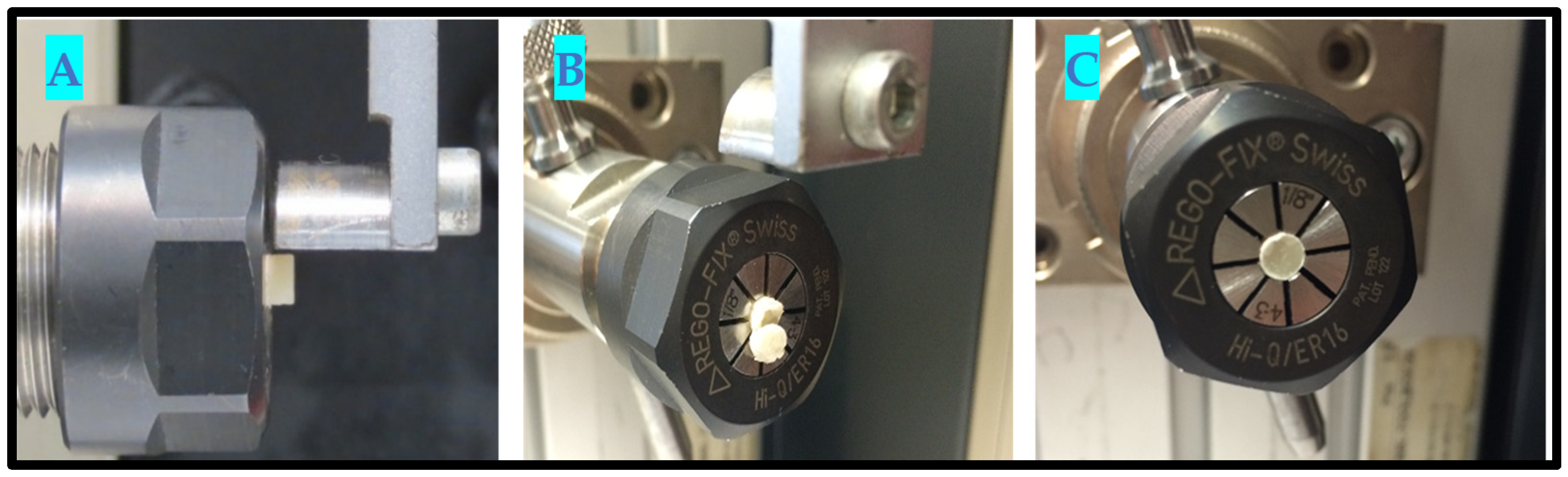
2.6. In Vitro Testing Machine Specs and Configuration
- Universal Testing Machine (Zwicki Z5.0, Zwick Roell Group, Ulm, Germany);
- 200-Newton load cell HP;
- 3–4-millimeter diameter clamping device;
- x-y axis moveable table;
- Alu t-slot plate.
2.7. In Vitro Testing Process Pictorial Description
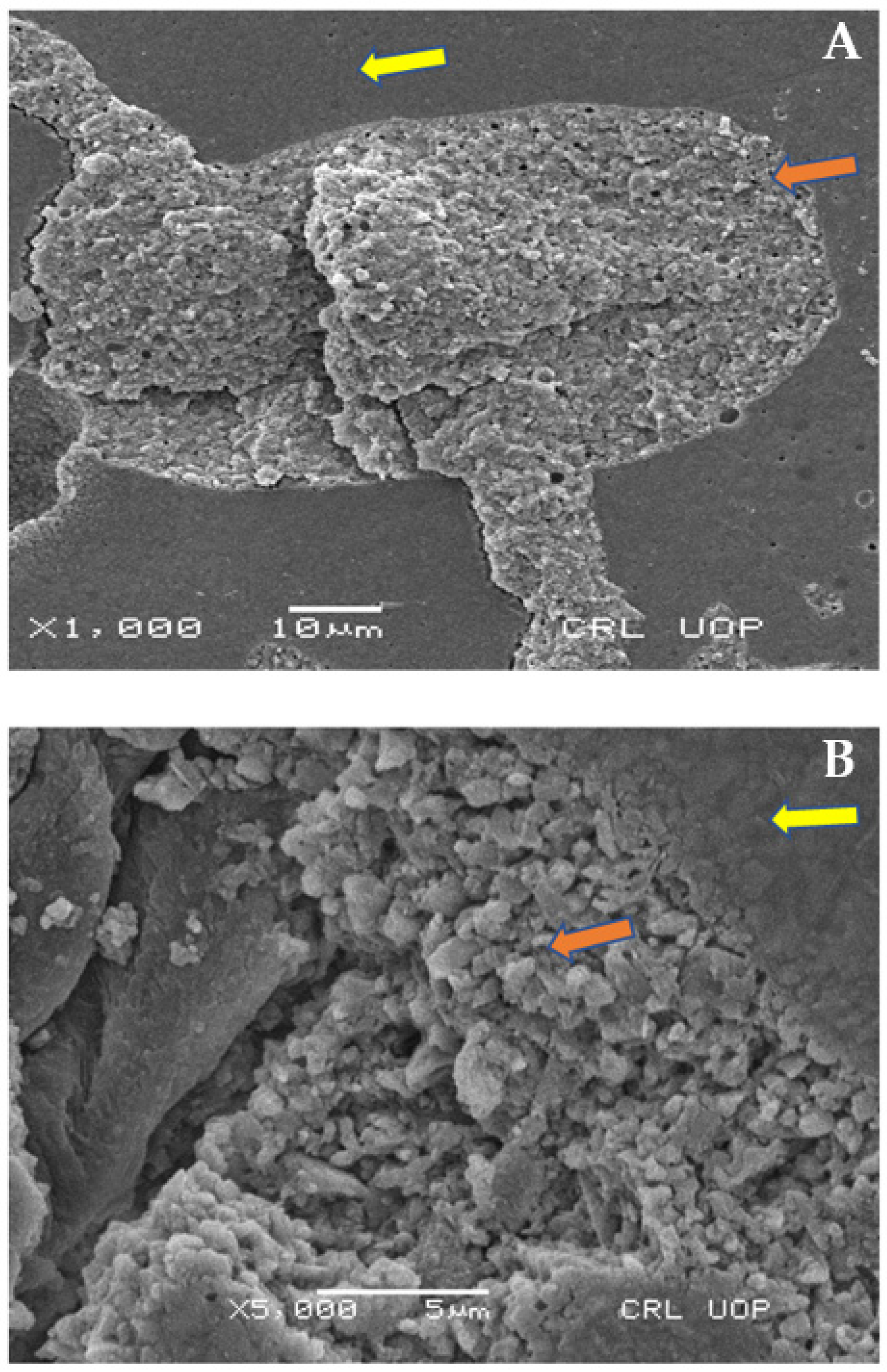
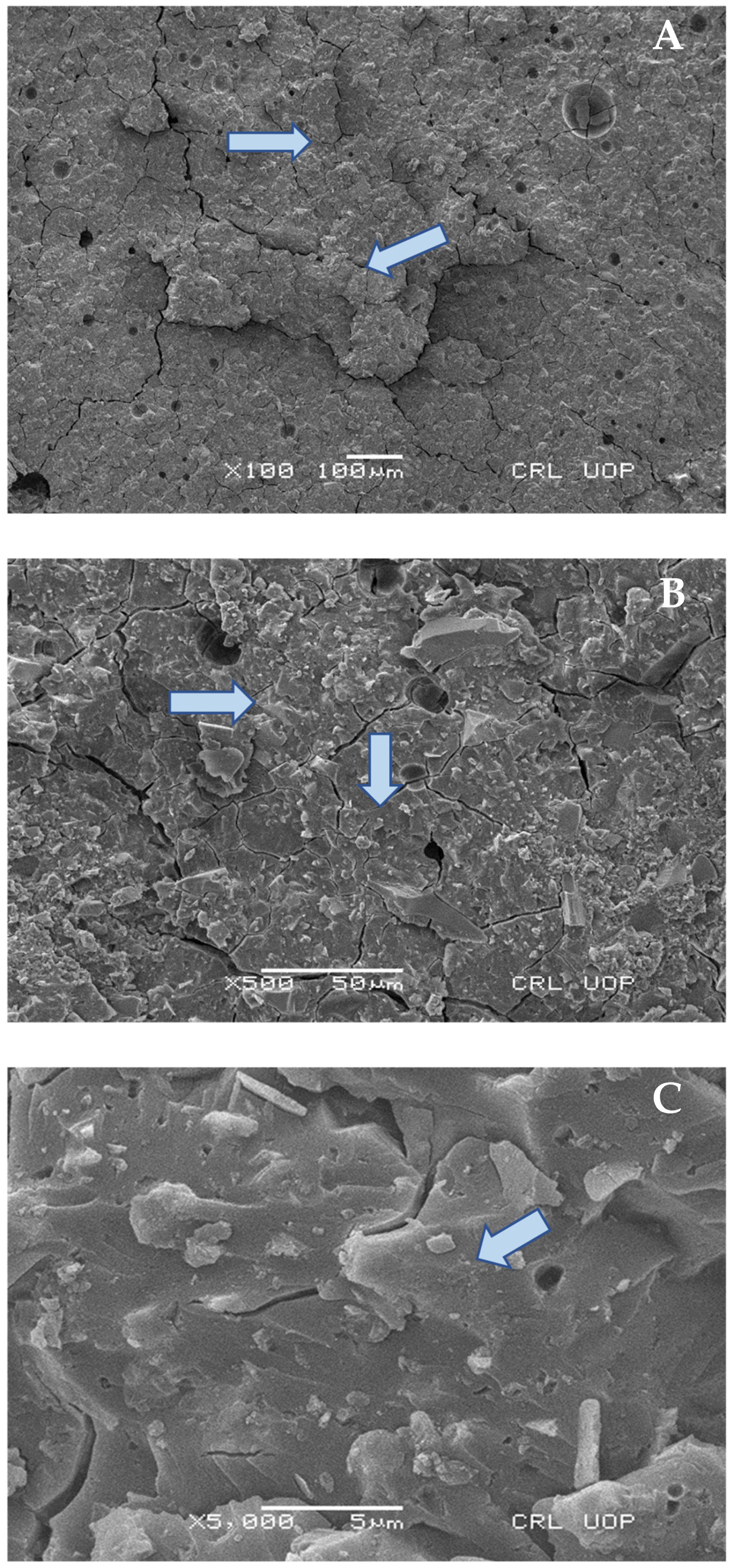
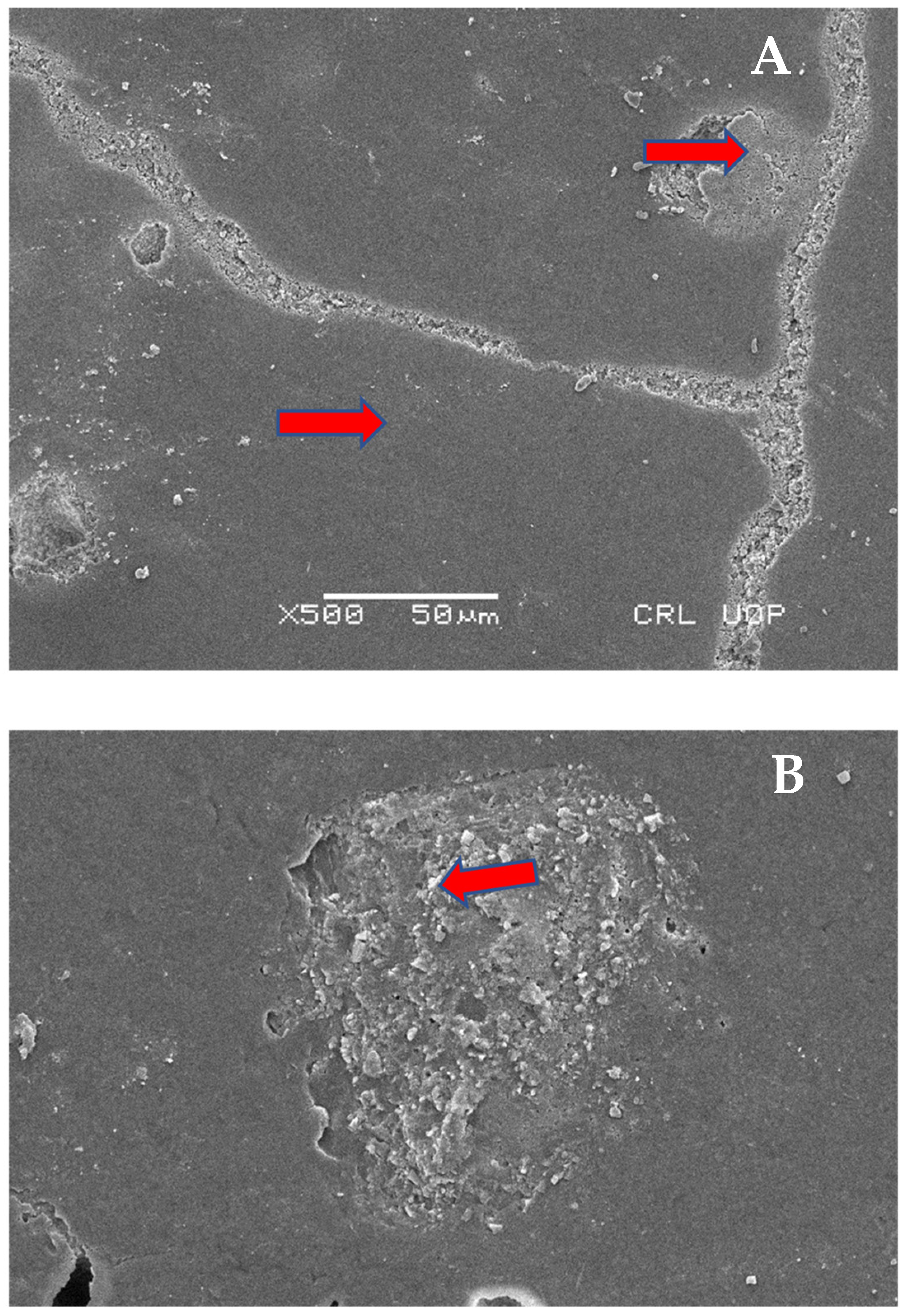
2.8. Scanning Electron Microscopy (SEM) Analysis
2.9. Statistical Analysis
3. Results
Scanning Electron Microscopy Images
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S.No | Abbreviation | Full Name/Description |
| 1 | ANOVA | One-Way Analysis of Variance |
| 2 | °C | degrees Centigrade |
| 3 | h | hour |
| 4 | ISO | International Standard Organization |
| 5 | LED | light emitting diode |
| 6 | GIC | glass ionomer cement |
| 7 | mm | millimeter |
| 8 | nm | nanometer |
| 9 | kPa | kilopascal |
| 10 | RC | resin-based omposites |
| 11 | s | seconds |
| 12 | TEFLON | polytetrafluoroethylene |
| 13 | SBS | shear bond strength |
References
- Umesi, D.C.; Oremosu, O.A.; Makanjuola, J.O. Amalgam phase down: Baseline data preceding implementation in Nigeria. Int. Dent. J. 2020, 70, 161–166. [Google Scholar] [CrossRef]
- Askar, H.; Krois, J.; Göstemeyer, G.; Schwendicke, F. Secondary caries risk of different adhesive strategies and restorative materials in permanent teeth: Systematic review and network meta-analysis. J. Dent. 2021, 104, 103541. [Google Scholar] [CrossRef] [PubMed]
- Latta, M.A.; Tsujimoto, A.; Takamizawa, T.; Barkmeier, W.W. Enamel and dentin bond durability of self-adhesive restorative materials. J. Adhes. Dent. 2020, 22, 99–105. [Google Scholar] [PubMed]
- Rathi, S.D.; Nikhade, P.; Chandak, M.; Motwani, N.; Rathi, C.; Chandak, M. Microleakage in Composite Resin Restoration-A Review Article. J. Evol. Med. Dent. Sci. 2020, 9, 1006–1011. [Google Scholar] [CrossRef]
- Çelik, Ç.; Bayraktar, Y.; Özdemir, B.E. Effect of Saliva Contamination on Microleakage of Open Sandwich Restorations. Acta Stomatol. Croat. Int. J. Oral Sci. Dent. Med. 2020, 54, 273–282. [Google Scholar] [CrossRef]
- Sadr, A.; Bakhtiari, B.; Hayashi, J.; Luong, M.N.; Chen, Y.W.; Chyz, G.; Chan, D.; Tagami, J. Effects of fiber reinforcement on adaptation and bond strength of a bulk-fill composite in deep preparations. Dent. Mater. 2020, 36, 527–534. [Google Scholar] [CrossRef]
- Kaup, M.; Dammann, C.H.; Schäfer, E.; Dammaschke, T. Shear bond strength of Biodentine, ProRoot MTA, glass ionomer cement and composite resin on human dentine ex vivo. Head Face Med. 2015, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Nujella, B.S.; Choudary, M.T.; Reddy, S.P.; Kumar, M.K.; Gopal, T. Comparison of shear bond strength of aesthetic restorative materials. Contemp. Clin. Dent. 2012, 3, 22. [Google Scholar] [CrossRef]
- Celik, E.; Çakıcı, F.; Sarıyılmaz, E.; Tulga, A.; Çakıcı, E.B.; Dede, D.Ö.; Polat, S. Evaluation of bond strength of resin nano-ceramic as post material. J. Stomatol. 2021, 74, 89–94. [Google Scholar] [CrossRef]
- Yushau, U.S.; Almofeez, L.; Bozkurt, A. Novel polymer nanocomposites comprising triazole functional silica for dental application. Silicon 2020, 12, 109–116. [Google Scholar] [CrossRef]
- Gupta, R.; Mahajan, S. Shear bond strength evaluation of resin composite bonded to GIC using different adhesives. J. Clin. Diagn. Res. 2015, 9, ZC27. [Google Scholar] [CrossRef] [PubMed]
- Babar, B.Z.; Amin, N.; Zia, M. Evaluation of shear bond strength of composite resin bonded to glass ionomer cement and resin-modified glass ionomer cement. Pak. Oral Dent. J. 2020, 40, 92–97. [Google Scholar]
- François, P.; Greenwall-Cohen, J.; Le Goff, S.; Ruscassier, N.; Attal, J.P.; Dursun, E. Shear bond strength and interfacial analysis of high-viscosity glass ionomer cement bonded to dentin with protocols including silver diamine fluoride. J. Oral Sci. 2020, 62, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Kheur, M.; Kantharia, N.; Iakha, T.; Kheur, S.; Husain, N.A.; Özcan, M. Evaluation of mechanical and adhesion properties of glass ionomer cement incorporating nano-sized hydroxyapatite particles. Odontology 2020, 108, 66–73. [Google Scholar] [CrossRef]
- Perdigão, J. Current perspectives on dental adhesion: (1) Dentin adhesion—not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Navimipour, E.J.; Oskoee, S.S.; Oskoee, P.A.; Bahari, M.; Rikhtegaran, S.; Ghojazadeh, M. Effect of acid and laser etching on shear bond strength of conventional and resin-modified glass-ionomer cements to composite resin. Lasers Med. Sci. 2012, 27, 305–311. [Google Scholar] [CrossRef]
- Sharafeddin, F.; Moradian, M.; Motamedi, M. Evaluation of shear bond strength of methacrylate-and silorane-based composite resin bonded to resin-modified glass-ionomer containing micro-and nano-hydroxyapatite. J. Dent. 2016, 17, 142. [Google Scholar]
- Bezerra, I.M.; Brito, A.C.; de Sousa, S.A.; Santiago, B.M.; Cavalcanti, Y.W.; de Almeida, L.D. Glass ionomer cements compared with composite resin in restoration of noncarious cervical lesions: A systematic review and meta-analysis. Heliyon 2020, 6, e03969. [Google Scholar] [CrossRef]
- Preethy, N.A.; Jeevanandan, G.; Govindaraju, L.; Subramanian, E.M. Comparison of Shear Bond Strength of Three Commercially Available Esthetic Restorative Composite Materials: An In Vitro Study. Int. J. Clin. Pediatric Dent. 2020, 13, 635. [Google Scholar]
- Mahrous, A.; Radwan, M.M.; Kamel, S.M. Micro-shear bond strength of novel MDP calcium-fluoride-releasing self-adhesive resin cement after thermocycling. Int. J. Periodontics Restor. Dent. 2020, 40, 445–455. [Google Scholar] [CrossRef]
- Becci, A.C.; Benetti, M.D.; Domingues, N.B.; Giro, E.M. Bond strength of a composite resin to glass ionomer cement using different adhesive systems. Rev. Odontol. UNESP 2017, 46, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Fattah, Z.; Jowkar, Z.; Rezaeian, S. Microshear bond strength of nanoparticle-incorporated conventional and resin-modified glass ionomer to caries-affected dentin. Int. J. Dent. 2021, 2021, 5565556. [Google Scholar] [CrossRef] [PubMed]
- Mehra, S.; Gupta, A.K.; Singh, B.P.; Kaur, M.; Kumar, A. To Evaluate Shear Bond Strength of Resin Composite to Theracal Lc, Biodentine, and Resin–Modified Glass Ionomer Cement and Mode of Fracture: An In Vitro Study. Dent. J. Adv. Stud. 2020, 8, 49–54. [Google Scholar] [CrossRef]
- Ansari, Z.J.; Panahandeh, N.; Shafiei, Z.S.; Baghban, A.A. Effect of self-etching adhesives on the bond strength of glass-ionomer cement. J. Dent. 2014, 11, 680. [Google Scholar]
- Sharafeddin, F.; Choobineh, M.M. Assessment of shear bond strength between Nano-filled composites bonded to Glass Ionomer cement using self-etched adhesive with different pHs and Total-etched adhesive. J. Dent Shiraz Univ Med. Sci. 2016, 17, 1–6. [Google Scholar]
- Klinke, T.; Daboul, A.; Turek, A.; Frankenberger, R.; Hickel, R.; Biffar, R. Clinical performance during 48 months of two current glass ionomer restorative systems with coatings: A randomized clinical trial in the field. Trials 2016, 17, 239. [Google Scholar] [CrossRef] [Green Version]
- Brzović-Rajić, V.; Miletić, I.; Gurgan, S.; Peroš, K.; Verzak, Ž.; Ivanišević-Malčić, A. Fluoride release from glass ionomer with nano filled coat and varnish. Acta Stomatol. Croatica. 2018, 52, 307. [Google Scholar] [CrossRef]
- Aslam, S.; Khan, S.A.; Sarwar, N.; Rafique, M.A.; Batool, A. Comparison of Microleakage in Chemically Bonded and Mechanically Bonded Res-torations After Using Chemomechanical Caries Removal Technique in Primary Teeth. Ann. King Edw. Med. Univ. 2021, 27, 355–361. [Google Scholar]
- Anna Luisa de Brito, P.A.; Isabel Cristina, O.L.; Clarissa Calil, B.O.; Ana Flávia Bissoto, C.A.; José Carlos Pettorossi, I.M.; Daniela Prócida, R.A. One year survival rate of Ketac Molar versus Vitro Molar for occlusoproximal ART restorations: A RCT. Braz. Oral Res. 2017, 6, 31. [Google Scholar] [CrossRef] [Green Version]


| Material | Type | Liquid | Powder | Brand |
|---|---|---|---|---|
| Ketac Molar™ Easy mix | glass ionomer | polialquenoic acid/tartaric acid/water | aluminum-calcium-lanthanum fluorosilicate glass copolymer (5% acrylic acid and maleic acid | 3M ESPE (St. Paul, MN, USA) |
| Nanocomposite Ceram X™ | polymethylmethacrylate-based resin composite | not supplied | nanoparticles and nanofillers as used in Prime & Bond NT combined with conventional glass fillers of 1µm | DENTSPLY SIRONA (Charlotte, NC, USA) |
| Nanocomposite Methacrylate Z350 | polymethylmethacrylate-based resin composite | not supplied | Bis-GMA, UDMA, TEGDMA, and Bis-EMA resins, PEGDMA, Zirconia/Silica cluster filler | 3M ESPE (St. Paul, MN, USA) |
| Microhybrid composite Spectrum™ | polymethylmethacrylate-based resin composite | not supplied | TEGDMA ethyl4(dimethylamino)benzoate butylated hydroxytoluene (BHT) UV stabilizer. barium-aluminium-borosilicate glass (mean particle size < 1 µm) | DENTSPLY SIRONA (Charlotte, NC, USA) |
| Material A No | Fmax N | Shear Bond Strength kPa | dL at Fmax mm | do mm | So mm2 | Failure Mode |
|---|---|---|---|---|---|---|
| 1.1 | 3.639 | 292.49 | 0.007 | 3.98 | 12.44 | pretest |
| 1.2 | 1.327 | 108.28 | 0.030 | 3.95 | 12.25 | adhesive |
| 1.3 | 15.14 | 1222.81 | 0.360 | 3.97 | 12.38 | mixed failure |
| 1.4 | 8.429 | 680.97 | 0.200 | 3.97 | 12.38 | adhesive |
| 1.5 | 25.57 | 2254.94 | 0.162 | 3.80 | 11.34 | cohesive |
| 1.6 | 42.35 | 3404.32 | 0.105 | 3.98 | 12.44 | mixed failure |
| 1.7 | 27.13 | 2367.17 | 0.127 | 3.82 | 11.46 | adhesive |
| 1.8 | 19.17 | 1548.74 | 0.177 | 3.97 | 12.38 | mixed failure |
| Material B No | Fmax N | Shear Bond Strength kPa | dL at Fmax mm | do mm | So mm2 | Failure Mode |
|---|---|---|---|---|---|---|
| 2.1 | 59.53 | 5009.21 | 0.181 | 3.97 | 11.88 | adhesive |
| 2.2 | 88.09 | 7450.54 | 0.462 | 3.88 | 11.82 | adhesive |
| 2.3 | 71.03 | 5680.66 | 0.380 | 3.99 | 12.50 | adhesive |
| 2.4 | 130.80 | 10,778.97 | 0.572 | 3.93 | 12.13 | adhesive |
| 2.5 | 65.33 | 5277.57 | 0.352 | 3.97 | 13.38 | adhesive |
| 2.6 | 30.22 | 2542.68 | 0.329 | 3.89 | 11.88 | adhesive |
| 2.7 | 95.84 | 7742.19 | 0.776 | 3.97 | 12.38 | adhesive |
| 2.8 | 49.26 | 4020.25 | 0.249 | 3.95 | 12.25 | cohesive |
| Material C No | Fmax N | Shear Bond Strength kPa | dL at Fmax mm | do mm | So mm | Failure Mode |
|---|---|---|---|---|---|---|
| 3.1 | 16.342 | 1800.61 | 0.029 | 3.96 | 12.23 | cohesive |
| 3.2 | 9.786 | 1600.56 | 0.491 | 3.88 | 12.99 | adhesive |
| 3.3 | 22.169 | 2401.68 | 0.261 | 3.91 | 12.11 | adhesive |
| 3.4 | 19.794 | 2134.89 | 0.537 | 3.96 | 12.09 | adhesive |
| 3.5 | 27.602 | 1965.34 | 0.248 | 3.90 | 11.99 | adhesive |
| 3.6 | 23.661 | 2012.56 | 0.239 | 3.86 | 12.88 | adhesive |
| 3.7 | 20.679 | 2289.65 | 0.219 | 3.55 | 12.33 | adhesive |
| 3.8 | 25.810 | 1589.62 | 0.431 | 3.82 | 12.11 | adhesive |
| Resin Composite | N | Mean and SD (MPa) | Group Comparison | p-Value |
|---|---|---|---|---|
| Methacrylate Z350 (3M ESPE) + GIC (Group A) | 08 | 1.484 ± 1.139 | A and B | d 0.001 |
| Ceram X (DENTSPLY) + GIC (Group B) | 08 | 6.062 ± 2.547 | A and C | e 0.824 |
| Conventional composite Spectrum (DENTSPLY) + GIC (Group C) | 08 | 1.974 ± 0.841 | B and C | d 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilgrami, A.; Maqsood, A.; Alam, M.K.; Ahmed, N.; Mustafa, M.; Alqahtani, A.R.; Alshehri, A.; Alqahtani, A.A.; Alghannam, S. Evaluation of Shear Bond Strength between Resin Composites and Conventional Glass Ionomer Cement in Class II Restorative Technique—An In Vitro Study. Materials 2022, 15, 4293. https://doi.org/10.3390/ma15124293
Bilgrami A, Maqsood A, Alam MK, Ahmed N, Mustafa M, Alqahtani AR, Alshehri A, Alqahtani AA, Alghannam S. Evaluation of Shear Bond Strength between Resin Composites and Conventional Glass Ionomer Cement in Class II Restorative Technique—An In Vitro Study. Materials. 2022; 15(12):4293. https://doi.org/10.3390/ma15124293
Chicago/Turabian StyleBilgrami, Afreen, Afsheen Maqsood, Mohammad Khursheed Alam, Naseer Ahmed, Mohammed Mustafa, Ali Robaian Alqahtani, Abdullah Alshehri, Abdullah Ali Alqahtani, and Shahad Alghannam. 2022. "Evaluation of Shear Bond Strength between Resin Composites and Conventional Glass Ionomer Cement in Class II Restorative Technique—An In Vitro Study" Materials 15, no. 12: 4293. https://doi.org/10.3390/ma15124293
APA StyleBilgrami, A., Maqsood, A., Alam, M. K., Ahmed, N., Mustafa, M., Alqahtani, A. R., Alshehri, A., Alqahtani, A. A., & Alghannam, S. (2022). Evaluation of Shear Bond Strength between Resin Composites and Conventional Glass Ionomer Cement in Class II Restorative Technique—An In Vitro Study. Materials, 15(12), 4293. https://doi.org/10.3390/ma15124293









