Preparation and Performance of Ternesite–Ye’elimite Cement
Abstract
1. Introduction
2. Experiment
2.1. Raw Materials
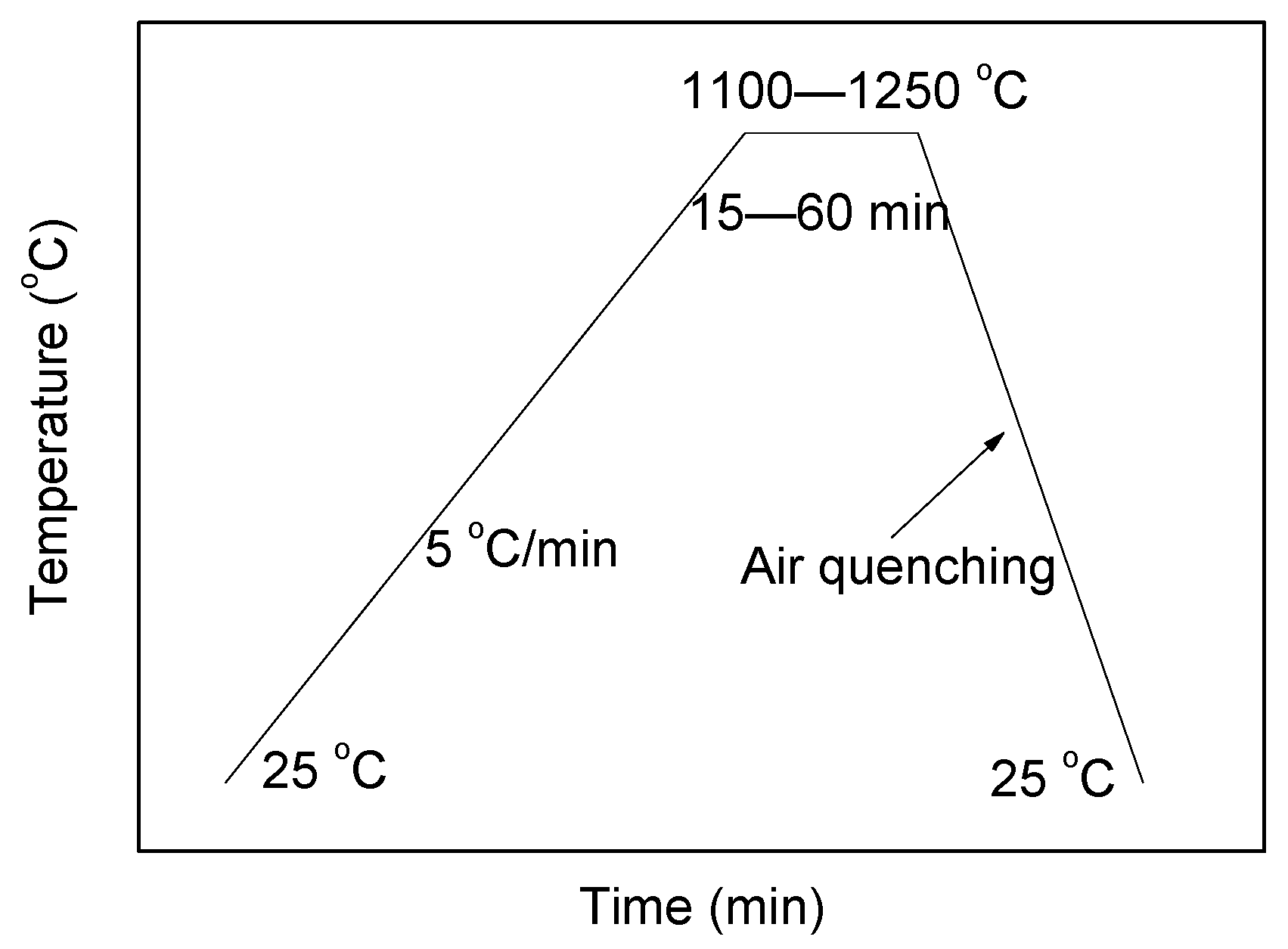
2.2. Synthesis of the TCSA Cement Clinkers
2.3. Testing Methods
3. Results and Discussion
3.1. Synthesis of the TCSA Cement Clinkers
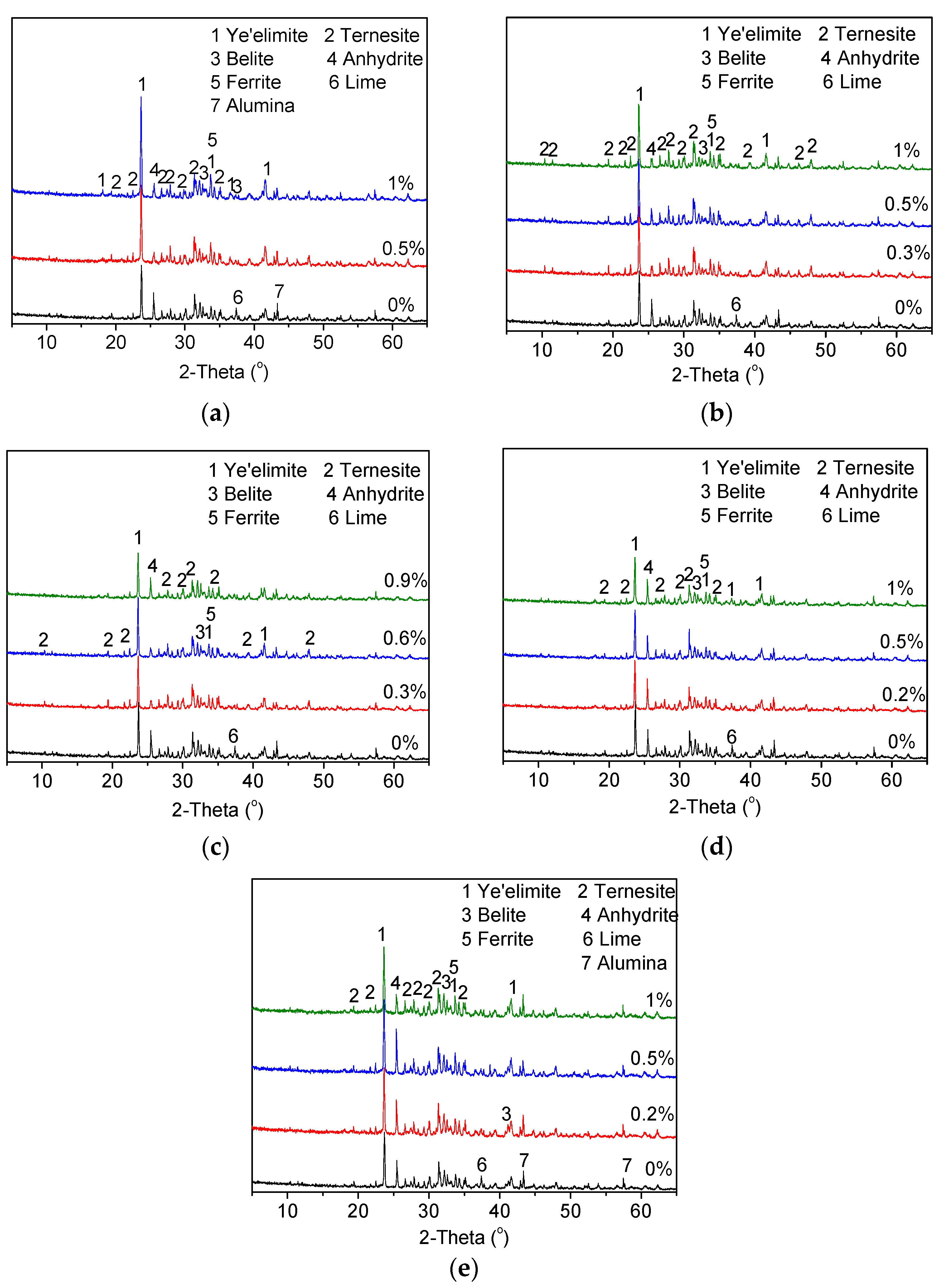
3.1.1. Influence of Dopants on the Coexistence of Ternesite and Ye’elimite
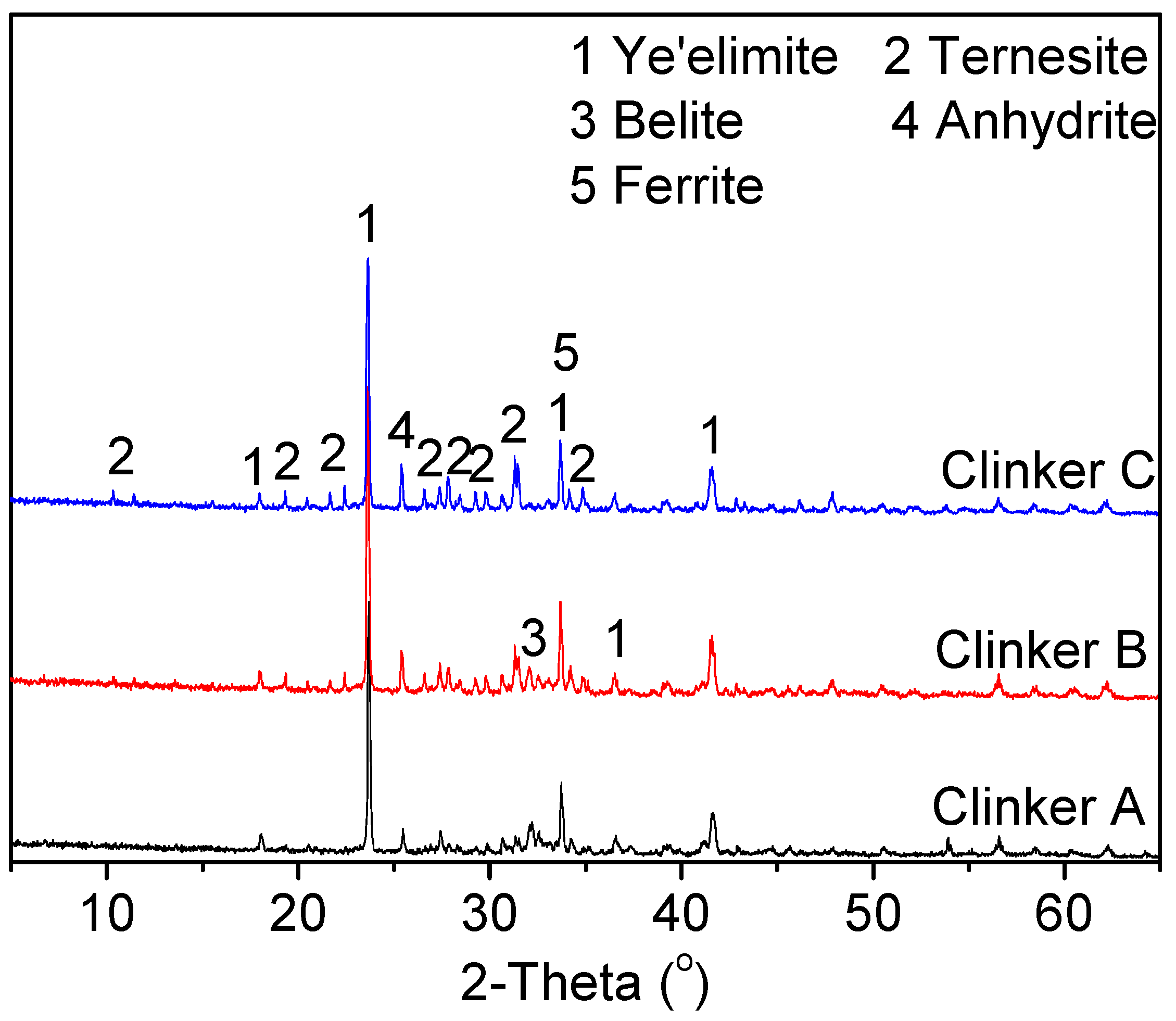
3.1.2. Influence of the Gypsum Content in the Raw Mixes on the Clinker Composition
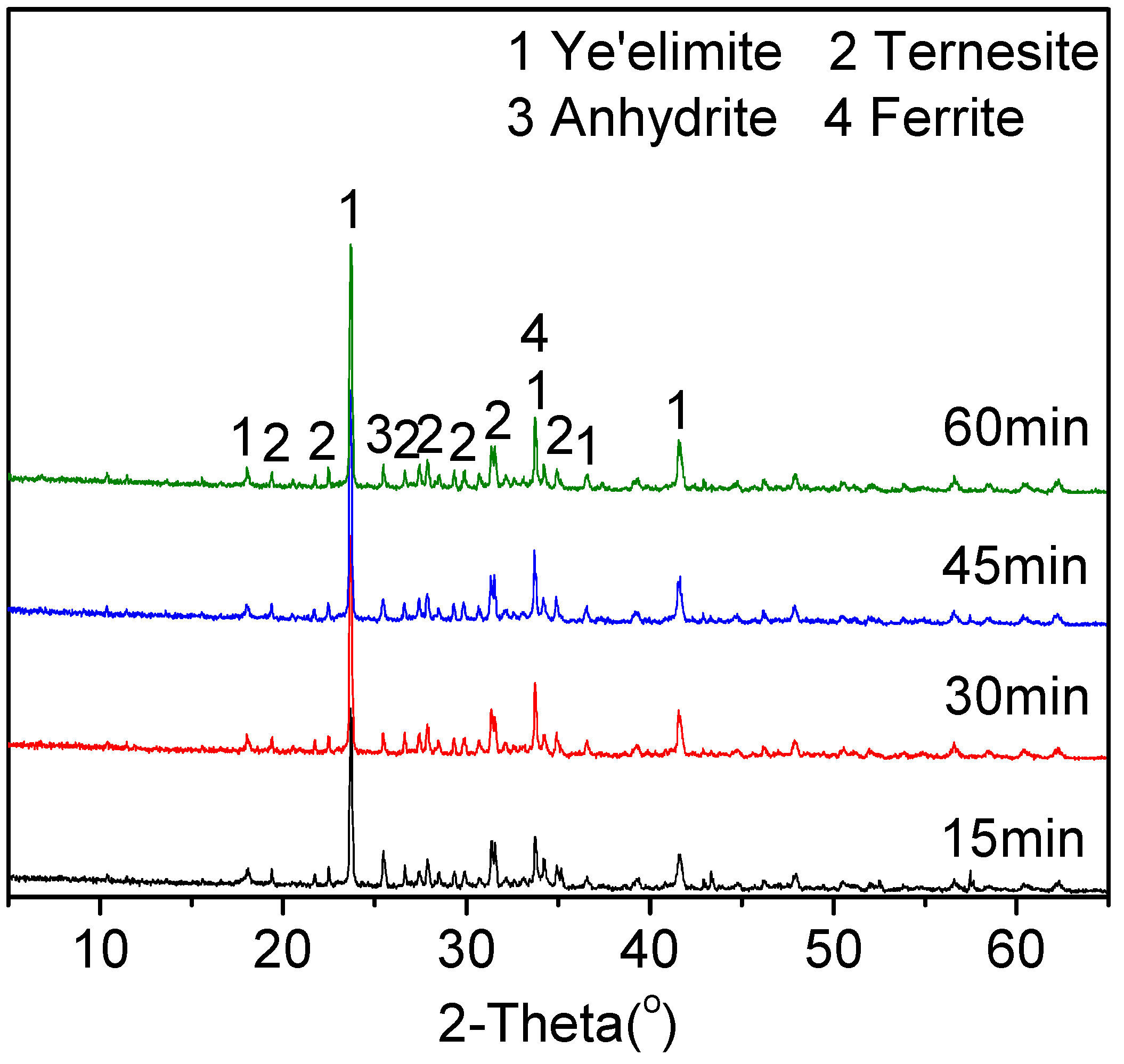
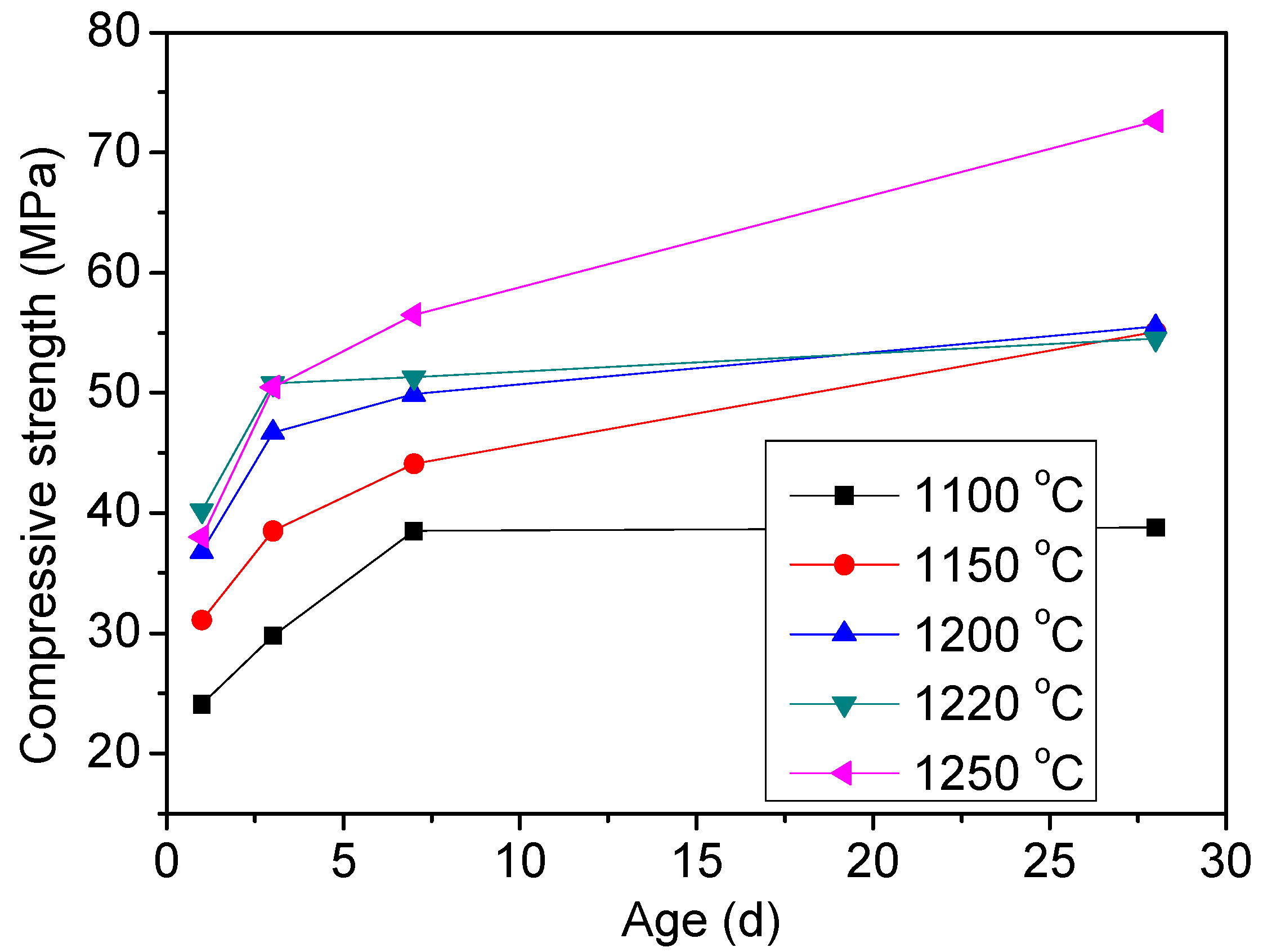
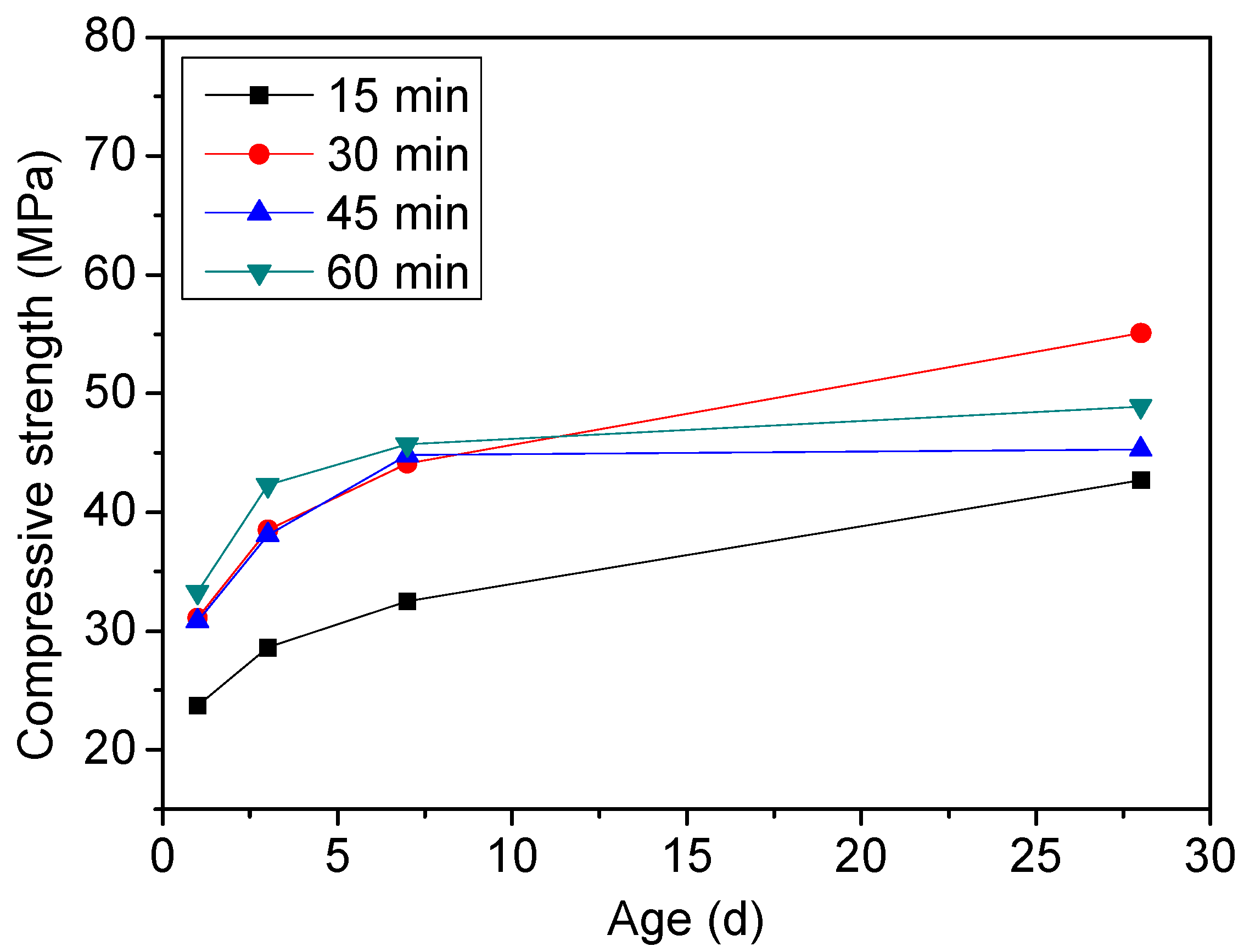
3.1.3. Influence of the Burning Conditions on the Clinker Composition
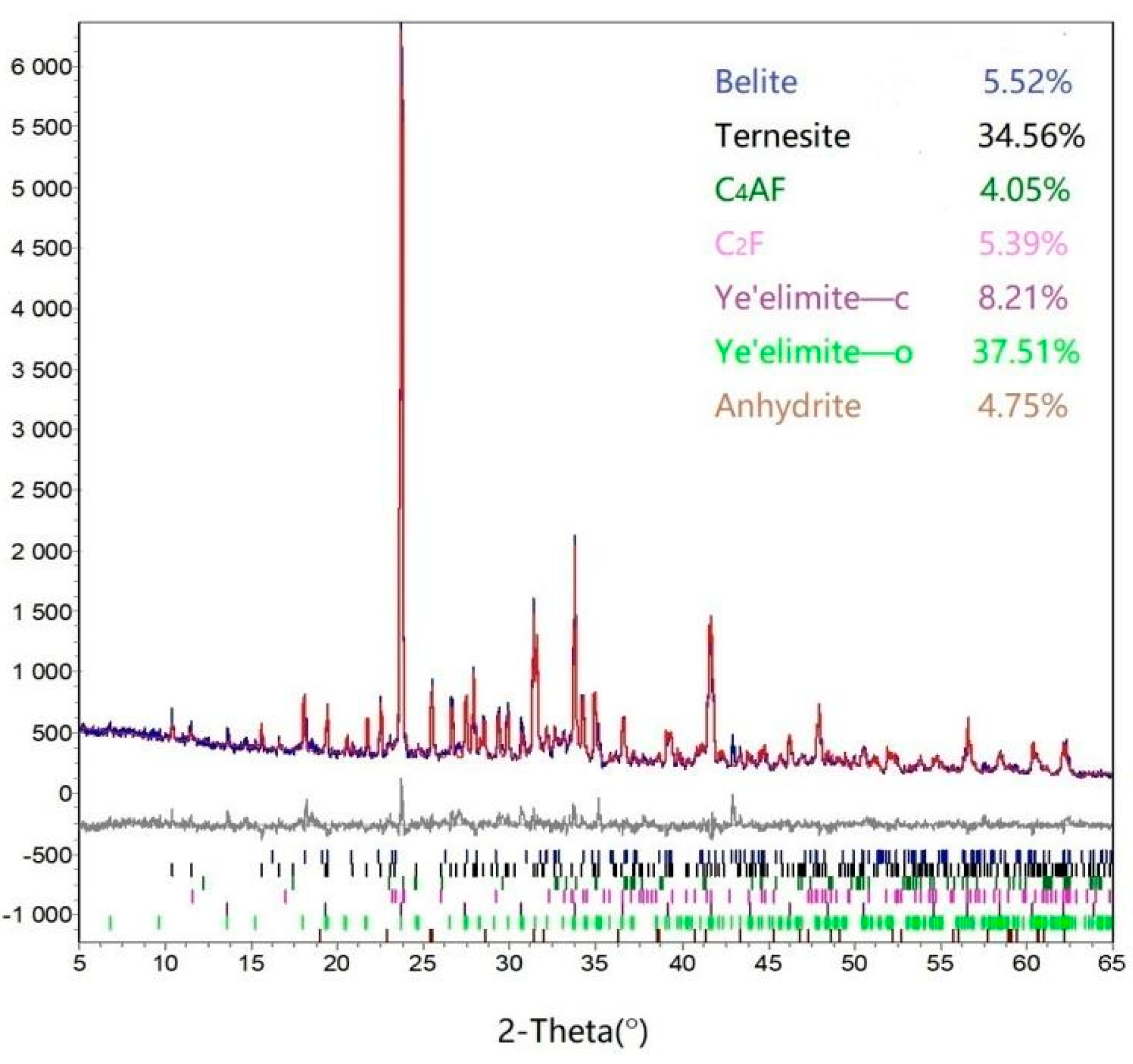
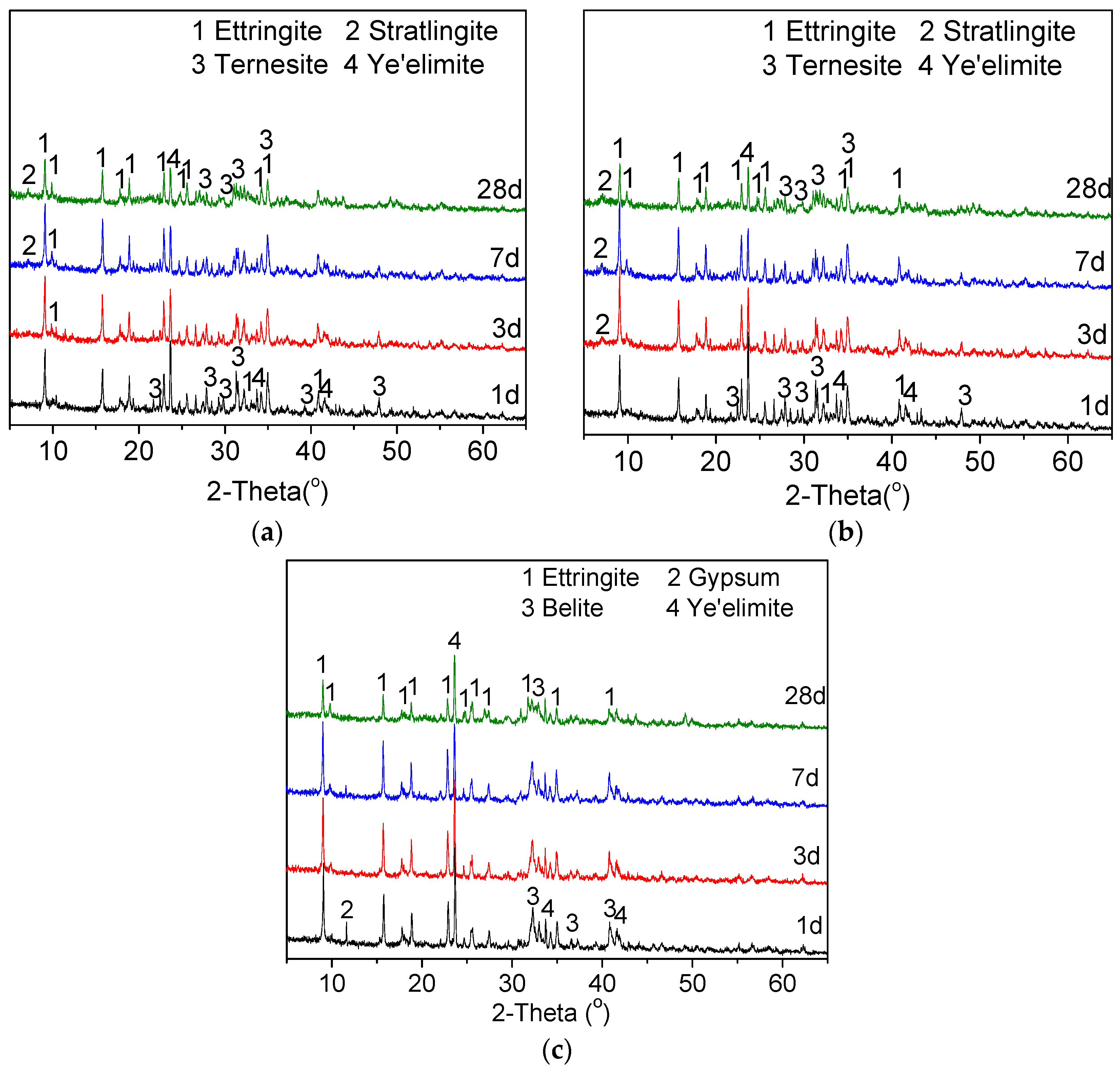
3.1.4. Characterization by XRD and SEM
3.2. Performance of the TCSA Cement
3.2.1. Setting Time
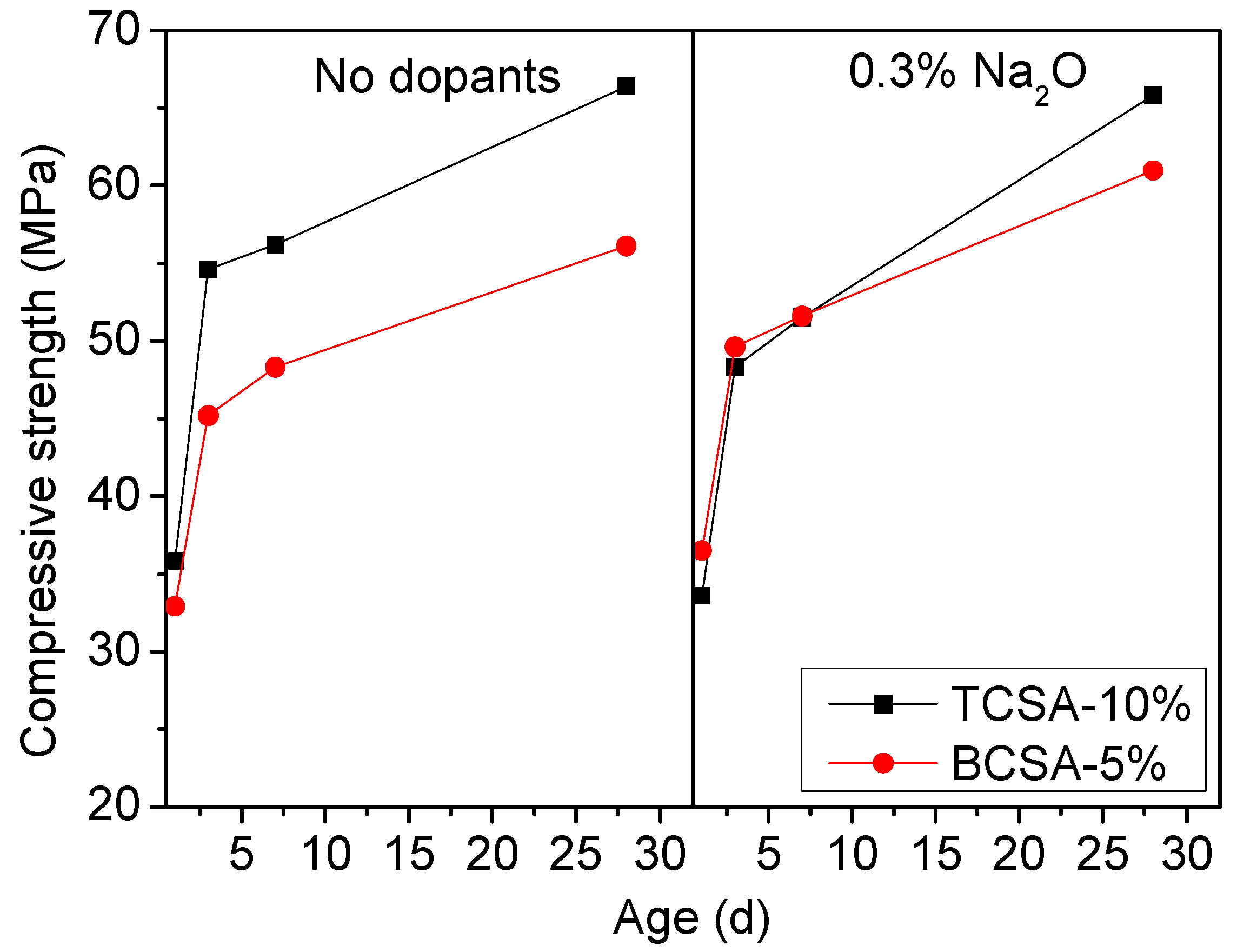
3.2.2. Compressive Strength
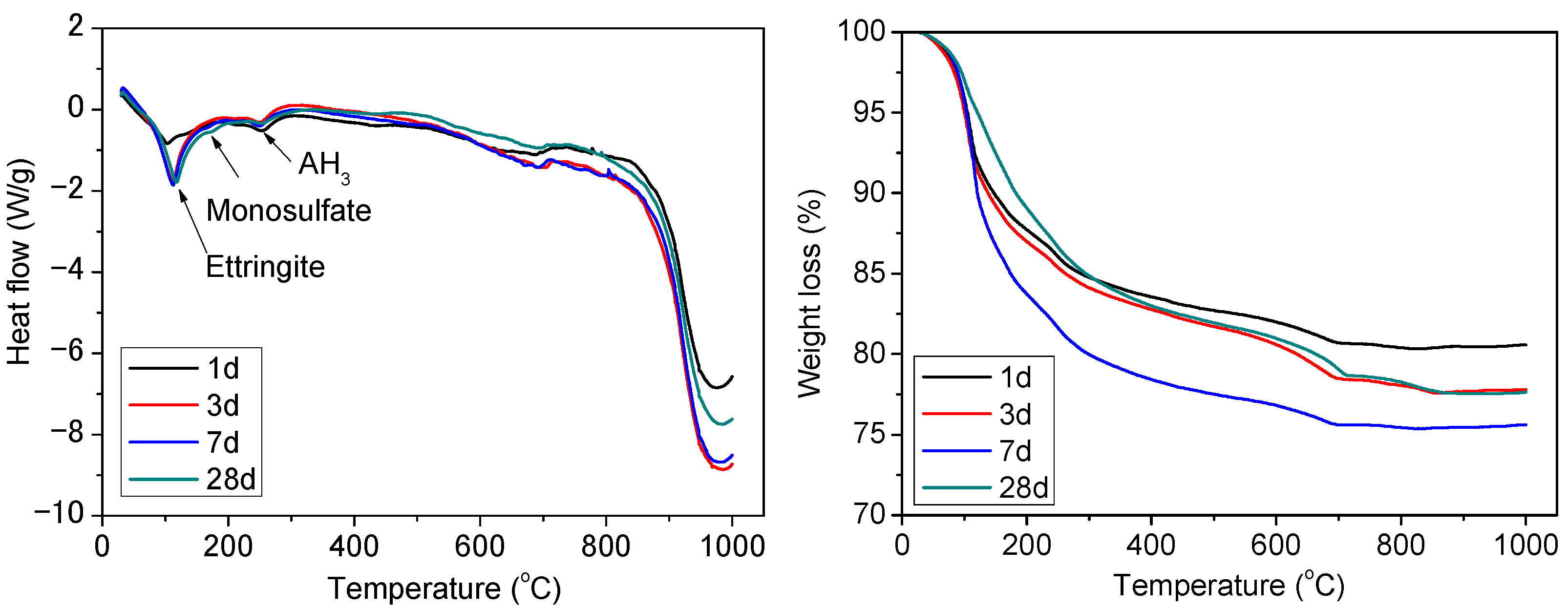
3.3. Hydration of TCSA Cement Pastes
4. Conclusions
- (1)
- Fe2O3 and MgO did not demonstrate a remarkable effect on the formation of ternesite. The addition of CaF2, P2O5 and Na2O can promote the formation and coexistence of ternesite and ye’elimite at 1150 °C. Na2O is the most effective dopant to facilitate the formation of ternesite.
- (2)
- A moderate gypsum content in the raw mixes for clinker B could facilitate the coexistence of ternesite and ye’elimite. A sintering temperature of 1150 °C and a retention time of 30 min were beneficial for the production of TCSA cement clinkers.
- (3)
- The TCSA cement clinkers exhibited shorter setting times than the BCSA cement clinkers. In the absence of dopants, TCSA cement exhibited greater compressive strength than BCSA cement. When Na2O was incorporated into the clinkers, the early strength gains of the two cements were similar. After 28 days of hydration, the compressive strength of TCSA cement showed a significant increase compared with BCSA cement.
- (4)
- The dissolution of ternesite could promote the formation of ettringite. The reactivity of belite was higher in TCSA cement due to the formation of strätlingite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, C.J.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production-present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Cleaner. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate-belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Berriel, S.; Favier, A.; Domínguez, E.; Machado, I.R.; Heierli, U.; Scrivener, K.; Hernández, F.; Habert, G. Assessing the environmental and economic potential of limestone calcined clay cement in Cuba. J. Clean. Prod. 2016, 124, 361–369. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Cuesta, A.; García-Maté, M.; Santacruz, I.; Losilla, E.R.; De la Torre, A.G.; León-Reina, L.; Aranda, M.A.G. Rietveld quantitative phase analysis of ye’elimite-containign cements. Cem. Concr. Res. 2012, 42, 960–971. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Ben Haha, M. CSA raw mix design: Effect on clinker formation and reactivity. Mater. Struct. 2015, 48, 3895–3911. [Google Scholar] [CrossRef]
- Janotka, I.; Krajèi, L.È. Resistance to freezing and thawing of mortar specimens made from sulphoaluminate-belite cement. Bull. Mater. Sci. 2000, 23, 521–527. [Google Scholar] [CrossRef][Green Version]
- Martín-Sedeno, M.A.; Cuberos, A.J.M.; De la Torre, A.G.; Alvarez-Pinazo, G.; Ordonez, L.M.; Gateshki, M.; Aranda, M. Aluminum-rich belite sulfoaluminate cements: Clinkering and early age hydration. Cem. Concr. Res. 2010, 40, 359–369. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Cuesta, A.; García-Mate, M.; Santacruz, I.; Losilla, E.R.; Sanfélix, S.G.; Fauth, F.; Aranda, M.A.G.; De la Torre, A.G. In-situ early-age hydration study of sulfobelite cements by synchrotron powder diffraction. Cem. Concr. Res. 2014, 56, 12–19. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Santacruz, I.; Leon-Reina, L.; Aranda, M.A.G.; De la Torre, A.G. Hydration reactions and mechanical strength developments of iron rich sulfobelite eco-cements. Ind. Eng. Chem. Res. 2013, 52, 16606–16614. [Google Scholar] [CrossRef]
- Wang, Y.M.; Su, M.Z.; Zhang, L. Sulfoaluminate Cement, 1st ed.; Beijing University of Technology Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Belz, G.; Beretka, J.; Marrocoli, M. Use of fly ash, blast furnace slag and chemical gypsum for the synthesis of calcium sulfoaluminate-based cements. Spec. Publ. ACI 1995, 153, 513–530. [Google Scholar]
- Makhmudova, V.; Iskandarova, M.; Ivanova, Y.; Chernev, G.; Ruziev, N.; Univ, J. Synthesis and properties of sulphoferrite calcium clinkers and low temperature cements on their basis. Chem. Technol. Metall. 2011, 46, 151–154. [Google Scholar]
- Beretka, J.; Cioffi, R.; Marrocoli, M.; Valenti, G.L. Energy-saving cements obtained from chemical gypsum and other industrial wastes. Waste. Manag. 1996, 16, 231–235. [Google Scholar] [CrossRef]
- Sherman, N.; Beretka, J.; Santoro, L.; Valenti, G.L. Long-term behavior of hydraulic binders based on calcium sulfoaluminate and calcium sulfosilicate. Cem. Concr. Res. 1995, 25, 113–126. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Ben Haha, M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.S.; Huang, Y.B.; Yang, D.Y. Synthesis of belite sulfoaluminate-ternesite cements with phosphogypsum. Cem. Concr. Compos. 2015, 63, 67–75. [Google Scholar] [CrossRef]
- Hanein, T.; Galan, I.; Glasser, F.P.; Skalamprinos, S.; Elhoweris, A.; Imbabi, M.S.; Bannerman, M.N. Stability of ternesite and the production at scale of ternesite-based clinkers. Cem. Concr. Res. 2017, 98, 91–100. [Google Scholar] [CrossRef]
- Ji, D.F.; Ma, S.H.; Li, W.F. Synthesis of belite-ye’elimite-ternesite cement clinker. Mater. Sci. Forum 2021, 1036, 223–229. [Google Scholar] [CrossRef]
- Li, Z.Y.; Guo, Y.; Hu, Y.Y.; Wang, X.; Qian, B.B.; Jiang, C.F. Preparation and performance of ternesite-ye’elimite clinker produced from steel slag at lower temperature. J. Renew. Mater. 2022, 10, 1–15. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Ben Haha, M. Mineralizer for Calcium Sulfoaluminate Ternesite Cements. European Patent EP3109215A1, 16 June 2016. [Google Scholar]
- Skalamprinos, S.; Jen, G.; Galan, I.; Whittaker, M.; Elhoweris, A.; Glasser, F. The synthesis and hydration of ternesite, Ca5(SiO4)2SO4. Cem. Concr. Res. 2018, 113, 27–40. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, P.F.; Chen, X.; Zhang, W.; Qian, J.S. Synthesis, characterization and hydration of ternesite. Constr. Build. Mater. 2021, 270, 121392. [Google Scholar] [CrossRef]
- Beretka, J.; Marrocoli, M.; Sherman, N.; Valenti, G.L. The influence of content and W/S ratio on the performance of calcium sulfoaluminate and calcium sulfosilicate. Cem. Concr. Res. 1996, 26, 1673–1681. [Google Scholar] [CrossRef]
- Dienemann, W.; Schmitt, D.; Bullerjahn, F.; Ben Haha, M. Belite-Calciumsulfoaluminate-Ternesite (BCT)-a new low carbon clinker technology. Cem. Inter. 2013, 11, 100–109. [Google Scholar]
- Pryce, M. Calicum sulphosilicate in lime-kiln wall coating. Min. Mag. 1972, 38, 968–971. [Google Scholar] [CrossRef]
- Santoro, L.; Garofano, R. Role of phosphogypsum in the hydration of calcium sulfoaluminate cement. Thermochim. Acta 1987, 116, 145–152. [Google Scholar] [CrossRef]
- Huang, Y.B.; Pei, Y.; Qian, J.S.; Gao, X.; Liang, J.; Duan, G.B.; Zhao, P.Q.; Lu, L.C.; Cheng, X. Bauxite free iron rich calcium sulfoaluminate cement: Preparation, hydration and properties. Constr. Build. Mater. 2020, 249, 118774. [Google Scholar] [CrossRef]
- Marroccoli, M.; Pace, M.L.; Telesca, A.; Valenti, G.L. Synthesis of calcium sulfoaluminate cements from Al2O3-rich by-products from aluminium manufacture. In Proceedings of the 2ed International Congress on Sustainable Construction Materials and Technologies, Ancona, Italy, 15–16 September 2010. [Google Scholar]
- Shen, Y.; Qian, J.S. Utilisation of phosphogypsum for sulfate-rich belite sulfoaluminate cement production. Adv. Cem. Res. 2015, 27, 515–525. [Google Scholar] [CrossRef]
- Pliego-Cuervo, Y.B.; Glasser, F.P. Role of sulfates in cement clinkering: The calcium silicosulfate phase. Cem. Concr. Res. 1978, 8, 455–460. [Google Scholar] [CrossRef]
- Selcuk, N.; Soner, I.; Selcuk, E. Synthesis of special cement with fluidized bed combustion ashes. Adv. Cem. Res. 2010, 22, 107–113. [Google Scholar] [CrossRef]
- Beretka, J.; Vito, B.D.; Santoro, L.; Sherman, N.; Valenti, G.L. Utilisation of industrial wastes and by-products for the synthesis of special cements. Resour. Conserv. Recycl. 1993, 9, 179–190. [Google Scholar] [CrossRef]
- Li, W.F.; Ji, D.P.; Shi, F.; Huang, X.F.; Ji, X.M.; Ma, S.H. Study on the synthesis of belite-ye’elimite-ternesite clinker. Constr. Build. Mater. 2022, 319, 126022. [Google Scholar] [CrossRef]
- Arjunan, P.; Silsbee, M.R.; Roy, D.M. Sulfoaluminate-belite cement from low-calcium fly ash and sulfur-rich and other industrial by-products. Cem. Concr. Res. 1999, 29, 1305–1311. [Google Scholar] [CrossRef]
- Huang, Y.B.; Qian, J.S.; Kang, X.J.; Yu, J.C.; Fan, Y.R.; Dang, Y.D.; Zhang, W.S.; Wang, S.D. Belite-calcium sulfoaluminate cement prepared with phosphogypsum: Influence of P2O5 and F on the clinker formation and cement performances. Constr. Build. Mater. 2019, 203, 432–442. [Google Scholar] [CrossRef]
- Perez-Bravo, R.; Alvarez-Pinazo, G.; Compana, J.M.; Santacruz, I.; Losilla, E.R.; Bruque, S.; De la Torre, A.G. Alite sulfoaluminate clinker: Rietveld mineralogical and SEM-EDX analysis. Adv. Cem. Res. 2013, 26, 10–20. [Google Scholar] [CrossRef]
- Lu, L.C.; Chang, J.; Cheng, X.; Liu, H.X.; Yuan, R.Z. Study on a cementing system taking alite-calcium barium sulphoaluminate as main minerals. J. Mater. Sci. 2005, 40, 4035–4438. [Google Scholar]
- Ma, B.; Ma, M.; Shen, X.D.; Li, X.R.; Wu, X.D. Compatibility between a polycarboxylate superplasticizer and the belite–rich sulfoaluminate cement: Setting time and the hydration properties. Constr. Build. Mater. 2014, 51, 47–54. [Google Scholar] [CrossRef]
- Kasselouri, V.; Tsakiridis, P.; Malami, C.; Georgali, B.; Alexandridou, C. A study on the hydration products of a non-expansive sulphoaluminate cement. Cem. Concr. Res. 1995, 25, 1726–1736. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, X.; Zhang, W.; Li, X.P.; Qian, J.S. Influence of ternesite on the properties of calcium sulfoaluminate cements blended with fly ash. Constr. Build. Mater. 2018, 193, 221–229. [Google Scholar] [CrossRef]
- Tambara, L.U.D.; Cheriaf, M.; Rocha, J.C.; Palomo, A.; Fernandez-Jimenez, A. Effect of alkali content on calcium sulfoaluminate (CSA) cement hydration. Cem. Concr. Res. 2020, 128, 105953. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, X.; Zhang, W.; Li, X.P. Effect of ternesite on the hydration and properties of calcium sulfoaluminate cement. J. Therm. Anal. Calorim. 2019, 136, 687–695. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Song, F.; Yu, Z.; Yang, F.; Lu, Y.; Liu, Y. Microstructure of amorphous aluminum hydroxide in belite-calcium sulfoaluminate cement. Cem. Concr. Res. 2015, 71, 1–6. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Bullerjahn, F.; Ben Haha, M. Effect of retarders on the early hydration of calcium-sulpho-aluminate (CSA) type cements. Cem. Concr. Res. 2016, 84, 62–75. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.C.; Moon, J. The effect of water and gypsum content on strätlingite formation in calcium sulfoaluminate-belite cement pastes. Constr. Build. Mater. 2018, 166, 712–722. [Google Scholar] [CrossRef]
- Paul, G.; Boccaleri, E.; Marchese, L.; Buzzi, L.; Canonico, F.; Gastaldi, D. Low temperature sulfoaluminate clinkers: The role of sulfates and silicates on the different hydration behavior. Constr. Build. Mater. 2021, 268, 121111. [Google Scholar] [CrossRef]
- Tobon, J.I.; Paya, J.; Borrachero, M.V.; Soriano, L.; Restrepo, O.J. Determination of the optimum parameters in the high resolution thermogravimetric analysis (HRTG) for cementitious materials. J. Therm. Anal. Calorim. 2012, 107, 233–239. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.M.; Lecomte, A.; Roux, A.; Le Rolland, B. Hydration of ordinary Portland cement and calcium sulfoaluminate cement blends. Cem. Concr. Compos. 2015, 56, 106–114. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, Y.; Shang, X.; Zhao, J.; Yu, X. Effects of Amorphous AH3 Phase on Mechanical Properties and Hydration Process of C4A3-CH2-CH-H2O System. Constr. Build. Mater. 2017, 133, 314–322. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements-Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]


| Materials | Limestone | Bauxite | Gypsum | Fly Ash |
|---|---|---|---|---|
| Oxide | ||||
| CaO | 44.34 | 0.38 | 32.28 | 4.73 |
| SiO2 | 9.83 | 12.38 | 2.02 | 53.56 |
| Al2O3 | 3.25 | 65.75 | 0.97 | 24.01 |
| Fe2O3 | 1.83 | 1.50 | 0.49 | 5.91 |
| MgO | 3.44 | 0.30 | 2.71 | 0.85 |
| SO3 | 1.06 | 0.16 | 39.50 | 0.51 |
| K2O | 0.16 | 1.23 | 0.12 | 1.64 |
| Na2O | 0.13 | 0.25 | - | 0.57 |
| TiO2 | 0.28 | 4.14 | 0.08 | 1.38 |
| LOI | 35.50 | 13.45 | 21.50 | 4.50 |
| Phase | A | B | C |
|---|---|---|---|
| 35 | 48 | 42 | |
| 40 | 40 | 40 | |
| C2S | 15 | 0 | 0 |
| C4AF | 10 | 10 | 10 |
| 0 | 2 | 8 |
| Clinker | Limestone | Bauxite | Fly Ash | Gypsum | Al2O3 |
|---|---|---|---|---|---|
| A | 59.1 | 21.5 | 1.1 | 18.3 | - |
| B | 53.8 | 20.6 | - | 24.5 | 1.1 |
| C | 50.3 | 16.9 | - | 29.0 | 3.8 |
| CaF2 | P2O5 | Fe2O3 | MgO | Na2O |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 0.5 | 0.3 | 0.2 | 0.2 | 0.3 |
| 1 | 0.5 | 0.5 | 0.5 | 0.6 |
| 1 | 1 | 1 | 0.9 |
| Phase | 0.5% CaF2 | 0.3% Na2O | 0.3% P2O5 | 0.5% Fe2O3 | 0.5% MgO |
|---|---|---|---|---|---|
| -o | 32.2 | 29.5 | 30.0 | 30.5 | 30.2 |
| -c | 6.9 | 11.3 | 9.6 | 10.8 | 6.7 |
| 25.3 | 28.5 | 27.8 | 16.3 | 18.5 | |
| C2S | 22.3 | 18.8 | 20.7 | 24.7 | 26.5 |
| C4AF | 5.7 | 6.2 | 5.9 | 6.2 | 5.3 |
| C2F | 3.9 | 3.5 | 3.2 | 4.3 | 3.7 |
| 3.7 | 2.2 | 2.8 | 5.6 | 6.7 | |
| f-CaO | - | - | - | 1.6 | 2.1 |
| Phase | 1100 °C | 1150 °C | 1200 °C | 1220 °C | 1250 °C |
|---|---|---|---|---|---|
| -o | 31.8 | 37.5 | 35.4 | 33.5 | 32.7 |
| -c | 5.3 | 8.2 | 7.9 | 8.8 | 9.5 |
| 25.7 | 34.6 | 29.2 | 10.3 | - | |
| C2S | 16.4 | 5.5 | 10.6 | 25.7 | 33.5 |
| C4AF | 5.6 | 4.0 | 3.8 | 4.3 | 4.6 |
| C2F | 4.4 | 5.4 | 5.8 | 4.8 | 4.3 |
| 9.6 | 4.8 | 7.3 | 12.6 | 15.4 | |
| f-CaO | 1.2 | - | - | - | - |
| Phase | 15 min | 30 min | 45 min | 60 min |
|---|---|---|---|---|
| -o | 31.8 | 37.5 | 35.9 | 35.8 |
| -c | 7.1 | 8.2 | 8.5 | 7.8 |
| 32.5 | 34.6 | 33.9 | 34.4 | |
| C2S | 11.7 | 5.5 | 6.5 | 6.7 |
| C4AF | 3.6 | 4.0 | 4.5 | 4.7 |
| C2F | 5.8 | 5.4 | 5.3 | 4.9 |
| 7.5 | 4.8 | 5.4 | 5.7 |
| Phase | TCSA (No Dopants) | TCSA (0.3% Na2O) | BCSA (No Dopants) | BCSA (0.3% Na2O) |
|---|---|---|---|---|
| -o | 36.0 | 37.5 | 38.8 | 39.9 |
| -c | 8.4 | 8.2 | 6.2 | 7.5 |
| 30.1 | 34.6 | - | - | |
| C2S | 8.2 | 5.5 | 29.8 | 28.8 |
| C4AF | 6.1 | 4.0 | 7.2 | 6.4 |
| C2F | 3.3 | 5.4 | 3.5 | 3.6 |
| 7.9 | 4.8 | 14.5 | 13.8 |
| Clinkers | Initial Setting Time (min) | Final Setting Time (min) |
|---|---|---|
| TCSA (no dopants) | 20 | 30 |
| TCSA (0.3% Na2O) | 17 | 20 |
| BCSA (no dopants) | 55 | 70 |
| BCSA (0.3% Na2O) | 35 | 50 |
| Phase | TCSA (1 d) | TCSA (3 d) | TCSA (28 d) | BCSA (1 d) | BCSA (3 d) | BCSA (28 d) |
|---|---|---|---|---|---|---|
| 7.7 | 5.5 | 4.2 | 11.1 | 9.8 | 7.5 | |
| 26.2 | 22.7 | 19.5 | - | - | - | |
| C2S | 4.6 | 2.1 | - | 25.5 | 25.3 | 24.6 |
| 0.5 | - | - | 2.7 | 1.3 | - | |
| 1.3 | - | - | 5.2 | 3.4 | - | |
| Ettringite | 33.8 | 36.3 | 30.6 | 30.7 | 33.6 | 31.2 |
| strätlingite | - | 3.4 | 6.7 | - | - | - |
| Amorphous | 25.4 | 28.2 | 30.6 | 23.9 | 26.2 | 30.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Chen, X.; Li, J.; Wang, P.; Qian, J. Preparation and Performance of Ternesite–Ye’elimite Cement. Materials 2022, 15, 4369. https://doi.org/10.3390/ma15124369
Shen Y, Chen X, Li J, Wang P, Qian J. Preparation and Performance of Ternesite–Ye’elimite Cement. Materials. 2022; 15(12):4369. https://doi.org/10.3390/ma15124369
Chicago/Turabian StyleShen, Yan, Xi Chen, Jiang Li, Peifang Wang, and Jueshi Qian. 2022. "Preparation and Performance of Ternesite–Ye’elimite Cement" Materials 15, no. 12: 4369. https://doi.org/10.3390/ma15124369
APA StyleShen, Y., Chen, X., Li, J., Wang, P., & Qian, J. (2022). Preparation and Performance of Ternesite–Ye’elimite Cement. Materials, 15(12), 4369. https://doi.org/10.3390/ma15124369





