Promotion Effect of Carboxymethyl Chitosan on Dental Caries via Intrafibrillar Mineralization of Collagen and Dentin Remineralization
Abstract
:1. Introduction
2. Materials and Methods
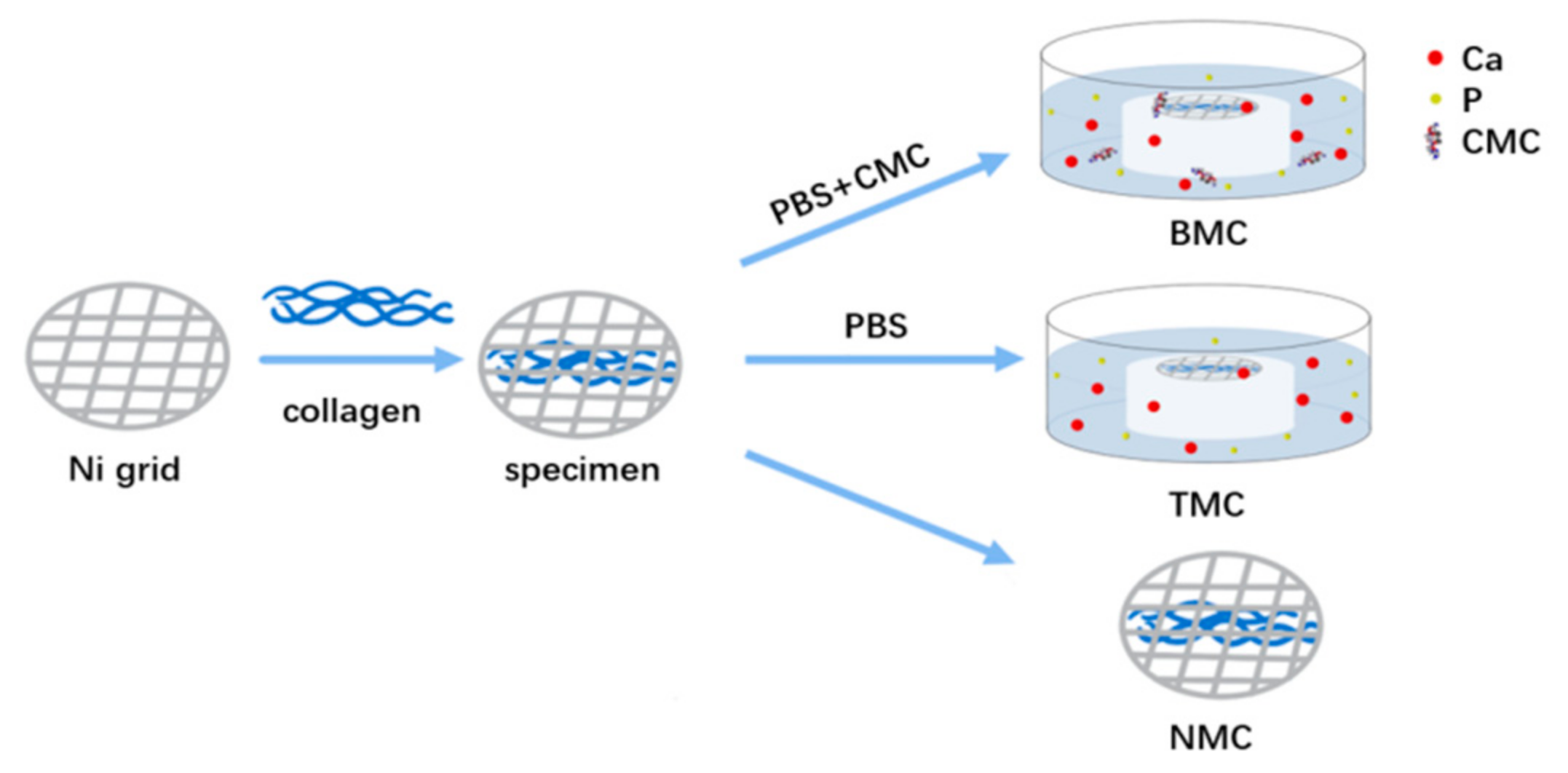
2.1. Mineralizing Media Preparation
2.2. Collagen Fibril Mineralization
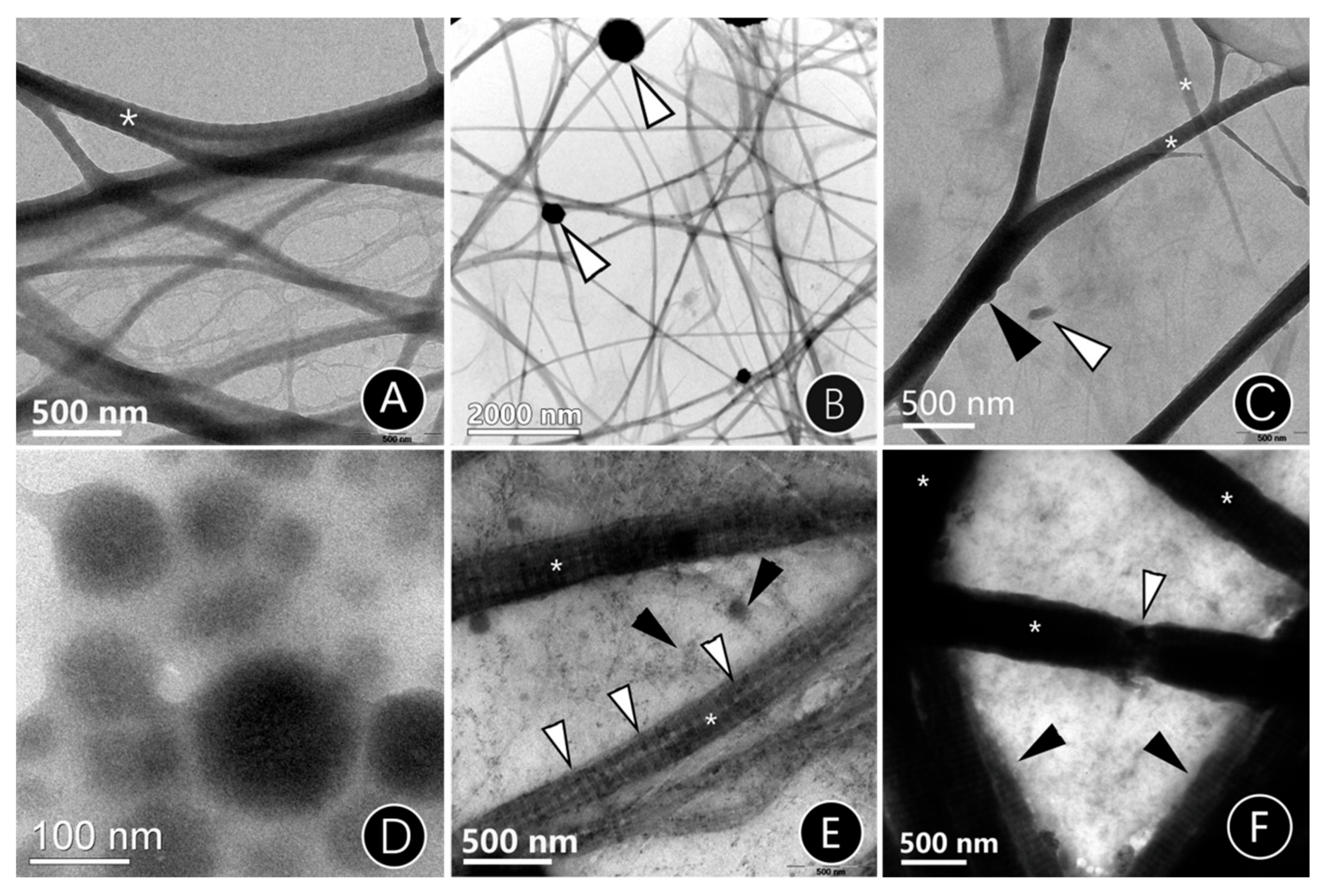
2.2.1. TEM of Mineralized Reconstituted Single-Layer Collagen Fibrils
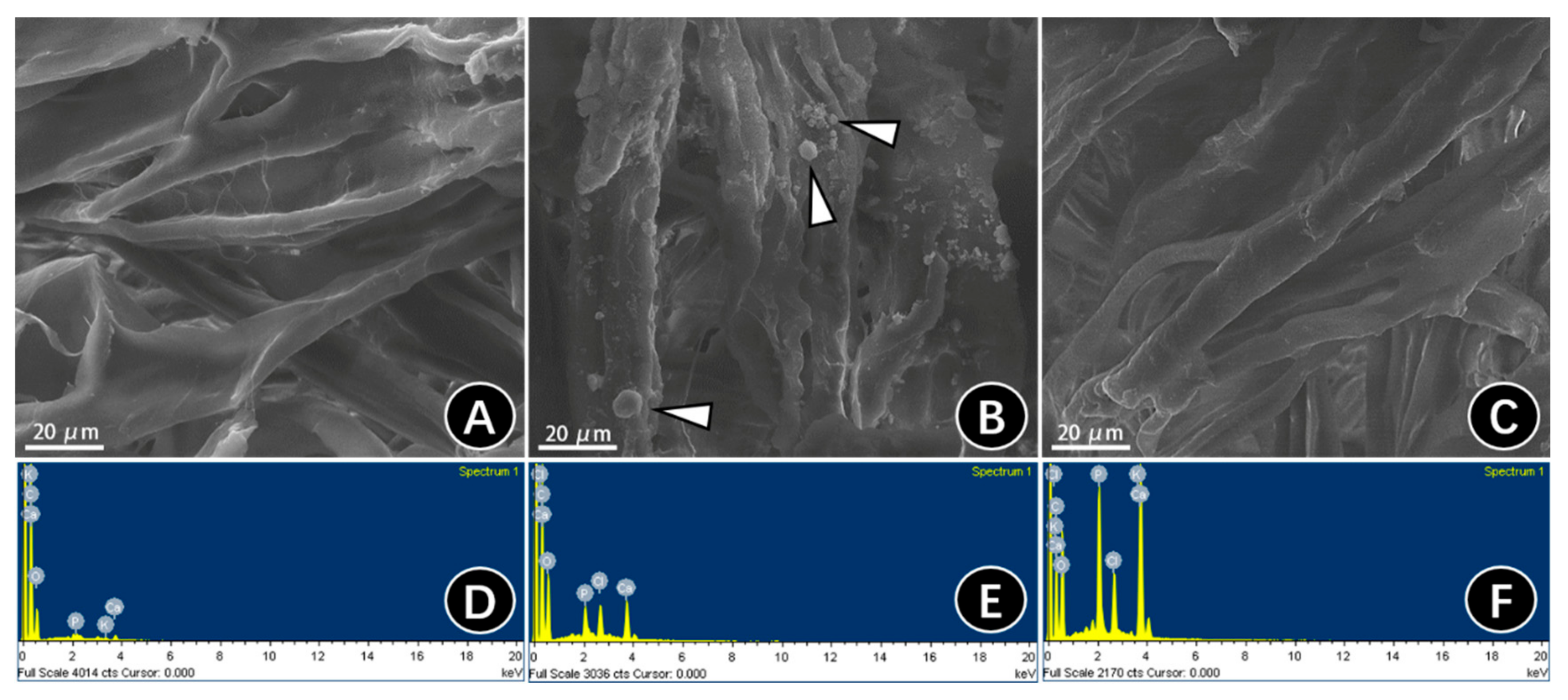
2.2.2. SEM and EDS of Mineralized Three-Dimensional Type-I Collagen Membranes
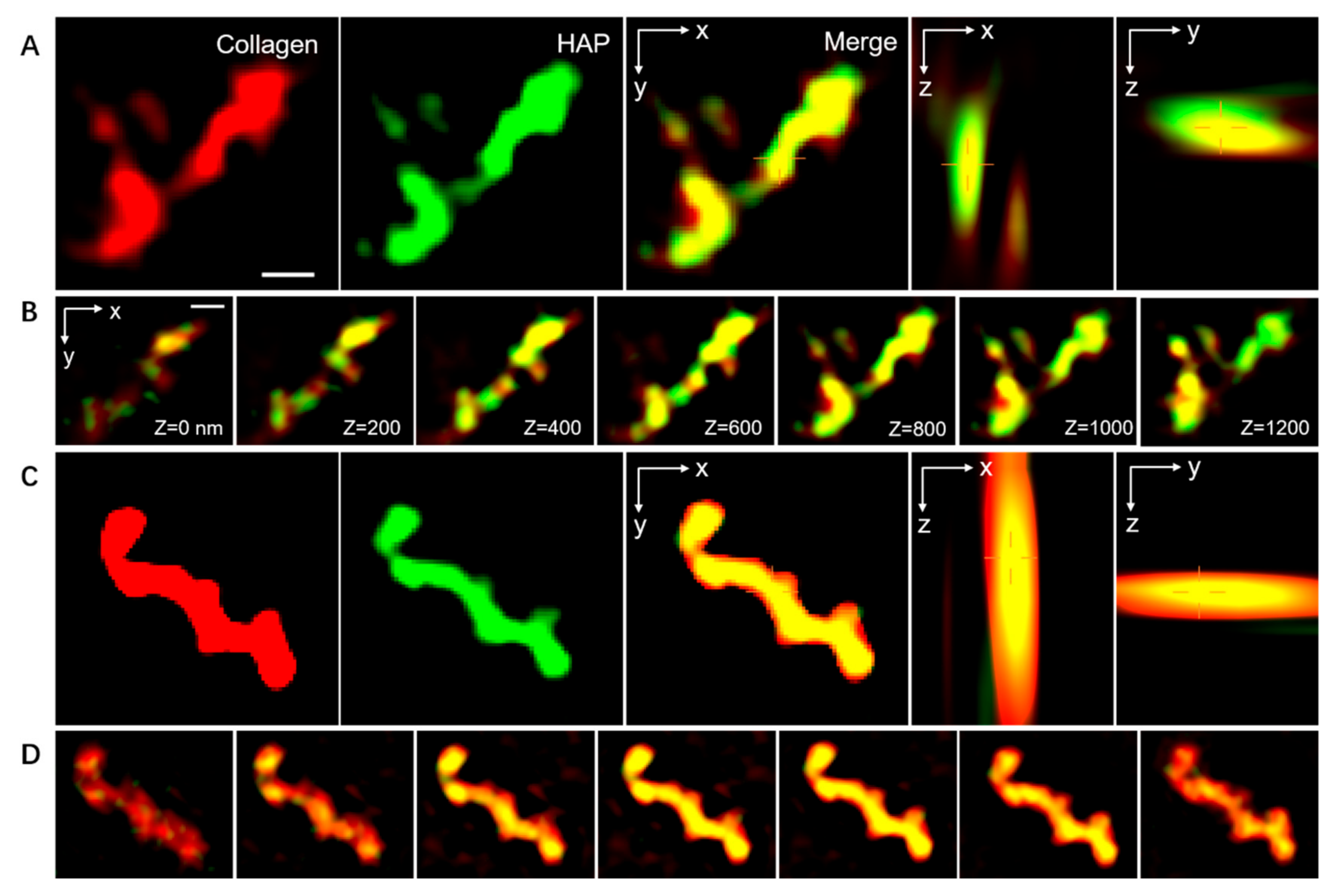
2.2.3. Spatial Distribution of the ACP within the Collagen Fibrils
2.3. Mineralization and Characterization of the Artificial Caries-Affected Dentin (ACAD) Model
2.3.1. Preparation and Mineralization of the ACAD model
2.3.2. Microhardness Test
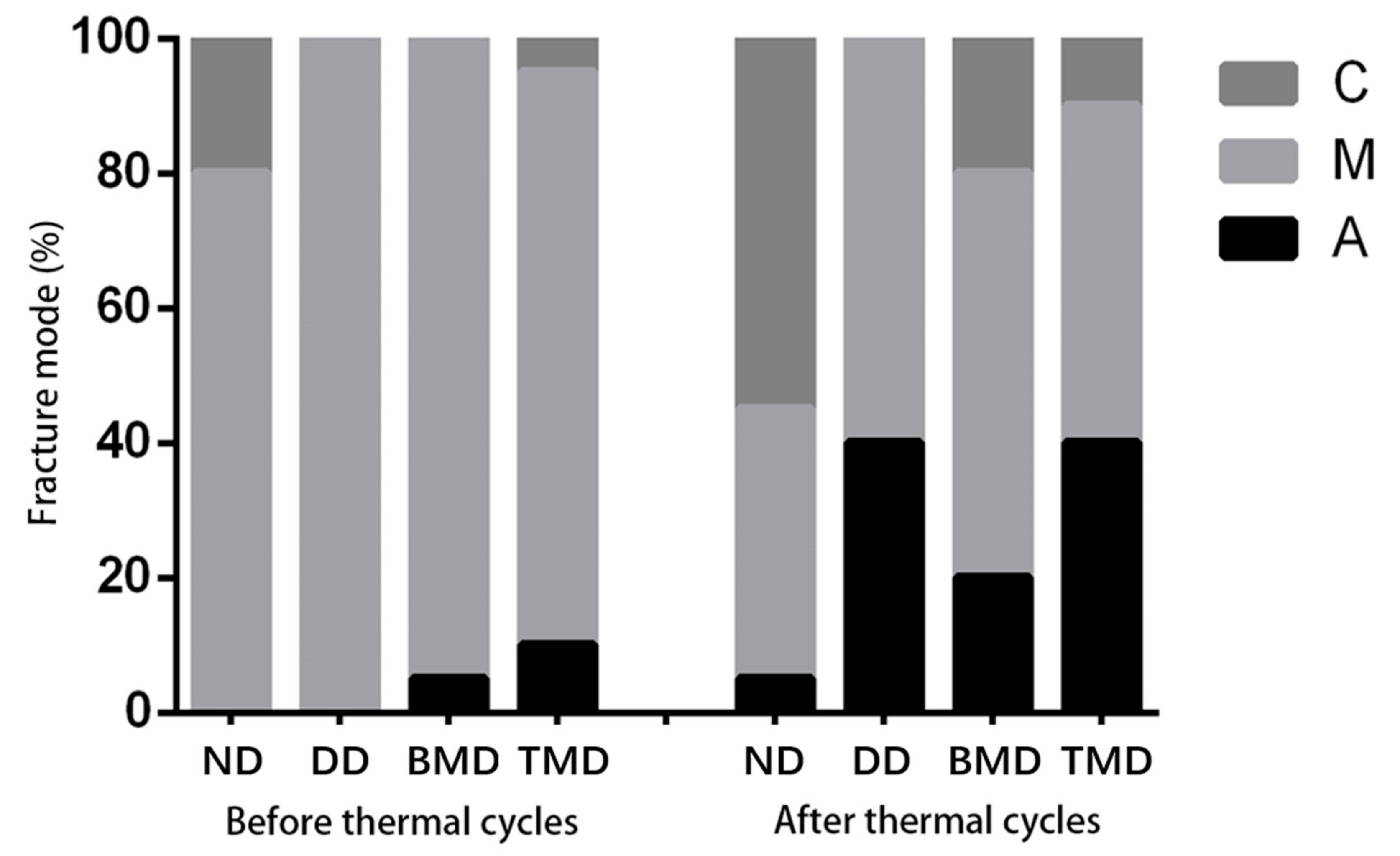
2.3.3. Microtensile Bond Strength (μTBS) Test and Failure Mode Analysis
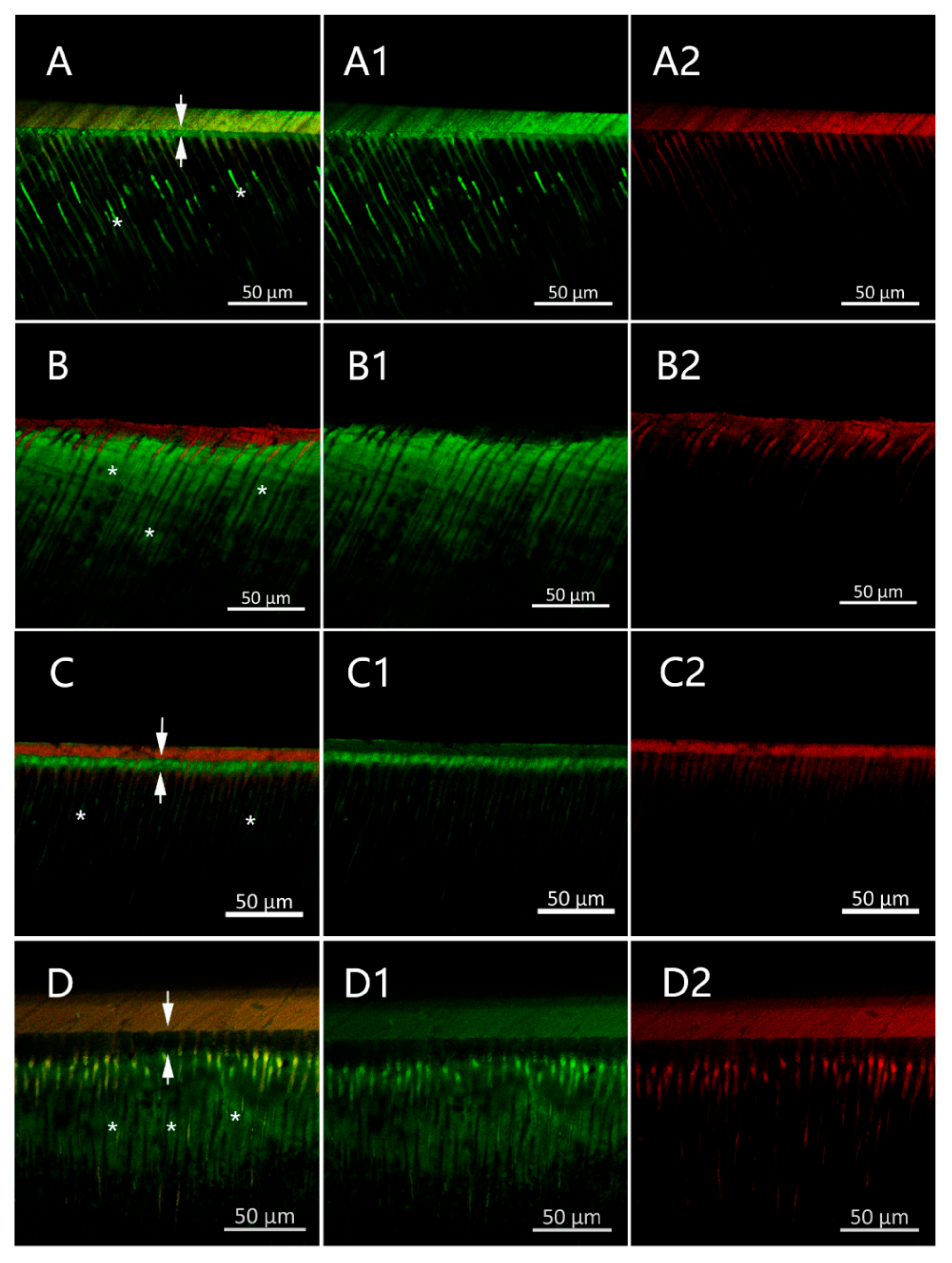
2.3.4. Confocal Laser Scanning Microscope (CLSM)
2.4. Statistical Analysis
3. Results
3.1. Effect of CMC as a Biomimetic Analog through 2D and 3D Collagen Models
3.1.1. The Mineralization Mode of Single-Layer Mineralized Collagen Fibrils
3.1.2. Surface Microtopography of Three-Dimensional Collagen
3.1.3. Location of Mineralization in Collagen Fibrils
3.2. CMC-Induced Dentin Remineralization Improves Dentin Bonding and Adhesive Durability
3.2.1. Hardness Evaluation
3.2.2. Microtensile Bond Strength (µTBS) and Failure Analysis
3.2.3. CMC-Induced Remineralization Influence on the Properties of the Resin–Dentin Adhesive Interfacial Microenvironment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Featherstone, J.D.B. The science and practice of caries prevention. J. Am. Dent. Assoc. 2000, 131, 887–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, W.; Konopka, S.; Kriwalsky, M.; Gaengler, P. Morphological analysis and chemical content of natural dentin carious lesion zones. Ann. Anat. Anat. Anz. 2003, 185, 419–424. [Google Scholar] [CrossRef]
- Sakoolnamarka, R.; Burrow, M.F.; Kubo, S.; Tyas, M. Morphological Study of Demineralized Dentine After Caries Removal Using Two Different Methods. Aust. Dent. J. 2002, 47, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.J. The advantages of minimally invasive dentistry. J. Am. Dent. Assoc. 2005, 136, 1563–1565. [Google Scholar] [CrossRef]
- Mashiko, R.; Inoue, G.; Nikaido, T.; Tagami, J. Morphological evaluation of artificial caries-affected dentin after applying FCP-COMPLEX. J. Oral Sci. 2017, 59, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Yiu, C.K.Y.; Kim, J.R.; Gu, L.; Kim, S.K.; Weller, R.N.; Pashley, D.H.; Tay, F.R. Failure of a Glass Ionomer to Remineralize Apatite-depleted Dentin. J. Dent. Res. 2010, 89, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Liu, W.; Ning, T.; Mei, M.L.; Li, Q.-L.; Lo, E.C.M.; Chu, C.H. A novel oligopeptide simulating dentine matrix protein 1 for biomimetic mineralization of dentine. Clin. Oral Investig. 2013, 18, 873–881. [Google Scholar] [CrossRef]
- Arsenault, A.L. Crystal-collagen relationships in calcified turkey leg tendons visualized by selected-area dark field electron microscopy. Calcif. Tissue Res. 1988, 43, 202–212. [Google Scholar] [CrossRef]
- Traub, W.; Arad, T.; Weiner, S. Three-dimensional ordered distribution of crystals in turkey tendon collagen fibers. Proc. Natl. Acad. Sci. USA 1989, 86, 9822–9826. [Google Scholar] [CrossRef] [Green Version]
- Fang, P.-A.; Conway, J.F.; Margolis, H.C.; Simmer, J.P.; Beniash, E. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proc. Natl. Acad. Sci. USA 2011, 108, 14097–14102. [Google Scholar] [CrossRef] [Green Version]
- Mai, S.; Kim, Y.; Kim, J.; Yiu, C.; Ling, J.; Pashley, D.; Tay, F. In vitro Remineralization of Severely Compromised Bonded Dentin. J. Dent. Res. 2010, 89, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Ye, Z.; Fok, A.; Holmes, B.N.; Espanol, M.; Ginebra, M.-P.; Aparicio, C. Effects of Molecular Weight and Concentration of Poly(Acrylic Acid) on Biomimetic Mineralization of Collagen. ACS Biomater. Sci. Eng. 2018, 4, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. The implications of recent advances in carboxymethyl chitosan based targeted drug delivery and tissue engineering applications. J. Control. Release 2014, 186, 54–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cao, S.; Wang, H.; Li, Y.; Kishen, A.; Deng, X.; Yang, X.; Wang, Y.; Cong, C.; Wang, H.; et al. Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an in vitro tooth model of deep caries. PLoS ONE 2015, 10, e0116553. [Google Scholar] [CrossRef]
- Huang, Z.; Qi, Y.; Zhang, K.; Gu, L.; Guo, J.; Wang, R.; Mai, S. Use of experimental-resin-based materials doped with carboxymethyl chitosan and calcium phosphate microfillers to induce biomimetic remineralization of caries-affected dentin. J. Mech. Behav. Biomed. Mater. 2019, 89, 81–88. [Google Scholar] [CrossRef]
- Wang, R.; Guo, J.; Lin, X.; Chen, S.; Mai, S. Influence of molecular weight and concentration of carboxymethyl chitosan on biomimetic mineralization of collagen. RSC Adv. 2020, 10, 12970–12981. [Google Scholar] [CrossRef]
- Yao, S.; Xu, Y.; Shao, C.; Nudelman, F.; Sommerdijk, N.A.J.M.; Tang, R. A Biomimetic Model for Mineralization of Type-I Collagen Fibrils. Methods Pharmacol. Toxicol. 2019, 1944, 39–54. [Google Scholar]
- Joves, G.J.; Inoue, G.; Sadr, A.; Nikaido, T.; Tagami, J. Nanoindentation hardness of intertubular dentin in sound, demineralized and natural caries-affected dentin. J. Mech. Behav. Biomed. Mater. 2014, 32, 39–45. [Google Scholar] [CrossRef]
- Li, X.; De Munck, J.; Yoshihara, K.; Pedano, M.; Van Landuyt, K.; Chen, Z.; Van Meerbeek, B. Re-mineralizing dentin using an experimental tricalcium silicate cement with biomimetic analogs. Dent. Mater. 2017, 33, 505–513. [Google Scholar] [CrossRef]
- Morresi, A.L.; D’Amario, M.; Capogreco, M.; Gatto, R.; Marzo, G.; D’Arcangelo, C.; Monaco, A. Thermal cycling for restorative materials: Does a standardized protocol exist in laboratory testing? A literature review. J. Mech. Behav. Biomed. Mater. 2014, 29, 295–308. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, M.J.; Kim, K.M.; Yang, S.Y.; Seo, J.Y.; Choi, S.H.; Kwon, J.S. Enamel Demineralization Resistance and Remineralization by Various Fluoride-Releasing Dental Restorative Materials. Materials 2021, 14, 4554. [Google Scholar] [CrossRef] [PubMed]
- Shioya, Y.; Tichy, A.; Yonekura, K.; Hasegawa, M.; Hatayama, T.; Ikeda, M.; Tagami, J.; Nakajima, M.; Hosaka, K. Sodium p-Toluenesulfinate Enhances the Bonding Durability of Universal Adhesives on Deproteinized Eroded Dentin. Polymers 2021, 13, 3901. [Google Scholar] [CrossRef] [PubMed]
- Pandya, M.; Diekwisch, T.G.H. Enamel biomimetics—Fiction or future of dentistry. Int. J. Oral Sci. 2019, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, C.Y.; Mei, M.L.; Li, Q.-L.; Lo, E.C.M.; Chu, C.H. Methods for Biomimetic Remineralization of Human Dentine: A Systematic Review. Int. J. Mol. Sci. 2015, 16, 4615–4627. [Google Scholar] [CrossRef]
- Qi, Y.P.; Nan, L.; Niu, L.-N.; Primus, C.M.; Ling, J.-Q.; Pashley, D.H.; Tay, F.R. Remineralization of artificial dentinal caries lesions by biomimetically modified mineral trioxide aggregate. Acta Biomater. 2012, 8, 836–842. [Google Scholar] [CrossRef] [Green Version]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Gower, L.B.; Odom, D.J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 2000, 210, 719–734. [Google Scholar] [CrossRef]
- Shao, C.; Zhao, R.; Jiang, S.; Yao, S.; Wu, Z.; Jin, B.; Yang, Y.; Pan, H.; Tang, R. Citrate Improves Collagen Mineralization via Interface Wetting: A Physicochemical Understanding of Biomineralization Control. Adv. Mater. 2018, 30, 1704876. [Google Scholar] [CrossRef]
- Wang, Z.; Ouyang, Y.; Wu, Z.; Zhang, L.; Shao, C.; Fan, J.; Zhang, L.; Shi, Y.; Zhou, Z.; Pan, H.; et al. A novel fluorescent adhesive-assisted biomimetic mineralization. Nanoscale 2018, 10, 18980–18987. [Google Scholar] [CrossRef]
- Wu, Q.; Mei, M.L.; Wu, X.; Shi, S.; Xu, Y.; Chu, C.H.; Chen, Y. Remineralising effect of 45S5 bioactive glass on artificial caries in dentine. BMC Oral Health 2020, 20, 49. [Google Scholar] [CrossRef]
- Song, Q.; Liu, X.C.; Ma, Y.X.; Wang, C.; Jiao, K.; Niu, L. Biomimetic remineralization of dentin. J. Prev. Treat. Stomatol. Dis. 2020, 28, 7. [Google Scholar]
- Schwendicke, F.; Frencken, J.E.; Bjørndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; Van Landuyt, K.; Banerjee, A.; Campus, G.; Doméjean, S.; et al. Carious Lesions: Consensus Recommendations on Carious Tissue Removal. Adv. Dent. Res. 2016, 28, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Mai, S.; Li, N.; Yiu, C.K.; Mao, J.; Pashley, D.H.; Tay, F.R. Differences between top-down and bottom-up approaches in mineralizing thick, partially demineralized collagen scaffolds. Acta Biomater. 2011, 7, 1742–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholtanus, J.; Purwanta, K.; Dogan, N.; Kleverlaan, C.J.; Feilzer, A.J. Microtensile Bond Strength of Three Simplified Adhesive Systems to Caries-affected Dentin. J. Adhes. Dent. 2010, 12, 273–278. [Google Scholar] [PubMed]
- Bacino, M.; Girn, V.; Nurrohman, H.; Saeki, K.; Marshall, S.J.; Gower, L.; Saeed, E.; Stewart, R.; Le, T.; Marshall, G.W.; et al. Integrating the PILP-mineralization process into a restorative dental treatment. Dent. Mater. 2019, 35, 53–63. [Google Scholar] [CrossRef]
- Saeki, K.; Chien, Y.-C.; Nonomura, G.; Chin, A.; Habelitz, S.; Gower, L.; Marshall, S.; Marshall, G.W. Recovery after PILP remineralization of dentin lesions created with two cariogenic acids. Arch. Oral Biol. 2017, 82, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Yang, H.; Yan, H.; Sun, Y.; Chen, X.; Guo, J.; Yue, J.; Huang, C. Quercetin as a simple but versatile primer in dentin bonding. RSC Adv. 2017, 7, 36392–36402. [Google Scholar] [CrossRef] [Green Version]
- Pashley, D.H.; Pashley, E.L.; Carvalho, R.M.; Tay, F.R. The effects of dentin permeability on restorative dentistry. Dent. Clin. N. Am. 2002, 46, 211–245. [Google Scholar] [CrossRef]
- Deng, D.; Yang, H.; Guo, J.; Chen, X.; Zhang, W.; Huang, C. Effects of different artificial ageing methods on the degradation of adhesive–dentine interfaces. J. Dent. 2014, 42, 1577–1585. [Google Scholar] [CrossRef]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Influence of phosphoproteins’ biomimetic analogs on remineralization of mineral-depleted resin–dentin interfaces created with ion-releasing resin-based systems. Dent. Mater. 2015, 31, 759–777. [Google Scholar] [CrossRef]
- Abuna, G.; Feitosa, V.P.; Correr, A.B.; Cama, G.; Giannini, M.; Sinhoreti, M.A.; Pashley, D.H.; Sauro, S. Bonding performance of experimental bioactive/biomimetic self-etch adhesives doped with calcium-phosphate fillers and biomimetic analogs of phosphoproteins. J. Dent. 2016, 52, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Chiari, M.; Rodrigues, M.C.; Xavier, T.A.; de Souza, E.M.; Arana-Chavez, V.E.; Braga, R.R. Mechanical properties and ion release from bioactive restorative composites containing glass fillers and calcium phosphate nano-structured particles. Dent. Mater. 2015, 31, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, L.; Mjör, I.A. Pulp-dentin biology in restorative dentistry. Part 4: Dental caries—Characteristics of lesions and pulpal reactions. Quintessence Int. 2001, 32, 717–736. [Google Scholar] [PubMed]
- Hanna, S.N.; Alfayate, R.P.; Prichard, J.; Ibrahim, S. Vital Pulp Therapy an Insight Over the Available Literature and Future Expectations. Eur. Endod. J. 2019, 5, 46–53. [Google Scholar]
| Vickers Hardness (MPa, Mean ± SD) | |
|---|---|
| ND | 75.1 ± 3.2 A |
| DD | 55.2 ± 2.9 B |
| BMD | 68.5 ± 2.5 C |
| TMD | 54.2 ± 2.8 B |
| F value | 252.735 |
| p-value | <0.001 |
| Before Thermal Cycling (Mean) | After Thermal Cycling (Mean) | F Value | p-Value | |
|---|---|---|---|---|
| ND | 35.9 (7.0) A1 | 45.1 (6.7) A2 | 18.33 | <0.001 |
| DD | 26.4 (5.9) B1 | 2.5 (2.2) B2 | 57.026 | <0.001 |
| BMD | 33.6 (4.3) A1 | 26.7 (9.1) C1 | 5.86 | 0.021 |
| TMD | 29.8 (8.3) A1 | 1.9 (2.4) B2 | 58.486 | <0.001 |
| F value | 8.197 | 110.839 | ||
| p-value | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Guo, J.; Huang, Z.; Mai, S. Promotion Effect of Carboxymethyl Chitosan on Dental Caries via Intrafibrillar Mineralization of Collagen and Dentin Remineralization. Materials 2022, 15, 4835. https://doi.org/10.3390/ma15144835
Zhang Q, Guo J, Huang Z, Mai S. Promotion Effect of Carboxymethyl Chitosan on Dental Caries via Intrafibrillar Mineralization of Collagen and Dentin Remineralization. Materials. 2022; 15(14):4835. https://doi.org/10.3390/ma15144835
Chicago/Turabian StyleZhang, Qi, Jiaxin Guo, Zihua Huang, and Sui Mai. 2022. "Promotion Effect of Carboxymethyl Chitosan on Dental Caries via Intrafibrillar Mineralization of Collagen and Dentin Remineralization" Materials 15, no. 14: 4835. https://doi.org/10.3390/ma15144835




