Characteristics and Application of Eugenol in the Production of Epoxy and Thermosetting Resin Composites: A Review
Abstract
:1. Introduction
2. Chemical Characterization of Eugenol Derived Resins and Auxiliary Components for Composites
2.1. Characteristics of Eugenol
2.2. Characterization of Eugenol Derived Compounds
3. Properties of Composites Based on Eugenol Derived Resins and Additives and Coupling Agents
3.1. Epoxy Resin Composites
3.2. Thermosetting Resins Composites
4. Discussion
5. Conclusions
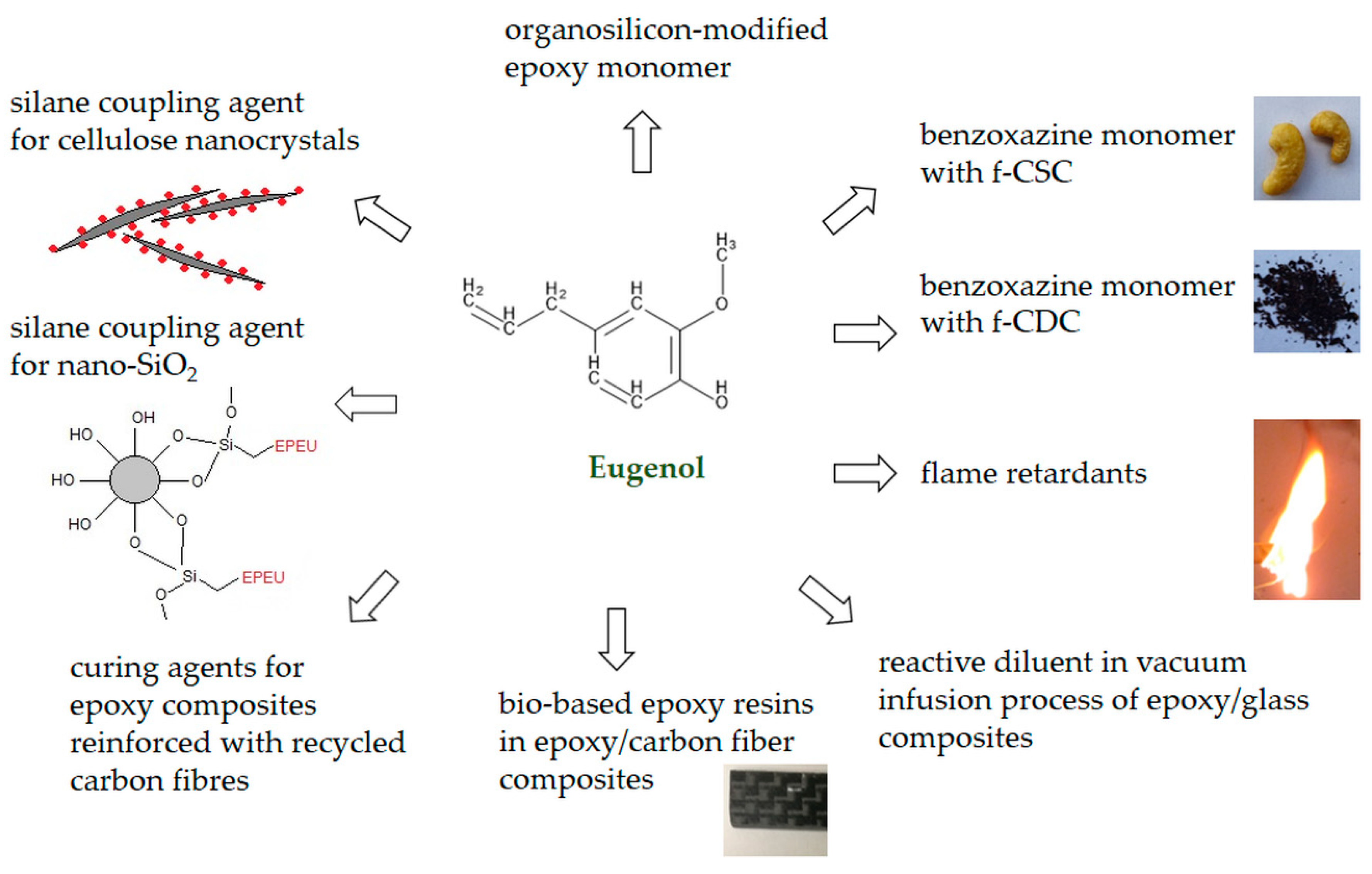
- Silane coupling agent based on eugenol, used for natural fillers such as cellulose nanocrystals, and nano-SiO2, leading to the improvement of thermo-mechanical properties of epoxy composites with their addition;
- Flame retardants such as diepoxy isoeugenol phenyl phosphate and a eugenol-based flame retardant containing phosphorus and silicon provide high flame retardancy to epoxy materials;
- Eugenol glycidyl ether is used as an effective diluent for epoxy-ester resins during vacuum infusion used in the production of epoxy composites with glass fiber;
- Diamine-allyl-eugenol is a hardener for epoxy resins based on resorcinol, providing higher gelling temperatures for this system and increasing the use of this material as a matrix for carbon fiber reinforced materials.
- Eugenol–benzoxazine monomer as a matrix for composites with carbon from cashew nut shells;
- Hetero structured benzoxazine monomer as a matrix for composites with functionalized cow dung carbon;
- Organosilicon-modified epoxy monomer with self-repairing properties;
- Epoxy resin (tris (2-methoxy-4-(oxiran-2-ylmethyl) phenyl) benzene-1,3,5-tricarboxylate) as a matrix for composites with carbon fibre.
Funding
Conflicts of Interest
References
- Polman, E.M.N.; Gruter, G.-J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Mysiukiewicz, O.; Kuzmin, A. Coffee Silverskin as a Multifunctional Waste Filler for High-Density Polyethylene Green Composites. J. Compos. Sci. 2021, 5, 44. [Google Scholar] [CrossRef]
- Ding, C.; Matharu, A.S. Recent Developments on Biobased Curing Agents: A Review of Their Preparation and Use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Datta, J.; Włoch, M. Selected biotrends in development of epoxy resins and their composites. Polym. Bull. 2014, 71, 3035–3049. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2016, 37, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, A.Q.; da Silva, L.F.M.; Abenojar, J.; Figueiredo, M.; Öchsner, A. Toughness of a brittle epoxy resin reinforced with micro cork particles: Effect of size, amount and surface treatment. Compos. Part B Eng. 2017, 114, 299–310. [Google Scholar] [CrossRef]
- Maiorana, A.; Spinella, S.; Gross, R.A. Bio-Based Alternative to the Diglycidyl Ether of Bisphenol A with Controlled Materials Properties. Biomacromolecules 2015, 16, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Mustata, F.; Tudorachi, N.; Rosu, D. Curing and thermal behavior of resin matrix for composites based on epoxidized soybean oil/diglycidyl ether of bisphenol A. Compos. Part B Eng. 2011, 42, 1803–1812. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Na, H.; Wu, Y.; Zheng, W.; Zhu, J. How a bio-based epoxy monomer enhanced the properties of diglycidyl ether of bisphenol A (DGEBA)/graphene composites. J. Mater. Chem. A 2013, 1, 5081. [Google Scholar] [CrossRef]
- Yim, Y.-J.; Rhee, K.Y.; Park, S.-J. Fracture toughness and ductile characteristics of diglycidyl ether of bisphenol-A resins modified with biodegradable epoxidized linseed oil. Compos. Part B Eng. 2017, 131, 144–152. [Google Scholar] [CrossRef]
- Ramon, E.; Sguazzo, C.; Moreira, P. A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace 2018, 5, 110. [Google Scholar] [CrossRef] [Green Version]
- Pin, J.-M.; Sbirrazzuoli, N.; Mija, A. From Epoxidized Linseed Oil to Bioresin: An Overall Approach of Epoxy/Anhydride Cross-Linking. ChemSusChem 2015, 8, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, T.; Liu, X.; Zhu, J. Research progress on bio-based thermosetting resins. Polym. Int. 2016, 65, 164–173. [Google Scholar] [CrossRef]
- Bobade, S.K.; Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Bio-Based Thermosetting Resins for Future Generation: A Review. Polym. Plast. Technol. Eng. 2016, 55, 1863–1896. [Google Scholar] [CrossRef]
- Rao, B.S.; Palanisamy, A. Synthesis of bio based low temperature curable liquid epoxy, benzoxazine monomer system from cardanol: Thermal and viscoelastic properties. Eur. Polym. J. 2013, 49, 2365–2376. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Gangireddy, C.S.R.; Wang, J.; Pan, Y.; Xing, W.; Song, L.; Hu, Y. Cardanol derived benzoxazine in combination with boron-doped graphene toward simultaneously improved toughening and flame retardant epoxy composites. Compos. Part A Appl. Sci. Manuf. 2019, 116, 13–23. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Liu, Y.-Y.; Weng, Y.; Li, Y.-D.; Zeng, J.-B. Sustainable Epoxy Vitrimers from Epoxidized Soybean Oil and Vanillin. ACS Sustain. Chem. Eng. 2020, 8, 15020–15029. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; He, J.; Li, Y.-D.; Zhao, X.-L.; Zeng, J.-B. Biobased epoxy vitrimer from epoxidized soybean oil for reprocessable and recyclable carbon fiber reinforced composite. Compos. Commun. 2020, 22, 100445. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; He, J.; Li, Y.-D.; Zhao, X.-L.; Zeng, J.-B. Biobased, reprocessable and weldable epoxy vitrimers from epoxidized soybean oil. Ind. Crops Prod. 2020, 153, 112576. [Google Scholar] [CrossRef]
- Yang, X.; Guo, L.; Xu, X.; Shang, S.; Liu, H. A fully bio-based epoxy vitrimer: Self-healing, triple-shape memory and reprocessing triggered by dynamic covalent bond exchange. Mater. Des. 2020, 186, 108248. [Google Scholar] [CrossRef]
- Nicolas, S.; Richard, T.; Dourdan, J.; Lemiègre, L.; Audic, J. Shape memory epoxy vitrimers based on waste frying sunflower oil. J. Appl. Polym. Sci. 2021, 138, 50904. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, P.; Dai, S.; Zhang, H.; Bi, L.; Jiang, J.; Chen, Y. Catalyst-free self-healing fully bio-based vitrimers derived from tung oil: Strong mechanical properties, shape memory, and recyclability. Ind. Crops Prod. 2021, 171, 113978. [Google Scholar] [CrossRef]
- Borah, N.; Karak, N. Tannic acid based bio-based epoxy thermosets: Evaluation of thermal, mechanical, and biodegradable behaviors. J. Appl. Polym. Sci. 2022, 139. [Google Scholar] [CrossRef]
- Fache, M.; Viola, A.; Auvergne, R.; Boutevin, B.; Caillol, S. Biobased epoxy thermosets from vanillin-derived oligomers. Eur. Polym. J. 2015, 68, 526–535. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkita, T. Fully biobased epoxy resin systems composed of a vanillin-derived epoxy resin and renewable phenolic hardeners. Eur. Polym. J. 2017, 92, 165–173. [Google Scholar] [CrossRef]
- Nabipour, H.; Niu, H.; Wang, X.; Batool, S.; Hu, Y. Fully bio-based epoxy resin derived from vanillin with flame retardancy and degradability. React. Funct. Polym. 2021, 168, 105034. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Liu, T.; Ou, R.; Lin, J.; Puglia, D.; Xu, P.; Wang, Q.; Dong, W.; Du, M.; et al. Design of Intrinsically Flame-Retardant Vanillin-Based Epoxy Resin for Thermal-Conductive Epoxy/Graphene Aerogel Composites. ACS Appl. Mater. Interfaces 2021, 13, 59341–59351. [Google Scholar] [CrossRef]
- Dai, J.; Teng, N.; Liu, J.; Feng, J.; Zhu, J.; Liu, X. Synthesis of bio-based fire-resistant epoxy without addition of flame retardant elements. Compos. Part B Eng. 2019, 179, 107523. [Google Scholar] [CrossRef]
- van de Pas, D.J.; Torr, K.M. Biobased Epoxy Resins from Deconstructed Native Softwood Lignin. Biomacromolecules 2017, 18, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C. (Charles) Synthesis of lignin-based epoxy resins: Optimization of reaction parameters using response surface methodology. RSC Adv. 2014, 4, 31745–31753. [Google Scholar] [CrossRef]
- Gioia, C.; Colonna, M.; Tagami, A.; Medina, L.; Sevastyanova, O.; Berglund, L.A.; Lawoko, M. Lignin-Based Epoxy Resins: Unravelling the Relationship between Structure and Material Properties. Biomacromolecules 2020, 21, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Nejad, S.; Özgüneş, H.; Başaran, N. Pharmacological and Toxicological Properties of Eugenol. Turkish J. Pharm. Sci. 2017, 14, 201–206. [Google Scholar] [CrossRef]
- Santiago, D.; Guzmán, D.; Ramis, X.; Ferrando, F.; Serra, À. New Epoxy Thermosets Derived from Clove Oil Prepared by Epoxy-Amine Curing. Polymers 2019, 12, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qing, Y.; Nan, H.; Luo, F.; Zhou, W. Nitrogen-doped graphene and titanium carbide nanosheet synergistically reinforced epoxy composites as high-performance microwave absorbers. RSC Adv. 2017, 7, 27755–27761. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Sun, L.; Zhang, Y.; Liu, Q.; Ru, C.; Zhang, W.; Zhao, C. Novel biobased epoxy resin thermosets derived from eugenol and vanillin. Polym. Degrad. Stab. 2019, 160, 45–52. [Google Scholar] [CrossRef]
- Chen, C.-H.; Tung, S.-H.; Jeng, R.-J.; Abu-Omar, M.M.; Lin, C.-H. A facile strategy to achieve fully bio-based epoxy thermosets from eugenol. Green Chem. 2019, 21, 4475–4488. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Wang, L.; Li, Y.; Liu, W.; Xin, J.; Zhang, J. Eugenol-Derived Biobased Epoxy: Shape Memory, Repairing, and Recyclability. Macromolecules 2017, 50, 8588–8597. [Google Scholar] [CrossRef]
- Kumar, B.; Agumba, D.O.; Pham, D.H.; Kim, H.C.; Kim, J. Recent progress in bio-based eugenol resins: From synthetic strategies to structural properties and coating applications. J. Appl. Polym. Sci. 2022, 139, 51532. [Google Scholar] [CrossRef]
- Krishnan, S.; Arumugam, H.; Chavali, M.; Muthukaruppan, A. High dielectric, low curing with high thermally stable renewable eugenol-based polybenzoxazine matrices and nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47050. [Google Scholar] [CrossRef]
- Nal, P.; Mestry, S.; Mapari, S.; Mhaske, S. Eugenol/vanillin-derived novel triarylmethane-based crosslinking agent for epoxy coating. Iran. Polym. J. 2019, 28, 685–695. [Google Scholar] [CrossRef]
- Gazzotti, S.; Hakkarainen, M.; Adolfsson, K.H.; Ortenzi, M.A.; Farina, H.; Lesma, G.; Silvani, A. One-Pot Synthesis of Sustainable High-Performance Thermoset by Exploiting Eugenol Functionalized 1,3-Dioxolan-4-one. ACS Sustain. Chem. Eng. 2018, 6, 15201–15211. [Google Scholar] [CrossRef]
- Chen, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-modified polysiloxanes as effective anticorrosion additives for epoxy resin coatings. RSC Adv. 2017, 7, 55967–55976. [Google Scholar] [CrossRef] [Green Version]
- Molina-Gutiérrez, S.; Vacche, S.D.; Vitale, A.; Ladmiral, V.; Caillol, S.; Bongiovanni, R.; Lacroix-Desmazes, P. Photoinduced polymerization of eugenol-derived methacrylates. Molecules 2020, 25, 3444. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahim, E.; Sanda, F.; Masuda, T. Synthesis and properties of novel eugenol-based polymers. Polym. Bull. 2004, 52, 93–100. [Google Scholar] [CrossRef]
- Rojo, L.; Barcenilla, J.M.; Vázquez, B.; González, R.; San Román, J. Intrinsically antibacterial materials based on polymeric derivatives of eugenol for biomedical applications. Biomacromolecules 2008, 9, 2530–2535. [Google Scholar] [CrossRef]
- Kim, M.-T.; Rhee, K.-Y. Flexural behavior of carbon nanotube-modified epoxy/basalt composites. Carbon Lett. 2011, 12, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Kayaci, F.; Ertas, Y.; Uyar, T. Enhanced thermal stability of eugenol by cyclodextrin inclusion complex encapsulated in electrospun polymeric nanofibers. J. Agric. Food Chem. 2013, 61, 8156–8165. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, X.; Chen, X.; Li, J.; Huang, Q.; Hu, Z.; Tu, Y. Self-healing polymers based on eugenol via combination of thiol-ene and thiol oxidation reactions. J. Polym. Res. 2016, 23, 110. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, M.; Wei, S. Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the Food Antisepsis Field. J. Food Sci. 2018, 83, 1476–1483. [Google Scholar] [CrossRef] [Green Version]
- Dighe, V.V.; Gursale, A.A.; Sane, R.T.; Menon, S.; Patel, P.H. Quantitative Determination of Eugenol from Cinnamomum tamala Nees and Eberm. Leaf Powder and Polyherbal Formulation Using Reverse Phase Liquid Chromatography. Chromatographia 2005, 61, 443–446. [Google Scholar] [CrossRef]
- Kumbar, S.M.; Shanbhag, G.V.; Halligudi, S.B. Synthesis of monoallyl guaiacol via allylation using HY zeolite. J. Mol. Catal. A Chem. 2006, 244, 278–282. [Google Scholar] [CrossRef]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef]
- Rojo, L.; Vazquez, B.; Parra, J.; López Bravo, A.; Deb, S.; San Roman, J. From Natural Products to Polymeric Derivatives of “Eugenol”: A New Approach for Preparation of Dental Composites and Orthopedic Bone Cements. Biomacromolecules 2006, 7, 2751–2761. [Google Scholar] [CrossRef]
- Harvey, B.G.; Guenthner, A.J.; Yandek, G.R.; Cambrea, L.R.; Meylemans, H.A.; Baldwin, L.C.; Reams, J.T. Synthesis and characterization of a renewable cyanate ester/polycarbonate network derived from eugenol. Polymer 2014, 55, 5073–5079. [Google Scholar] [CrossRef]
- Cheng, C.; Li, J.; Yang, F.; Li, Y.; Hu, Z.; Wang, J. Renewable eugenol-based functional polymers with self-healing and high temperature resistance properties. J. Polym. Res. 2018, 25, 57. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Vargas Méndez, L.Y. Synthesis of eugenol-based monomers for sustainable epoxy thermoplastic polymers. J. Appl. Polym. Sci. 2022, 139, 52237. [Google Scholar] [CrossRef]
- Yang, Y.; Song, L.X. Study on the Inclusion Compounds of Eugenol with α-, β-, γ- and Heptakis (2,6-di-O-methyl)-β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 27–33. [Google Scholar] [CrossRef]
- Ayoubi, R.; Wali, S.; Singh, G.B. The UV and FTIR Fingerprint of Ocimum kilimandscharicum Guerke Essential oil: A Eugenol-Rich Chemo Type. Int. J. Innov. Res. Sci. Stud. 2022, 5, 1–9. [Google Scholar] [CrossRef]
- Miao, J.-T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased epoxy resin derived from eugenol with excellent integrated performance and high renewable carbon content. Polym. Int. 2018, 67, 1194–1202. [Google Scholar] [CrossRef]
- Dai, J.; Yang, S.; Teng, N.; Liu, Y.; Liu, X.; Zhu, J.; Zhao, J. Synthesis of Eugenol-Based Silicon-Containing Benzoxazines and Their Applications as Bio-Based Organic Coatings. Coatings 2018, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Capricho, J.C.; Fox, B.; Hameed, N. Multifunctionality in Epoxy Resins. Polym. Rev. 2020, 60, 1–41. [Google Scholar] [CrossRef]
- Aziz, T.; Zheng, J.; Jamil, M.I.; Fan, H.; Ullah, R.; Iqbal, M.; Ali, A.; Khan, F.U.; Ullah, A. Enhancement in Adhesive and Thermal Properties of Bio-based Epoxy Resin by Using Eugenol Grafted Cellulose Nanocrystals. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3290–3300. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, X.; Cao, J.; Chen, R.; Aziz, T.; Fan, H.; Bittencourt, C. Behavior of epoxy resin filled with nano-SiO2 treated with a Eugenol epoxy silane. J. Appl. Polym. Sci. 2021, 138. [Google Scholar] [CrossRef]
- Vaithilingam, S.; ThangavelRavivarman, R.; Muthukaruppan, A. Development of cashew nut shell carbon reinforced thiourea based biophenolic benzoxazine-epoxy composites: High performance biobased coating materials. Polym. Compos. 2020, 41, 1950–1961. [Google Scholar] [CrossRef]
- Selvaraj, V.; Raghavarshini, T.R.; Alagar, M. Design and development of bio-carbon reinforced hetero structured biophenolics polybenzoxazine-epoxy hybrid composites for high performance applications. J. Polym. Res. 2021, 28, 1–13. [Google Scholar] [CrossRef]
- Li, W.; Xiao, L.; Wang, Y.; Chen, J.; Nie, X. Self-healing silicon-containing eugenol-based epoxy resin based on disulfide bond exchange: Synthesis and structure-property relationships. Polymer 2021, 229, 123967. [Google Scholar] [CrossRef]
- Reddy, K.S.K.; Gao, W.-J.; Chen, C.-H.; Juang, T.-Y.; Abu-Omar, M.M.; Lin, C.-H. Degradation of Thermal-Mechanically Stable Epoxy Thermosets, Recycling of Carbon Fiber, and Reapplication of the Degraded Products. ACS Sustain. Chem. Eng. 2021, 9, 5304–5314. [Google Scholar] [CrossRef]
- Pourchet, S.; Sonnier, R.; Ben-Abdelkader, M.; Gaillard, Y.; Ruiz, Q.; Placet, V.; Plasseraud, L.; Boni, G. New Reactive Isoeugenol Based Phosphate Flame Retardant: Toward Green Epoxy Resins. ACS Sustain. Chem. Eng. 2019, 7, 14074–14088. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Zhang, T.; Li, J.; Tan, J.; Zhang, M.; Zhou, Y.; Zhu, X. Facile Synthesis of Eugenol-Based Phosphorus/Silicon-Containing Flame Retardant and Its Performance on Fire Retardancy of Epoxy Resin. ACS Appl. Polym. Mater. 2022, 4, 1794–1804. [Google Scholar] [CrossRef]
- Yue, L.; Maiorana, A.; Patel, A.; Gross, R.; Manas-Zloczower, I. A sustainable alternative to current epoxy resin matrices for vacuum infusion molding. Compos. Part A Appl. Sci. Manuf. 2017, 100, 269–274. [Google Scholar] [CrossRef]
- Mattar, N.; de Anda, A.R.; Vahabi, H.; Renard, E.; Langlois, V. Resorcinol-Based Epoxy Resins Hardened with Limonene and Eugenol Derivatives: From the Synthesis of Renewable Diamines to the Mechanical Properties of Biobased Thermosets. ACS Sustain. Chem. Eng. 2020, 8, 13064–13075. [Google Scholar] [CrossRef]
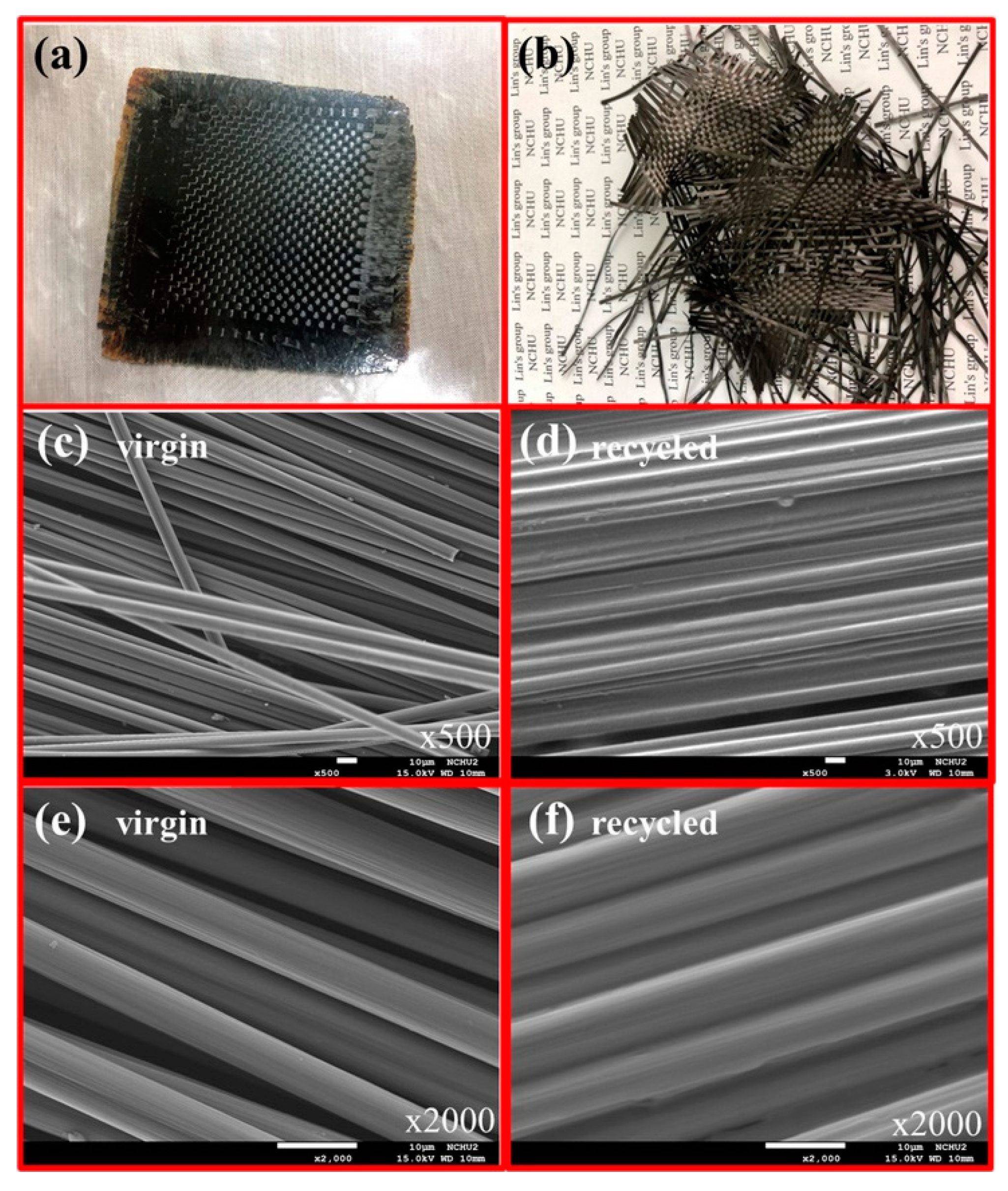
- Mattar, N.; Langlois, V.; Renard, E.; Rademacker, T.; Hübner, F.; Demleitner, M.; Altstädt, V.; Ruckdäschel, H.; Rios de Anda, A. Fully Bio-Based Epoxy-Amine Thermosets Reinforced with Recycled Carbon Fibers as a Low Carbon-Footprint Composite Alternative. ACS Appl. Polym. Mater. 2021, 3, 426–435. [Google Scholar] [CrossRef]
- Jeyapragash, R.; Srinivasan, V.; Sathiyamurthy, S. Mechanical properties of natural fiber/particulate reinforced epoxy composites–A review of the literature. Mater. Today Proc. 2020, 22, 1223–1227. [Google Scholar] [CrossRef]
- Reddy, P.V.; Reddy, R.V.S.; Rajendra Prasad, P.; Mohana Krishnudu, D.; Reddy, R.M.; Rao, H.R. Evaluation of Mechanical and Wear Performances of Natural Fiber Reinforced Epoxy Composites. J. Nat. Fibers 2020, 19, 2218–2231. [Google Scholar] [CrossRef]
- Barczewski, M.; Matykiewicz, D.; Szostak, M. The effect of two-step surface treatment by hydrogen peroxide and silanization of flax/cotton fabrics on epoxy-based laminates thermomechanical properties and structure. J. Mater. Res. Technol. 2020, 9, 13813–13824. [Google Scholar] [CrossRef]
- Anne, B. Environmental-Friendly Biodegradable Polymers and Composites. In Integrated Waste Management-Volume I; InTech: London, UK, 2011. [Google Scholar]
- Li, C.; Fan, H.; Aziz, T.; Bittencourt, C.; Wu, L.; Wang, D.-Y.; Dubois, P. Biobased Epoxy Resin with Low Electrical Permissivity and Flame Retardancy: From Environmental Friendly High-Throughput Synthesis to Properties. ACS Sustain. Chem. Eng. 2018, 6, 8856–8867. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, C.-M.; Juang, T.-Y.; Abu-Omar, M.M.; Lin, C.-H. The reaction of activated esters with epoxides for self-curable, highly flexible, A2B2- and A3B3-type epoxy compounds. Polym. Chem. 2019, 10, 3983–3995. [Google Scholar] [CrossRef]
- Modjinou, T.; Versace, D.-L.; Abbad-Andallousi, S.; Bousserrhine, N.; Dubot, P.; Langlois, V.; Renard, E. Antibacterial and antioxidant bio-based networks derived from eugenol using photo-activated thiol-ene reaction. React. Funct. Polym. 2016, 101, 47–53. [Google Scholar] [CrossRef]
- Fan, M.; Weclawski, B. Long natural fibre composites. In Advanced High Strength Natural Fibre Composites in Construction; Elsevier: Amsterdam, The Netherlands, 2017; pp. 141–177. [Google Scholar]
- Zhang, Z.; Zhou, Y.; Cai, L.; Xuan, L.; Wu, X.; Ma, X. Synthesis of eugenol-functionalized polyhedral oligomer silsesquioxane for low-k bismaleimide resin combined with excellent mechanical and thermal properties as well as its composite reinforced by silicon fiber. Chem. Eng. J. 2022, 439, 135740. [Google Scholar] [CrossRef]
- Shibata, M.; Tetramoto, N.; Imada, A.; Neda, M.; Sugimoto, S. Bio-based thermosetting bismaleimide resins using eugenol, bieugenol and eugenol novolac. React. Funct. Polym. 2013, 73, 1086–1095. [Google Scholar] [CrossRef]
- Ning, Y.; Li, D.; Wang, M.; Jiang, L. Bio-resourced eugenol derived phthalonitrile resin for high temperature composite. J. Appl. Polym. Sci. 2021, 138, 50721. [Google Scholar] [CrossRef]
- Wu, Y.; Fei, M.; Chen, T.; Qiu, R.; Liu, W. Biocomposites from bamboo fibers and palm oil-based thermosets: Effects of natural phenolic cross-linkers. Compos. Commun. 2020, 22, 100489. [Google Scholar] [CrossRef]
- Paolillo, S.; Bose, R.K.; Santana, M.H.; Grande, A.M. Intrinsic Self-Healing Epoxies in Polymer Matrix Composites (PMCs) for Aerospace Applications. Polymers 2021, 13, 201. [Google Scholar] [CrossRef]
- Dey, P.; Ray, S. An Overview of the Recent Trends in Manufacturing of Green Composites–Considerations and Challenges. Mater. Today Proc. 2018, 5, 19783–19789. [Google Scholar] [CrossRef]
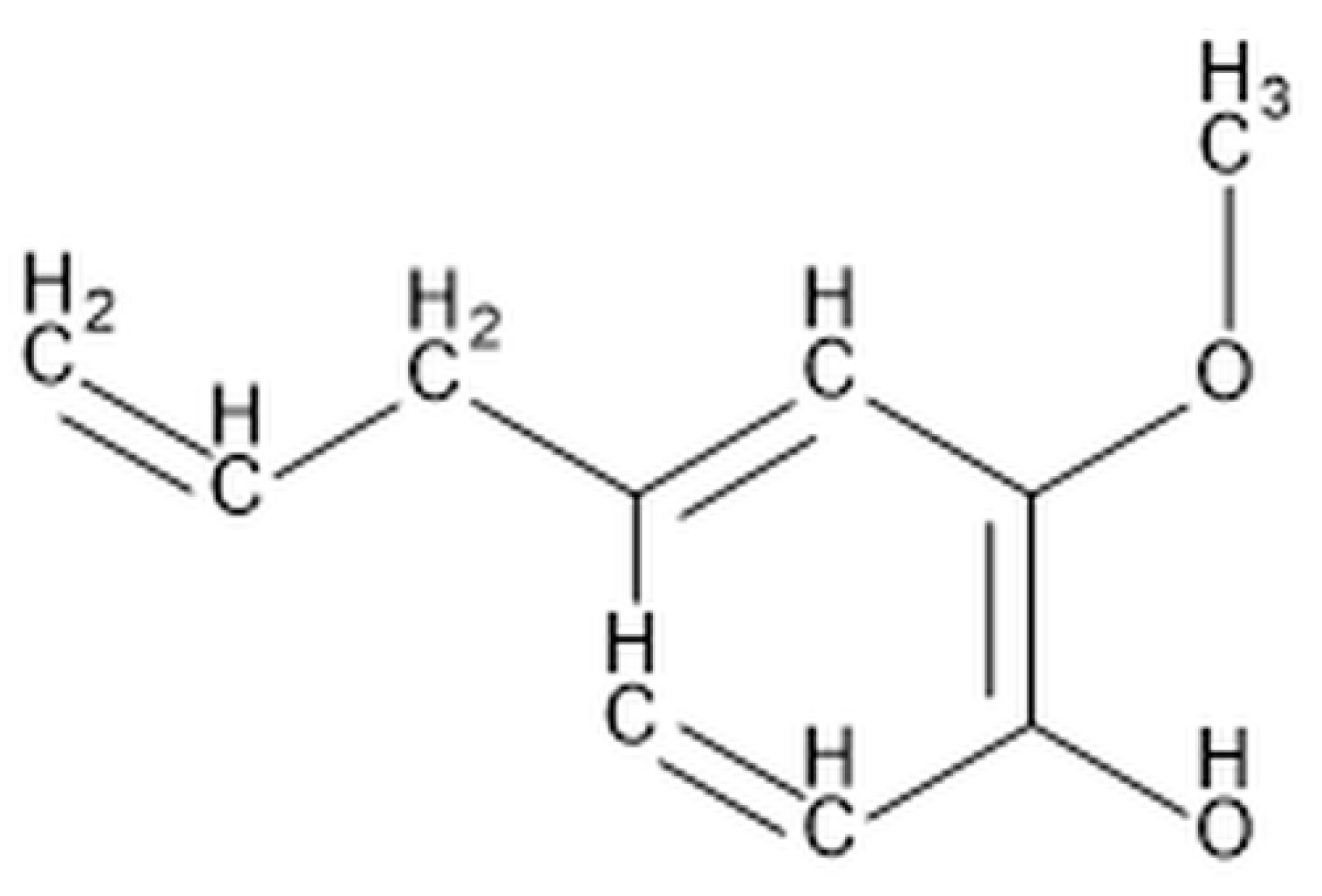
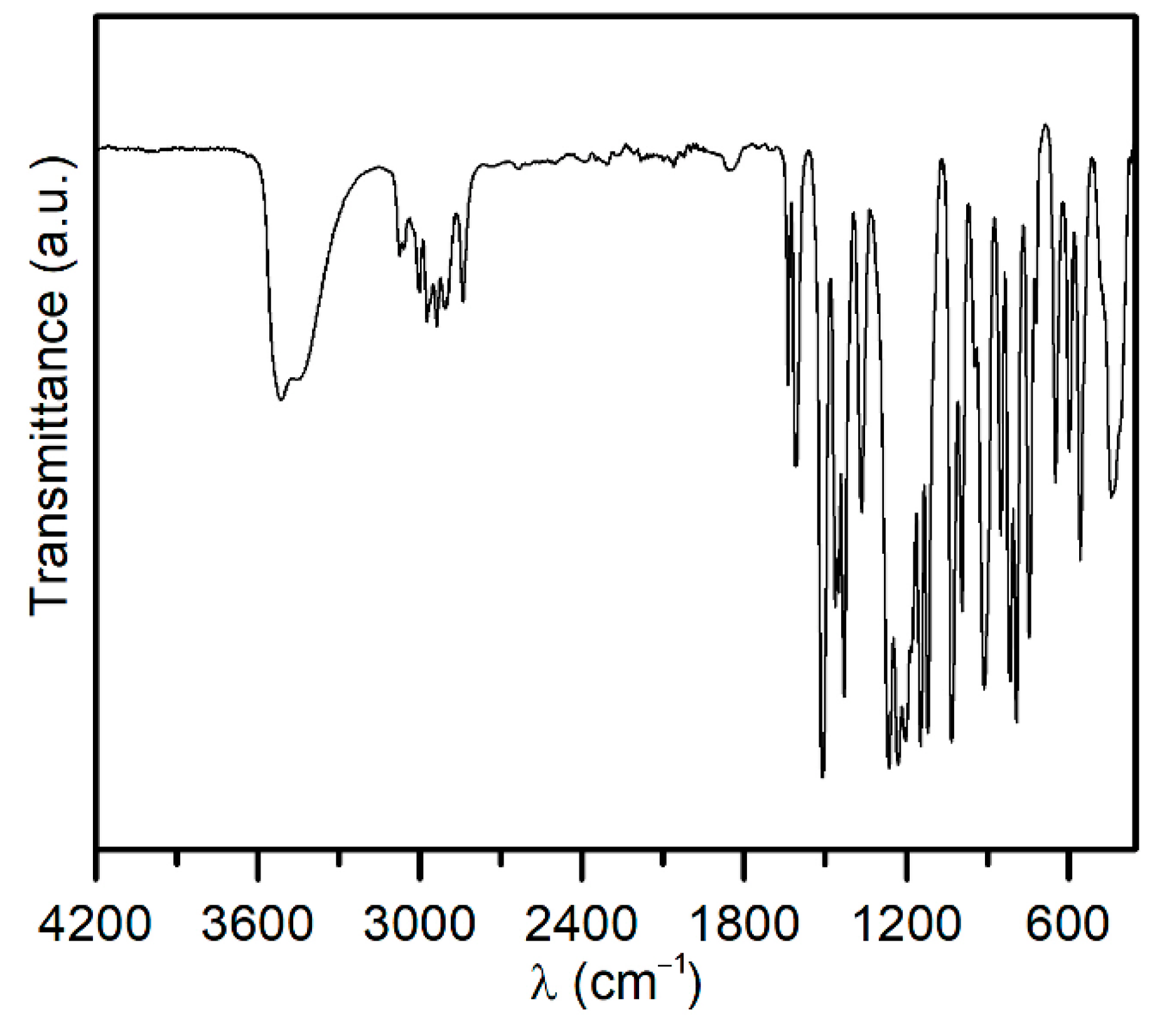
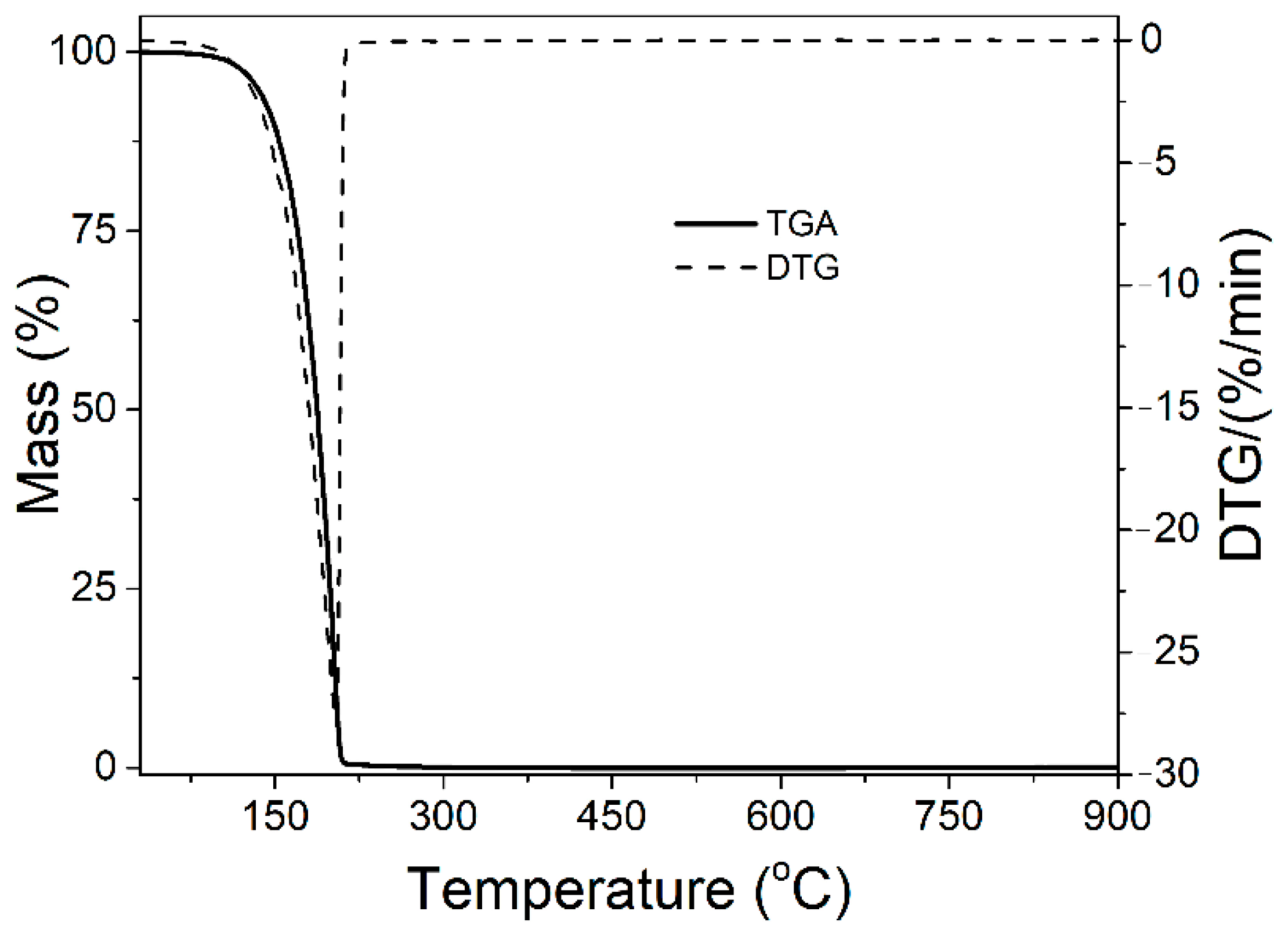
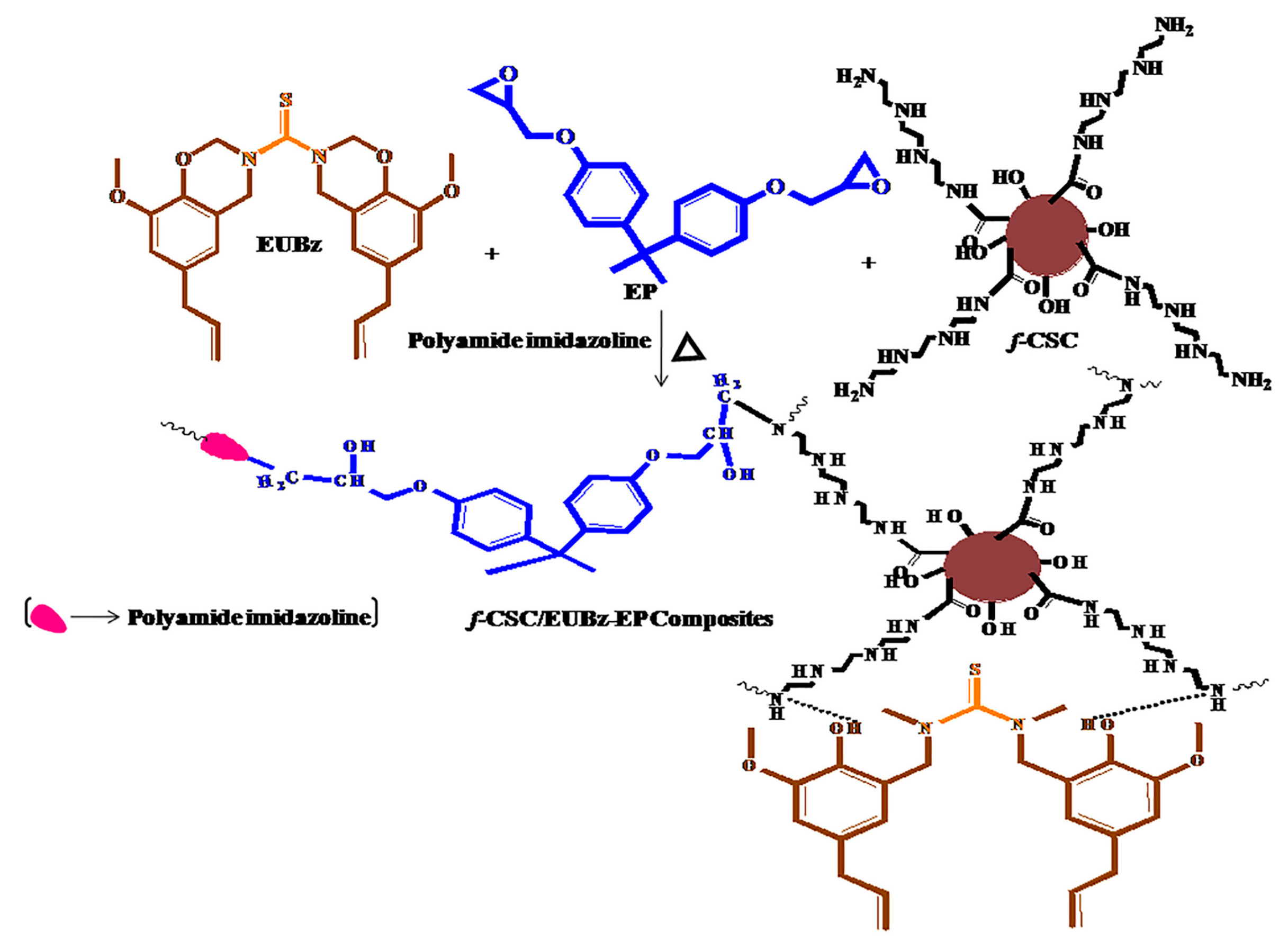
| Name | Type | Synthesis Method | Ref. |
|---|---|---|---|
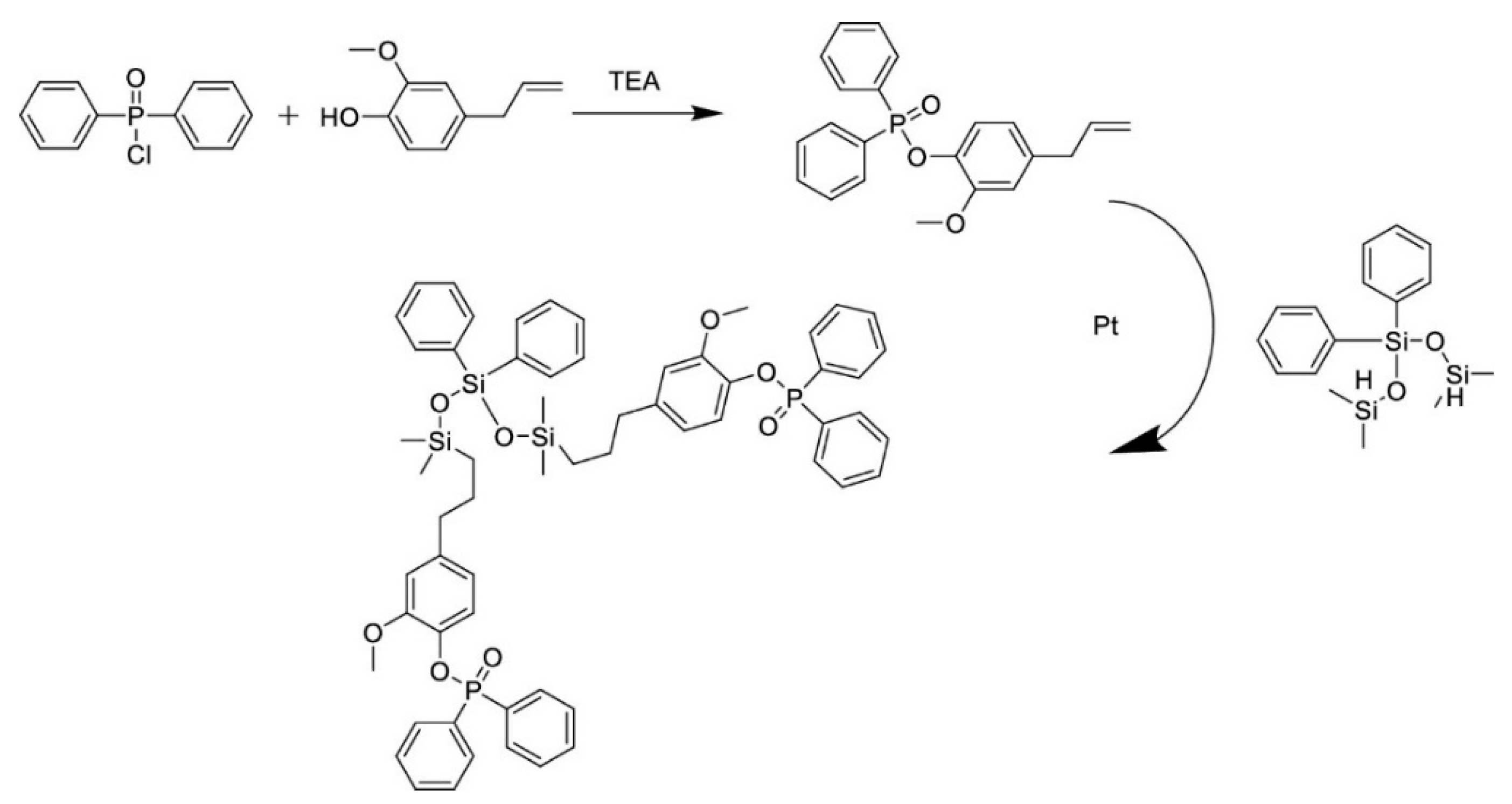
| eugenol based silicone coupling agent | coupling agent | hydrosilylation between triethoxysilane and 3-(4-allyl-2-methoxyphenoxy) propylene 1,2-oxide | [62,63] |
| eugenol–benzoxazine monomer (EBUz) | monomer | thiourea reaction | [64] |
| hetero structured benzoxazine monomer (HSBBz) | monomer | diphenyldiaminomethane reaction | [65] |
| organosilicon-modified epoxy monomer | monomer | melting polymerization of the epoxy group and amidogen with the disulfide bond | [66] |
| eugenol-based epoxy resins | monomer |
| [67] |
| diepoxy-isoeugenol phenyl phosphate (DEpiEPP) | flame retardant |
| [68] |
| eugenol-based flame retardant containing phosphorus (P) and silicon (Si) | flame retardant |
| [69] |
| eugenol monoglycidyl ether (GE) | diluent | glycidylation reaction | [70] |
| diamine-allyl-eugenol (DAAE) | curing agent |
| [71,72] |
| Matrix/ Curing Agent | Type of Filler | Eugenol Derivative | Filler wt. % | Producing Method | Improved Properties | Ref. |
|---|---|---|---|---|---|---|
| bio-based epoxy/ TETA | CNC | eugenol-based silane coupling agent (EBSCA) | 1%, 3%, 5% | sonication, mixing, casting | tensile strength, storage modulus, degradability | [62] |
| epoxy (DGEBA)/ (IPDA) | SiO2 | eugenol epoxy silane (EUPCP), | 1%, 2%, 4% | sonication, mixing, casting | storage modulus, Tg temperature, thermal stability, flexural strength, impact strength | [63] |
| epoxy (DGEBA)/ polyamide imidazoline | f-CSC | eugenol–benzoxazine monomer (EUBz) | 1%, 3%, 5% | mixing, casting | thermal stability, char yield, Tg temperature, anticorrosion futures | [64] |
| epoxy (DGEBA)/ polyamide imidazoline | f-CDC | hetero structured benzoxazine monomer (HSBBz) | 1%, 3%, 5% | mixing, casting | thermal stability, char yield, Tg temperature, anticorrosion futures antibacterial properties | [65] |
| epoxy(DGEBA)/ CA bio-glycidyl ether of epoxyisougenol (GEEpiE)/CA | flame retardant | diepoxy-isoeugenol phenyl phosphate (DEpiEPP) | 1%, 2%, 3%, 4.3% of P | mixing, casting | flame retardancy char yield, | [68] |
| epoxy (DGEBA)/ DDM | flame retardant | a eugenol-based flame retardant with phosphorus (P) and silicon (Si) groups (EGN-Si/P) | 0.5% Si/0.37% P%; 1% Si/0.74% P; 2% Si/1.48% P | mixing, casting | flame retardancy. limiting oxygen index, impact strength | [69] |
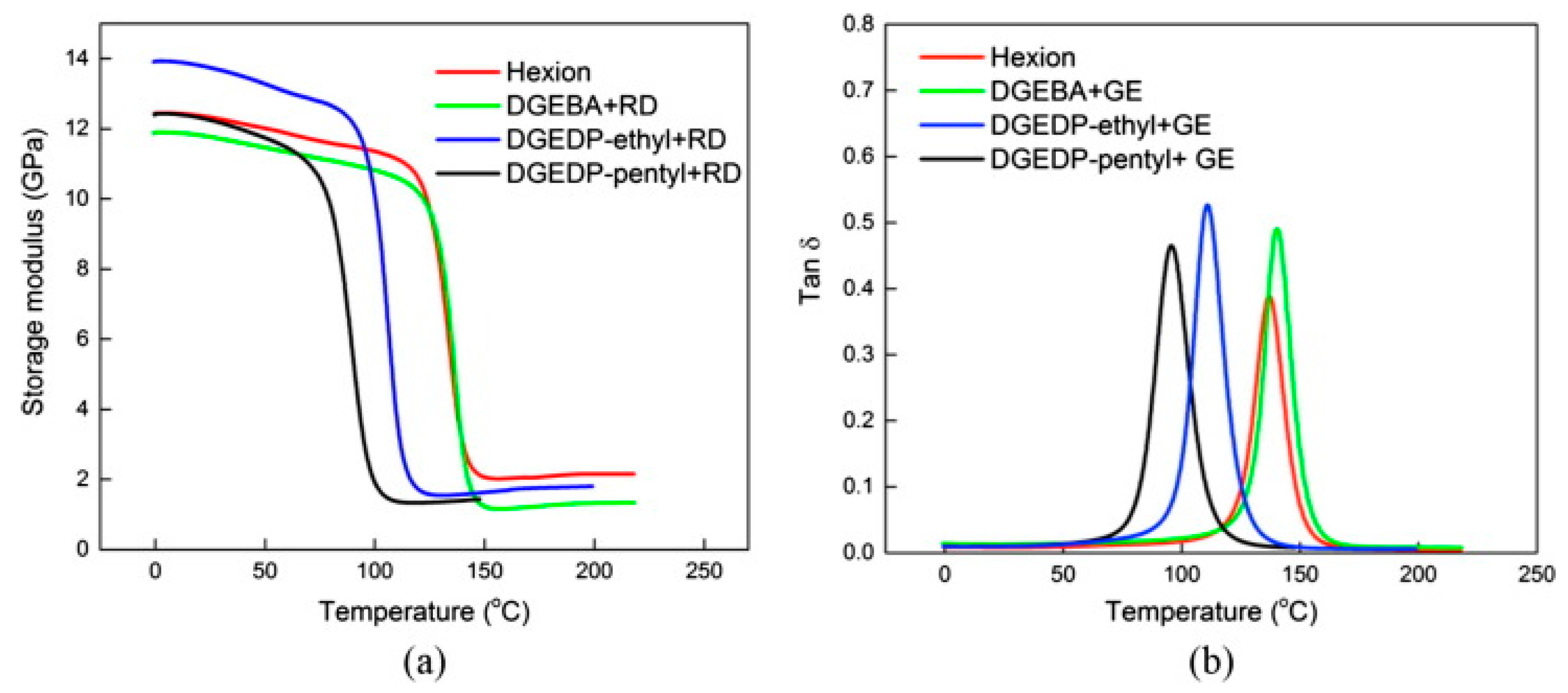
| biobase diphenolate diglycidyl ethers (DGEDP)/ (IPDA) | E-glass fibre mats | eugenol monoglycidyl ether (GE) as a diluent | 12 layers of glass mats, | vacuum infusion | viscosity reduction, gel time extension, storage modulus, flexural modulus, flexural strength, | [70] |
| tris(2-methoxy-4-(oxiran-2-ylmethyl)phenyl) benzene-1,3,5-tricarboxylate/ DMAP | carbon fibre | eugenol-based epoxy resins | 6 layers of carbon fabric | mixing, immersing, pressing | degradability, rigidity, thermal stability, | [67] |
| resorcinol diglycidyl ether (RE)/(DAAE) | short carbon fibre | diamine-allyl-eugenol (DAAE) as curing agent | fiber volume content 27–32% | vacuum assisted resin infusion | tensile strength, bending strength thermal resistance, | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matykiewicz, D.; Skórczewska, K. Characteristics and Application of Eugenol in the Production of Epoxy and Thermosetting Resin Composites: A Review. Materials 2022, 15, 4824. https://doi.org/10.3390/ma15144824
Matykiewicz D, Skórczewska K. Characteristics and Application of Eugenol in the Production of Epoxy and Thermosetting Resin Composites: A Review. Materials. 2022; 15(14):4824. https://doi.org/10.3390/ma15144824
Chicago/Turabian StyleMatykiewicz, Danuta, and Katarzyna Skórczewska. 2022. "Characteristics and Application of Eugenol in the Production of Epoxy and Thermosetting Resin Composites: A Review" Materials 15, no. 14: 4824. https://doi.org/10.3390/ma15144824
APA StyleMatykiewicz, D., & Skórczewska, K. (2022). Characteristics and Application of Eugenol in the Production of Epoxy and Thermosetting Resin Composites: A Review. Materials, 15(14), 4824. https://doi.org/10.3390/ma15144824







