Antibacterial Activity and Cell Responses of Vancomycin-Loaded Alginate Coating on ZSM-5 Scaffold for Bone Tissue Engineering Applications
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
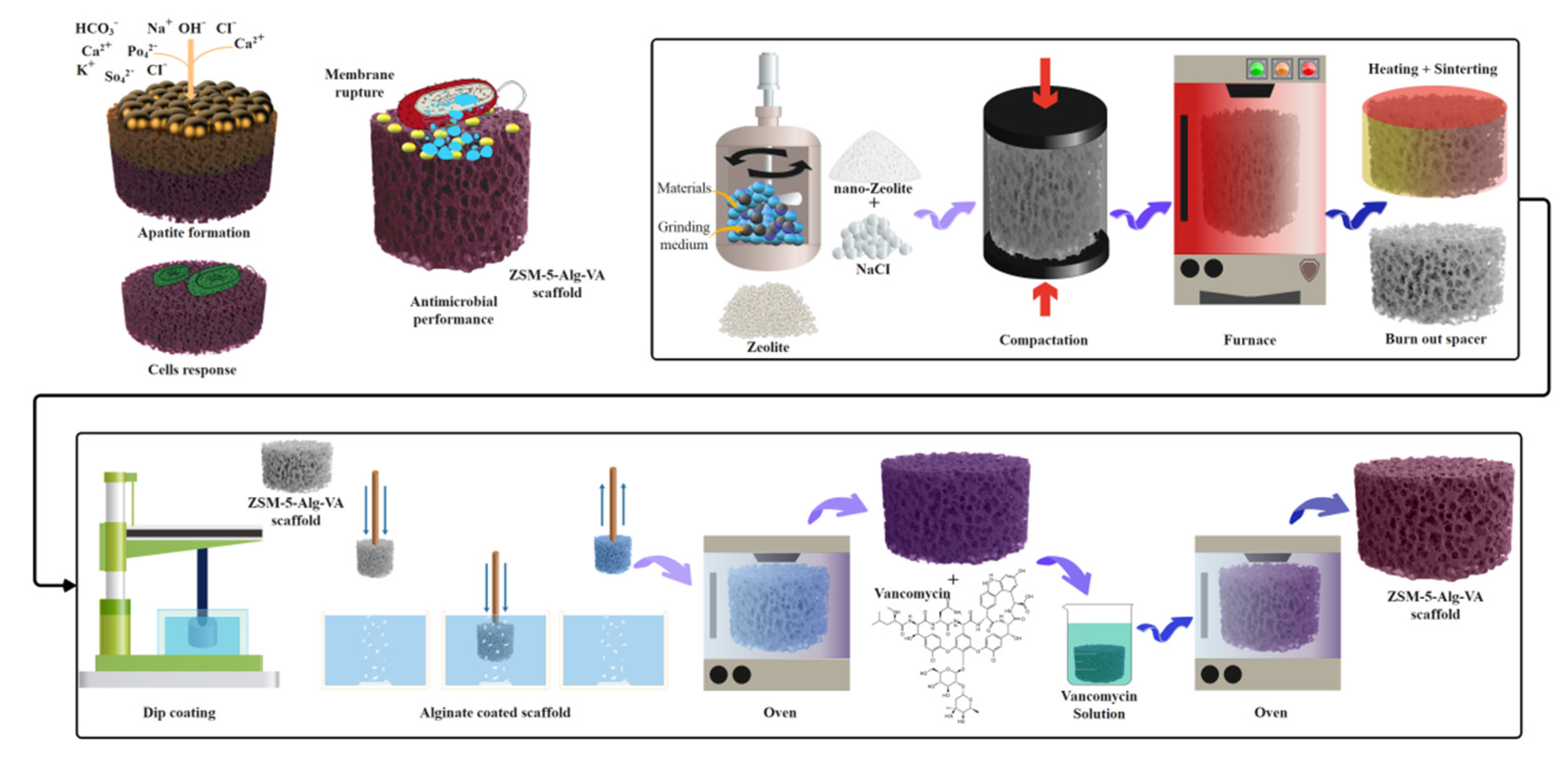
2.2. Fabrication of ZSM-5 Scaffolds
2.3. Preparation of Drug-Loaded Alg Coated ZSM-5 Scaffolds
2.4. Characterization of ZSM-5 Scaffolds
2.5. Machinal Properties of ZSM-5 Scaffolds
2.6. In Vitro Drug Release Evaluation
2.7. In Vitro Bioactivity Properties of ZSM-5 Scaffolds
2.8. Antibacterial Activity Evaluation of ZSM-5 Scaffolds
2.9. Cell Culture
2.9.1. In Vitro Cytocompatibility of Scaffolds
2.9.2. In Vitro Cell Attachment Evaluation
2.9.3. Osteogenic Differentiation Evaluation
2.10. Statistical Analysis
3. Results and Discussion
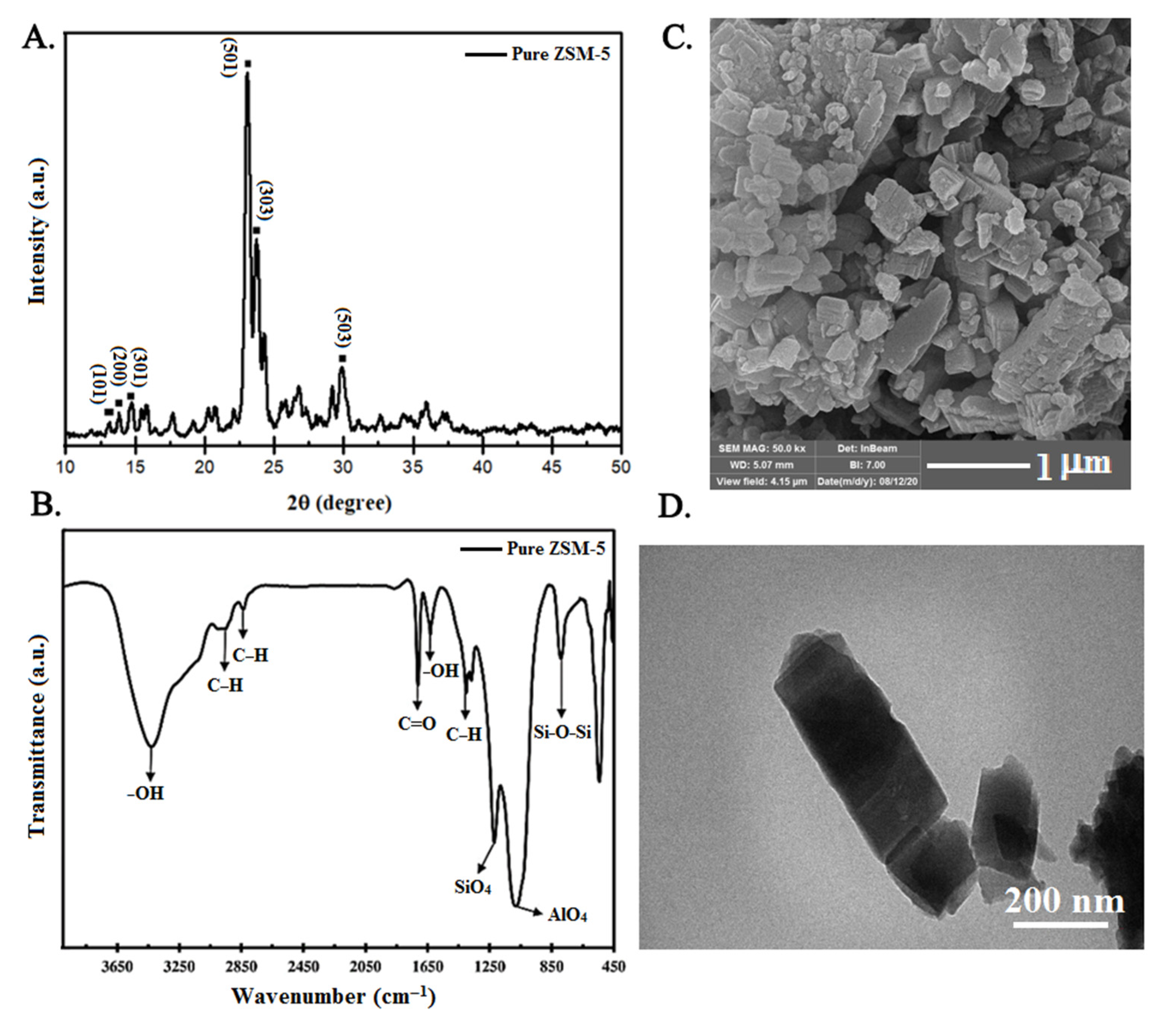
3.1. Characterization of ZSM-5 Nanopowder
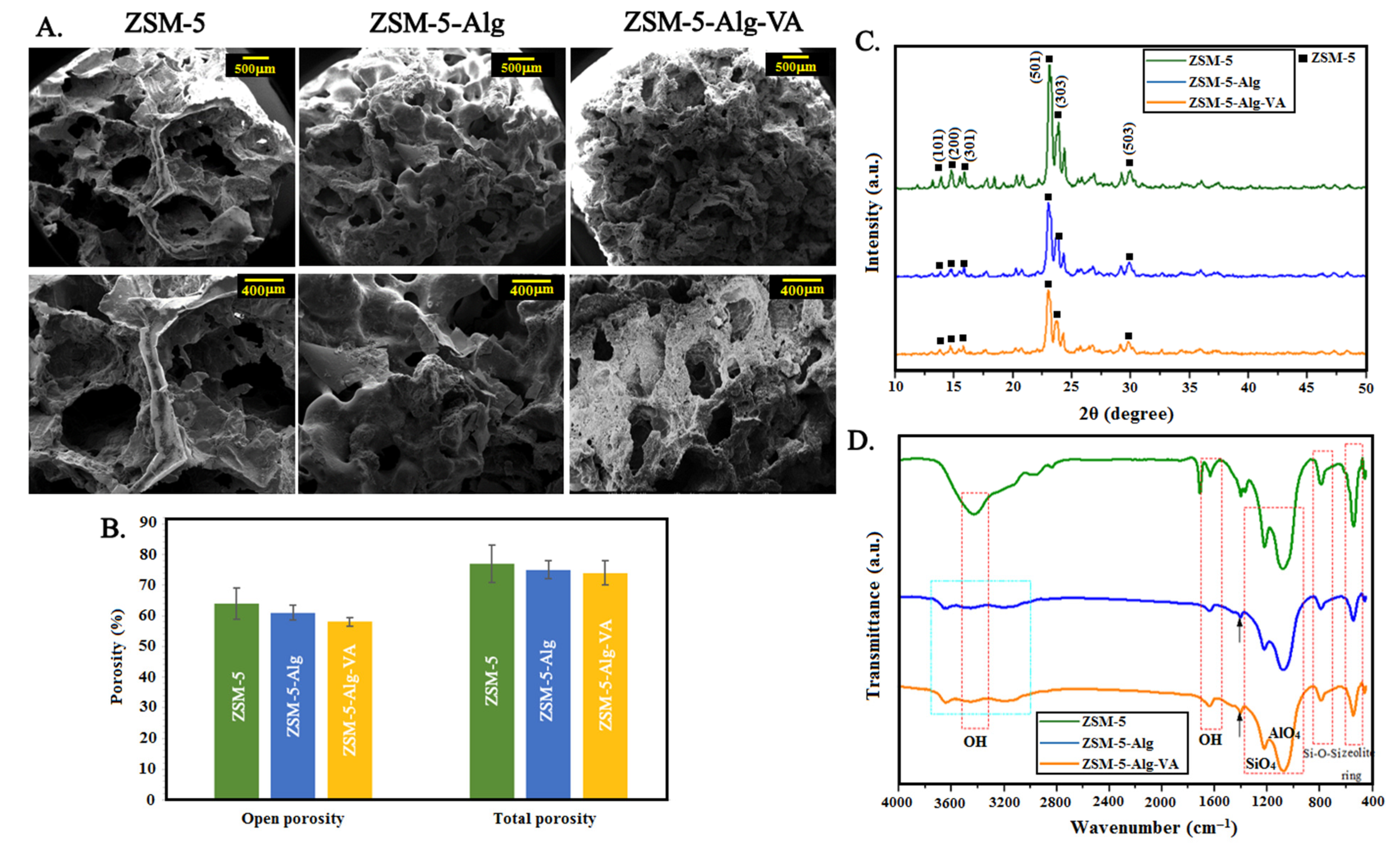
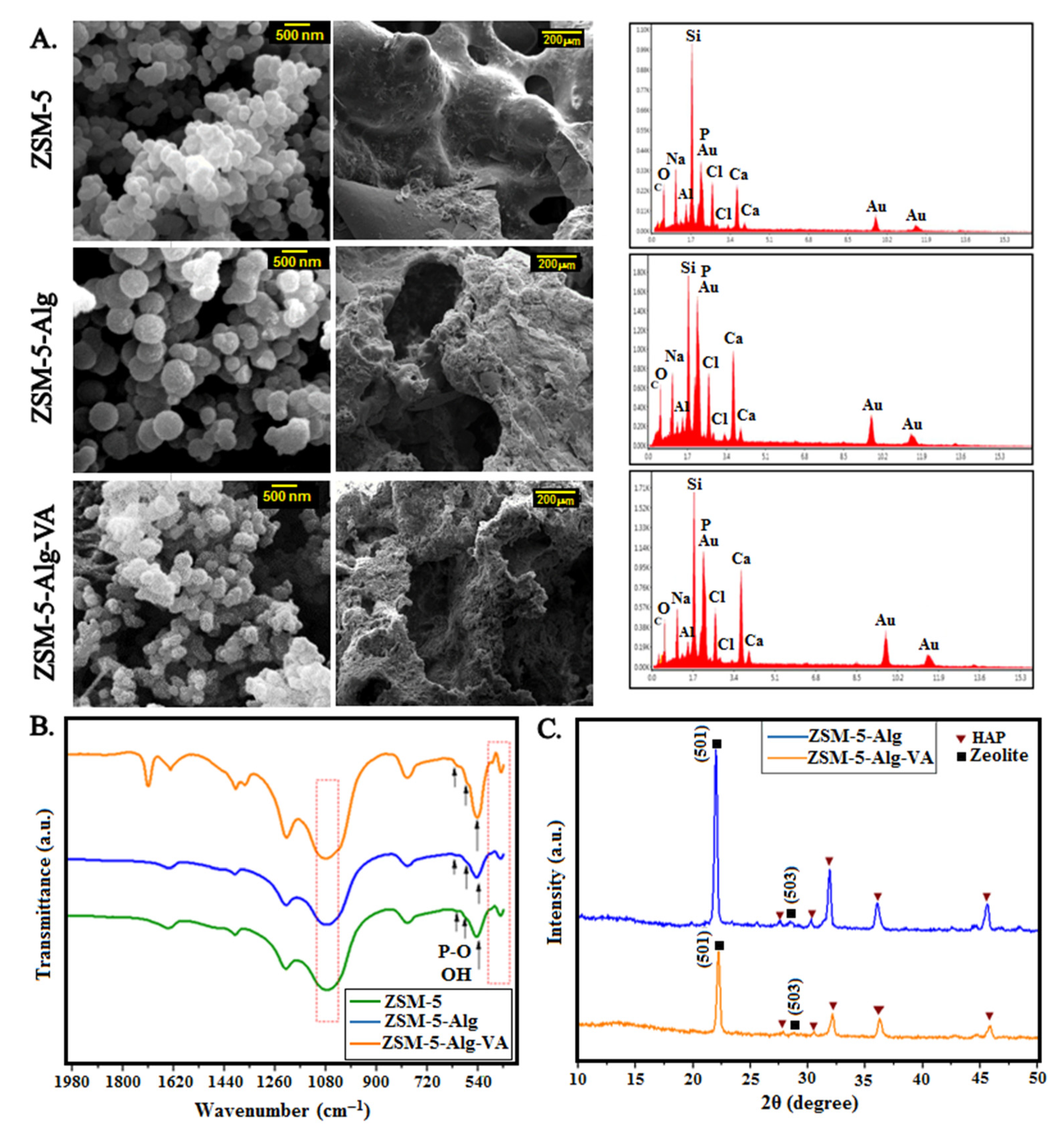
3.2. Physico-Chemical Characterization of ZSM-5 Scaffolds
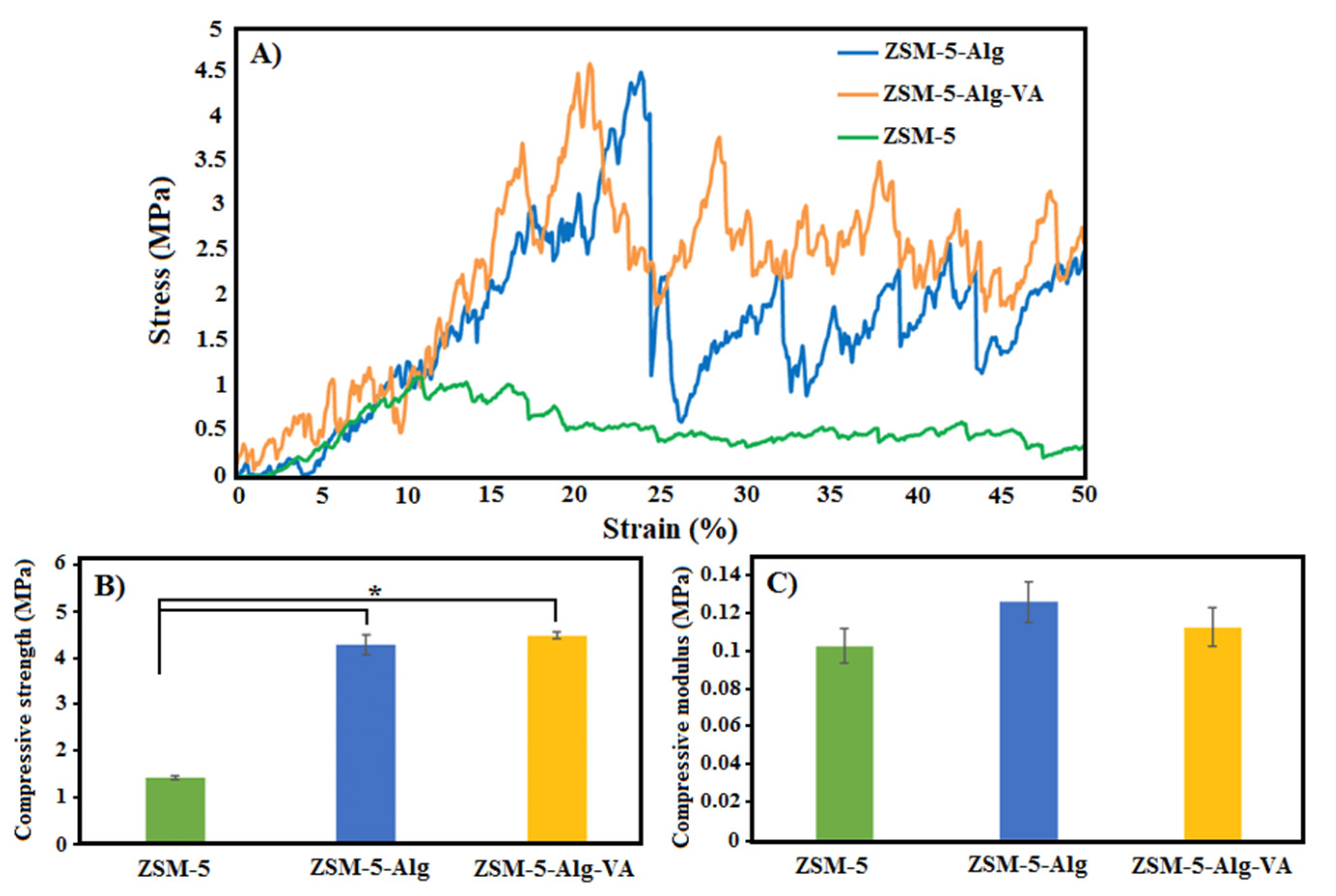
3.3. Mechanical Characterization of ZSM-5 Scaffolds
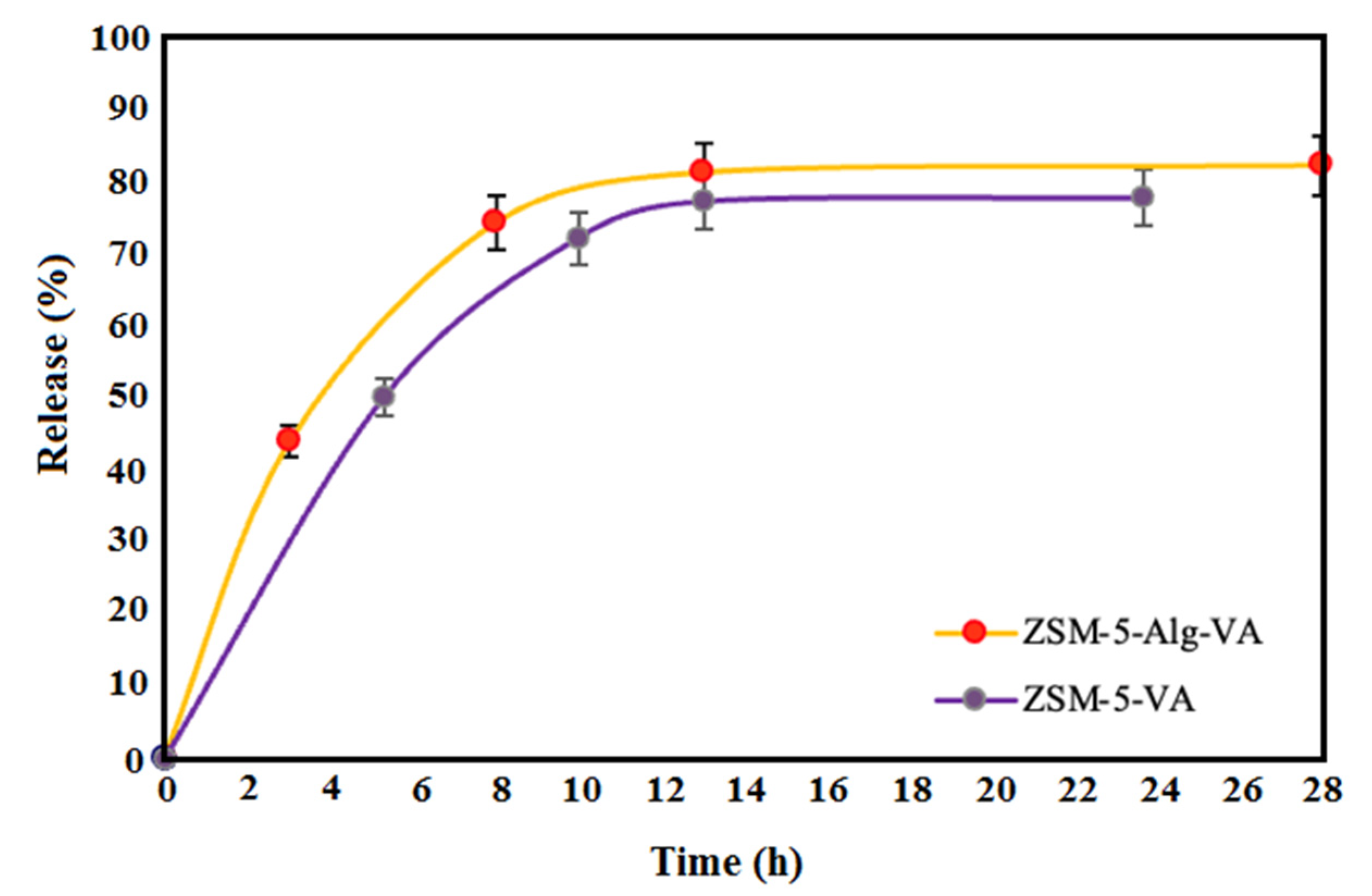
3.4. Drug Release Evaluation
3.5. In Vitro Bioactivity of Scaffolds
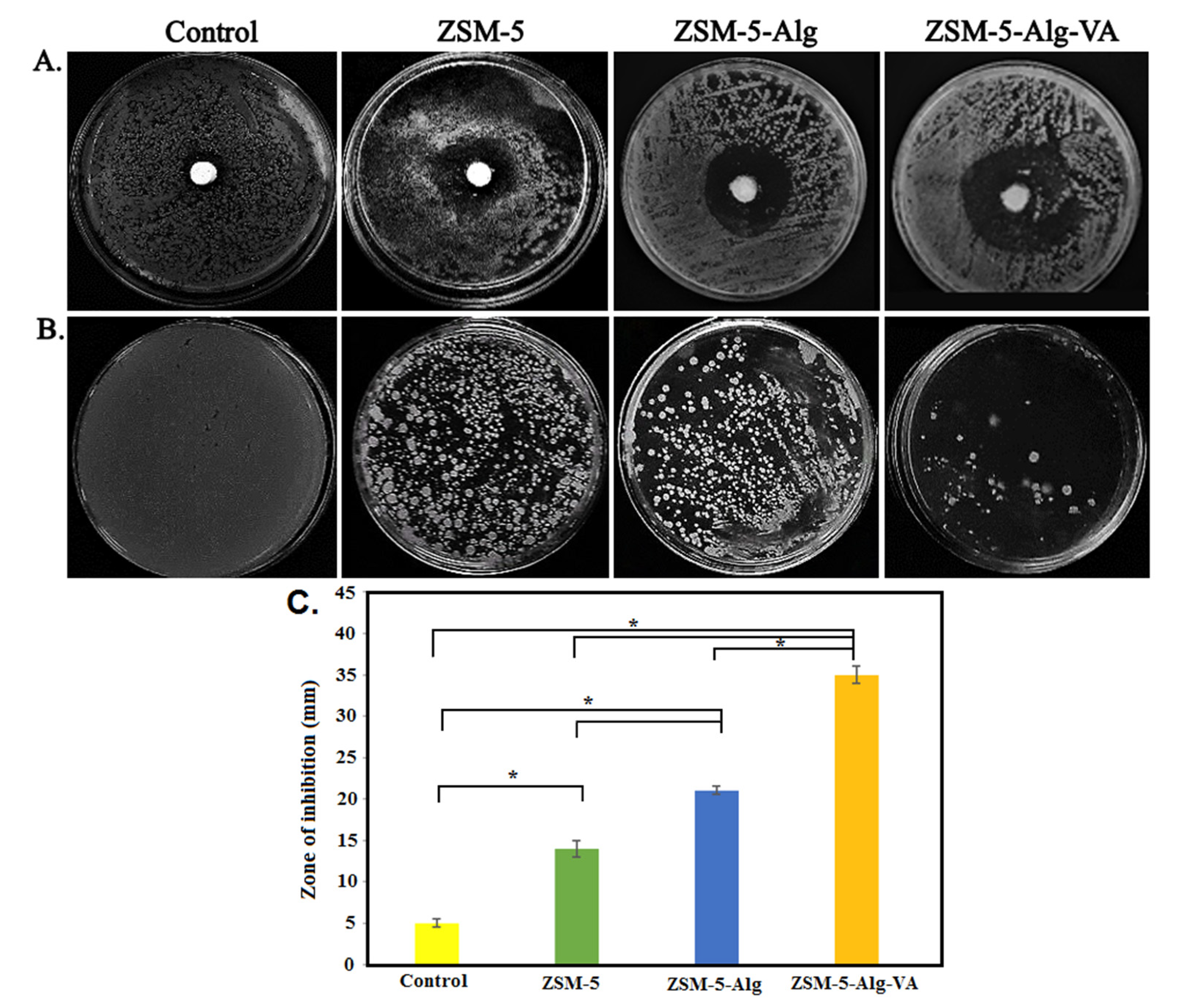
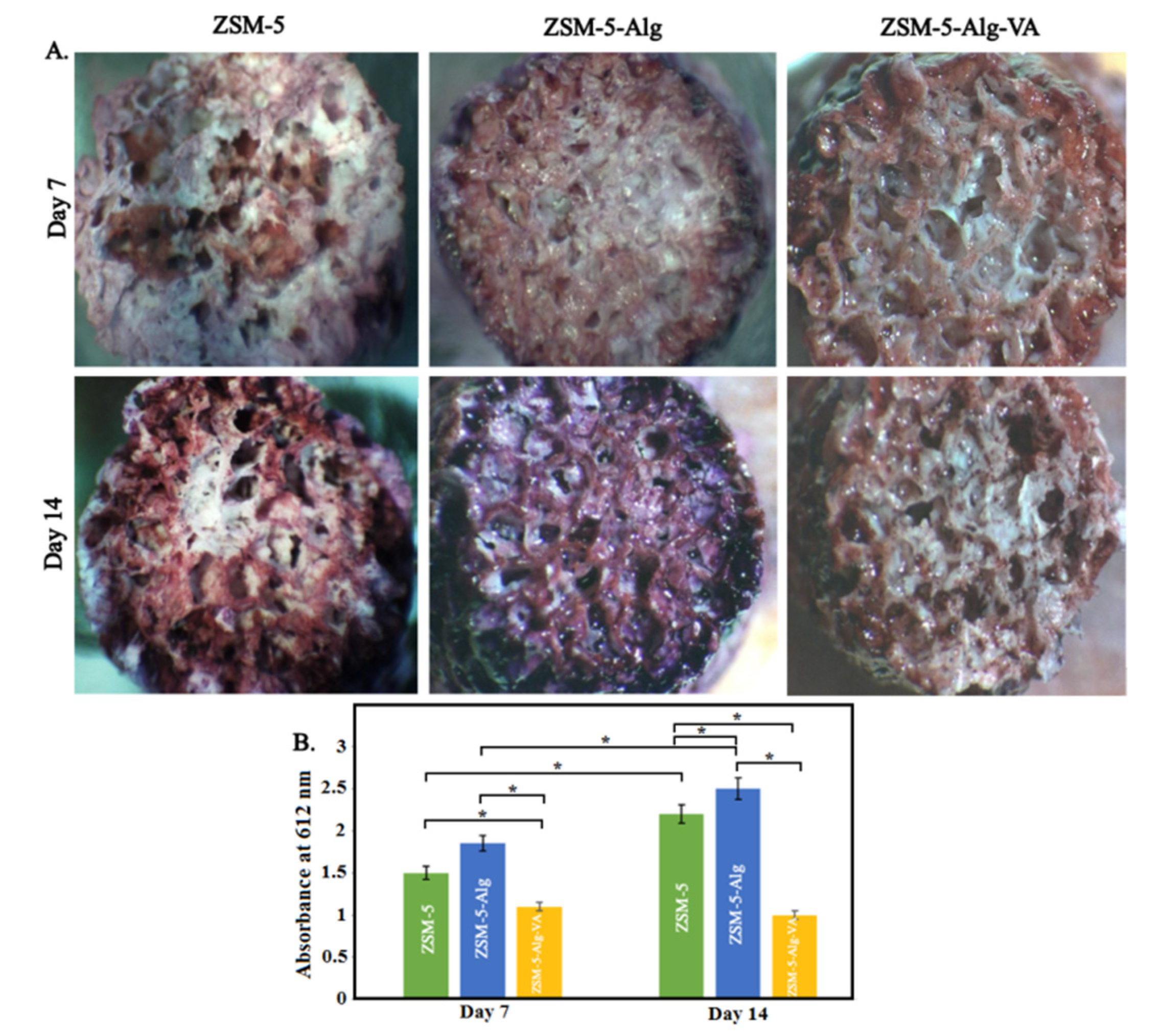
3.6. Antibacterial Activity of Scaffolds
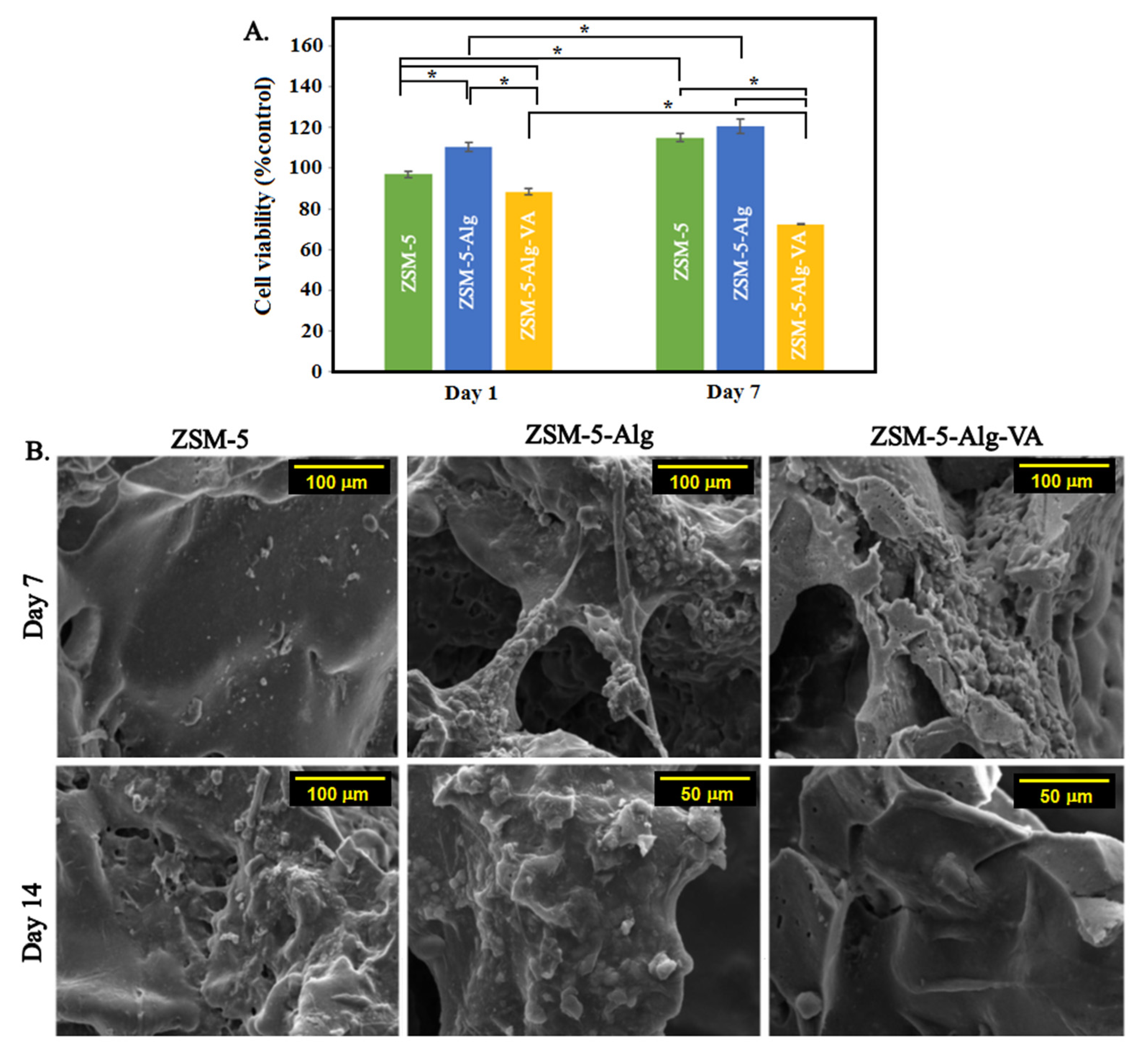
3.7. In Vitro Cytocompatibility of Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bedi, R.S.; Chow, G.; Wang, J.; Zanello, L.; Yan, Y.S. Bioactive materials for regenerative medicine: Zeolite-hydroxyapatite bone mimetic coatings. Adv. Eng. Mater. 2012, 14, 200–206. [Google Scholar] [CrossRef]
- Abdellahi, M.; Najafinezhad, A.; Ghayour, H.; Saber-Samandari, S.; Khandan, A. Preparing diopside nanoparticle scaffolds via space holder method: Simulation of the compressive strength and porosity. J. Mech. Behav. Biomed. Mater. 2017, 72, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [Green Version]
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef] [PubMed]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef]
- Platas-Iglesias, C.; Vander Elst, L.; Zhou, W.; Muller, R.N.; Geraldes, C.F.; Maschmeyer, T.; Peters, J.A. Zeolite GdNaY nanoparticles with very high relaxivity for application as contrast agents in magnetic resonance imaging. Chem. A Eur. J. 2002, 8, 5121–5131. [Google Scholar] [CrossRef]
- Yu, L.; Shang, X.; Chen, H.; Xiao, L.; Zhu, Y.; Fan, J. A tightly-bonded and flexible mesoporous zeolite-cotton hybrid hemostat. Nat. Commun. 2019, 10, 1932. [Google Scholar] [CrossRef]
- Servatan, M.; Zarrintaj, P.; Mahmodi, G.; Kim, S.-J.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M. Zeolites in drug delivery: Progress, challenges and opportunities. Drug Discov. Today 2020, 25, 642–656. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Dowaidar, M.; Langel, Ü. Carbonized chitosan encapsulated hierarchical porous zeolitic imidazolate frameworks nanoparticles for gene delivery. Microporous Mesoporous Mater. 2020, 302, 110200. [Google Scholar] [CrossRef]
- Zavareh, S.; Farrokhzad, Z.; Darvishi, F. Modification of zeolite 4A for use as an adsorbent for glyphosate and as an antibacterial agent for water. Ecotoxicol. Environ. Saf. 2018, 155, 1–8. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Mahmodi, G.; Manouchehri, S.; Mashhadzadeh, A.H.; Khodadadi, M.; Servatan, M.; Ganjali, M.R.; Azambre, B.; Kim, S.J.; Ramsey, J.D. Zeolite in tissue engineering: Opportunities and challenges. MedComm 2020, 1, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Serati-Nouri, H.; Jafari, A.; Roshangar, L.; Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Biomedical applications of zeolite-based materials: A review. Mater. Sci. Eng. C 2020, 116, 111225. [Google Scholar] [CrossRef]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine—A review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Mooney, D.J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998, 16, 224–230. [Google Scholar] [CrossRef]
- Lee, D.S.H.; Pai, Y.; Chang, S. Effect of Thermal Treatment of the Hydroxyapatite Powders on the Micropore and Microstructure of Porous Biphasic Calcium Phosphate Composite Granules. J. Biomater. Nanobiotechnol. 2013, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Freeze-drying as a novel biofabrication method for achieving a controlled microarchitecture within large, complex natural biomaterial scaffolds. Adv. Healthc. Mater. 2017, 6, 1700598. [Google Scholar] [CrossRef]
- Kazemzadeh Narbat, M.; Orang, F.; Solati Hashtjin, M.; Goudarzi, A. Fabrication of porous hydroxyapatite-gelatin composite scaffolds for bone tissue engineering. Iran. Biomed. J. 2006, 10, 215–223. [Google Scholar]
- Prasad, A.; Sankar, M.R.; Katiyar, V. State of art on solvent casting particulate leaching method for orthopedic scaffoldsfabrication. Mater. Today Proc. 2017, 4, 898–907. [Google Scholar] [CrossRef]
- Vayer, M.; Pineau, A.; Warmont, F.; Roulet, M.; Sinturel, C. Constrained crystallization of poly (L-lactic acid) in thin films prepared by dip coating. Eur. Polym. J. 2018, 101, 332–340. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [Green Version]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Frith, J.E.; Dehghan-Manshadi, A.; Attar, H.; Kent, D.; Soro, N.D.M.; Bermingham, M.J.; Dargusch, M.S. Mechanical properties and biocompatibility of porous titanium scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2017, 75, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Reyes, E.A.A.; Patin, C.A.L.; Diaz, B.J.; Lefebvre, L.P. Structural characterization and mechanical evaluation of bioactive glass 45S5 foams obtained by a powder technology approach. J. Am. Ceram. Soc. 2012, 95, 3776–3780. [Google Scholar] [CrossRef]
- Araújo, M.; Viveiros, R.; Philippart, A.; Miola, M.; Doumett, S.; Baldi, G.; Perez, J.; Boccaccini, A.; Aguiar-Ricardo, A.; Verné, E. Bioactivity, mechanical properties and drug delivery ability of bioactive glass-ceramic scaffolds coated with a natural-derived polymer. Mater. Sci. Eng. C 2017, 77, 342–351. [Google Scholar] [CrossRef]
- Erol, M.; Mouriňo, V.; Newby, P.; Chatzistavrou, X.; Roether, J.; Hupa, L.; Boccaccini, A.R. Copper-releasing, boron-containing bioactive glass-based scaffolds coated with alginate for bone tissue engineering. Acta Biomater. 2012, 8, 792–801. [Google Scholar] [CrossRef]
- Mouriño, V.; Newby, P.; Boccaccini, A.R. Preparation and Characterization of Gallium Releasing 3-D Alginate Coated 45S5 Bioglass® Based Scaffolds for Bone Tissue Engineering. Adv. Eng. Mater. 2010, 12, B283–B291. [Google Scholar] [CrossRef]
- Keshavarz, M.; Alizadeh, P. On the role of alginate coating on the mechanical and biological properties of 58S bioactive glass scaffolds. Int. J. Biol. Macromol. 2021, 167, 947–961. [Google Scholar] [CrossRef]
- Torres, A.; Gaspar, V.; Serra, I.; Diogo, G.; Fradique, R.; Silva, A.; Correia, I. Bioactive polymeric–ceramic hybrid 3D scaffold for application in bone tissue regeneration. Mater. Sci. Eng. C 2013, 33, 4460–4469. [Google Scholar] [CrossRef]
- Darley, E.S.; MacGowan, A.P. Antibiotic treatment of gram-positive bone and joint infections. J. Antimicrob. Chemother. 2004, 53, 928–935. [Google Scholar] [CrossRef]
- Smith, R.L.; Schurman, D.; Kajiyama, G.; Mell, M.; Gilkerson, E. The effect of antibiotics on the destruction of cartilage in experimental infectious arthritis. J. Bone Jt. Surgery. Am. Vol. 1987, 69, 1063–1068. [Google Scholar] [CrossRef]
- Owens, C.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef]
- Hoque, J.; Bhattacharjee, B.; Prakash, R.G.; Paramanandham, K.; Haldar, J. Dual function injectable hydrogel for controlled release of antibiotic and local antibacterial therapy. Biomacromolecules 2018, 19, 267–278. [Google Scholar] [CrossRef]
- Du, M.; Huang, L.; Peng, M.; Hu, F.; Gao, Q.; Chen, Y.; Liu, P. Preparation of vancomycin-loaded alginate hydrogel coating on magnesium alloy with enhanced anticorrosion and antibacterial properties. Thin Solid Film. 2020, 693, 137679. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Najafinezhad, A.; Hadisi, Z.; Iqbal, N.; Daroonparvar, M.; Sharif, S.; Ismail, A.F.; Akbari, M.; RamaKrishna, S.; Berto, F. Characterization and biological properties of nanostructured clinoenstatite scaffolds for bone tissue engineering applications. Mater. Chem. Phys. 2021, 259, 123969. [Google Scholar] [CrossRef]
- Saheban, M.; Bakhsheshi-Rad, H.; Kasiri-Asgarani, M.; Hamzah, E.; Ismail, A.; Aziz, M.; Dayaghi, E. Effect of zeolite on the corrosion behavior, biocompatibility and antibacterial activity of porous magnesium/zeolite composite scaffolds. Mater. Technol. 2019, 34, 258–269. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Rajabi, N.; Kharaziha, M.; Emadi, R.; Zarrabi, A.; Mokhtari, H.; Salehi, S. An adhesive and injectable nanocomposite hydrogel of thiolated gelatin/gelatin methacrylate/Laponite® as a potential surgical sealant. J. Colloid Interface Sci. 2020, 564, 155–169. [Google Scholar] [CrossRef]
- Golafshan, N.; Kharaziha, M.; Fathi, M. Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon 2017, 111, 752–763. [Google Scholar] [CrossRef]
- Karadzic, I.; Vucic, V.; Jokanovic, V.; Debeljak-Martacic, J.; Markovic, D.; Petrovic, S.; Glibetic, M. Effects of novel hydroxyapatite-based 3D biomaterials on proliferation and osteoblastic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 350–357. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Hamzah, E.; Ismail, A.; Aziz, M.; Karamian, E.; Iqbal, N. Bioactivity, in-vitro corrosion behavior, and antibacterial activity of silver–zeolites doped hydroxyapatite coating on magnesium alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 1553–1562. [Google Scholar] [CrossRef]
- Aloulou, H.; Bouhamed, H.; Ghorbel, A.; Ben Amar, R.; Khemakhem, S. Elaboration and characterization of ceramic microfiltration membranes from natural zeolite: Application to the treatment of cuttlefish effluents. Desalin Water Treat 2017, 59, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Roeffaers, M.B.; Ameloot, R.; Baruah, M.; Uji-i, H.; Bulut, M.; De Cremer, G.; Müller, U.; Jacobs, P.A.; Hofkens, J.; Sels, B.F. Morphology of large ZSM-5 crystals unraveled by fluorescence microscopy. J. Am. Chem. Soc. 2008, 130, 5763–5772. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Rodriguez, M.; Pena, P.; De Aza, A.; De Aza, S.; Ferraz, M.; Monteiro, F. Physical characterization of hydroxyapatite porous scaffolds for tissue engineering. Mater. Sci. Eng. C 2009, 29, 1510–1514. [Google Scholar] [CrossRef]
- Cheng, M.-q.; Wahafu, T.; Jiang, G.-f.; Liu, W.; Qiao, Y.-q.; Peng, X.-c.; Cheng, T.; Zhang, X.-l.; He, G.; Liu, X.-y. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 2016, 6, 24134. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.C.; Chian, K.S.; Leong, K.F. Cryogenic prototyping of chitosan scaffolds with controlled micro and macro architecture and their effect on in vivo neo-vascularization and cellular infiltration. J. Biomed. Mater. Res. Part A 2010, 94, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Philippart, A.; Boccaccini, A.R.; Fleck, C.; Schubert, D.W.; Roether, J.A. Toughening and functionalization of bioactive ceramic and glass bone scaffolds by biopolymer coatings and infiltration: A review of the last 5 years. Expert Rev. Med. Devices 2015, 12, 93–111. [Google Scholar] [CrossRef]
- Mohamad Yunos, D.; Bretcanu, O.; Boccaccini, A.R. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 2008, 43, 4433–4442. [Google Scholar] [CrossRef]
- Bertolla, L.; Dlouhý, I.; Philippart, A.; Boccaccini, A. Mechanical reinforcement of Bioglass®-based scaffolds by novel polyvinyl-alcohol/microfibrillated cellulose composite coating. Mater. Lett. 2014, 118, 204–207. [Google Scholar] [CrossRef]
- Peroglio, M.; Gremillard, L.; Chevalier, J.; Chazeau, L.; Gauthier, C.; Hamaide, T. Toughening of bio-ceramics scaffolds by polymer coating. J. Eur. Ceram. Soc. 2007, 27, 2679–2685. [Google Scholar] [CrossRef]
- Pezzotti, G.; Asmus, S. Fracture behavior of hydroxyapatite/polymer interpenetrating network composites prepared by in situ polymerization process. Mater. Sci. Eng. A 2001, 316, 231–237. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Sánchez, M.; Gamero, P.; Cortés, D. Bioactivity assessment of ZSM-5 type zeolite functionalized with silver or zinc. Mater. Lett. 2012, 74, 250–253. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Hadisi, Z.; Hamzah, E.; Ismail, A.; Aziz, M.; Kashefian, M. Drug delivery and cytocompatibility of ciprofloxacin loaded gelatin nanofibers-coated Mg alloy. Mater. Lett. 2017, 207, 179–182. [Google Scholar] [CrossRef]
- Yunos, D.; Ahmad, Z.; Salih, V.; Boccaccini, A.R. Stratified scaffolds for osteochondral tissue engineering applications: Electrospun PDLLA nanofibre coated Bioglass®-derived foams. J. Biomater. Appl. 2013, 27, 537–551. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Nooeaid, P.; Roether, J.A.; Schubert, D.W.; Boccaccini, A.R. Preparation and characterization of vancomycin releasing PHBV coated 45S5 Bioglass®-based glass–ceramic scaffolds for bone tissue engineering. J. Eur. Ceram. Soc. 2014, 34, 505–514. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Boughton, P.; Zreiqat, H. Improvement of mechanical and biological properties of porous CaSiO3 scaffolds by poly (D, L-lactic acid) modification. Acta Biomater. 2008, 4, 343–353. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Akkapeddi, P.; Manjunath, G.B.; Haldar, J. Membrane active vancomycin analogues: A strategy to combat bacterial resistance. J. Med. Chem. 2014, 57, 4558–4568. [Google Scholar] [CrossRef]
- Karakeçili, A.; Topuz, B.; Korpayev, S.; Erdek, M. Metal-organic frameworks for on-demand pH controlled delivery of vancomycin from chitosan scaffolds. Mater. Sci. Eng. C 2019, 105, 110098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Gu, J.; Yang, H.; Nie, J.; Ma, G. Electrodeposition of alginate/chitosan layer-by-layer composite coatings on titanium substrates. Carbohydr. Polym. 2014, 103, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhsheshi-Rad, H.; Hamzah, E.; Ismail, A.; Aziz, M.; Hadisi, Z.; Kashefian, M.; Najafinezhad, A. Novel nanostructured baghdadite-vancomycin scaffolds: In-vitro drug release, antibacterial activity and biocompatibility. Mater. Lett. 2017, 209, 369–372. [Google Scholar] [CrossRef]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, R.; Li, D.; Zhang, Z.-Y.; Liu, G.; Liang, H.; Qin, Y.; Yu, J.; Li, Y. Fabrication of bioactive 3D printed porous titanium implants with Sr ion-incorporated zeolite coatings for bone ingrowth. J. Mater. Chem. B 2018, 6, 3254–3261. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, M.; Wang, Y. Immobilization of laccase by alginate–chitosan microcapsules and its use in dye decolorization. World J. Microbiol. Biotechnol. 2007, 23, 159–166. [Google Scholar] [CrossRef]
- Ghosh, M.; Halperin-Sternfeld, M.; Grinberg, I.; Adler-Abramovich, L. Injectable alginate-peptide composite hydrogel as a scaffold for bone tissue regeneration. Nanomaterials 2019, 9, 497. [Google Scholar] [CrossRef] [Green Version]
- Jang, C.H.; Cho, Y.B.; Choi, C.H.; Jang, Y.S.; Jung, W.-K.; Lee, H.; Kim, G.H. Effect of umbilical cord serum coated 3D PCL/alginate scaffold for mastoid obliteration. Int. J. Pediatric Otorhinolaryngol. 2014, 78, 1061–1065. [Google Scholar] [CrossRef]
- Senila, L.; Hoaghia, A.; Moldovan, A.; Török, I.A.; Kovacs, D.; Simedru, D.; Tomoiag, C.H.; Senila, M. The Potential Application of Natural Clinoptilolite-Rich Zeolite as Support for Bacterial Community Formation for Wastewater Treatment. Materials 2022, 15, 3685. [Google Scholar] [CrossRef]
- Król, M.; Syguła-Cholewińska, J.; Sawoszczuk, T. Zeolite-Supported Aggregate as Potential Antimicrobial Agents in Gypsum Composites. Materials 2022, 15, 3305. [Google Scholar] [CrossRef] [PubMed]
- Pahlevanzadeh, F.; Mokhtari, H.; Bakhsheshi-Rad, H.R.; Emadi, R.; Kharaziha, M.; Valiani, A.; Poursamar, S.A.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Recent trends in three-dimensional bioinks based on alginate for biomedical applications. Materials 2020, 13, 3980. [Google Scholar] [CrossRef] [PubMed]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. Carbon nanotubes (CNTs)-reinforced magnesium-based matrix composites: A comprehensive review. Materials 2020, 13, 4421. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, W.; Zhao, X.; Sheng, X.; Hu, Z.; Wang, Q.; Chen, Z.; Wang, S.; Zhang, X.; Wang, X. Efficient Adsorption-Assisted Photocatalysis Degradation of Congo Red through Loading ZIF-8 on KI-Doped TiO2. Materials 2022, 15, 2857. [Google Scholar] [CrossRef]
- Montesano, G.; Cappelletti, P.; Caputo, D.; Liguori, B.; Campanile, A.; Rispoli, C. Mineralogical and Technological Characterization of Zeolites from Basin and Range as Pozzolanic Addition of Cement. Materials 2022, 15, 2684. [Google Scholar] [CrossRef] [PubMed]
- Neag, E.; Stupar, Z.; Torok, A.I.; Surupaceanu, I.; Senila, M.; Cadar, O. Exploring the Properties of Micronized Natural Zeolitic Volcanic Tuff as Cosmetic Ingredient. Materials 2022, 15, 2405. [Google Scholar] [CrossRef]
- Nassrullah, H.; Anis, S.F.; Lalia, B.S.; Hashaikeh, R. Cellulose Nanofibrils as a Damping Material for the Production of Highly Crystalline Nanosized Zeolite Y via Ball Milling. Materials 2022, 15, 2258. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, H.; Zhang, F.; Ji, K.; Liu, P.; Liu, Z.; Zhang, K. Effect of Preparation Method on the Catalytic Performance of HZSM-5 Zeolite Catalysts in the MTH Reaction. Materials 2022, 15, 2206. [Google Scholar] [CrossRef]
- Thomas, M.; Osińska, M.; Ślosarczyk, A. Long-Term Behavior of Cement Mortars Based on Municipal Solid Waste Slag and Natural Zeolite—A Comprehensive Physico-Mechanical, Structural and Chemical Assessment. Materials 2022, 15, 1001. [Google Scholar] [CrossRef]
- Tontisirin, S.; Phalakornkule, C.; Sa-ngawong, W.; Sirisawat, S. Magnetic Induction Assisted Heating Technique in Hydrothermal Zeolite Synthesis. Materials 2022, 15, 689. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslani, Z.; Nazemi, N.; Rajabi, N.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Kasiri-Asgarani, M.; Najafinezhad, A.; Ismail, A.F.; Sharif, S.; Berto, F. Antibacterial Activity and Cell Responses of Vancomycin-Loaded Alginate Coating on ZSM-5 Scaffold for Bone Tissue Engineering Applications. Materials 2022, 15, 4786. https://doi.org/10.3390/ma15144786
Aslani Z, Nazemi N, Rajabi N, Kharaziha M, Bakhsheshi-Rad HR, Kasiri-Asgarani M, Najafinezhad A, Ismail AF, Sharif S, Berto F. Antibacterial Activity and Cell Responses of Vancomycin-Loaded Alginate Coating on ZSM-5 Scaffold for Bone Tissue Engineering Applications. Materials. 2022; 15(14):4786. https://doi.org/10.3390/ma15144786
Chicago/Turabian StyleAslani, Z., N. Nazemi, N. Rajabi, M. Kharaziha, H. R. Bakhsheshi-Rad, M. Kasiri-Asgarani, A. Najafinezhad, A. F. Ismail, S. Sharif, and F. Berto. 2022. "Antibacterial Activity and Cell Responses of Vancomycin-Loaded Alginate Coating on ZSM-5 Scaffold for Bone Tissue Engineering Applications" Materials 15, no. 14: 4786. https://doi.org/10.3390/ma15144786






