Evaluation of Sediments from the River Drava and Their Potential for Further Use in the Building Sector
Abstract
:1. Introduction
2. Materials and Methods
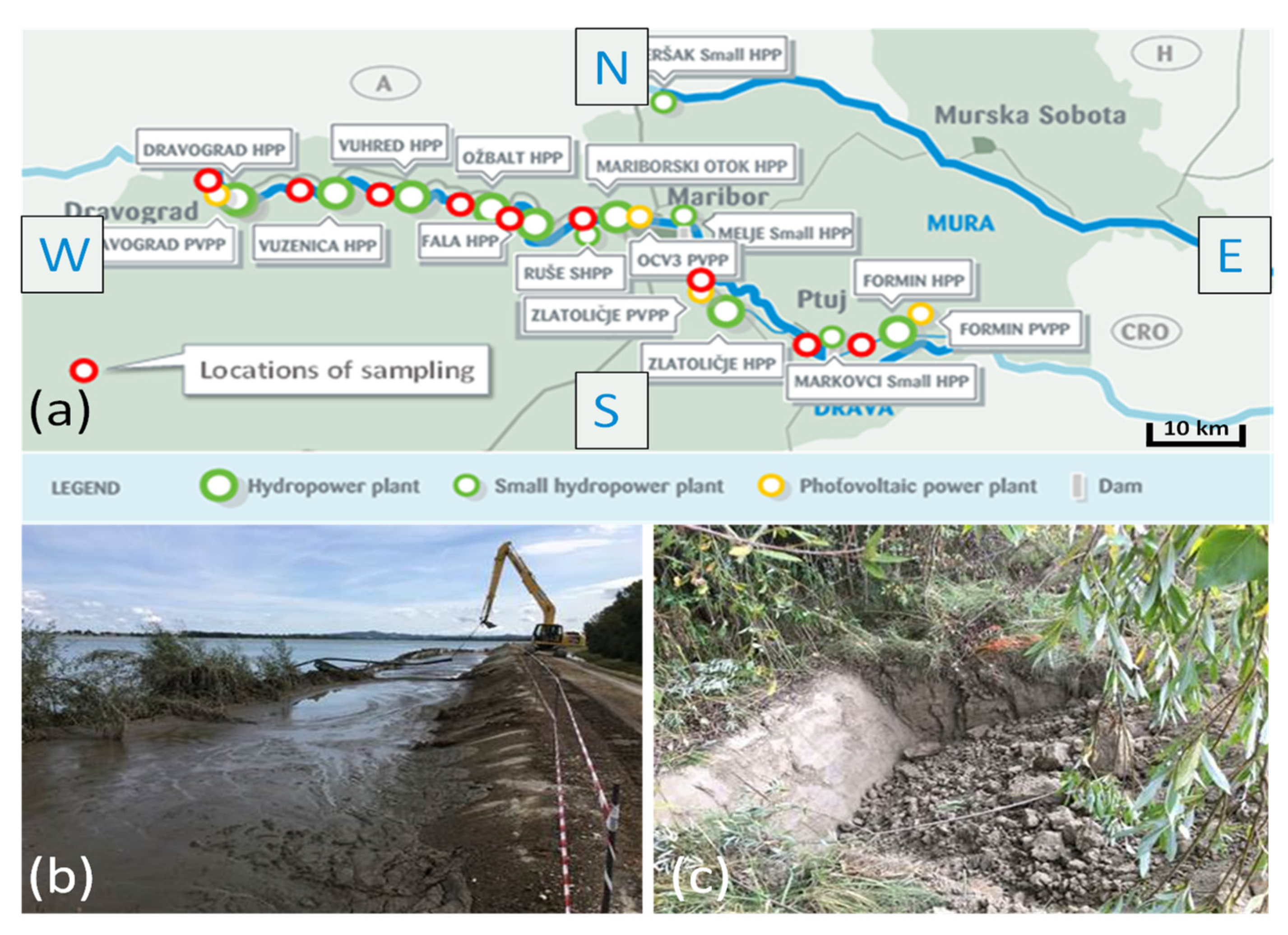
2.1. Sampling of Sediments
2.2. Analysis for Further Use
2.2.1. Determining the Contents of Water and Loss on Ignition
2.2.2. Determining the Pb, Zn, and Cd Content
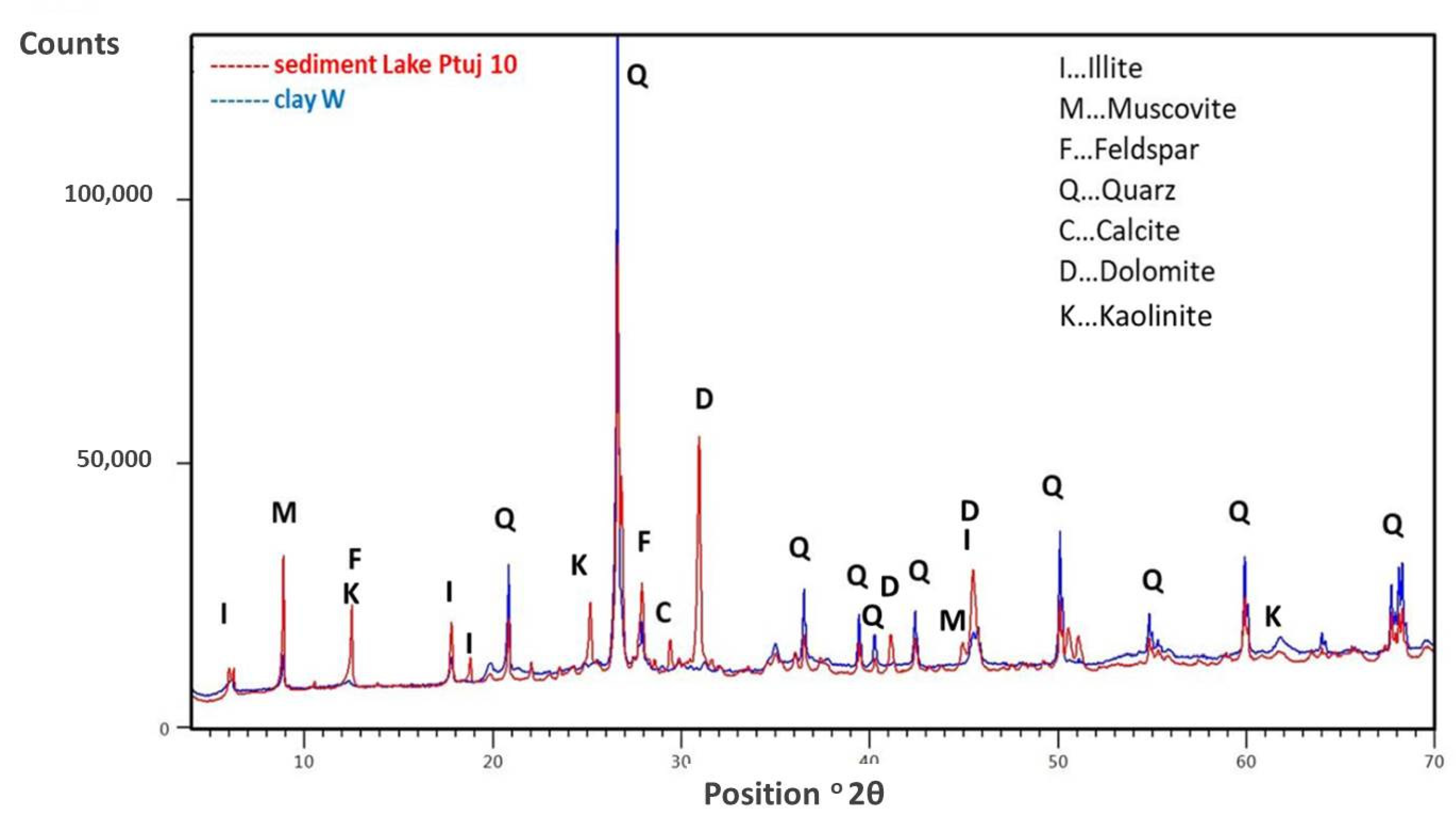
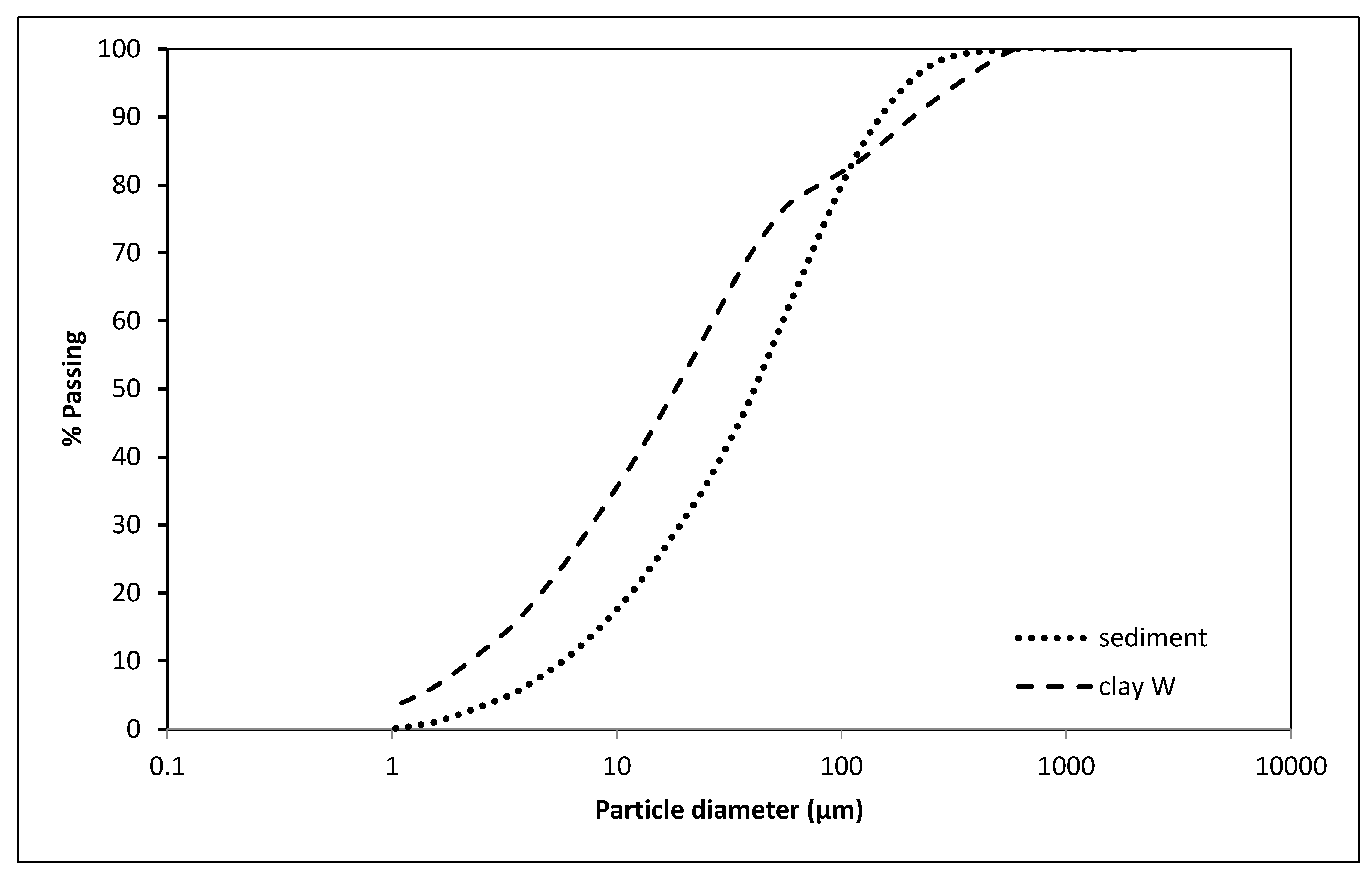
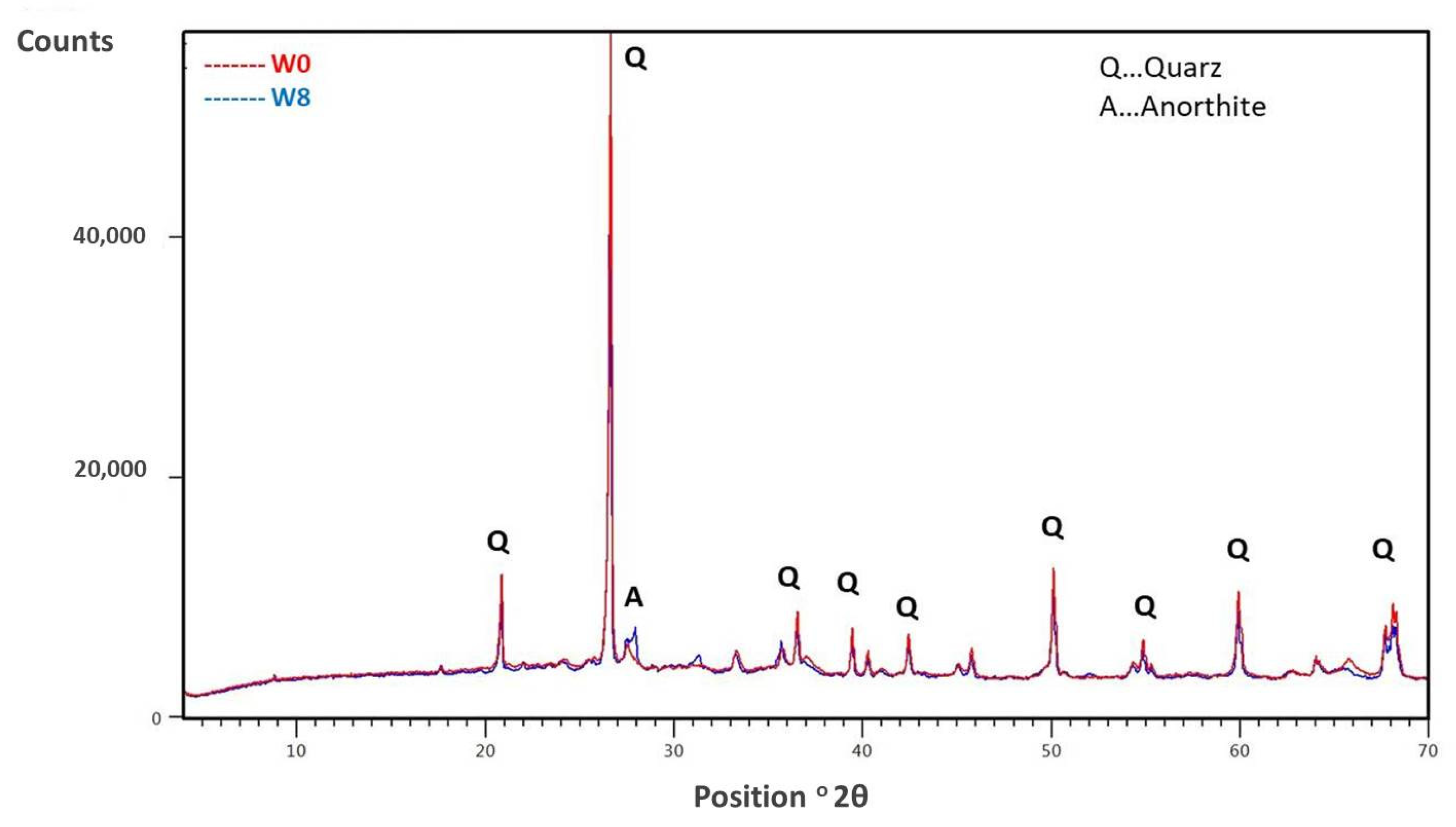
2.2.3. Chemical, Mineralogical, and Total Organic Compounds (TOC) Analysis of the Sediment and Clay
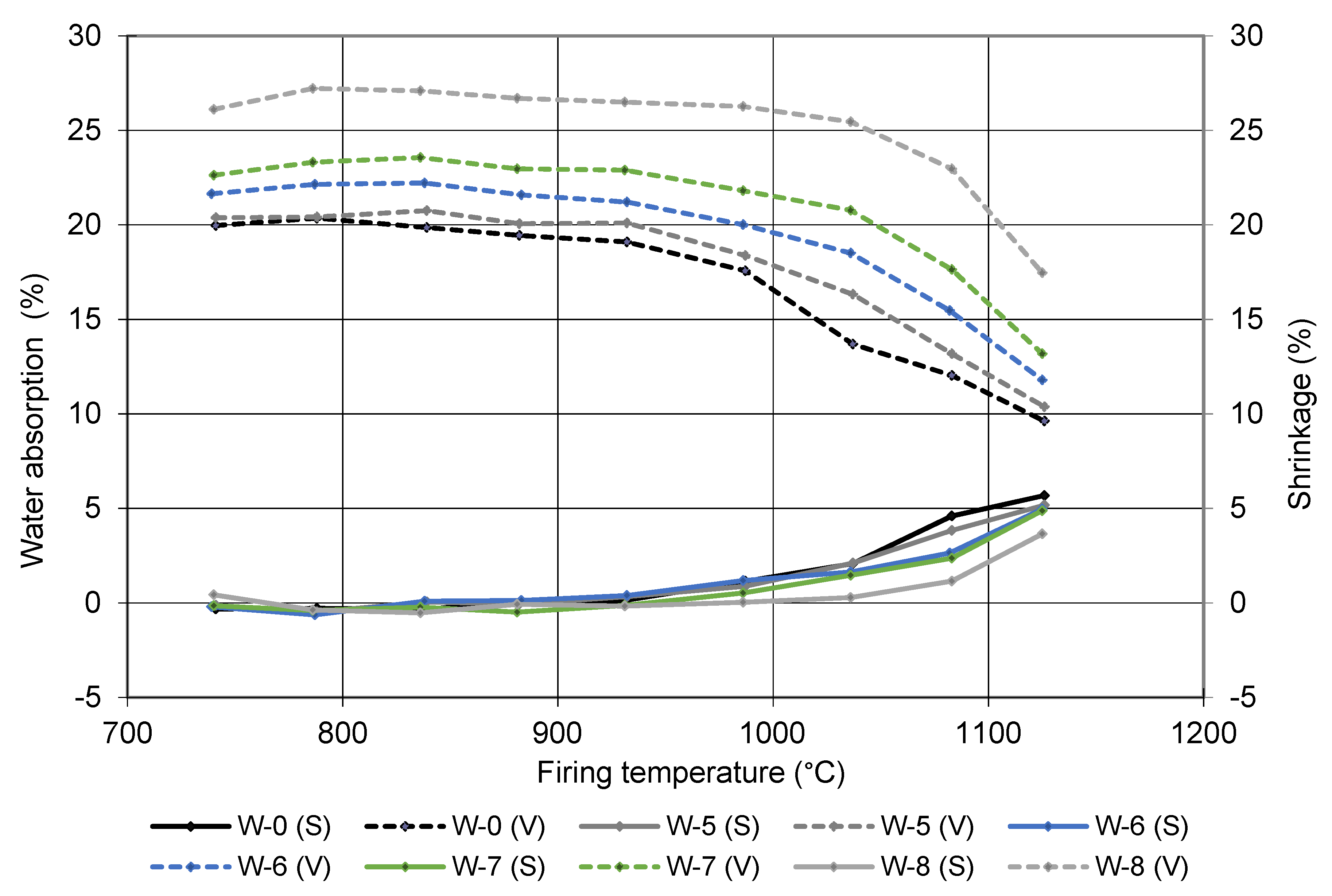
2.2.4. Firing Shrinkage, Density, Water Absorption, Bending Strength, and Compressive Strength of Fired Brick-Sediment Samples
2.2.5. Leaching Test in Water
2.2.6. Weather Resistance and Frost Resistance
3. Results and Discussion
3.1. Analysis of Potentially Harmful Substances in the Sediments
3.1.1. Water and Organic Matter Content
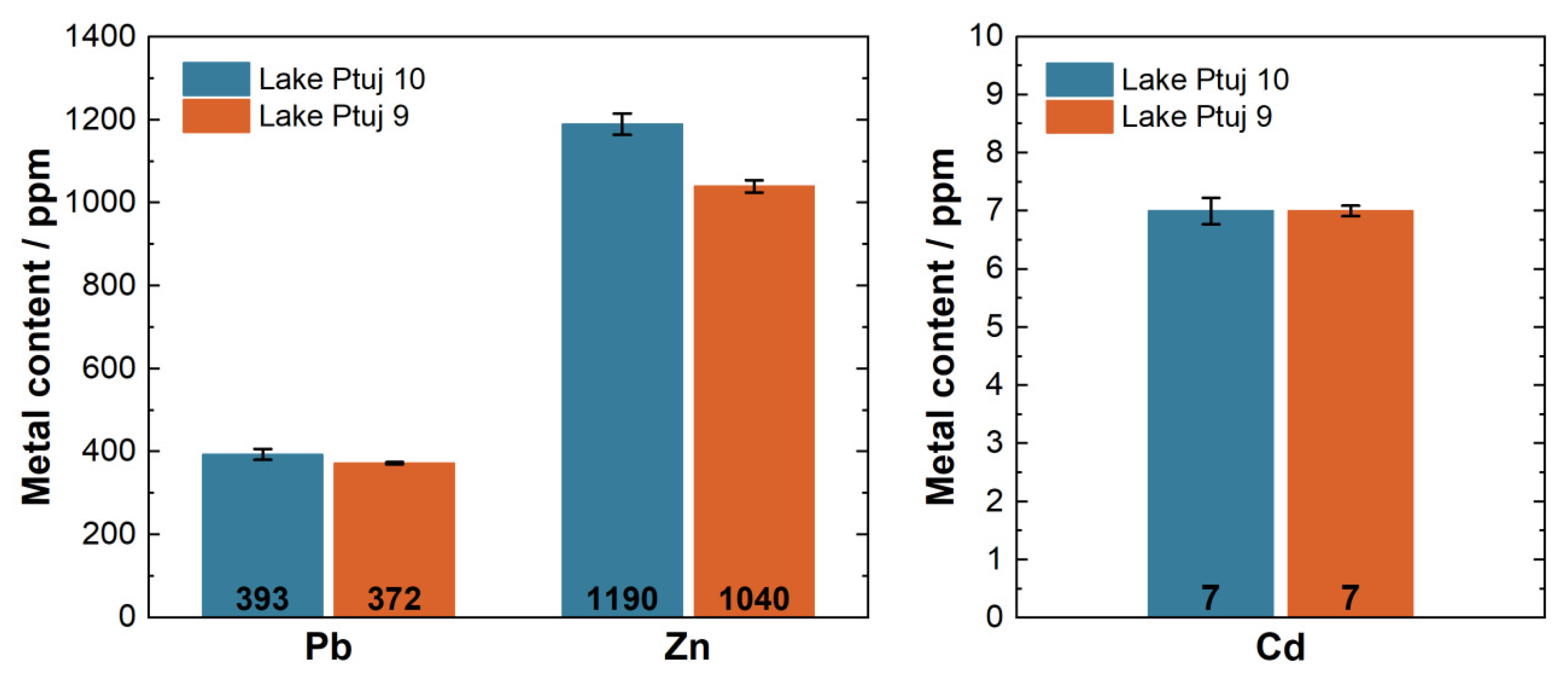
3.1.2. Pb, Zn, and Cd in the Lake Ptuj 9 and Lake Ptuj 10 Samples
3.2. Evaluating the Potential Use of Sediments for the Production of Clay Bricks
- -
- The clay content,
- -
- The free silica and carbonate content,
- -
- The particle size distribution,
- -
- The moisture content per wet mass at moulding (plasticity),
- -
- The shrinkage on drying, and
- -
- The properties after firing.
3.3. Evaluating the Potential Use of Sediment for Geotechnical Purposes
3.3.1. Geomechanical Properties of the Sediment
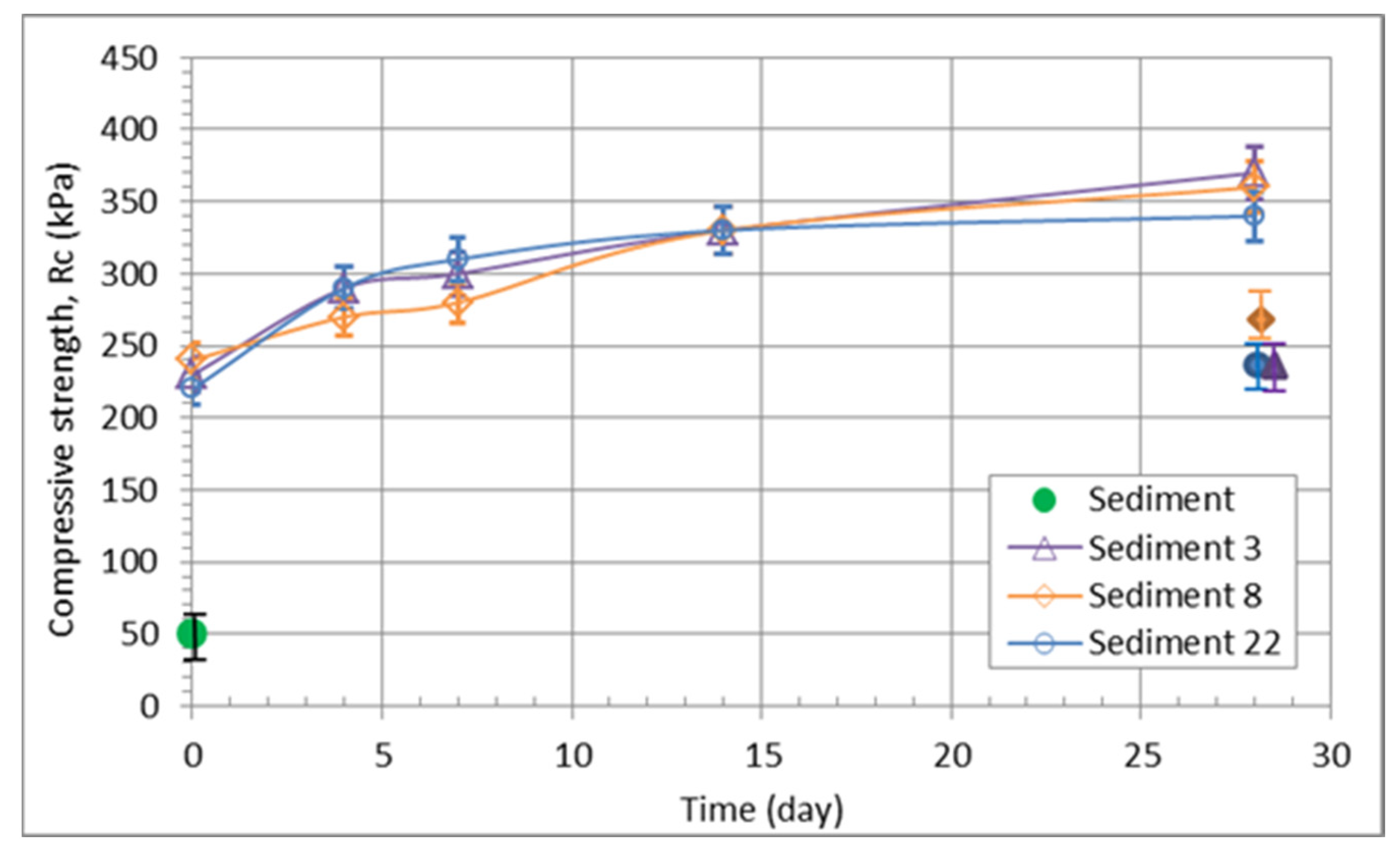
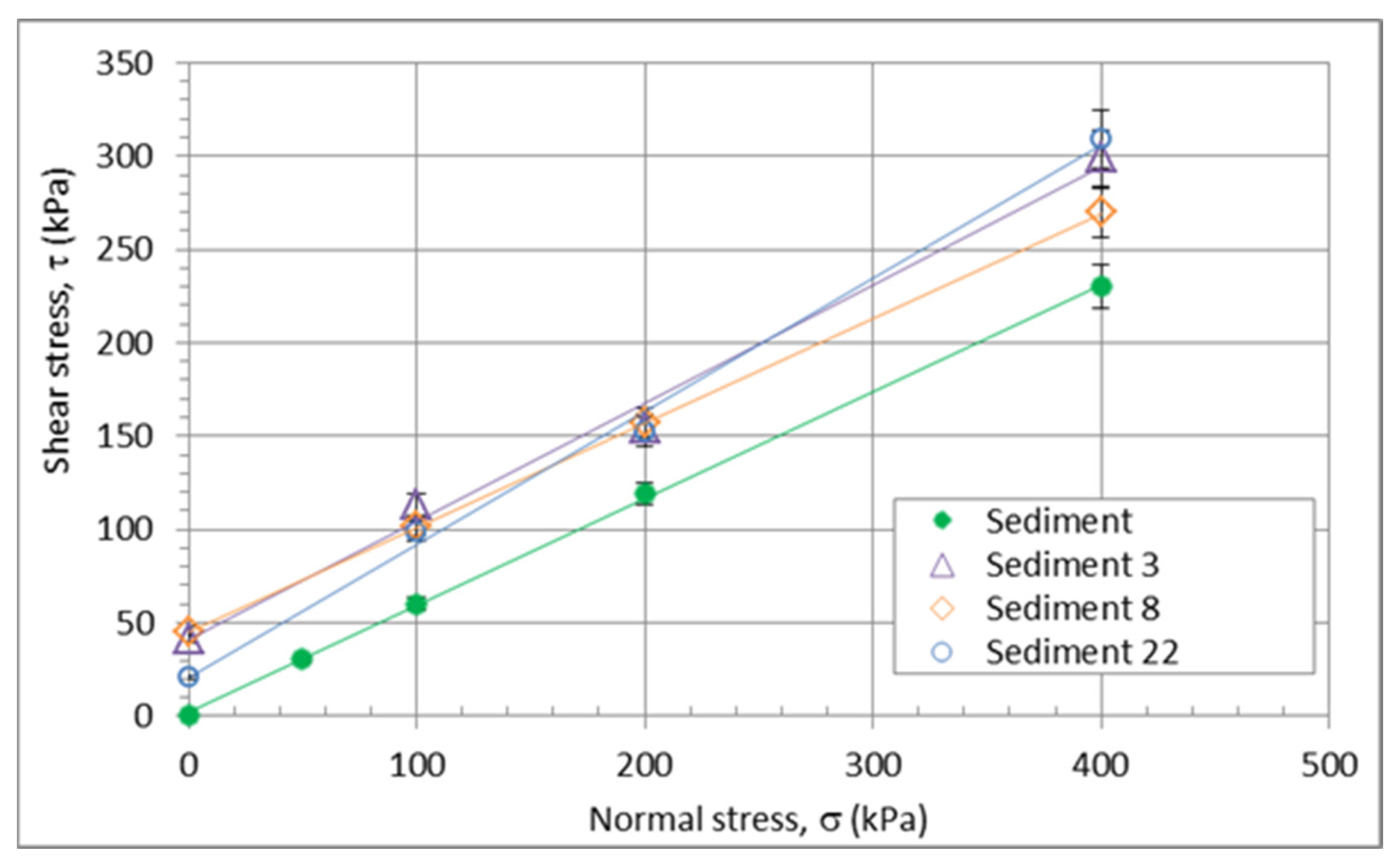
3.3.2. Geomechanical Properties of the Sediment/Quicklime Mixture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basson, G.R. Sedimentation and Sustainable Use of Reservoirs and River Systems. Draft Icold Bulletin; International Commission on Large Dams (ICOLD): Paris, France, 2009; 187p. [Google Scholar]
- Li, Y.; Liao, X.; Li, W. Combined sieving and washing of multi-metal-contaminated soils using remediation equipment: A pilot-scale demonstration. J. Clean. Prod. 2019, 212, 81–89. [Google Scholar] [CrossRef]
- Jurkovič, J.; Muhić-Šarac, T.; Kolar, M. Chemical characterisation of acid mine drain-age from an abandoned gold mine site. In YISAC 2014, Proceedings of the 21st Young Investigators’ Seminar on Analytical Chemistry, Pardubice, Czech Republic, 25–28 June 2014; Metelka, R., Ed.; University of Pardubice: Pardubice, Czech Republic, 2014; Volume 108, pp. s165–s170. [Google Scholar]
- Jurković, J.; Babajić, E.; Muhič-Šarac, T.; Kolar, M.; Kazlagić, A. Gold, silver and iron in iron oxy-hydroxide precipitate formed in process of acid mine drainage. J. Min. Environ. 2020, 11, 335–346. [Google Scholar] [CrossRef]
- Šajn, R.; Halamić, J.; Peh, Z.; Galović, L.; Alijagić, J. Assessment of the natural and anthropogenic sources of chemical elements in alluvial soils from the Drava River using multivariate statistical methods. J. Geochem. Explor. 2011, 110, 278–289. [Google Scholar] [CrossRef]
- Gosar, M.; Miler, M. Anthropogenic metal loads and their sources in stream sediments of the Meža River catchment area (NE Slovenia). Appl. Geochem. 2011, 26, 1855–1866. [Google Scholar] [CrossRef]
- Miler, M.; Gosar, M. Characteristics and potential environmental influences of mine waste in the area of the closed Mežica Pb–Zn mine (Slovenia). J. Geochem. Explor. 2012, 112, 152–160. [Google Scholar] [CrossRef]
- Osseo-Asare, K. Semiconductor electrochemistry and hydrometallurgical dissolution processes. Hydrometallurgy 1992, 29, 61–90. [Google Scholar] [CrossRef]
- Courtin-Nomade, A.; Waltzing, T.; Evrard, C.; Soubrand, M.; Lenain, J.F.; Ducloux, E.; Ghorbel, S.; Grosbois, C.; Bril, H. Arsenic and lead mobility: From tailing materials to the aqueous compartment. Appl. Geochem. 2016, 64, 10–21. [Google Scholar] [CrossRef]
- Farrow, L.A.; Graedel, T.E.; Leygraf, C. Gildes model studies of aqueous chemistry. II. The corrosion of zinc in gaseous exposure chambers. Corros. Sci. 1996, 38, 2181–2199. [Google Scholar] [CrossRef]
- Huš, S.; Kolar, M.; Kranjc, P. Separation of heavy metals from water by functionalized glycidyl methacrylate poly (high internal phase emulsions). J. Chromatogr. A 2016, 1437, 168–175. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. An evaluation of technologies for the heavy metal remediation of dredged sediments. J. Hazard. Mater. 2001, 85, 145–163. [Google Scholar] [CrossRef]
- Singh, A.; Prasad, S.M. Remediation of Heavy Metal Contaminated Ecosystem: An Overview on Technology Advancement. Int. J. Environ. Sci. Technol. 2015, 12, 353–366. [Google Scholar] [CrossRef]
- Akcil, A.; Erust, C.; Ozdemiroglu, S.; Fonti, V.; Beolchini, F. A Review of Approaches and Techniques Used in Aquatic Contaminated Sediments: Metal Removal and Stabilization by Chemical and Biotechnological Processes. J. Clean. Prod. 2015, 86, 24–36. [Google Scholar] [CrossRef]
- Lasheen, M.; Ammar, N. Ex Situ Remediation Technology for Heavy Metals in Contaminated Sediment. Desalin. Water Treat. 2016, 57, 827–834. [Google Scholar] [CrossRef]
- Peng, J.-F.; Song, Y.-H.; Yuan, P.; Cui, X.-Y.; Qiu, G.-L. The Remediation of Heavy Metals Contaminated Sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef]
- Ma, T.; Sheng, Y.; Mwng, Y.; Sun, J. Multistage remediation of heavy metal contaminated river sediments in a mining region based on particle size. Chemosphere 2019, 225, 83–92. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil Washing for Metal Removal: A Review of Physical/Chemical Technologies and Field Applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef]
- Ducman, V.; Kopar, T. The influence of different waste additions to clay-product mixtures = Vpliv različnih odpadkov na izhodno surovino za proizvodnjo opečnih izdelkov. Mater. Tehnol. 2007, 41, 289–293. [Google Scholar]
- Baksa, P.; Cepak, F.; Kovačič Lukman, R.; Ducman, V. An evaluation of marine sediments in terms of their usability in the brick industry: Case study Port of Koper. J. Sustain. Dev. Energy Water Environ. Syst. 2017, 6, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Hamer, K.; Karius, V. Brick production with dredged harbour sediments. An industrial-scale experiment. Waste Manag. 2002, 22, 521–530. [Google Scholar] [CrossRef]
- Chiang, K.Y.; Chien, K.L.; Hwang, S.J. Study on the characteristics of building bricks produced from reservoir sediment. J. Hazard. Mater. 2008, 159, 499–504. [Google Scholar] [CrossRef]
- Samara, M.; Lafhaj, Z.; Chapiseau, C. Valorization of stabilized river sediments in fired clay bricks: Factory scale experiment. J. Hazard. Mater. 2009, 163, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Cappuyns, V.; Deweirt, V.; Rousseau, S. Dredged sediments as a resource for brick production: Possibilities and barriers from a consumers’ perspective. Waste Manag. 2015, 38, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mymrin, V.; Stella, J.C.; Scremim, C.B.; Pan, R.C.; Sanches, F.G.; Alekseev, K.; Pedroso, D.E.; Molinetti, A.; Fortini, O.M. Utilization of sediments dredged from marine ports as a principal component of composite material. J. Clean. Prod. 2017, 142, 4041–4049. [Google Scholar] [CrossRef]
- Karius, V.; Hamer, K. pH and grain-size variation in leaching tests with bricks made of harbour sediments compared to commercial bricks. Sci. Total Environ. 2001, 278, 73–85. [Google Scholar] [CrossRef]
- Tajudin, S.A.A.; Azmi, M.A.M.; Nabila, A.T.A. Stabilization/Solidification Remediation Method for Contaminated Soil: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2016, 136, 012043. [Google Scholar] [CrossRef] [Green Version]
- Aldaood, A.; Bouasker, M.; Al-Mukhtar, M. Impact of wetting–drying cycles on the microstructure and mechanics. Eng. Geol. 2014, 174, 11–21. [Google Scholar] [CrossRef]
- Chew, S.; Kamruzzaman, A.; Lee, F. Physicochemical and engineering behavior of cement treated clays. J. Geotech. Geoenviron. Eng. 2004, 130, 696–706. [Google Scholar] [CrossRef]
- SIST EN 15936:2012; Sludge, Treated Biowaste, Soil and Waste-Determination of Total Organic Carbon (TOC) by Dry Combustion. European Committee for Standardization: Brussels, Belgium, 2012; p. 28.
- SIST EN 1744-3:2002; Tests for Chemical Properties of Aggregates-Part 3: Preparation of Eluates by Leaching of Aggregates. European Committee for Standardization: Brussels, Belgium, 2002; p. 24.
- Decree on Waste Regulation (Annex 5). Available online: http://www.pisrs.si/Pis.web/pregledPredpisa?id=URED7011 (accessed on 8 September 2021).
- Decree on Soil Pollution by Waste Input. Available online: http://www.pisrs.si/Pis.web/pregledPredpisa?id=URED4791 (accessed on 7 September 2021).
- Vogt, S. Methods of evaluation for raw material suitability and body optimization (Part 2). Ziegelind. Int.-Brick Tile Ind. Int. 2015, 68, 21–27. [Google Scholar]
- Elgamouz, A.; Tijani, N.; Shehadi, I.; Hasan, K.; Al-Farooq Kawam, M. Characterization of the firing behaviour of an illite-kaolinite clay mineral and its potential use as membrane support. Heliyon 2019, 5, e02281. [Google Scholar] [CrossRef] [Green Version]
- Rathossi, C.; Pontikes, Y. Effect of firing temperature and atmosphere on ceramics made of NW Peloponnese clay sediments: Part II. Chemistry of pyrometamorphic minerals and comparison with ancient ceramics. J. Eur. Ceram. Soc. 2010, 30, 1853–1866. [Google Scholar] [CrossRef]
- SIST EN ISO 17892-1; Geotechnical Investigation and Testing-Laboratory Testing of Soil-Part 1: Determination of Water Content. European Committee for Standardization: Brussels, Belgium, 2014; p. 22.
- SIST EN ISO 17892-3; Geotechnical Investigation and Testing-Laboratory Testing of Soil-Part 3: Determination of Particle Density. European Committee for Standardization: Brussels, Belgium, 2016; p. 24.
- SIST EN ISO 17892-12; Geotechnical Investigation and Testing-Laboratory Testing of Soil-Part 12: Determination of Liquid and Plastic Limits. European Committee for Standardization: Brussels, Belgium, 2018; p. 40.
- SIST EN ISO 17892-4; Geotechnical Investigation and Testing-Laboratory Testing of Soil-Part 4: Determination of Particle Size Distribution. European Committee for Standardization: Brussels, Belgium, 2017; p. 44.
- SIST EN ISO 14688-2; Geotechnical Investigation and Testing-Identification and Classification of soil-Part 2: Principles for a Classification. European Committee for Standardization: Brussels, Belgium, 2018; p. 123.
- SIST EN 13286-2; Unbound and Hydraulically Bound Mixtures-Part 2: Test Methods for Laboratory Reference Density and Water Content-Proctor Compaction. European Committee for Standardization: Brussels, Belgium, 2010; p. 44.
- SIST EN ISO 17892-7; Geotechnical Investigation and Testing-Laboratory Testing of Soil-Part 7: Unconfined Compression Test. European Committee for Standardization: Brussels, Belgium, 2018; p. 22.
- SIST EN ISO 17892-5; Geotechnical Investigation and Testing-Laboratory Testing of soil-Part 5: Incremental Loading Oedometer Test. European Committee for Standardization: Brussels, Belgium, 2017; p. 38.
- SIST EN ISO 17892-10; Geotechnical Investigation and Testing-Laboratory Testing of Soil-Part 10: Direct Shear Tests. European Committee for Standardization: Brussels, Belgium, 2019; p. 36.
- Ghobadi, M.H.; Babazadeh, R.; Abdilor, Y. Utilization of lime for Stabilizing Marly Soils and Investigating the Effect of pH Variations on shear Strength Parameters. J. Eng. Geol. 2014, 8, 1939–1962. [Google Scholar]
- Nguyen, T.T.M.; Rabbanifar, S.; Brake, N.A.; Qian, Q.; Kibodeaux, K.; Crochet, H.E.; Oruji, S.; Whitt, R.; Farrow, J.; Belaire, B.; et al. Stabilization of Silty Clayey Dredged Material. J. Mater. Civ. Eng. 2018, 30, 04018199. [Google Scholar] [CrossRef]
- Wang, D.; Zentar, R. Shear Strength Behavior of Cement/Lime-Solidified Dunkirk Sediments by Fall Cone Tests and Vane Shear Tests. Geotech. Test. J. 2013, 36, 155–162. [Google Scholar] [CrossRef]
- Furlan, A.P.; Razakamanantsoa, A.; Ranaivomanana, H.; Amiri, O.; Levacher, D.; Deneele, D. Effect of Fly Ash on microstructural and resistance characteristics of dredged sediment stabilized with lime and cement. Constr. Build. Mater. 2021, 272. [Google Scholar] [CrossRef]
- Silitonga, E.; Levacher, D.; Mezazigh, S. Utilization of fly ash for stabilization of marine dredged sediments. Eur. J. Environ. Civ. Eng. 2010, 14, 253–265. [Google Scholar] [CrossRef]
- TSPI PG 05.200:2021; Earthworks-Classification of Geological Materials in Earthworks-Part 1-Classification of Soils. Ministry of Infrastructure: Ljubljana, Slovenia, 2021. (In Slovenian)


| Sample | Water (%) | RSD Water (%) | LOI at 950 °C (wt%) | RSD LOI (%) |
|---|---|---|---|---|
| Dravograd 1 | 29.2 | 3.3 | 12.3 | 2.8 |
| Dravograd 2 | 29.3 | 1.7 | 12.2 | 1.9 |
| Vuhred 1 | 27.2 | 8.1 | 10.8 | 13.0 |
| Vuhred 2 | 21.8 | 13.8 | 6.2 | 16.8 |
| Melje 1 | 56.9 | 4.6 | 6.8 | 2.9 |
| Melje 2 | 30.9 | 13.9 | 7.9 | 9.8 |
| Čolnarna 1 | 24.8 | 2.7 | 9.3 | 5.5 |
| Čolnarna 2 | 23.8 | 5.4 | 8.3 | 3.0 |
| Mariborski otok 1 | 27.6 | 1.9 | 13.4 | 1.3 |
| Mariborski otok 2 | 28.2 | 1.0 | 11.2 | 0.7 |
| Ožbalt 1 | 67.1 | 0.4 | 7.0 | 7.6 |
| Ožbalt 2 | 71.3 | 0.6 | 7.0 | 1.8 |
| Lake Ptuj fresh | 42.1 | 1.9 | 8.5 | 0.4 |
| Lake Ptuj 1 | 35.5 | 0.5 | 10.0 | 2.1 |
| Lake Ptuj 2 | 29.8 | 5.8 | 10.8 | 2.2 |
| Lake Ptuj 3 | 32.9 | 1.1 | 10.7 | 0.8 |
| Lake Ptuj 4 | 27.7 | 2.2 | 10.8 | 2.1 |
| Lake Ptuj 5 | 33.5 | 5.5 | 10.4 | 2.2 |
| Lake Ptuj 6 | 30.8 | 3.0 | 11.2 | 0.9 |
| Lake Ptuj 7 | 30.5 | 9.5 | 11.1 | 1.1 |
| Lake Ptuj 8 | 32.9 | 5.9 | 10.8 | 2.9 |
| Lake Ptuj 9 | 11.5 | 3.8 | 12.4 | 1.4 |
| Lake Ptuj 10 | 30.4 | 1.7 | 10.7 | 1.3 |
| W0 | Lake Ptuj 10 | |
|---|---|---|
| Na2O | 0.85 | 1.46 |
| MgO | 1.21 | 6.33 |
| Al2O3 | 20.22 | 18.74 |
| SiO2 | 66.30 | 53.28 |
| P2O5 | 0.09 | 0.28 |
| SO3 | 0.00 | 0.58 |
| K2O | 2.14 | 2.73 |
| CaO | 0.54 | 8.46 |
| TiO2 | 1.22 | 0.94 |
| V2O5 | 0.03 | 0.03 |
| Cr2O3 | 0.01 | 0.02 |
| MnO | 0.10 | 0.10 |
| Fe2O3 | 6.93 | 6.52 |
| ZnO | 0.01 | 0.19 |
| As2O3 | 0.10 | 0.11 |
| Rb2O | 0.02 | 0.02 |
| SrO | 0.01 | 0.03 |
| ZrO2 | 0.07 | 0.04 |
| others | 0.22 | 0.18 |
| TOC | 1.8 | 3.4 |
| LOI at 550 °C | 9.1 | 7.2 |
| LOI at 950 °C | 10.9 | 15.8 |
| Designation | Addition of Sediment from Lake Ptuj 10 (wt%) | Shrinkage after Drying (%) | Shrinkage after Firing (%) | Density (g/cm³) | Water Absorption (%) | Bending Strength (MPa) | Compressive Strength (MPa) |
|---|---|---|---|---|---|---|---|
| W-0 | 0 | 8.5 | 1.3 | 1.76 | 17.2 | 11.2 | 33 |
| W-5 | 10 | 8.9 | 1.1 | 1.73 | 18.1 | 13.7 | 31 |
| W-6 | 20 | 9.2 | 0.6 | 1.69 | 19.9 | 13.6 | 35 |
| W-7 | 30 | 9.2 | 0.4 | 1.66 | 21.4 | 10.7 | 32 |
| W-8 | 50 | 9.2 | 0.0 | 1.52 | 25.1 | 7.1 | 25 |
| Zn (ppb) | Cd (ppb) | Pb (ppb) | |
|---|---|---|---|
| W-0 | 0.52 | 0.04 | <0.01 |
| W-8 | 1.27 | 0.03 | 0.35 |
| legal limit | 35.0 | 0.25 | 3.5 |
| Property | Standard | Value |
|---|---|---|
| Initial Moisture Content (w) (wt%) 1 | SIST EN ISO 17892-1 [37] | 42–60 |
| Specific Gravity (γs) (Mg/m3) | SIST EN ISO 17892-3 [38] | 2.69–2.70 |
| Liquid Limit (wL) (%) | SIST EN ISO 17892-12 [39] | 49.1–69.0 |
| Plastic Limit (wP) (%) | SIST EN ISO 17892-12 [39] | 41.9–49.0 |
| Consistency Index (Ic) (-) | SIST EN ISO 17892-12 [39] | 0.4–1.6 |
| Particle Size Distribution: | ||
| Particle (<2.0 mm) (%) | SIST EN ISO 17892-4 [40] | 90–100 |
| Particle (<0.063 mm) (%) | SIST EN ISO 17892-4 [40] | 53.9–61.4 |
| Particle (<0.002 mm) (%) | SIST EN ISO 17892-4 [40] | 3.8–4.4 |
| Classification | SIST EN ISO 14688-2 [41] | mSi–hSi |
| Optimum Water Content—Standard Proctor Test (wopt) (%) | SIST EN 13286-2 [42] | 29.5 |
| Maximum Dry Density—Standard Proctor Test (ρd,max) (Mg/m3) | SIST EN 13286-2 [42] | 1.33 |
| Unconfined Composite Strength After Compaction (qu) (MPa) | SIST EN ISO 17892-7 [43] | 0.05 |
| Eodometer Modulus (MPa) | SIST EN ISO 17982-5 [44] | 2.55 |
| Shear Resistance: | ||
| Friction Angle (f’) (°) | SIST EN ISO 17892-10 [45] | 30.5 |
| Cohesion (c’) (kPa) | SIST EN ISO 17892-10 [46] | 0.6 |
| Mixture Designation | Average Moisture Content of the Sediment w, (wt%) | Quicklime Content (wt%) * | Optimal Water Content Standard Proctor Test wopt (wt%) | Maximum Dry Density—Standard Proctor Test ρd,max (mg/m3) |
|---|---|---|---|---|
| Sediment | 60 | 0 | 29.5 | 1.33 |
| Sediment 3 | 33 | 3 | 27.1 | 1.42 |
| Sediment 8 | 43 | 8 | 24.4 | 1.52 |
| Sediment 22 | 53 | 22 | 26.3 | 1.43 |
| Mixture Designation | Unconfined Compressive Strength, Rc (MPa) | Shear Strength | Weather Resistance | Frost Resistance | Oedometer Modulus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 days | After 4 days | After 7 days | After 14 days | After 28 days | Saturated, 28 days | Friction angle, f’ (°) | Cohesion, c’ (kPa) | R (-) | - | Eoed,200 kPa (MPa) | |
| Sediment | 0.05 | 30.5 | 0.6 | - | - | 2.55 | |||||
| Sediment 3 | 0.23 | 0.29 | 0.30 | 0.33 | 0.37 | 0.21 | 32.5 | 41 | 0.86 | middle to high | 11.2 |
| Sediment 8 | 0.24 | 0.27 | 0.28 | 0.33 | 0.36 | 0.24 | 29.0 | 45 | 0.85 | middle to high | 12.5 |
| Sediment 22 | 0.22 | 0.29 | 0.31 | 0.33 | 0.34 | 0.21 | 35.5 | 21 | 0.78 | middle to high | 13.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ducman, V.; Bizjak, K.F.; Likar, B.; Kolar, M.; Robba, A.; Imperl, J.; Božič, M.; Gregorc, B. Evaluation of Sediments from the River Drava and Their Potential for Further Use in the Building Sector. Materials 2022, 15, 4303. https://doi.org/10.3390/ma15124303
Ducman V, Bizjak KF, Likar B, Kolar M, Robba A, Imperl J, Božič M, Gregorc B. Evaluation of Sediments from the River Drava and Their Potential for Further Use in the Building Sector. Materials. 2022; 15(12):4303. https://doi.org/10.3390/ma15124303
Chicago/Turabian StyleDucman, Vilma, Karmen Fifer Bizjak, Barbara Likar, Mitja Kolar, Ana Robba, Jernej Imperl, Mojca Božič, and Boštjan Gregorc. 2022. "Evaluation of Sediments from the River Drava and Their Potential for Further Use in the Building Sector" Materials 15, no. 12: 4303. https://doi.org/10.3390/ma15124303
APA StyleDucman, V., Bizjak, K. F., Likar, B., Kolar, M., Robba, A., Imperl, J., Božič, M., & Gregorc, B. (2022). Evaluation of Sediments from the River Drava and Their Potential for Further Use in the Building Sector. Materials, 15(12), 4303. https://doi.org/10.3390/ma15124303








