Effects of Selected Pigments on the Properties of Silicone Resin-Based Paints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
- Zinc–iron bronze Fe2O3ZnO (Kremer Pigmente GmbH & Co., Aischstetten, Germany)—a spinel pigment obtained under high temperature from iron oxides (II) and (III) and zinc oxide, characterized by high heat resistance, high UV resistance, and low absorption of solar radiation through the entire spectrum. It is classified as a “cool” pigment, able to resist surface heating under sunlight.
- Iron red (Kremer Pigmente GmbH & Co., Aischstetten, Germany)—iron oxide α-Fe2O3, a red pigment, known by the color index PR101.
- Nicosia green (Kremer Pigmente GmbH & Co., Aischstetten, Germany)—a mixture of earth pigments containing compounds of iron, aluminum, magnesium, and potassium, known by the color index PG23.
- Bohemian earth (Kremer Pigmente GmbH & Co., Aischstetten, Germany)—PG23 excavated at a different location, containing additional silicon and calcium compounds (Fe, Al, Si, K, Mg, Ca). The shade and hue of PG23 may vary due to composition differences, depending on the excavation site.
- Commercial green GX11—a pigment paste based on cobalt green pigment Co2TiO4. PG50—a spinel pigment obtained under high temperature from cobalt (II) oxide and titanium (IV) oxide.
- Commercial red RX10—iron red, PR101, in the form of a pigment paste.
- Commercial red RH6—organic red pigment, known by the color index PR254.
- P60 (Piotrowice Sp. Z o.o., Piotrowice, Poland)—standard dolomite filler used in the tested paint.
2.2. Sample Preparation
2.3. Test Methods
- Density measurements were performed using a density cup with a volume of 100.088 cm3 to measure the weight of each sample, followed by density calculations according to the formula given in PN-EN ISO 2811-1:2012 [31]:where m is sample mass and V is volume. Each sample was measured three times. The results were averaged.
- Viscosity measurements were performed according to PN-EN ISO 2555:2011 [32] using a Brookfield (Middleboro, MA, USA) DV-II+ Pro viscometer at 20 rpm with a spindle for highly viscous liquids. Each sample was measured immediately after incorporating the pigment into the paint.
- Gloss measurement was performed using a GLS glossmeter (Gdańsk, Poland) at angles of 60° and 85°, since the base paint is supposed to be matt, according to PN-EN ISO 2813:2014-11 [33].
- The rub-out test was performed by gently rubbing the surface of the applied paint with a fingertip after short time periods (1, 5, and 10 min after application) [34]. The rub-out test was used to analyze the compatibility of the pigment with the material by observing the occurrence of flocculation.
- The wet-scrub resistance test was performed for 200 cycles of scrub on an Elcometer 1720 (Manchester, UK) using a sponge and a 5% solution of sodium dodecylbenzenesulfonate (ABSNa) as a wetting agent. The samples were prepared by the application of a 300 µm thick coating of each paint on a dedicated scrub test substrate. The coatings were conditioned for 28 days at room temperature to ensure complete drying. The dry samples were measured using a thickness meter and placed in the scrub resistance tester. After the test, the samples were rinsed with water and placed in an air dryer at temperature of 50 °C for 7 days. Subsequently, the thicknesses of the coatings was measured again. Scrub resistance class was determined based on loss of coating thickness (PN-EN 13300:2002 standard) [35].
- Adhesion to the substrate was measured using the TQC cross-cut adhesion test (Hadley, UK). Samples were applied to cardboard using a 300-µm applicator and left for 28 days at room temperature to dry out. Next, the surface of each sample was cut vertically and horizontally, with an angle of 90° between cuts. Adhesion to the substrate was determined based on chipping at the incision edges.
- Water absorption tests were conducted according to the PN-C-81521:1976 standard. Two equal layers of paint were applied to a limestone substrate with a 24-h wait between layers. The dimensions of the limestone substrate were 11.5 × 11 × 2.5 cm. The samples were left to dry out for 28 days at room temperature. The edges and sides of the limestone were covered with silicone to seal any areas not covered with paint. Two samples were prepared for each pigment. The samples were conditioned by dipping in water for 72 h, followed by drying in an air dryer in 50 °C for 72 h and resting for 24 h in room temperature. The samples were then weighed and placed in a container filled with water up to half the height of the sides of the samples. The samples were placed on a piece of sponge with the surface covered with paint facing down, to ensure contact with water throughout the test. After 24 h, the samples were weighed again and left to dry at 50 °C for another 24 h. The process was repeated three times. The water uptake factor was calculated according to the formula [36].where W24 is the water absorbance factor after 24 h, m24 is the sample mass after 24 h in water, m0 is the sample mass before dipping in water and S is the surface of the sample covered with paint.
- Vapor permeability was measured using the wet-cup method, according to PN-EN ISO 7783:2018-11 [37]. Samples were prepared by applying two equal layers of paint on an oval substrate, with a 24-h wait between layers. The samples were left to dry out for 28 days. Measuring cups were filled with a saturated solution of anhydrous ammonium dihydrogen phosphate, leaving space of about 30 mm between the surface of the solution and the sample. The samples were placed on top of the measuring cups, sealed, and weighed. The edges of each sample were sealed with rubber bands to ensure vapor circulation exclusively through the sample. The cups were placed in a chamber with forced air circulation and weighed every 24 h for 4 days. The linearity of the graph shows that the test has been correctly executed.
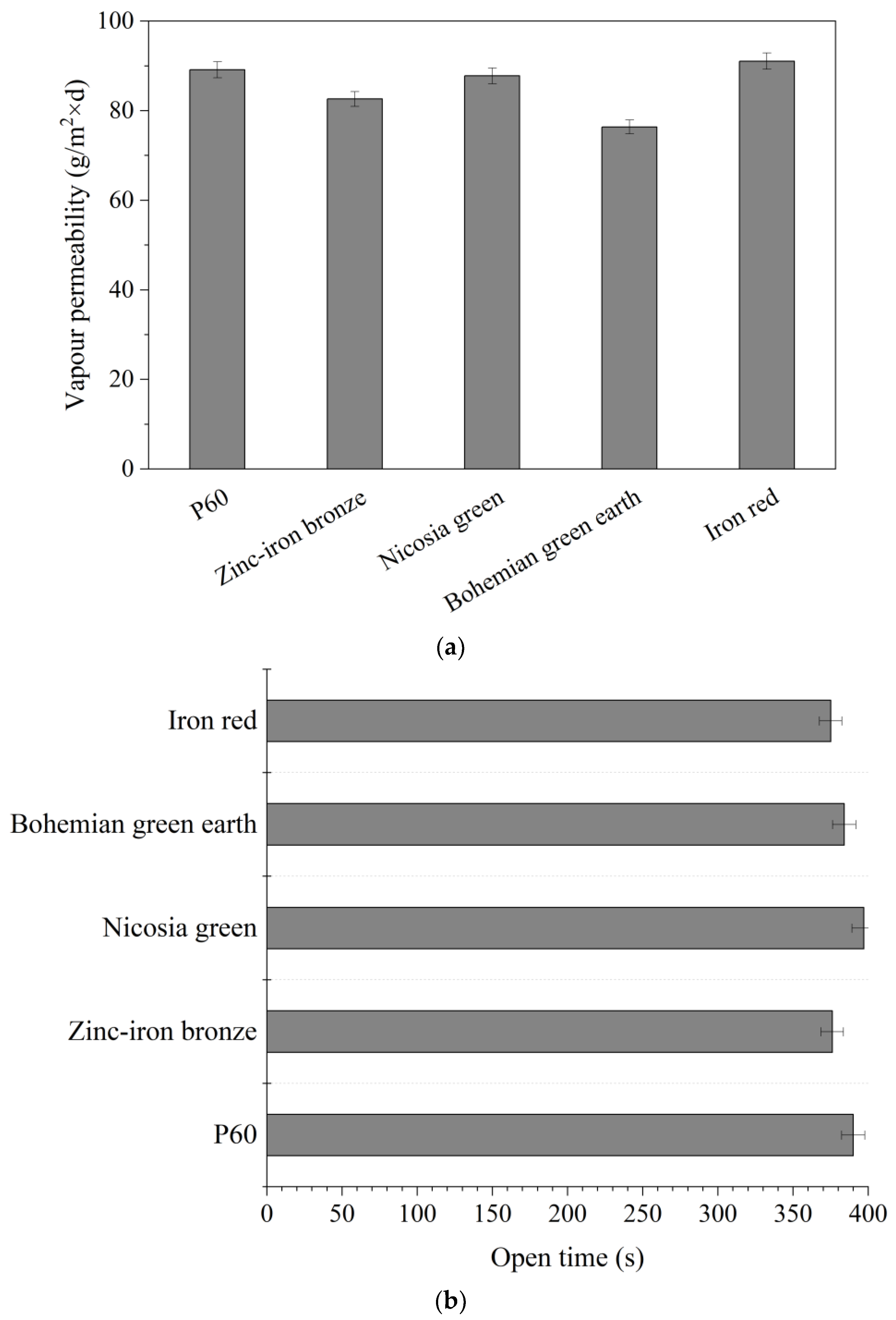
- Open time measurements were conducted using a Q-Lab open time tester (Westlake, OH, USA) equipped with a rod for scratching the surface of the sample and a timescale alongside the sample. As the scratching rod moves steadily over the surface, the timescale makes it possible to monitor the time passing. Coatings were applied with a 200-µm applicator on a glass plate. The measurements lasted for 15 min, during which the scratching rod moved through the sample to the point where it no longer damaged the forming film. Each sample was measured separately immediately after application [34].
- Contrast measurements (mating) were conducted using an X-Rite Color i5 (Grand Rapids, MI, USA) spectrophotometer according to PN-EN ISO 2814:2006 [38]. Samples were prepared by applying a 300-µm film of paint on black-white contrast cards. The samples were conditioned for 28 days at room temperature. Mating ability was determined by measuring Lab values over white and black fields and calculating the ΔE values.
- UV color stability was measured using a X-Rite Color i5 (Grand Rapids, MI, USA) spectrophotometer. The lab values were compared with reference samples using ΔE, according to PN-EN ISO 16474-3:2014_02 [39]. The samples were conditioned using a Q-Lab xenotest chamber (Westlake, OH, USA), where they were exposed to xenon arc lamps imitating full spectrum solar irradiance for 28 days at room temperature. Two series of measurements were conducted: ageing for 150 h and aging for 500 h. After that time, the lab values of the samples were measured
- Particle size measurements were conducted using the light scattering method with a Zetasizer device (Malvern, UK). Samples were prepared by mixing 0.1 g of the pigment in 100 g of water. Ultrasound was used to maximize pigment dispersal. The samples were placed in measuring cuvettes and transferred to the Zetasizer device.
3. Results and Discussion
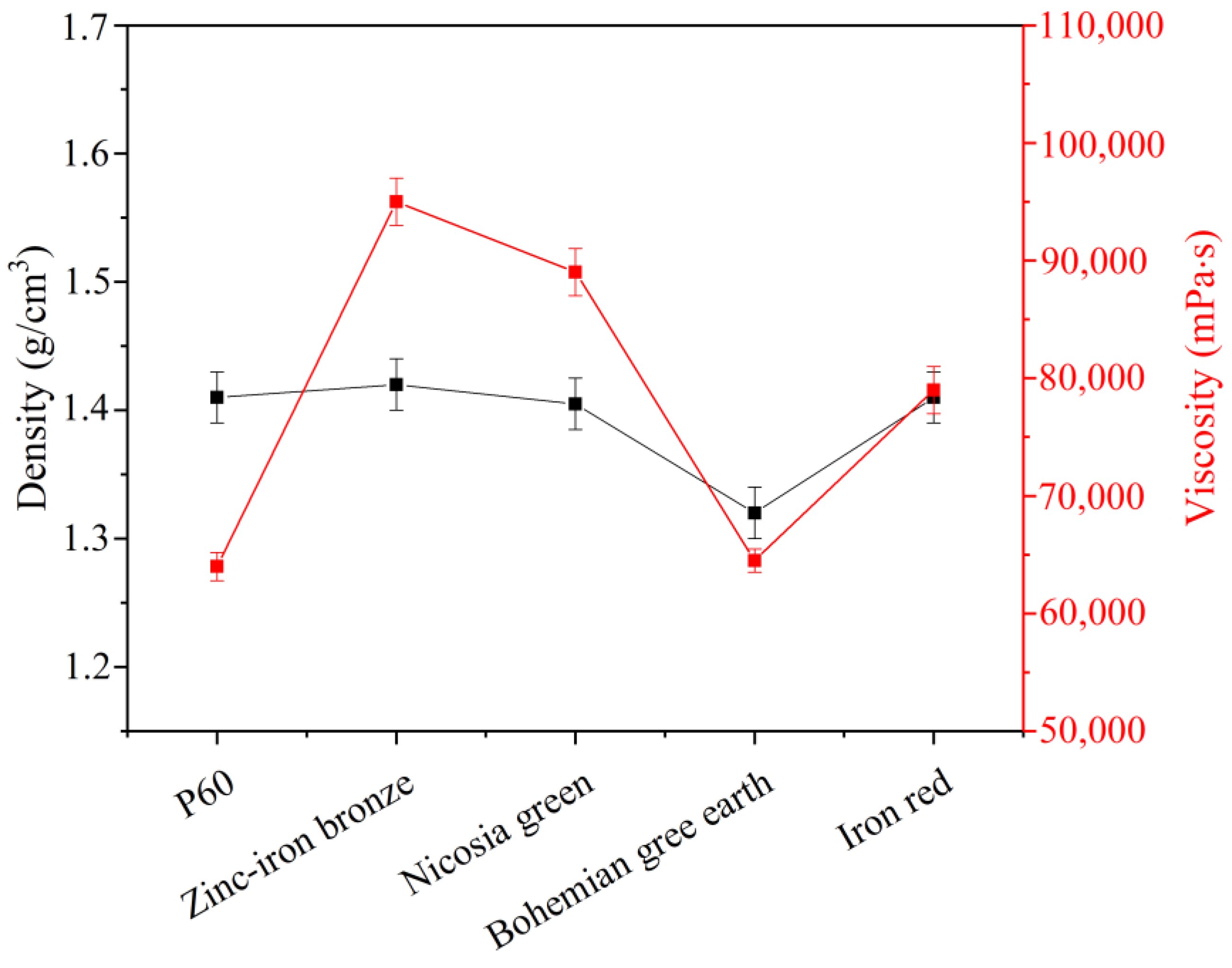
3.1. Density and Viscosity
3.2. Gloss, Rub-Out Test and Substrate Adhesion
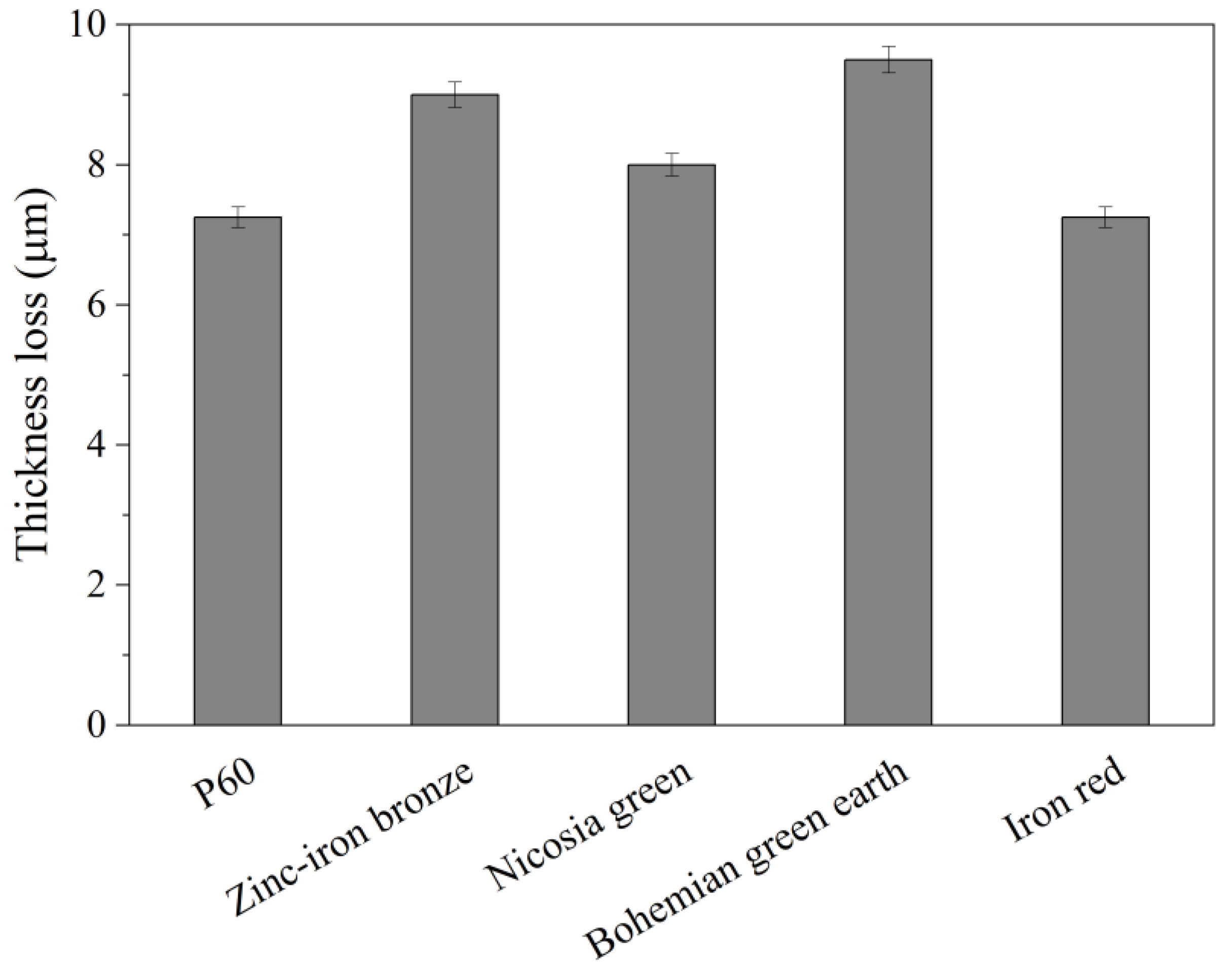
3.3. Scrub Test
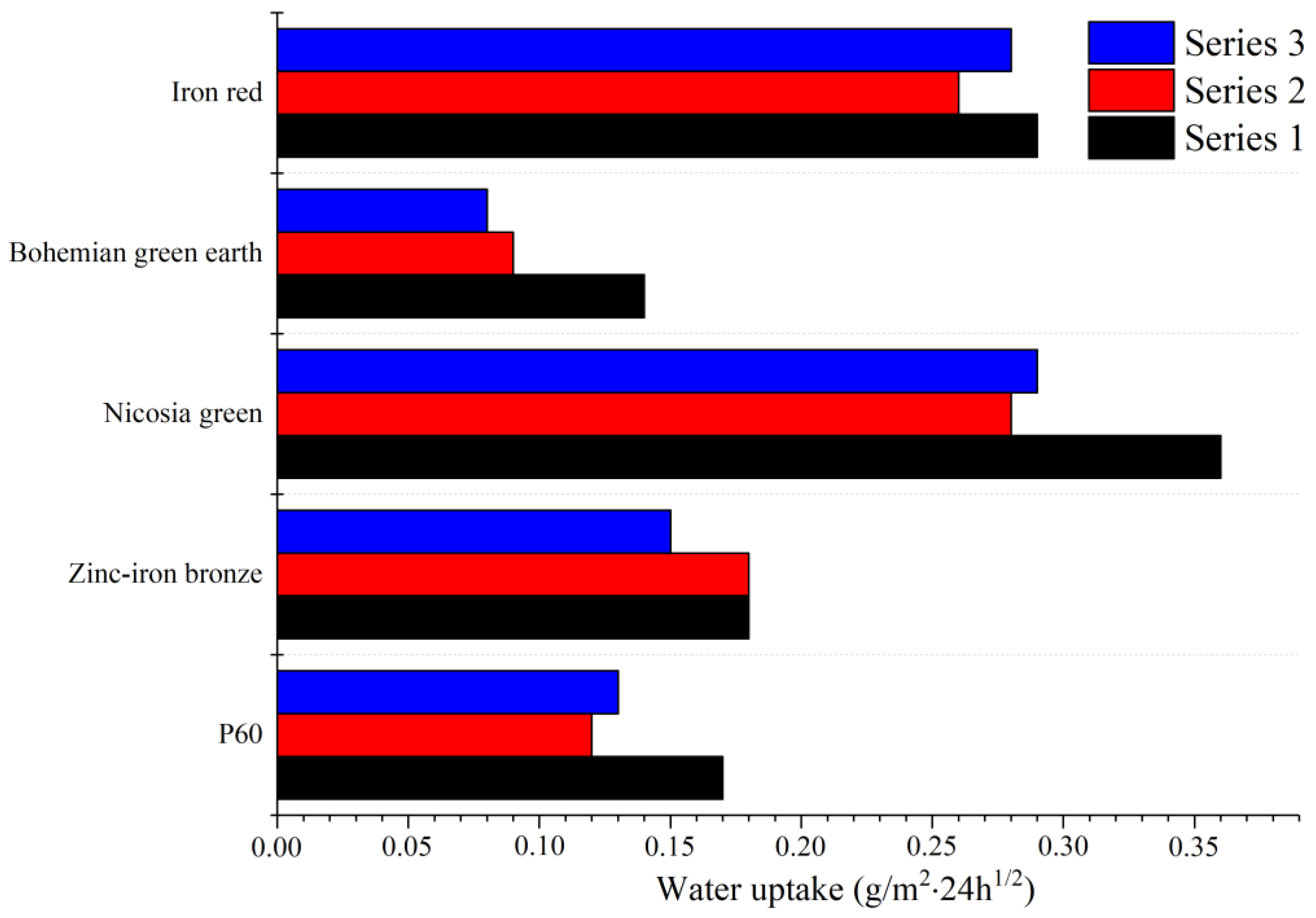
3.4. Water Absorption
3.5. Vapor Permeability
3.6. Contrast
3.7. UV Aging
3.8. New Paint Formulation (Viscosity Measurements, Storage Stability, Wet-Scrub Resistance)
3.9. Rheological Profile of the Newly-Formulated Paint
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pigment | |||||
|---|---|---|---|---|---|
| Particle Radius [nm] | P60 | Zinc-Iron Bronze | Iron Red | Nicosia Green | Bohemian Green Earth |
| Measurement 1 | 137.6 | 146.2 | 156.8 | 145.5 | 160.1 |
| Measurement 2 | 158.0 | 148.7 | 138.6 | 144.0 | 148.9 |
References
- Hansen, M.K.; Larsen, M.; Cohr, K. Waterborne paints: A review of their chemistry and toxicology and the results of determinations made during their use. Scand. J. Work Environ. Health 1987, 13, 473–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overbeek, A.; Bückmann, F.; Martin, E.; Steenwinkel, P.; Annable, T. New generation decorative paint technology. Prog. Org. Coat. 2003, 48, 125–139. [Google Scholar] [CrossRef]
- Bardage, S.L.; Bjurman, J. Adhesion of waterborne paints to wood. J. Coat. Technol. Res. 1998, 70, 39–47. [Google Scholar] [CrossRef]
- Roselli, S.N.; Romagnoli, R.; Deyá, C. The anti-corrosion performance of water-borne paints in long term tests. Prog. Org. Coat. 2017, 109, 172–178. [Google Scholar] [CrossRef]
- Reuvers, A.J. Control of rheology of water-borne paints using associative thickeners. Prog. Org. Coat. 1999, 35, 171–181. [Google Scholar] [CrossRef]
- Nakayama, Y. Polymer blend systems for water-borne paints. Prog. Org. Coat. 1998, 33, 108–116. [Google Scholar] [CrossRef]
- Bellotti, N.; Romagnoli, R.; Quintero, C.; Domínguez-Wong, C.; Ruiz, F.; Deyá, C. Nanoparticles as antifungal additives for indoor water borne paints. Prog. Org. Coat. 2015, 86, 33–40. [Google Scholar] [CrossRef]
- Zawada, E. Paints and Varnishes, 1st ed.; Publishing Cooperation “Czytelnik”: Warsaw, Poland, 1950. [Google Scholar]
- Park, S.A.; Ahn, S.Y.; Choi, K.Y. Functional microbial pigments isolated from Chryseobacterium and Deinococcus species for bio-paint application. Biotechnol. Bioprocess Eng. 2020, 25, 394–402. [Google Scholar] [CrossRef]
- Chen, M.C.; Koh, P.W.; Ponnusamy, V.K.; Lee, S.L. Titanium dioxide and other nanomaterials based antimicrobial additives in functional paints and coatings. Prog. Org. Coat. 2022, 163, 106660. [Google Scholar] [CrossRef]
- D’alessandro, O.; Byrne, C.E.; Selmi, G.; Deyá, C. Anticorrosive paint with a modified zeolite as functional pigment for SAE 1010 steel protection. Pigment Resin Technol. 2020, 50, 136–145. [Google Scholar] [CrossRef]
- Meenakshi, P.; Selvaraj, M. Bismuth titanate as an infrared reflective pigment for cool roof coating. Sol. Energy Mater. Sol. Cells 2018, 174, 530–537. [Google Scholar] [CrossRef]
- Thejus, P.K.; Krishnapriya, K.V.; Nishanth, K.G. A cost-effective intense blue colour inorganic pigment for multifunctional cool roof and anticorrosive coatings. Sol. Energy Mater. Sol. Cells 2021, 219, 110778. [Google Scholar] [CrossRef]
- Yuan, J.; Zhou, S.; Wu, L.; You, B. Organic Pigment Particles Coated with Titania via Sol−Gel Process. J. Phys. Chem. B 2006, 110, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Van Olphen, H. Maya Blue: A Clay-Organic Pigment? Science 1996, 154, 645–646. [Google Scholar] [CrossRef]
- Dreuw, A.; Plötner, J.; Lorenz, L.; Wachtveitl, J.; Djanhan, J.E.; Brüning, J.; Metz, T.; Bolte, M.; Schmidt, M.U. Molecular Mechanism of the Solid-State Fluorescence Behavior of the Organic Pigment Yellow 101 and Its Derivatives. Angew. Chem. Int. Ed. 2005, 44, 7783–7786. [Google Scholar] [CrossRef]
- Yan, J.; Huang, J.; Zhang, T.; Tian, H.; Yu, J.; Zhang, L.; Zhang, Y. Investigation of the microstructure, cation distribution and optical properties of nanoscale NixMg1-xAl2O4 spinel pigments. Ceram. Int. 2019, 45, 14073–14083. [Google Scholar] [CrossRef]
- Sadek, H.E.H.; Khattab, R.M.; Gaber, A.A.; Zawrah, M.F. Nano Mg1−xNixAl2O4 spinel pigments for advanced applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 125, 353–358. [Google Scholar] [CrossRef]
- Fernández, A.L.; de Pablo, L. Formation and the colour development in cobalt spinel pigments. Pigment Resin Technol. 2002, 31, 350–356. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Butola, B.S.; Joshi, M. Application of thermochromic colorants on textiles: Temperature dependence of colorimetric properties. Color. Technol. 2013, 129, 232–237. [Google Scholar] [CrossRef]
- Hu, J.; Yu, X.B. Measurement of Wavelength and Temperature-Dependent Optical Properties of Thermochromic Pigments. Appl. Spectrosc. 2017, 72, 297–304. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, B.; Wu, K. Preparation of reversible thermochromic building coatings and their properties. J. Coat. Technol. Res. 2002, 72, 67–71. [Google Scholar] [CrossRef]
- Mazzari, R.; Dainese, U.; Marsili, G. Copper phthalocyanine pigment blue interior fading. Durability evaluation of copper phthalocyanine pigment blue in interior architectural paints. Farby I Lakiery 2015, 3, 3–9. [Google Scholar]
- Karakaş, F.; Hassas, B.V.; Celik, M.S. Effect of precipitated calcium carbonate additions on waterborne paints at different pigment volume concentrations. Prog. Org. Coat. 2015, 83, 64–70. [Google Scholar] [CrossRef]
- Tressmann, D.M.G.A.; Pedroti, L.G.; de Carvalho, A.F.; Ribeiro, J.C.L.; de Paula Cardoso, F.; Lopes, M.M.S.; de Oliveira, A.F.; Ferreira, S.O. Research into the use of marble waste as mineral filler in soil pigment-based paints and as an active pigment in waterborne paints. Constr. Build. Mater. 2020, 241, 117976. [Google Scholar] [CrossRef]
- Shah, P.; Agrawal, N.; Ravuru, N.R.; Patel, S. Ultrafine titanium-dioxide (rutile) based nano-crystalline dispersions as a pigment for waterborne coatings. Prog. Color Color. Coat. 2022, 15, 305–318. [Google Scholar] [CrossRef]
- Wainwright, I.N.M.; Helwig, K.; Rolandi, D.S.; Gradin, C.; Podesta, M.M.; Onetto, M.; Aschero, C.A. Rock paintings conservation and pigment analysis at Cueva de las Manos and Cerro de losIndios, Santa Cruz (Patagonia), Argentina. In Proceedings of the ICOM Committee for Conservation, 13th Triennial Meeting II, Rio de Janeiro, Brazil, 22–27 September 2002; Vontobel, R., Ed.; James & James Ltd.: London, UK, 2002; pp. 582–589. [Google Scholar]
- Kremer Pigmente Online Shop. Available online: https://shop.kremerpigments.com/en/ (accessed on 13 May 2022).
- Maslennikova, G.N. Pigments of spinel type. Glass Ceram. 2001, 58, 216–220. [Google Scholar] [CrossRef]
- Kalendova, A. Application of spinel pigments in anticorrosive heat-resistant coatings. Pigment Resin Technol. 2000, 29, 164–172. [Google Scholar] [CrossRef]
- PN-EN ISO 2811-1:2012; Paints and Varnishes—Determination of Density—Part 1: Pycnometric Method. Polish Norm, International Organization for Standardization: Warsaw, Poland, 2012.
- PN-EN ISO 2555:2011; Plastics—Polymers in the Liquid State, Emulsion or Dispersion—Determination of Apparent Viscosity by Brookfield Method. Polish Norm, International Organization for Standardization: Warsaw, Poland, 2011.
- PN-EN ISO 2813:2014-11; Paints and Varnishes—Determination of the Gloss Value at an Angle of 20 Degrees, 60 Degrees and 85 Degrees. Polish Norm, International Organization for Standardization: Warsaw, Poland, 2014.
- Bancken, E.L.J. Paints and Varnishes Volume 1—General Test Methods; NEN. International Organization for Standardization: Geneve, Switzerland, 1994; pp. 123–129. [Google Scholar]
- PN-EN 13300:2002; Farbyilakiery—Water-Based Varnish Products and Coating Systems for Interior Walls and Ceilings—Classification. Polish Norm, International Organization for Standardization: Warsaw, Poland, 2002.
- PN-C-81521:1976; Paint Products—Testing the Resistance of Paint Coatings to Water and Determining Water Absorption. Polish Norm, International Organization for Standardization: Warsaw, Poland, 1976.
- PN-EN ISO 7783:2018-11; Paints and Varnishes—Determination of Water Vapor Permeability Properties—Vessel Method. Polish Norm, International Organization for Standardization: Warsaw, Poland, 2018.
- PN-EN ISO 2814:2006; Paints and Varnishes—Comparison of the Contrast Ratio (Opacity) of Paints of the Same Type and Color. Polish Norm, International Organization for Standardization: Warsaw, Poland, 2006.
- PN-EN ISO 16474-3:2014_02; Paints and Varnishes—Methods of Exposure to Laboratory Light Sources—Part 3: UV Fluorescent Lamps. Polish Norm, International Organization for Standardization: Warsaw, Poland, 2014.
- Deka, A.; Dey, N. Rheological studies of two component high build epoxy and polyurethane based high performance coatings. J. Coat. Technol. Res. 2013, 10, 305–315. [Google Scholar] [CrossRef]
- Jaehnichen, K.; Frank, J.; Pleul, D.; Simon, F. A study of paint adhesion to polymeric substrates. J. Adhes. Sci. Technol. 2003, 17, 1635–1654. [Google Scholar] [CrossRef]
- De Oliveira, M.P.; Silva, C.R.; Muller Guerrini, L. Effect of itaconic acid on the wet scrub resistance of highly pigmented paints for architectural coatings. J. Coat. Technol. Res. 2011, 8, 439–447. [Google Scholar] [CrossRef]
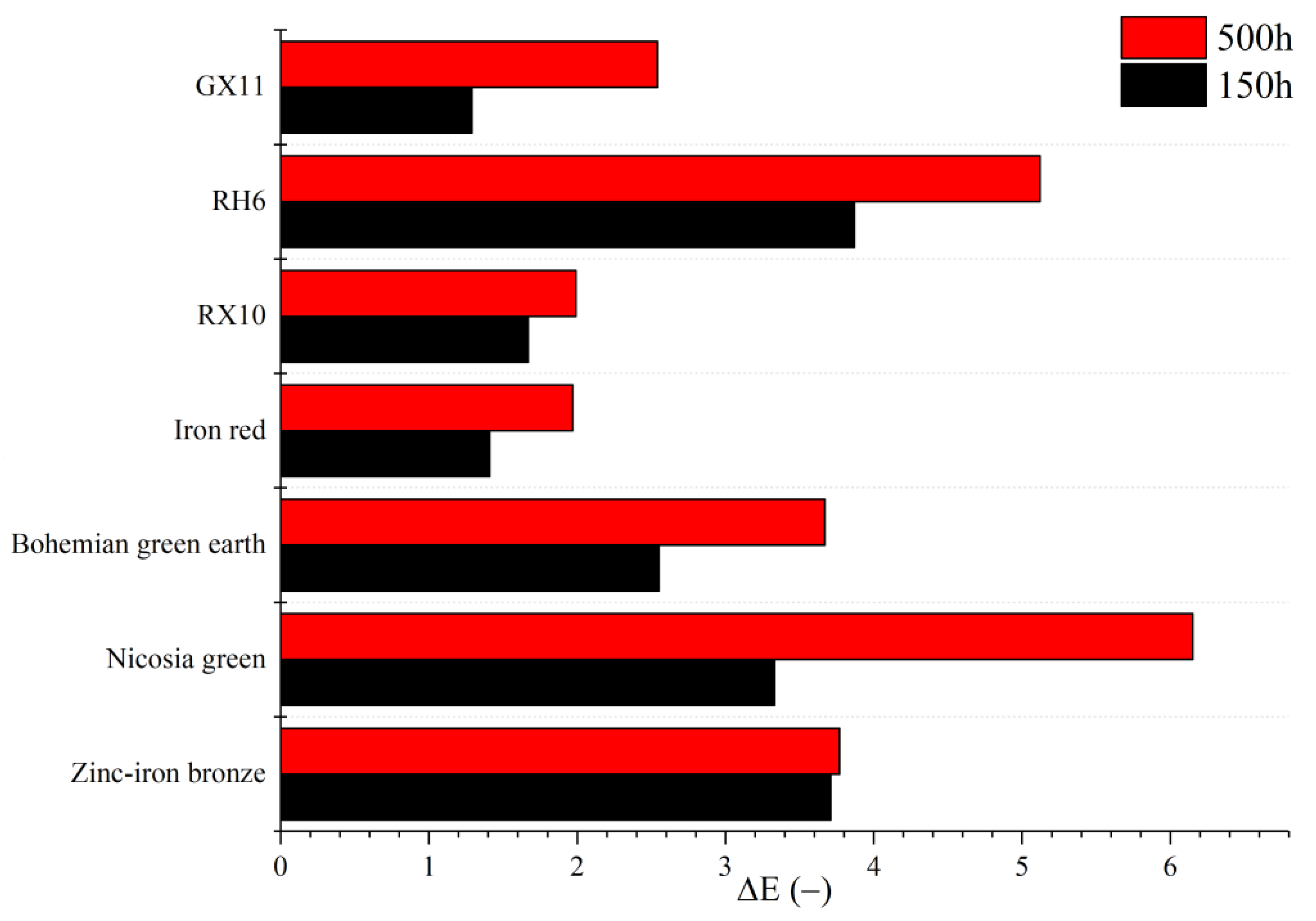
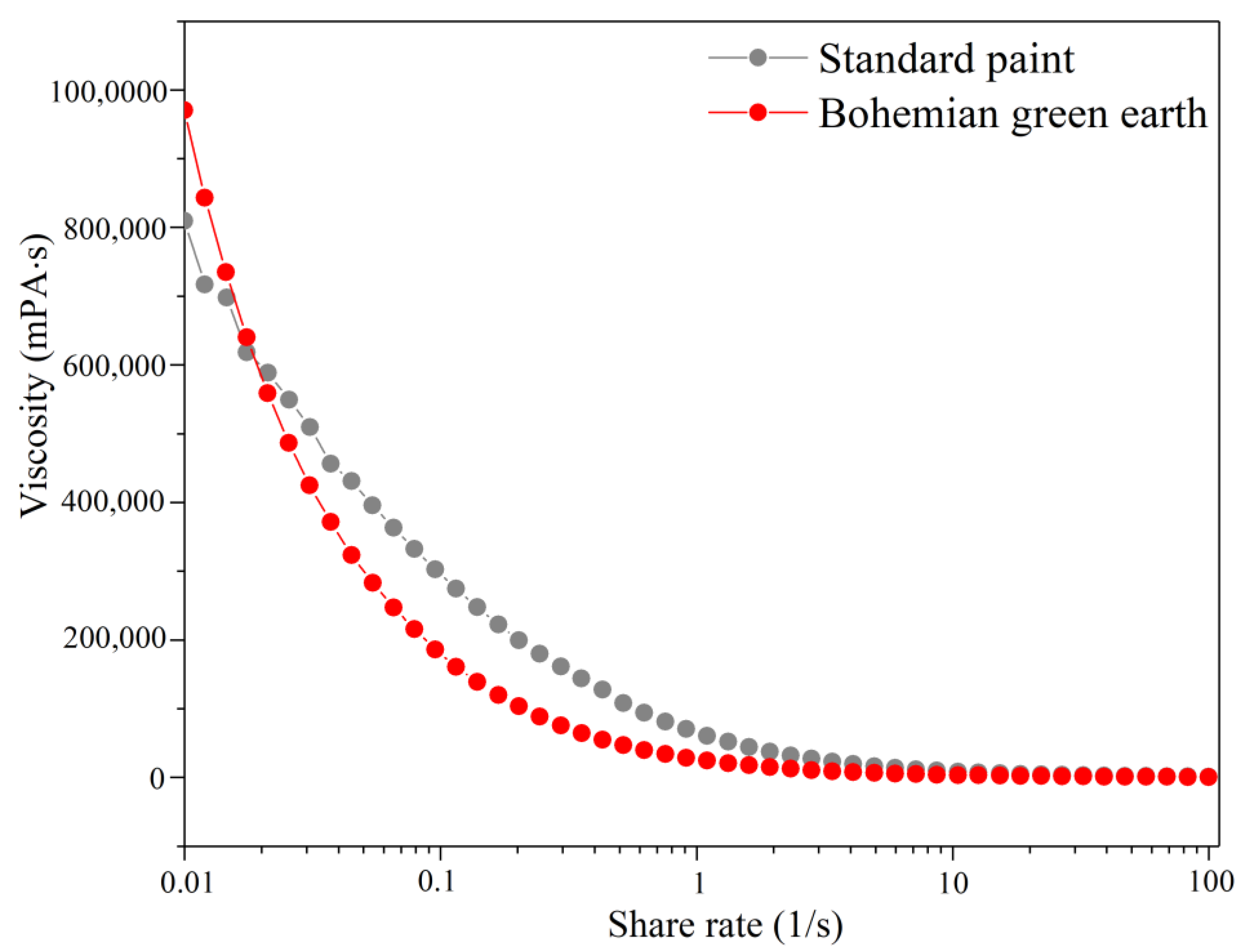
| Pigment | Gloss at 60 [GU] | Gloss at 85 [GU] | Exfoliation [%] | Adhesion Class |
|---|---|---|---|---|
| Iron Red | 0.6 | 0.5 | <5 | 1 |
| Bohemian green earth | 1.5 | 0.6 | <5 | 1 |
| Nicosia green | 1.5 | 0.6 | 0 | 0 |
| Zinc–iron bronze | 1.0 | 0.6 | 0 | 0 |
| P60 | 1.4 | 0.5 | 0 | 0 |
| Pigment | P60 | Zinc-Iron Bronze | Iron Red | Nicosia Green | Bohemian Green Earth | RX10 | RH6 | GX11 |
|---|---|---|---|---|---|---|---|---|
| Contrast [dE] | 0.52 | 0.06 | 0.10 | 0.12 | 0.17 | 0.14 | 0.27 | 0.98 |
| Reference Paint | Modified Paint | |
|---|---|---|
| Component | Weight Share [%] | |
| Water | 20.0 | 20.0 |
| Antifoam emulsion | 0.2 | 0.2 |
| Biocide | 1.0 | 1.0 |
| Coalescent | 1.6 | 1.6 |
| Titanium dioxide | 3.0 | 3.0 |
| Dolomite filler | 42.0 | 37.0 |
| Acrylic resin | 28.0 | 28.0 |
| Silicone resin | 3.0 | 3.0 |
| Cellulose thickener | 0.5 | 0.5 |
| Acrylic thickener | 0.7 | 0.7 |
| Bohemian green earth | 0.0 | 5.0 |
| Reference Paint | Modified Paint | |
|---|---|---|
| Initial viscosity [mPas] | 15.63 | 15.93 |
| Viscosity after 24 h [mPas] | 16.10 | 16.20 |
| Viscosity after storage [mPas] | 17.03 | 17.13 |
| Sedimentation | no | no |
| Thickness [µm] | ||||
|---|---|---|---|---|
| Before Scrub | After Scrub | Loss | Class | |
| Reference paint a | 192.00 | 185.00 | ||
| Reference paint b | 187.00 | 180.00 | ||
| Reference paint c | 190.00 | 178.00 | ||
| Reference paint d | 190.00 | 179.00 | ||
| Average | 189.75 | 180.50 | 9.25 | II |
| Modified paint a | 197.00 | 190.00 | ||
| Modified paint b | 205.00 | 189.00 | ||
| Modified paint c | 190.00 | 184.00 | ||
| Modified paint d | 191.00 | 190.00 | ||
| Average | 195.75 | 188.25 | 7.50 | II |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisowski, J.; Szadkowski, B.; Marzec, A. Effects of Selected Pigments on the Properties of Silicone Resin-Based Paints. Materials 2022, 15, 4961. https://doi.org/10.3390/ma15144961
Lisowski J, Szadkowski B, Marzec A. Effects of Selected Pigments on the Properties of Silicone Resin-Based Paints. Materials. 2022; 15(14):4961. https://doi.org/10.3390/ma15144961
Chicago/Turabian StyleLisowski, Jakub, Bolesław Szadkowski, and Anna Marzec. 2022. "Effects of Selected Pigments on the Properties of Silicone Resin-Based Paints" Materials 15, no. 14: 4961. https://doi.org/10.3390/ma15144961
APA StyleLisowski, J., Szadkowski, B., & Marzec, A. (2022). Effects of Selected Pigments on the Properties of Silicone Resin-Based Paints. Materials, 15(14), 4961. https://doi.org/10.3390/ma15144961






