New Heterotrinuclear CuIILnIIICuII (Ln = Ho, Er) Compounds with the Schiff Base: Syntheses, Structural Characterization, Thermal and Magnetic Properties
Abstract
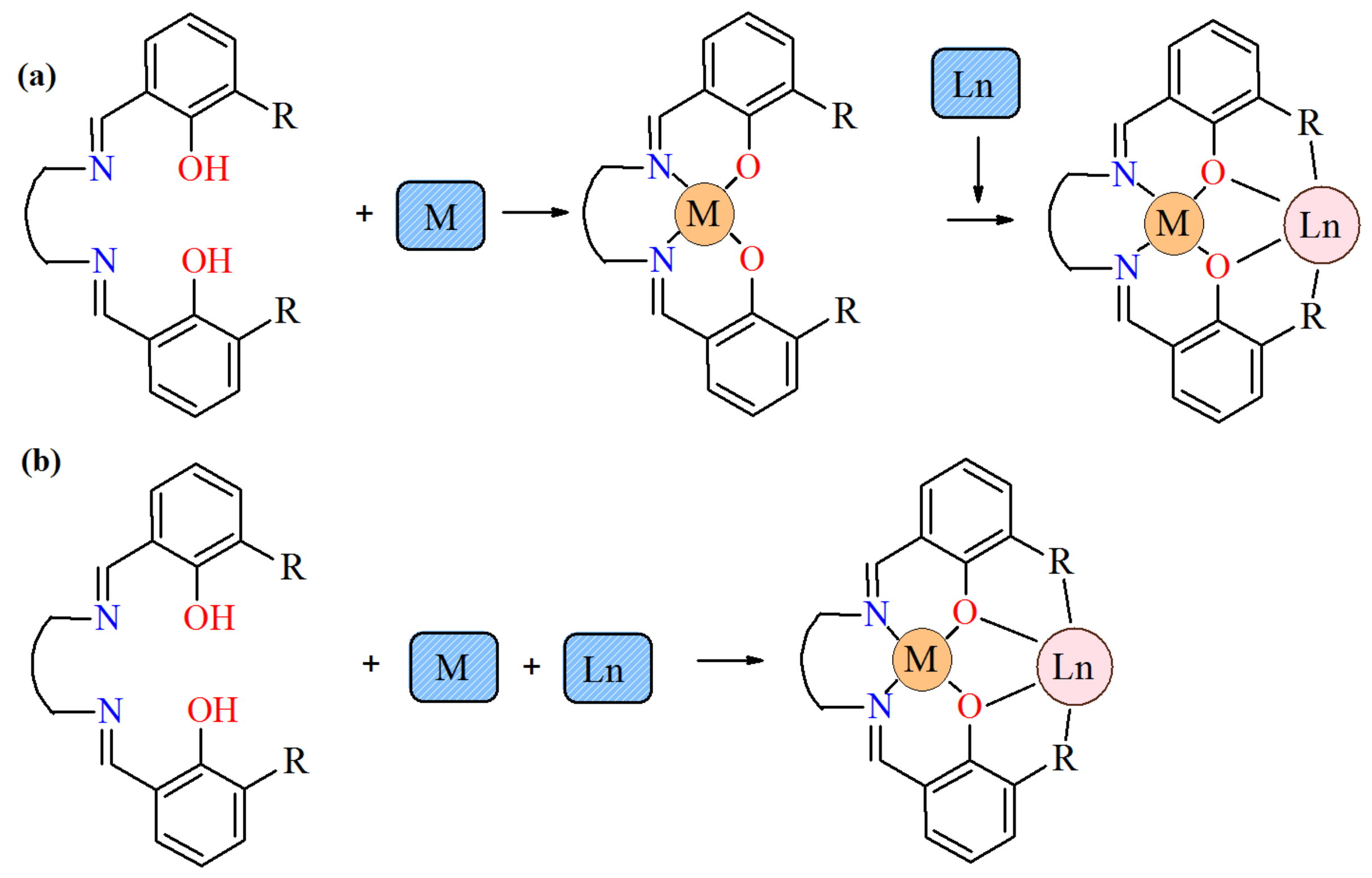
:1. Introduction
2. Materials and Methods
2.1. Materials
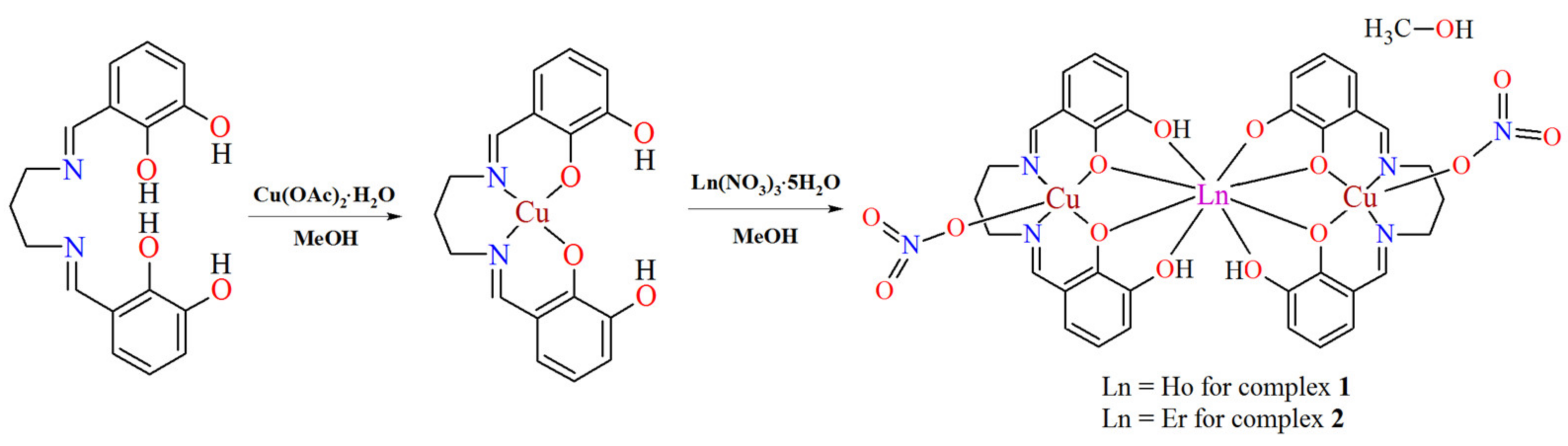
2.2. Synthesis
2.2.1. N,N’-Bis(2,3-dihydroxybenzylidene)-1,3-diaminopropane (H4L)
2.2.2. Complexes [Cu2Ln(H2L)(HL)(NO3)2]·MeOH (1, 2)
Complex [Cu2Ho(H2L)(HL)(NO3)2]·MeOH (1)
Complex [Cu2Er(H2L)(HL)(NO3)2]·MeOH (2)
2.3. Methods
X-ray Crystal Structure Determination
3. Results and Discussion
3.1. Infrared Spectra
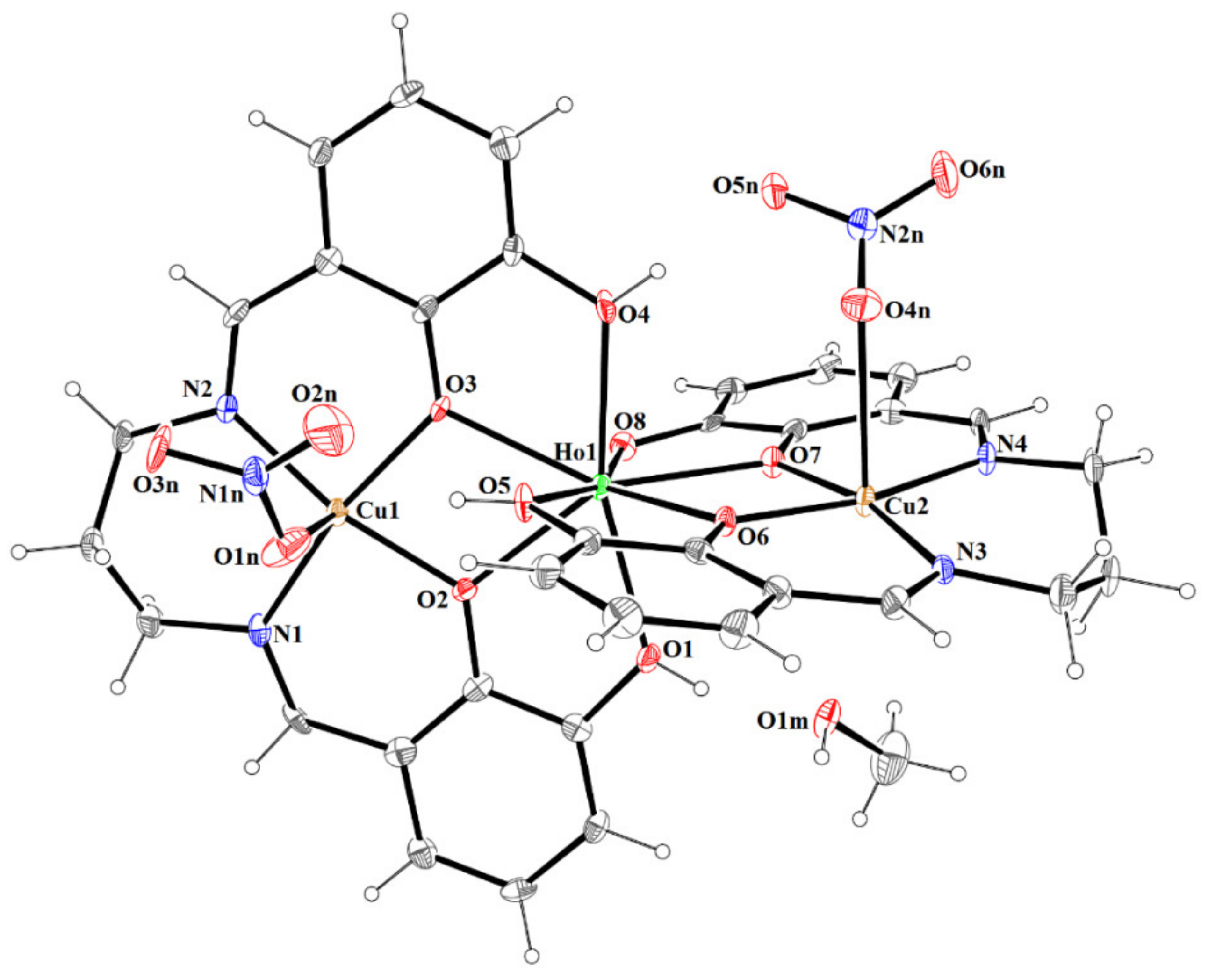
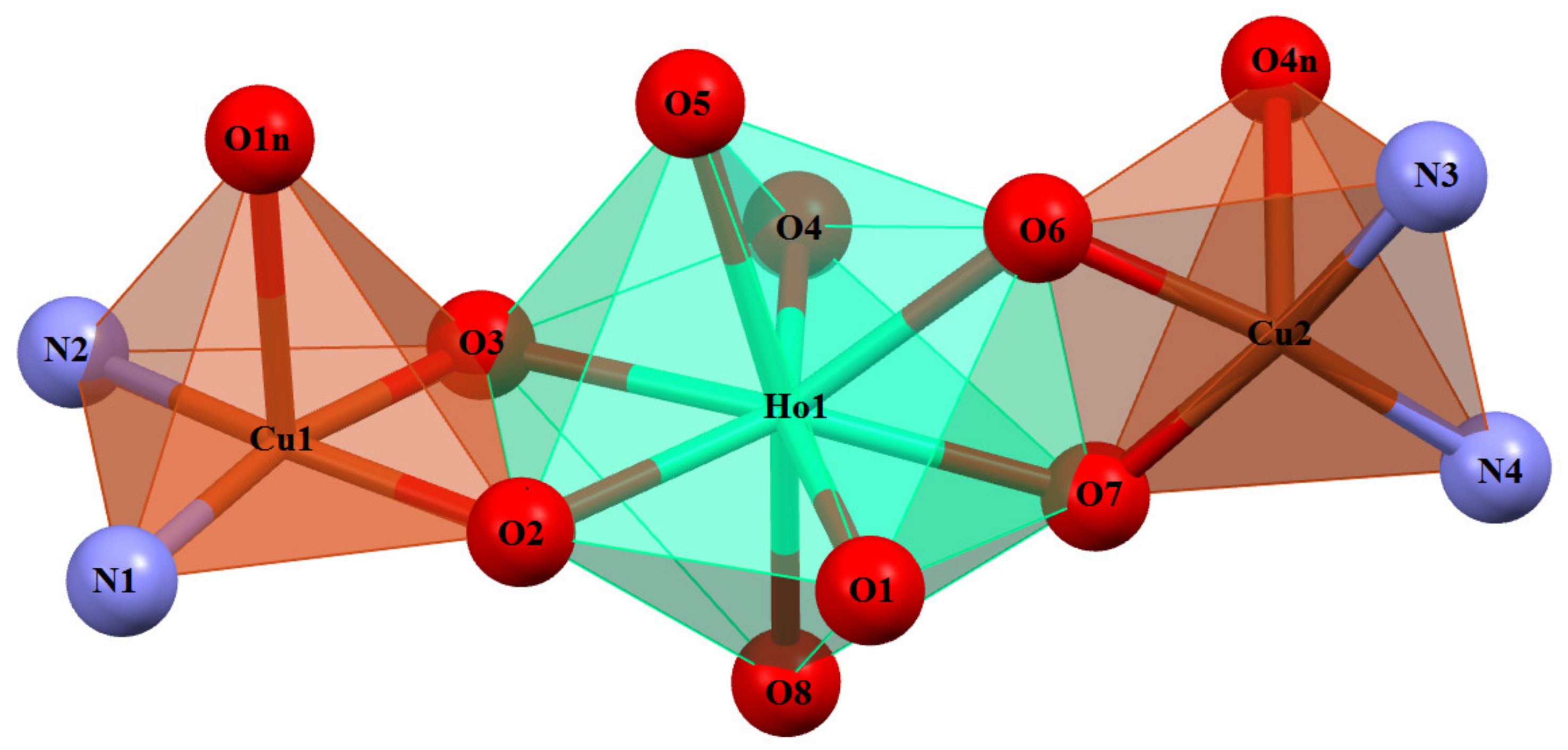
3.2. Crystal and Molecular Structure
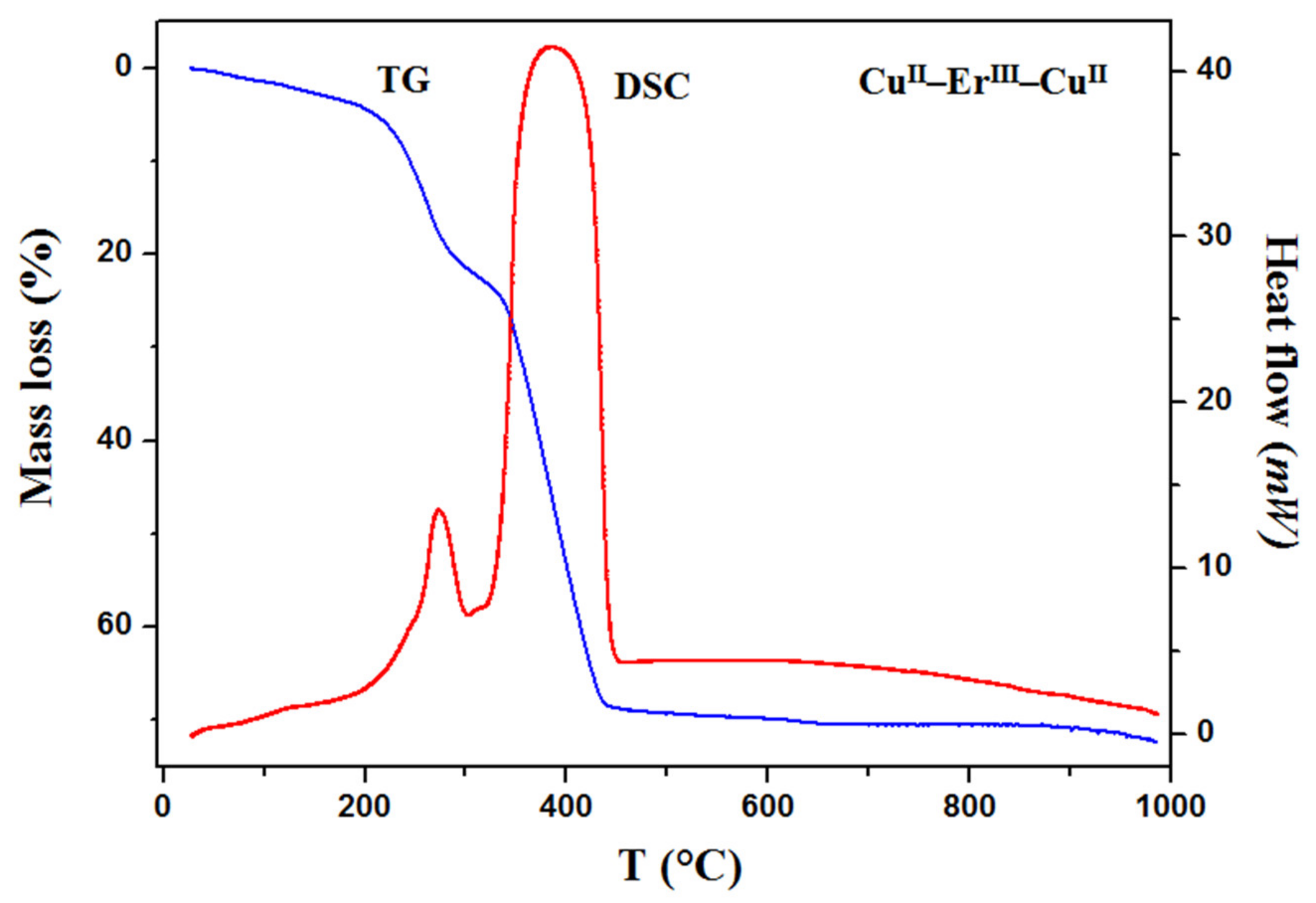
3.3. Thermal Analysis
3.4. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dey, A.; Bag, P.; Kalita, P.; Chandrasekhar, V. Heterometallic CuII–LnIII complexes: Single molecule magnets and magnetic refrigerants. Coord. Chem. Rev. 2021, 432, 213707. [Google Scholar] [CrossRef]
- Liua, K.; Shia, W.; Chenga, P. Toward heterometallic single-molecule magnets: Synthetic strategy, structures and properties of 3d–4f discrete complexes. Coord. Chem. Rev. 2015, 289–290, 74–122. [Google Scholar] [CrossRef]
- Im, S.Y.; Park, S.J.; Im, H.J.; Lee, S.W. Conversion of Ni–Nd and Ni–Tb compartment compounds into one-dimensional coordination polymers or tetranuclear dimers. Polyhedron 2016, 117, 231–243. [Google Scholar] [CrossRef]
- Buta, I.; Shova, S.; Ilies, S.; Manea, F.; Andruh, M.; Costisor, O. Mono- and oligonuclear complexes based on a o-vanillin derived Schiff-base ligand: Synthesis, crystal structures, luminescent and electrochemical properties. J. Mol. Struct. 2022, 1248, 131439. [Google Scholar] [CrossRef]
- Ishida, T.; Watanabe, R.; Fujiwara, K.; Okazawa, A.; Kojima, N.; Tanaka, G.; Yoshii, S.; Nojiri, H. Exchange coupling in TbCu and DyCu single-molecule magnets and related lanthanide and vanadium analogs. Dalton Trans. 2012, 41, 13609–13619. [Google Scholar] [CrossRef]
- Ghosh, M.; Sepay, S.; Schollmeyer, D.; Sakiyama, H.; Mikuriya, M.; Mal, D.; Gayen, A.; Seikh, M.M.; Saha, S. Spacers directed self-assembly of heterobimetallic copper(II)-lanthanide(III) [Ln = Nd and Gd] moieties: Syntheses, structural diversities and magnetic properties. Polyhedron 2022, 216, 115718. [Google Scholar] [CrossRef]
- Cristóvão, B.; Osypiuk, D.; Miroslaw, B.; Bartyzel, A. Heterometallic di- and trinuclear CuIILnIII (LnIII = La, Ce, Pr, Nd) complexes with an alcohol-functionalized compartmental Schiff base ligand: Syntheses, crystal structures, thermal and magnetic studies. Polyhedron 2020, 188, 114703. [Google Scholar] [CrossRef]
- Huang, X.-C.; Zhao, X.-H.; Shao, D.; Wang, H.-Y. Syntheses, structures, and magnetic properties of a family of end-on azido-bridged CuII–LnIII complexes. Dalton Trans. 2017, 46, 7232–7241. [Google Scholar] [CrossRef]
- Karmakar, M.; Roy, S.; Chattopadhyay, S. Synthesis, structure and properties of homo- and hetero-trinuclear complexes of salicylaldehyde-based di-Schiff bases. Polyhedron 2022, 215, 115652. [Google Scholar] [CrossRef]
- Apostol, A.A.; Mihalache, I.; Mocanu, T.; Tutunaru, O.; Pachiu, C.; Gavrila, R.; Maxim, C.; Andruh, M. Luminescent [ZnIILnIII] complexes anchored on graphene: Synthesis and crystal structures of [ZnIIEuIII] and [ZnIITbIII] complexes decorated with pyrene groups. Appl. Organomet. Chem. 2021, 35, e6126. [Google Scholar] [CrossRef]
- Wen, H.-R.; Bao, J.; Liu, S.-J.; Liu, C.-M.; Zhang, C.-W.; Tang, Y.-Z. Temperature-controlled polymorphism of chiral CuII–LnIII dinuclear complexes exhibiting slow magnetic relaxation. Dalton Trans. 2015, 44, 11191–11201. [Google Scholar] [CrossRef] [PubMed]
- Corredoira-Vázquez, J.; Fondo, M.; Sanmartín-Matalobos, J.; Taboada, P.; García-Deibe, A.M. Filling tricompartmental igands with GdIII and ZnII ions: Some structural and MRI studies. Crystals 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Kori, D.; Dote, Y.; Koikawa, M.; Yamada, Y. Syntheses, crystal structures, and solid-state photoluminescence properties of heterotrinuclear Zn2Ln (Ln: La, Sm, Eu, Tb) complexes derived from 1,4-diaminobutane-based N2O4 compartmental ligand. Polyhedron 2019, 170, 612–621. [Google Scholar] [CrossRef]
- Gheorghe, R.; Cucos, P.; Andruh, M.; Costes, J.P.; Donnadieu, B.; Shova, S. Oligonuclear 3d–4f complexes as tectons in designing supramolecular solid-state architectures: Impact of the nature of linkers on the structural diversity. Chem. Eur. J. 2006, 12, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-W.; Tian, Y.-M.; Tao, J.; Chen, P.; Li, H.-P.; Zhang, Y.-Q.; Yan, P.-F.; Sun, W.-B. Modulation of the coordination environment around the magnetic easy axis leads to significant magnetic relaxations in a series of 3d–4f Schiff complexes. Inorg. Chem. 2018, 57, 8065–8077. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Titiš, J.; Valigura, D.; Boča, R.; Mohanta, S. Bis-phenoxido and bis-acetato bridged heteronuclear {CoIIIDyIII} single molecule magnets with two slow relaxation branches. Dalton Trans. 2016, 45, 7510–7520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, M.-X.; Sui, Y.; Nfor, E.N.; Wang, Z.-X. Two 1D homochiral heterometallic chains: Crystal structures, spectra, ferroelectricity and ferromagnetic properties. RSC Adv. 2020, 10, 7004–7010. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, A.; Das, C.; Langley, S.K.; Murray, K.S.; Srivastava, A.K.; Shanmugam, M. Heteronuclear Ni(II)–Ln(III ) (Ln = La, Pr, Tb, Dy) complexes: Synthesis and single-molecule magnet behavior. Dalton Trans. 2016, 45, 3616–3626. [Google Scholar] [CrossRef]
- Gebrezgiabher, M.; Bayeh, Y.; Gebretsadik, T.; Gebreslassie, G.; Elemo, F.; Thomas, M.; Linert, W. Lanthanide-based single-molecule magnets derived from Schiff base ligands of salicylaldehyde derivatives. Inorganics 2020, 8, 66. [Google Scholar] [CrossRef]
- Andruh, M.; Ramade, I.; Codjovi, E.; Guillou, O.; Kahn, O.; Trombe, J.C. Crystal structure and magnetic properties of [Ln2Cu4] hexanuclear clusters (where Ln = trivalent lanthanide). Mechanism of the gadolinium(III)-copper(II) magnetic interaction. J. Am. Chem. Soc. 1993, 115, 1822–1829. [Google Scholar] [CrossRef]
- Zou, H.-H.; Sheng, L.-B.; Liang, F.-P.; Chena, Z.-L.; Zhang, Y.-Q. Experimental and theoretical investigations of four 3d–4f butterfly single-molecule magnets. Dalton Trans. 2015, 44, 18544–18552. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, T.; Jose, R.; Remya, P.R.; Rajaraman, G. Theoretical studies on trinuclear {MnIII2GdIII} and tetranuclear {MnIII2GdIII2} clusters: Magnetic exchange, mechanism of magnetic coupling, magnetocaloric effect, and magneto–structural correlations. Inorg. Chem. 2019, 58, 11927–11940. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Liu, X.; Tian, J.; Yan, S. Three series of heterometallic NiII–LnIII Schiff base complexes: Synthesis, crystal structures and magnetic characterization. Dalton Trans. 2017, 46, 12558. [Google Scholar] [CrossRef]
- Ahmed, N.; Das, C.; Vaidya, S.A.; Srivastava, K.; Langley, S.K.; Murra, K.S.; Shanmugam, M. Probing the magnetic and magnetothermal properties of M(II)–Ln(III) complexes (where M (II)= Ni or Zn; Ln (III)= La or Pr or Gd). Dalton Trans. 2014, 43, 17375–17384. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Beg, M.F.; Rajaraman, G. Role of magnetic exchange interactions in the magnetization relaxation of {3d–4f} single-molecule magnets: A theoretical perspective. Chem. Eur. J. 2016, 22, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Bhunia, P.; Ichihashi, K.; Ishida, T.; Ghosh, A. SMM behaviour of heterometallic dinuclear CuIILnIII (Ln = Tb and Dy) complexes derived from N2O3 donor unsymmetrical ligands. New J. Chem. 2020, 44, 6197–6205. [Google Scholar] [CrossRef]
- Fu, X.-X.; Wang, H.-L.; Zou, H.-H.; Quan, B.-H.; Li, B.; Liang, F.-P. Structures and magnetic properties of three heterobimetallic 3d–4f hexanuclear complexes. J. Clust. Sci. 2017, 28, 3229–3239. [Google Scholar] [CrossRef]
- Cristóvão, B.; Miroslaw, B. Asymmetry in propeller-like trinuclear diphenoxo-bridged CuII–LnIII–CuII (Ln = La, Pr, Nd) Schiff base complexes—Synthesis, structure and magnetic properties. J. Coord. Chem. 2015, 68, 1602–1615. [Google Scholar] [CrossRef]
- Cristóvão, B.; Pełka, R.; Miroslaw, B. A novel hexanuclear CuII4–GdIII2 cluster obtained from heterotrinuclear building blocks. Inorg. Chem. Commun. 2015, 54, 81–84. [Google Scholar] [CrossRef]
- Cristóvão, B.; Miroslaw, B. Tautomerism of a compartmental Schiff base ligand and characterization of a new heterometallic CuII–DyIII complex—Synthesis, structure and magnetic properties. Inorg. Chem. Commun. 2015, 52, 64–68. [Google Scholar] [CrossRef]
- Zeyrek, C.T.; Elmali, A.; Elerman, Y. Magnetic characterization, synthesis and crystal structure of a heterodinuclear CuIIGdIII Schiff base complex bridged by the two phenolic oxygen atoms. J. Mol. Struct. 2005, 740, 47–52. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishers Inc.: New York, NY, USA, 1993. [Google Scholar]
- Agilent Technologies Ltd. CrysAlis PRO; Agilent Technologies Ltd.: Oxfordshire, UK, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Cristóvão, B.; Kłak, J.; Miroslaw, B. Synthesis and characterization of CuII–LnIII (Ln = Ho, Tm, Yb or Lu) complexes with N2O4-donor Schiff base ligand. J. Coord. Chem. 2014, 67, 2728–2746. [Google Scholar] [CrossRef]
- Singh, B.K.; Prakash, A.; Rajour, H.K.; Bhojak, N.; Adhikari, D. Spectroscopic characterization and biological activity of Zn(II), Cd(II), Sn(II) and Pb(II) complexes with Schiff base derived from pyrrole-2-carboxaldehyde and 2-amino phenol. Spectrochim. Acta A Mol. Biomol. 2010, 76, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, B.; Osypiuk, D.; Miroslaw, B.; Bartyzel, A. Syntheses, crystal structures, thermal and magnetic properties of new heterotrinuclear CuII–LnIII–CuII complexes incorporating N2O4-donor Schiff base ligands. Polyhedron 2018, 144, 225–233. [Google Scholar] [CrossRef]
- Anitha, C.; Sheela, C.D.; Tharmaraj, P.; Sumathi, S. Spectroscopic studies and biological evaluation of some transition metal complexes of azo Schiff-base ligand derived from (1-phenyl-2,3-dimethyl-4-aminopyrazol-5-one) and 5-((4-chlorophenyl)diazenyl)-2-hydroxybenzaldehyde. Spectrochim. Acta A Mol. Biomol. 2012, 96, 493–500. [Google Scholar] [CrossRef]
- Cristóvão, B.; Hnatejko, Z. Lanthanide(III) compounds with the N2O4 Schiff base—Synthesis, spectral, thermal, magnetic and luminescence properties. J. Mol. Struct. 2015, 1088, 50–55. [Google Scholar] [CrossRef]
- Osypiuk, D.; Cristóvão, B.; Bartyzel, A. New coordination compounds of CuII with Schiff base ligands—Crystal structure, thermal, and spectral investigations. Crystals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Miroslaw, B.; Osypiuk, D.; Cristóvão, B.; Głuchowska, H. Symmetry in recognition of supramolecular synthons–competition between hydrogen bonding and coordination bond in multinuclear CuII–4f complexes with bicompartmental Schiff base ligand. Symmetry 2019, 11, 460. [Google Scholar] [CrossRef] [Green Version]
- Olea-Román, D.; Bélanger-Desmarais, N.; Flores-Álamo, M.; Bazán, C.; Thouin, F.; Reber, C.; Castillo-Blum, S.E. Spectroscopic studies of lanthanide complexes of varying nuclearity based on a compartmentalized ligand. Dalton Trans. 2015, 44, 17175–17188. [Google Scholar] [CrossRef] [Green Version]
- Datta, A.; Clegg, J.K.; Huang, J.-H.; Pevec, A.; Garribba, E.; Fondo, M. Hydroxo-bridged 1-D coordination polymer of Cu(II) incorporating with salicyladimine precursor: Spectral and temperature dependent magneto structural correlation. Inorg. Chem. Commun. 2012, 24, 216–220. [Google Scholar] [CrossRef]
- Bartyzel, A. Synthesis, thermal behaviour and some properties of CuII complexes with N,O donor Schiff bases. J. Therm. Anal. Calorim. 2018, 131, 1221–1236. [Google Scholar] [CrossRef] [Green Version]
- Zarei, L.; Asadi, Z.; Dusek, M.; Eigner, V. Homodinuclear Ni(II) and Cu(II) Schiff base complexes derived from O-vanillin with a pyrazole bridge: Preparation, crystal structures, DNA and protein (BSA) binding, DNA cleavage, molecular docking and cytotoxicity study. J. Photochem. Photobiol. A Chem. 2019, 374, 145–160. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Skrobanska, M.; Zabiszak, M.; Walesa-Chorab, M.; Kubicki, M.; Jastrzab, R. Coordination properties of N,N′-bis(5-methylsalicylidene)-2-hydroxy-1,3-propanediamine with d- and f-electron ions: Crystal structure, stability in solution, spectroscopic and spectroelectrochemical studies. RSC Adv. 2018, 8, 30994–31007. [Google Scholar] [CrossRef] [Green Version]
- Bruno, I.J.; Cole, J.C.; Kessler, M.; Luo, J.; Motherwell, W.D.S.; Purkis, L.H.; Smith, B.R.; Taylor, R.; Cooper, R.I.; Harris, S.E.; et al. Retrieval of crystallographically-derived molecular geometry information. J. Chem. Inf. Comput. Sci. 2004, 44, 2133–2144. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Senapati, T.; Dey, A.; Das, S.; Kalisz, M.; Clerac, R. Cyclo- and carbophosphazene-supported ligands for the assembly of heterometallic (Cu2+/Ca2+, Cu2+/Dy3+, Cu2+/Tb3+) complexes: Synthesis, structure, and magnetism. Inorg. Chem. 2012, 51, 2031–2038. [Google Scholar] [CrossRef]
- Kashyap, S.; Singh, U.P.; Singh, A.K.; Kumar, P.; Singh, S.P. Synthesis and structural studies of some copper-benzoate complexes. Transit. Met. Chem. 2013, 38, 573–585. [Google Scholar] [CrossRef]
- Yang, H.; Meng, Y.-X.; Tian, H.-Q.; Li, D.-C.; Zeng, S.-Y.; Song, Y.; Dou, J.-M. Investigating the effect of lanthanide radius and diamagnetic linkers on the framework of metallacrown complexes. Dalton Trans. 2020, 49, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.-M.; An, X.-X.; Liu, C.; Long, H.-T.; Zhao, L. Insights into crystal structures, supramolecular architectures and antioxidant activities of self-assembled fluorescent hetero-multinuclear [Cu (II)-Ln (III)] (Ln = La, Ce, Pr and Nd) salamo-like complexes. Appl. Organomet. Chem. 2020, 34, e5980. [Google Scholar] [CrossRef]
- Mathews, I.I.; Manohar, H. Synthesis, spectral and structural studies on metal complexes of Schiff bases involving vitamin B6 and histamine. J. Chem. Soc. Dalton Trans. 1991, 2289–2294. [Google Scholar] [CrossRef]
- Bereau, V.; Dhers, S.; Costes, J.-P.; Duhayon, C.; Sutter, J.-P. Syntheses, structures, and magnetic properties of symmetric and dissymmetric ester-functionalized 3d-4f Schiff base complexes. Eur. J. Inorg. Chem. 2018, 2018, 66–73. [Google Scholar] [CrossRef]
- Haribabu, P.; Patil, Y.P.; Hussain Reddy, K.; Nethaji, M. Synthesis, crystal structure, DNA interaction and cleavage activities of mononuclear and trinuclear copper(II) complexes. Transit. Met.Chem. 2014, 39, 167–175. [Google Scholar] [CrossRef]
- Singh, Y.P.; Patel, R.N.; Singh, Y.; Choquesillo-Lazarte, D.; Butcher, R.J. Classical hydrogen bonding and stacking of chelate rings in new copper(II) complexes. Dalton Trans. 2017, 46, 2803–2820. [Google Scholar] [CrossRef]
- Li, G.; Jones, C.A.; Grassian, V.H.; Larsen, S.C. Selective catalytic reduction of NO2 with urea in nanocrystalline NaY zeolite. J. Catal. 2005, 234, 401–413. [Google Scholar] [CrossRef]
- Blake, A.J.; Cherepanov, V.A.; Dunlop, A.A.; Grant, C.M.; Milne, P.E.Y.; Rawson, J.M.; Winpenny, R.E.P. Synthesis, Crystal structures and thermal decomposition studies of a series of copper-lanthanoid complexes of 6-methyl-2-pyridone. J. Chem. Soc. Dalton Trans. 1994, 1, 2719–2727. [Google Scholar] [CrossRef]
- Ahmed, N.; Sharma, T.; Spillecke, L.; Koo, C.; Ansari, K.U.; Tripathi, S.; Caneschi, A.; Klingeler, R.; Rajaraman, G.; Shanmugam, M. Probing the origin of ferro-/afntiferromagnetic exchange interactions in Cu(II)–4f complexes. Inorg. Chem. 2022, 61, 5572–5587. [Google Scholar] [CrossRef]
- Mahapatra, P.; Ghosh, S.; Koizumi, N.; Kanetomo, T.; Ishida, T.; Drew, M.G.B.; Ghosh, A. Structural variations in (CuL)2Ln complexes of a series of lanthanide ions with a salen-type unsymmetrical Schiff base(H2L): Dy and Tb derivatives as potential single-molecule magnets. Dalton Trans. 2017, 46, 12095. [Google Scholar] [CrossRef]
- Ghosh, S.; Gómez García, C.J.; Clemente-Juan, J.M.; Ghosh, A. Key role of size and electronic configuration on the sign and strength of the magnetic coupling in a series of Cu2Ln trimers (Ln = Ce, Gd, Tb, Dy and Er). Magnetochemistry 2016, 2, 2. [Google Scholar] [CrossRef] [Green Version]




| Compound | 1 | 2 |
|---|---|---|
| CCDC | 2165783 | 2165782 |
| Temperature K | 120(2) | 298(2) |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/c | P21/c |
| a (Å) | 8.5856(3) | 8.5422(5) |
| b (Å) | 30.3312(12) | 30.3435(15) |
| c (Å) | 14.1441(6) | 14.1234(7) |
| β (°) | 101.114(4) | 101.081(5) |
| Volume (Å3) | 3614.2(2) | 3592.5(3) |
| Z | 4 | 4 |
| Calculated density (g cm−3) | 1.970 | 1.986 |
| μ (mm−1) | 3.419 | 3.573 |
| Absorption correction | multi-scan | multi-scan |
| F(000) | 2128 | 2132 |
| Crystal size (mm) | 0.22 × 0.10 × 0.05 | 0.20 × 0.08 × 0.05 |
| θ range (°) | 2.49 to 26.37 | 2.43 to 26.37 |
| Reflections collected/unique | 17840/7403 | 17336/7613 |
| Rint | 0.0575 | 0.0648 |
| Data/restraints/parameters | 7403/3/545 | 7613/2/545 |
| GooF on F2 | 1.032 | 1.015 |
| Final R indices [I > 2σ(I)] | R1 = 0.0461, wR2 = 0.0734 | R1 = 0.0516, wR2 = 0.0837 |
| R indices (all data) | R1 = 0.0761, wR2 = 0.0833 | R1 = 0.0862, wR2 = 0.0968 |
| Largest diff. peak/hole, e Å−3 | 0.961/−0.892 | 2.001/−1.554 |
| H4L | 1 | 2 | Proposed Assignments |
|---|---|---|---|
| 3192 | ν(OH) + ν(N–H) | ||
| 2929 | 2924 | ν(OH) + ν(CHas) | |
| 1632 | 1618 | 1616 | ν(C=N) |
| 1540, 1517 | 1569 | 1570 | ν(C=C) |
| 1446 | 1467 | 1465 | ν(C=C) + ν(N–O)complex |
| 1394 | 1402 | 1404 | ν(C–H) + ν(CCC) |
| 1355 | 1365 | 1356 | δ(O–H) |
| 1285 | 1287 | ω(C–H) +δ(O–H) + ν(N–O) | |
| 1233 | 1251 | 1248 | ν(C–O) |
| 1218 | 1219 | ν(C–O) | |
| 1189 | 1168 | 1167 | δ(O–H) |
| 1126 | 1125 | 1119 | ν(C–C) + tw(C–H) |
| 1088 | 1087 | ν(C–O)methanol | |
| 1064 | 1069 | 1069 | δ(C–H) + skeletal |
| 1024 | 1024 | ν(N–O) | |
| 896 | ρ(C–H) + δ(CCC) | ||
| 865 | 863 | 863 | γ(O–H) |
| 782 | 781 | γ(C–H) + ν(N–O) | |
| 711 | 741 | 734 | γ(C–H) |
| 639 | 641 | δ(C=C) + ring deform. | |
| 614 | 615 | ring deform. | |
| 556 | 558 | ν(M–O) | |
| 538 | ν(M–N) |
| Bond Lengths (Å) | |||
|---|---|---|---|
| 1 | 2 | ||
| Cu(1)–N(1) | 1.983(5) | Cu(1)–N(1) | 1.977(5) |
| Cu(1)–N(2) | 1.978(4) | Cu(1)–N(2) | 1.976(5) |
| Cu(1)–O(1n) | 2.417(4) | Cu(1)–O(1n) | 2.409(6) |
| Cu(1)–O(2) | 1.946(3) | Cu(1)–O(2) | 1.957(4) |
| Cu(1)–O(3) | 1.952(4) | Cu(1)–O(3) | 1.944(4) |
| Cu(2)–N(3) | 1.971(4) | Cu(2)–N(3) | 1.959(5) |
| Cu(2)–N(4) | 1.971(4) | Cu(2)–N(4) | 1.976(5) |
| Cu(2)–O(6) | 1.929(3) | Cu(2)–O(6) | 1.913(4) |
| Cu(2)–O(7) | 1.920(4) | Cu(2)–O(7) | 1.932(4) |
| Cu(2)–O(4n) | 2.644(4) | Cu(2)–O(4n) | 2.649(5) |
| Ho(1)–O(1) | 2.360(4) | Er(1)–O(1) | 2.340(4) |
| Ho(1)–O(2) | 2.320(4) | Er(1)–O(2) | 2.303(4) |
| Ho(1)–O(3) | 2.316(3) | Er(1)–O(3) | 2.319(4) |
| Ho(1)–O(4) | 2.355(3) | Er(1)–O(4) | 2.347(4) |
| Ho(1)–O(5) | 2.439(4) | Er(1)–O(5) | 2.221(5) |
| Ho(1)–O(6) | 2.343(4) | Er(1)–O(6) | 2.304(4) |
| Ho(1)–O(7) | 2.308(4) | Er(1)–O(7) | 2.325(4) |
| Ho(1)–O(8) | 2.242(3) | Er(1)–O(8) | 2.409(5) |
| Ho(1)–Cu(1) | 3.4977(8) | Er(1)–Cu(1) | 3.4871(7) |
| Ho(1)–Cu(2) | 3.4939(8) | Er(1)–Cu(2) | 3.4831(7) |
| Cu(1)–N(1) | 1.978(5) | ||
| Angles(°) | |||
| Cu(1)–O(2)–Ho(1) | 109.83(14) | Cu(1)–O(2)–Er(1) | 109.65(19) |
| Cu(1)–O(3)–Ho(1) | 109.76(15) | Cu(1)–O(3)–Er(1) | 109.48(18) |
| O(2)–Cu(1)–O(3) | 76.98(14) | O(2)–Cu(1)–O(3) | 77.08(17) |
| Cu(2)–O(6)–Ho(1) | 109.36(15) | Cu(2)–O(6)–Er(1) | 111.0(2) |
| Cu(2)–O(7)–Ho(1) | 111.13(15) | Cu(2)–O(7)–Er(1) | 109.47(19) |
| O(6)–Ho(1)–O(7) | 62.17(12) | O(6)–Er(1)–O(7) | 62.28(15) |
| O(6)–Cu(2)–O(7) | 77.20(15) | O(6)–Cu(2)–O(7) | 77.04(17) |
| O(2)–Ho(1)–O(3) | 63.12(12) | O(2)–Er(1)–O(3) | 63.45(14) |
| φa | 5.10 | φc | 5.23 |
| φb | 3.40 | φd | 3.84 |
| Refcode | Cu-Onitrate Bond Length [Å] | Reference |
|---|---|---|
| AZIROS | 2.626(4) | [28] |
| AZIRIM | 2.646(4) | [28] |
| CERZAD | 2.785(4) | [40] |
| CAWYEG | 2.833(7) | [51] |
| DIFTET | 2.759(4) | [52] |
| FUBTEE | 2.714(5) | [53] |
| HUYYOS | 2.741(3) | [54] |
| KOCMOE | 2.643(2) | [55] |
| MESBAQ | 2.718(1) | [56] |
| MESBEU | 2.734(6) | [56] |
| MIWLEL | 2.762(4) | [57] |
| SAJTAB | 2.701(2) | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristóvão, B.; Osypiuk, D.; Bartyzel, A. New Heterotrinuclear CuIILnIIICuII (Ln = Ho, Er) Compounds with the Schiff Base: Syntheses, Structural Characterization, Thermal and Magnetic Properties. Materials 2022, 15, 4299. https://doi.org/10.3390/ma15124299
Cristóvão B, Osypiuk D, Bartyzel A. New Heterotrinuclear CuIILnIIICuII (Ln = Ho, Er) Compounds with the Schiff Base: Syntheses, Structural Characterization, Thermal and Magnetic Properties. Materials. 2022; 15(12):4299. https://doi.org/10.3390/ma15124299
Chicago/Turabian StyleCristóvão, Beata, Dariusz Osypiuk, and Agata Bartyzel. 2022. "New Heterotrinuclear CuIILnIIICuII (Ln = Ho, Er) Compounds with the Schiff Base: Syntheses, Structural Characterization, Thermal and Magnetic Properties" Materials 15, no. 12: 4299. https://doi.org/10.3390/ma15124299
APA StyleCristóvão, B., Osypiuk, D., & Bartyzel, A. (2022). New Heterotrinuclear CuIILnIIICuII (Ln = Ho, Er) Compounds with the Schiff Base: Syntheses, Structural Characterization, Thermal and Magnetic Properties. Materials, 15(12), 4299. https://doi.org/10.3390/ma15124299








