Pyrolysis Combustion Characteristics of Epoxy Asphalt Based on TG-MS and Cone Calorimeter Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Test Methods
- (1)
- TG-MS
- (2)
- Cone Calorimeter Test
3. Results and Analysis
3.1. TG-MS Analysis
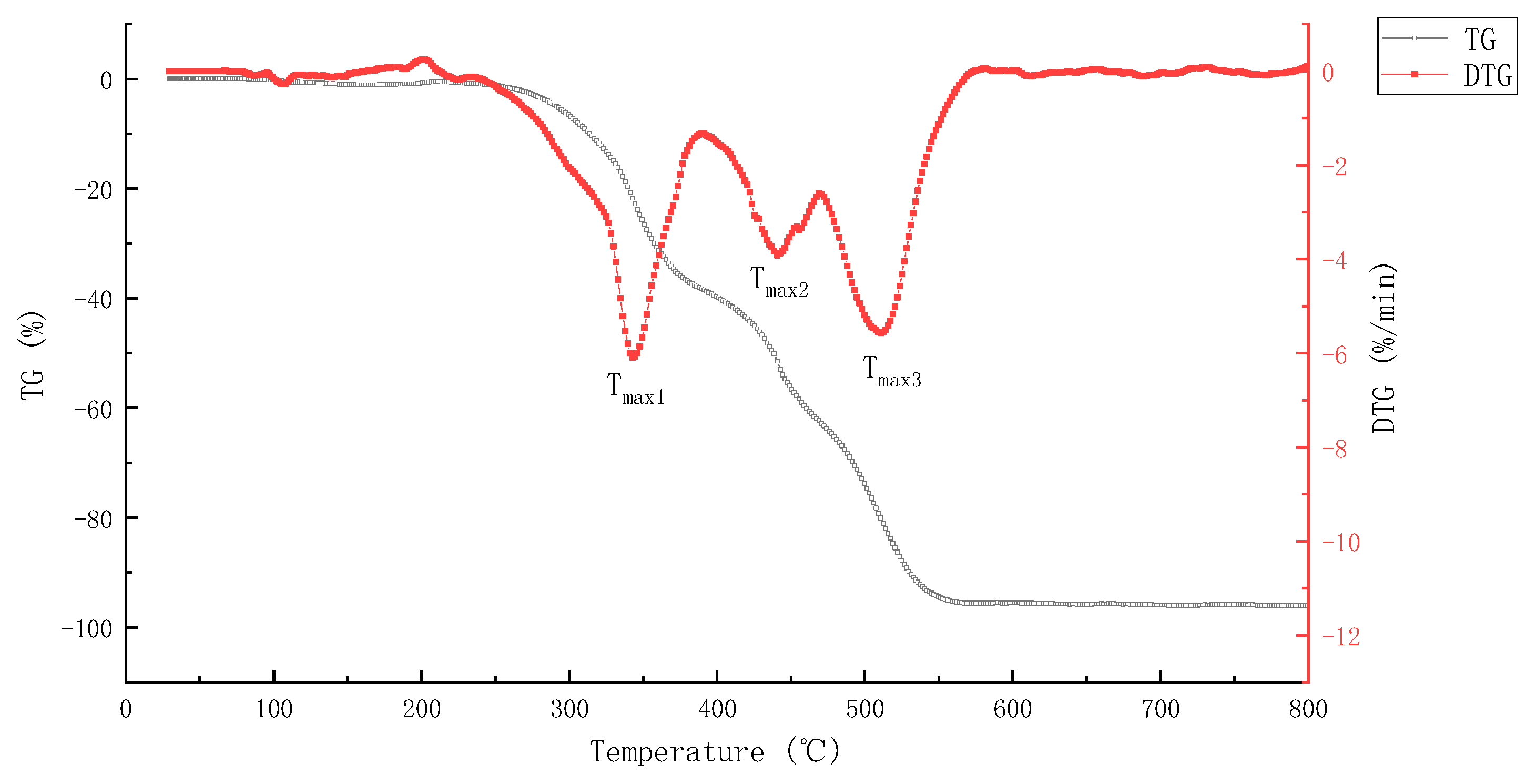
3.1.1. TG Analysis
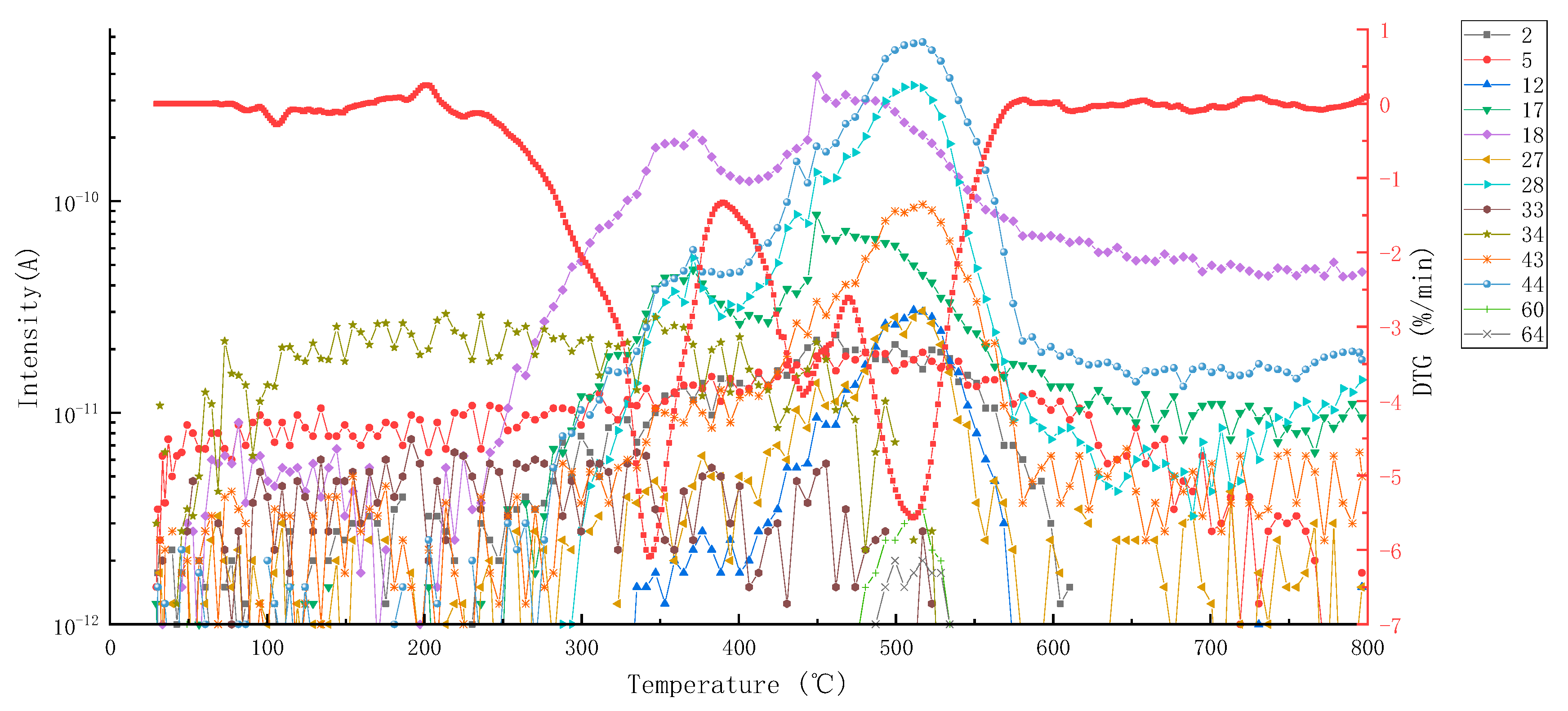
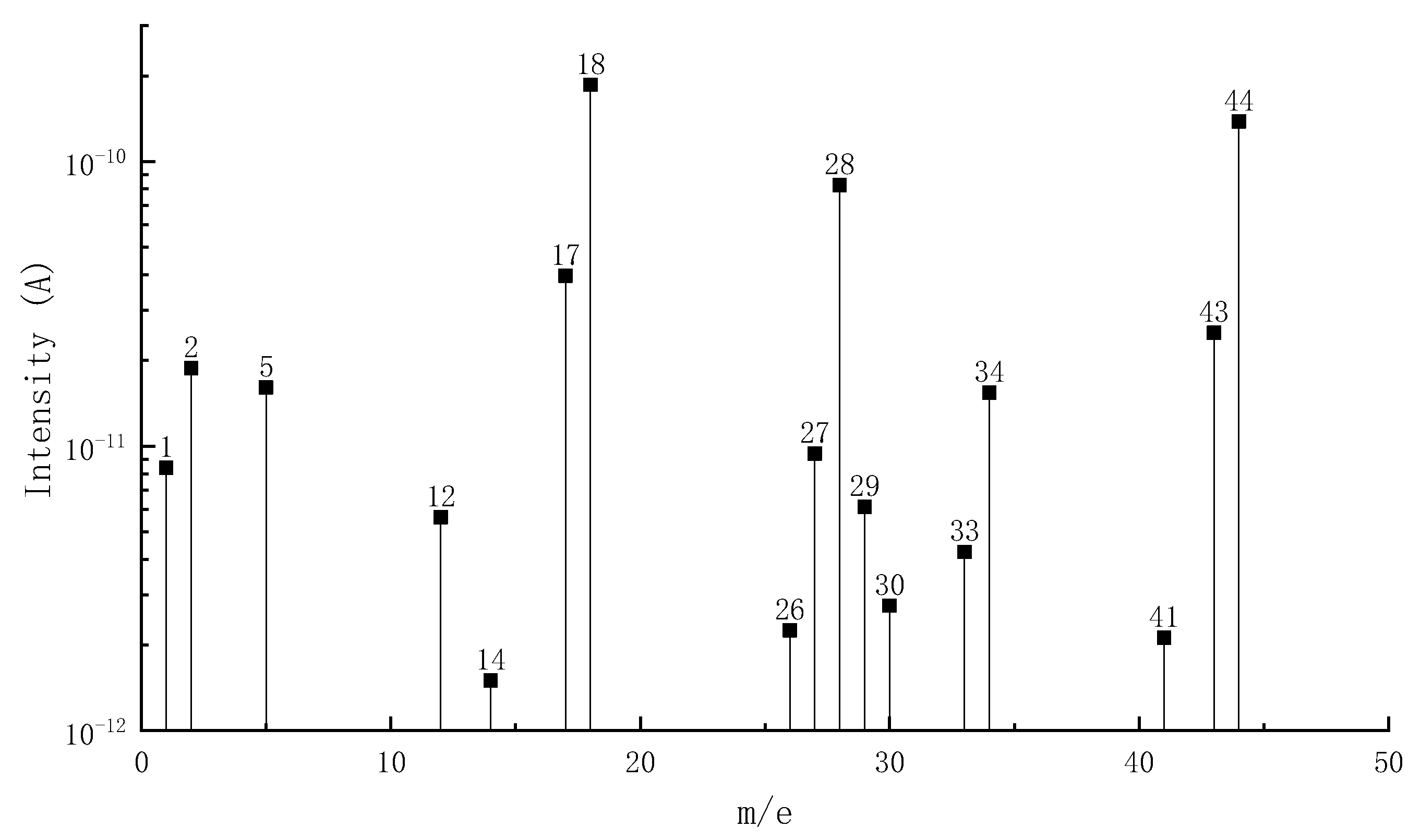
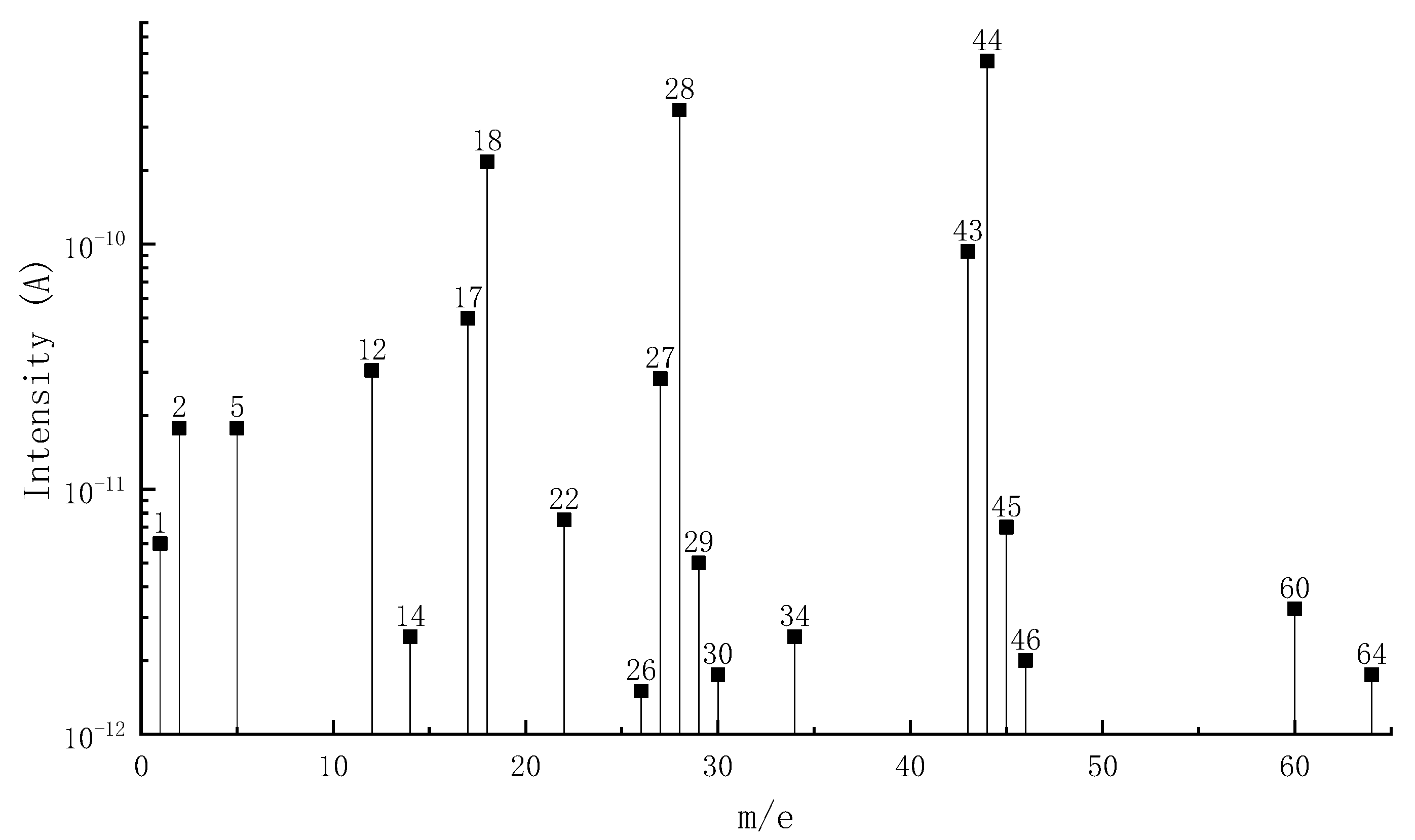
3.1.2. MS Analysis
3.2. Cone Calorimeter Test Analysis
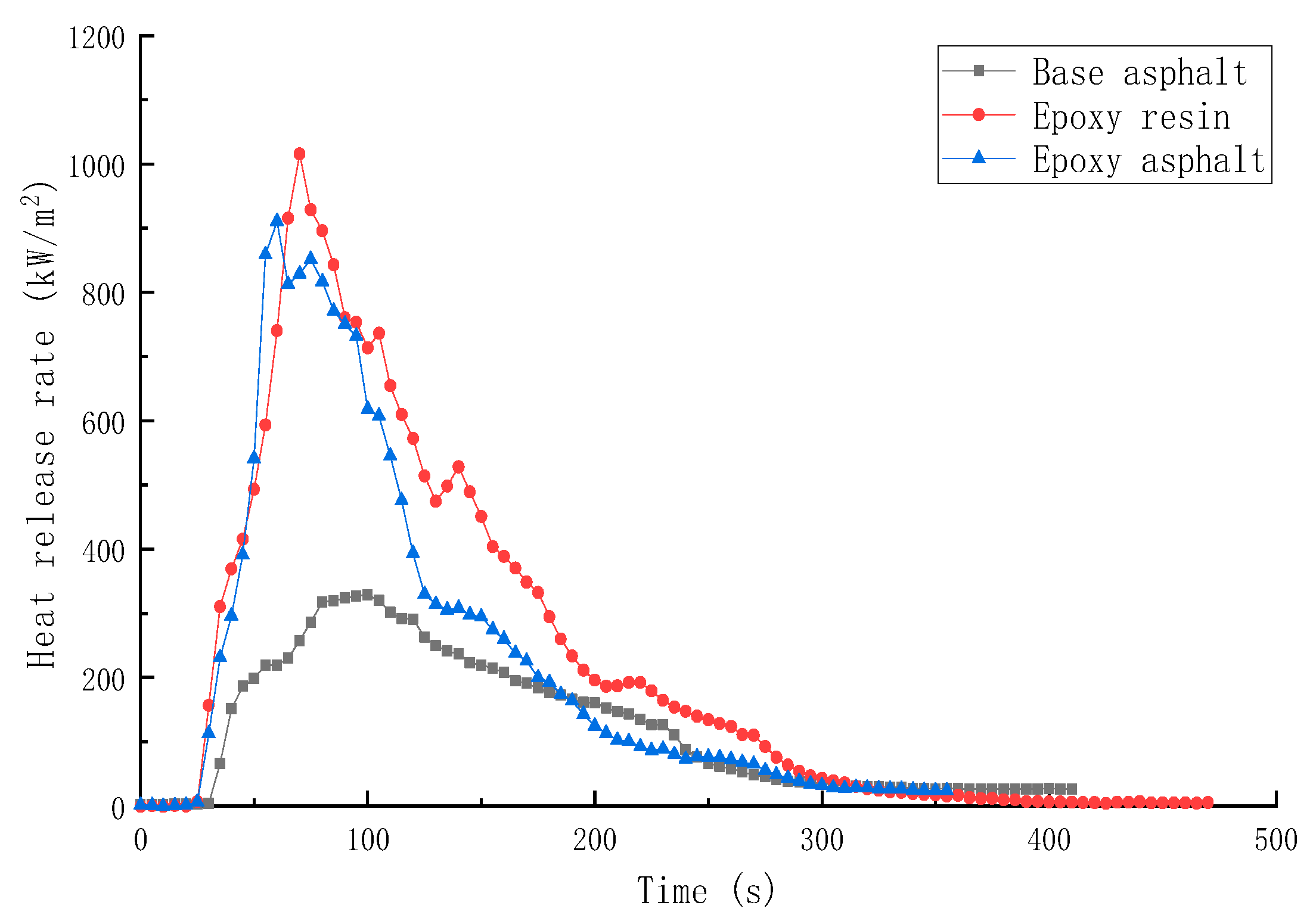
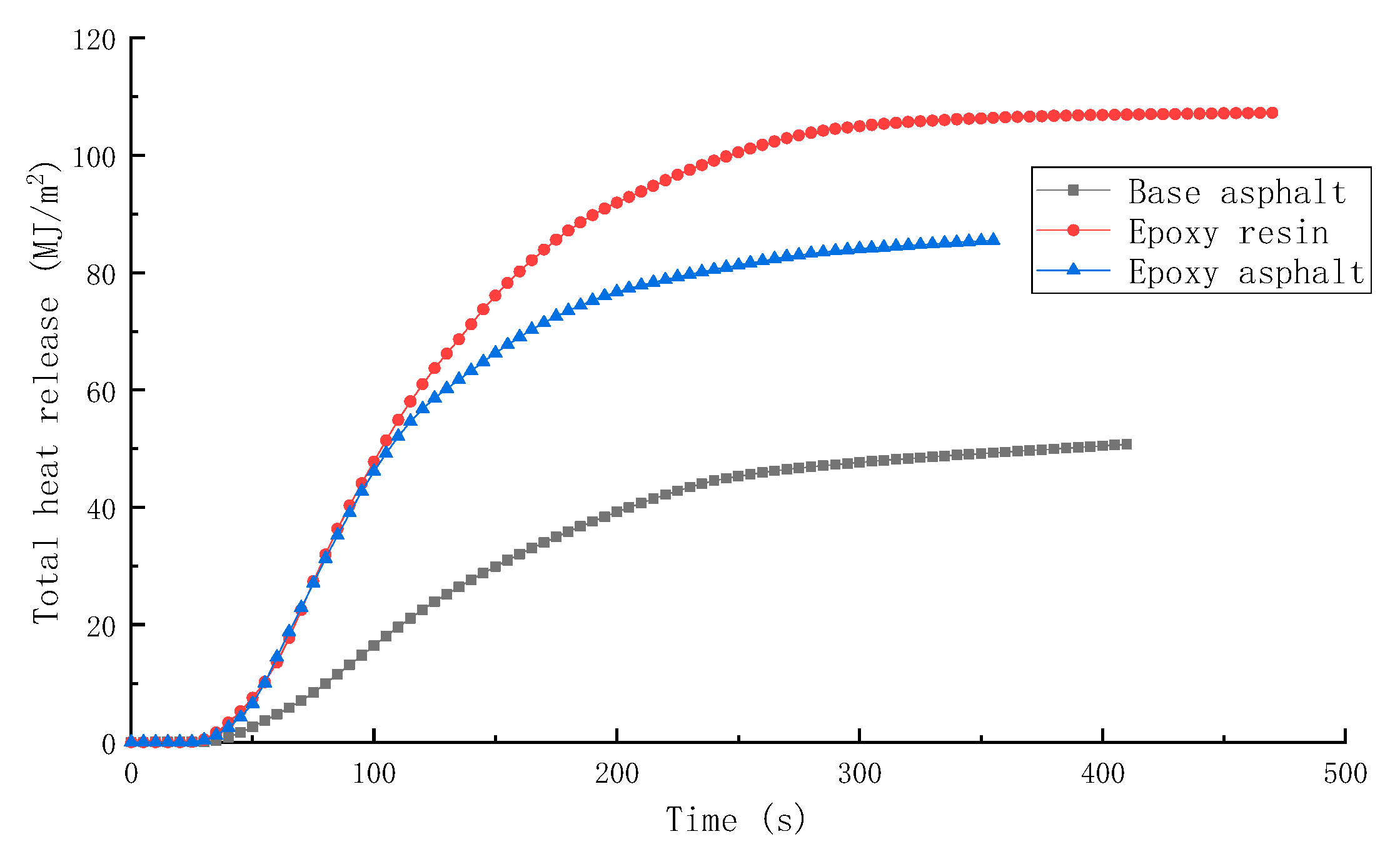
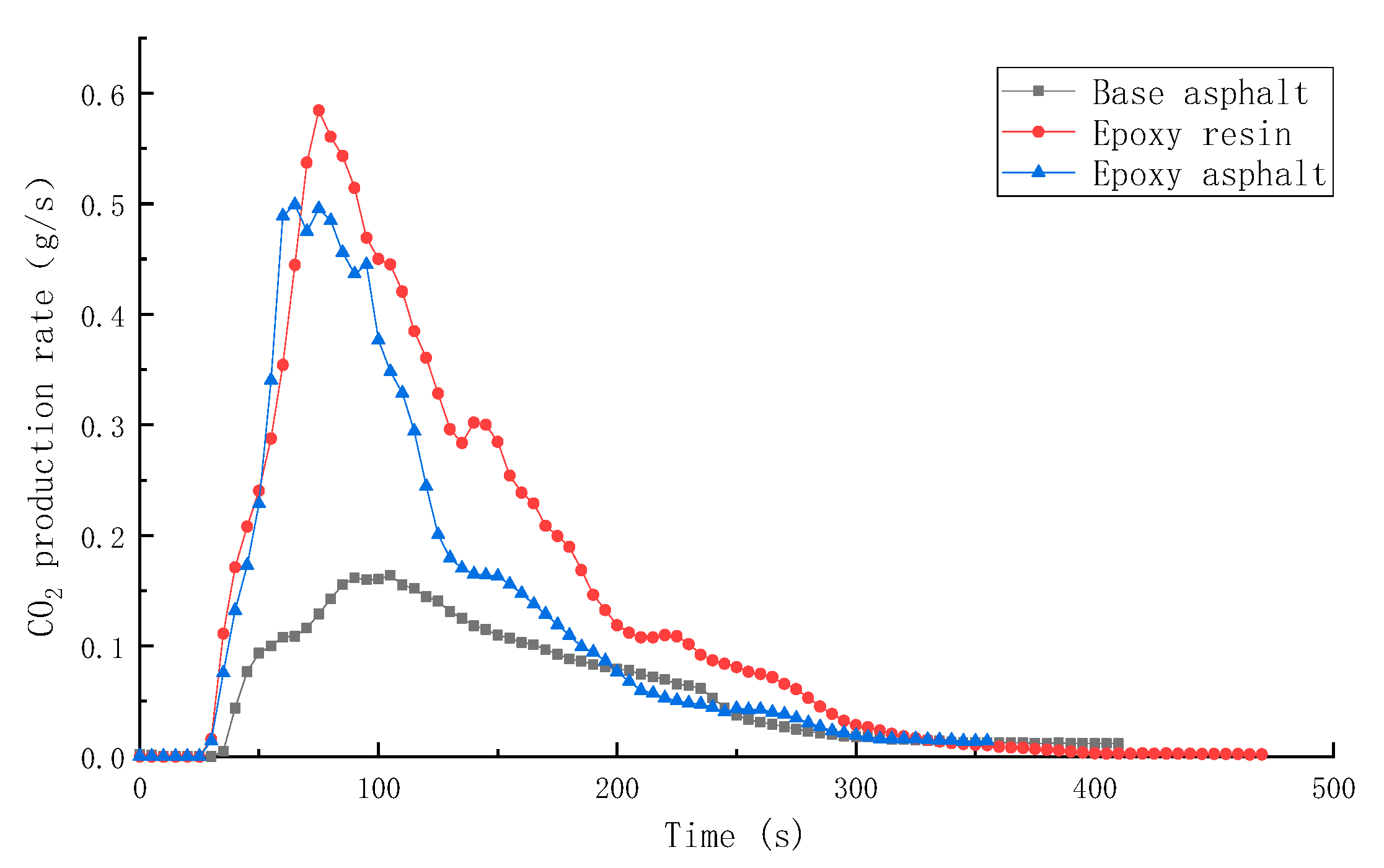
3.2.1. Combustion Heat Release Performance of Different Materials
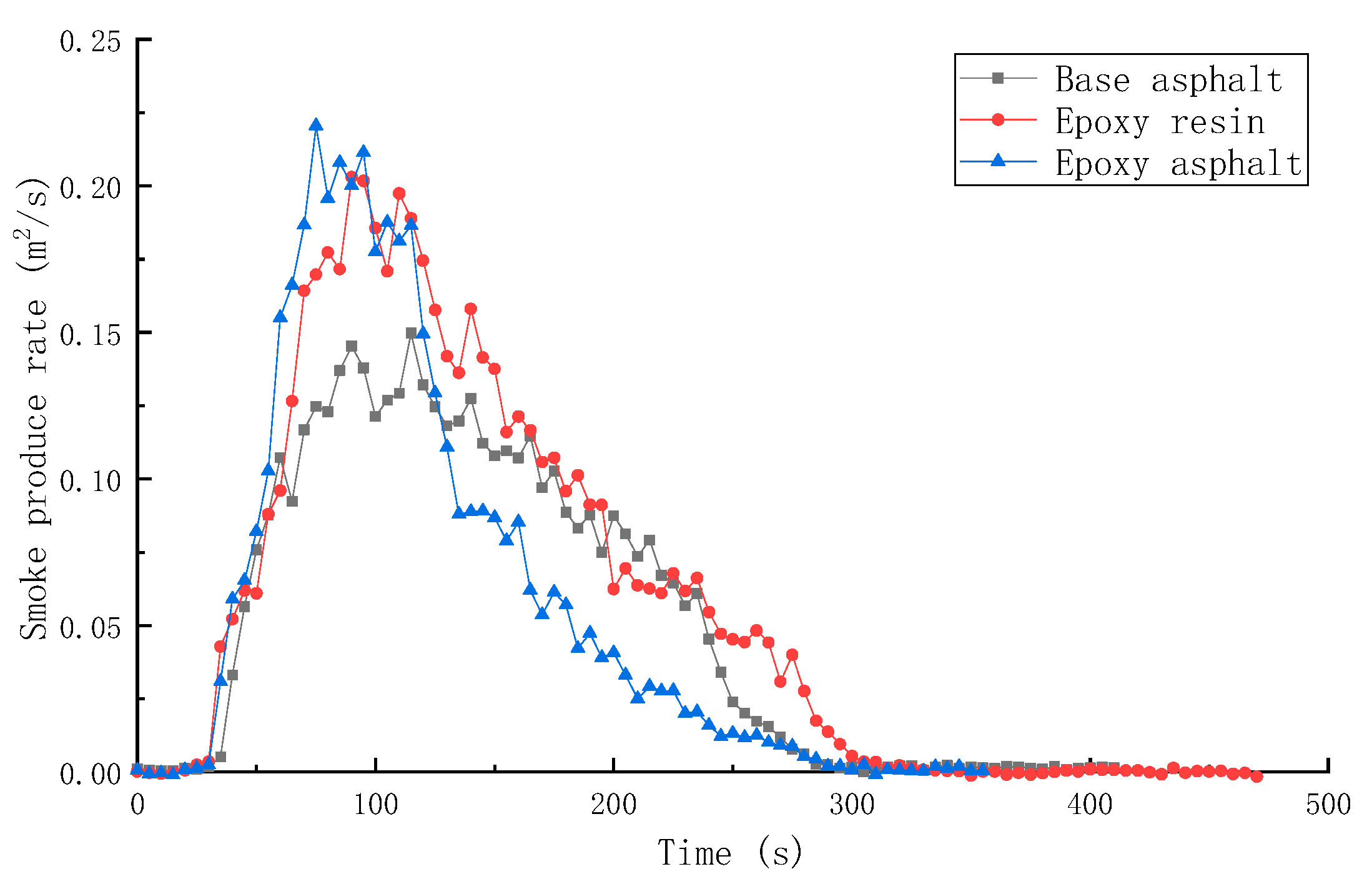
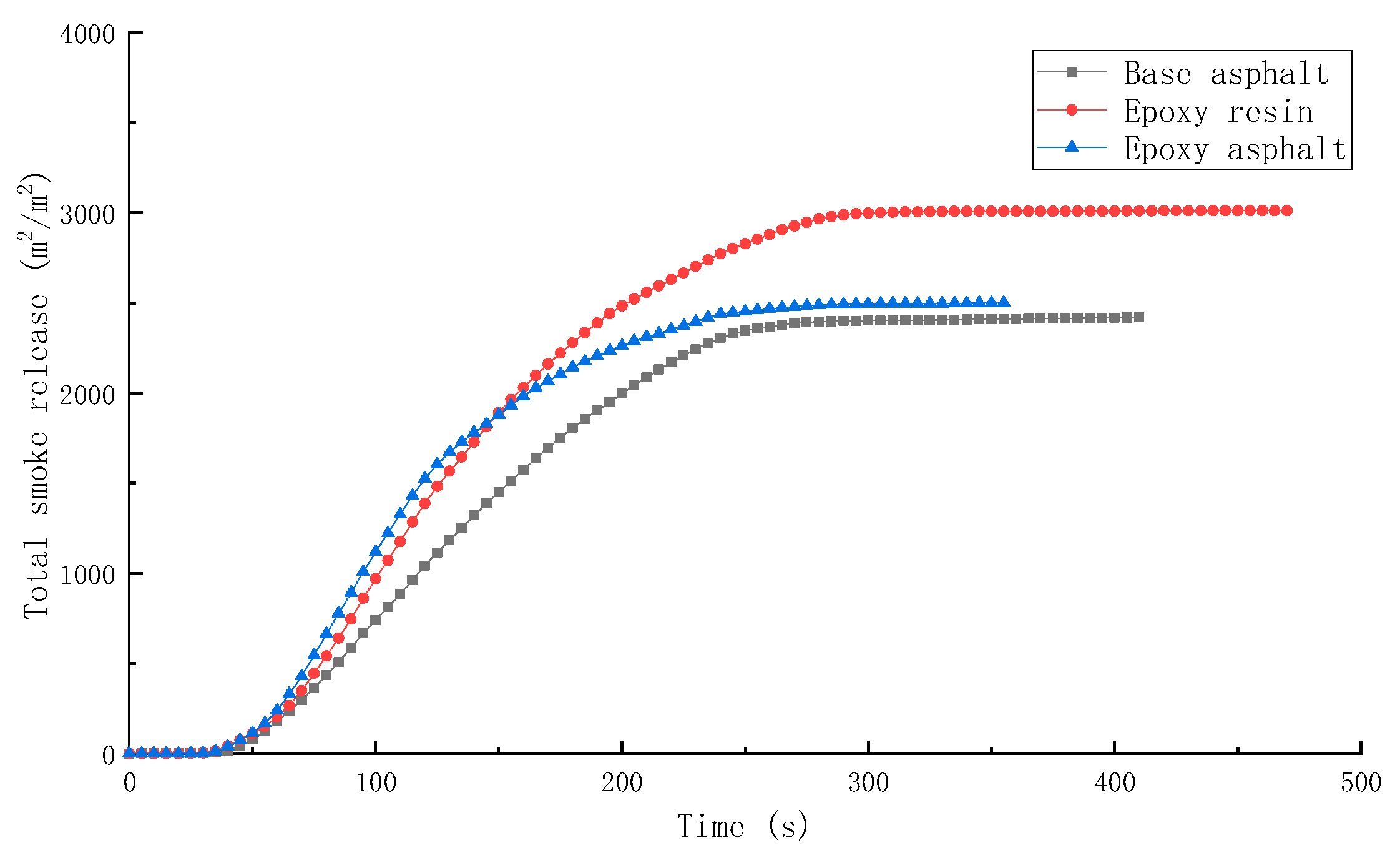
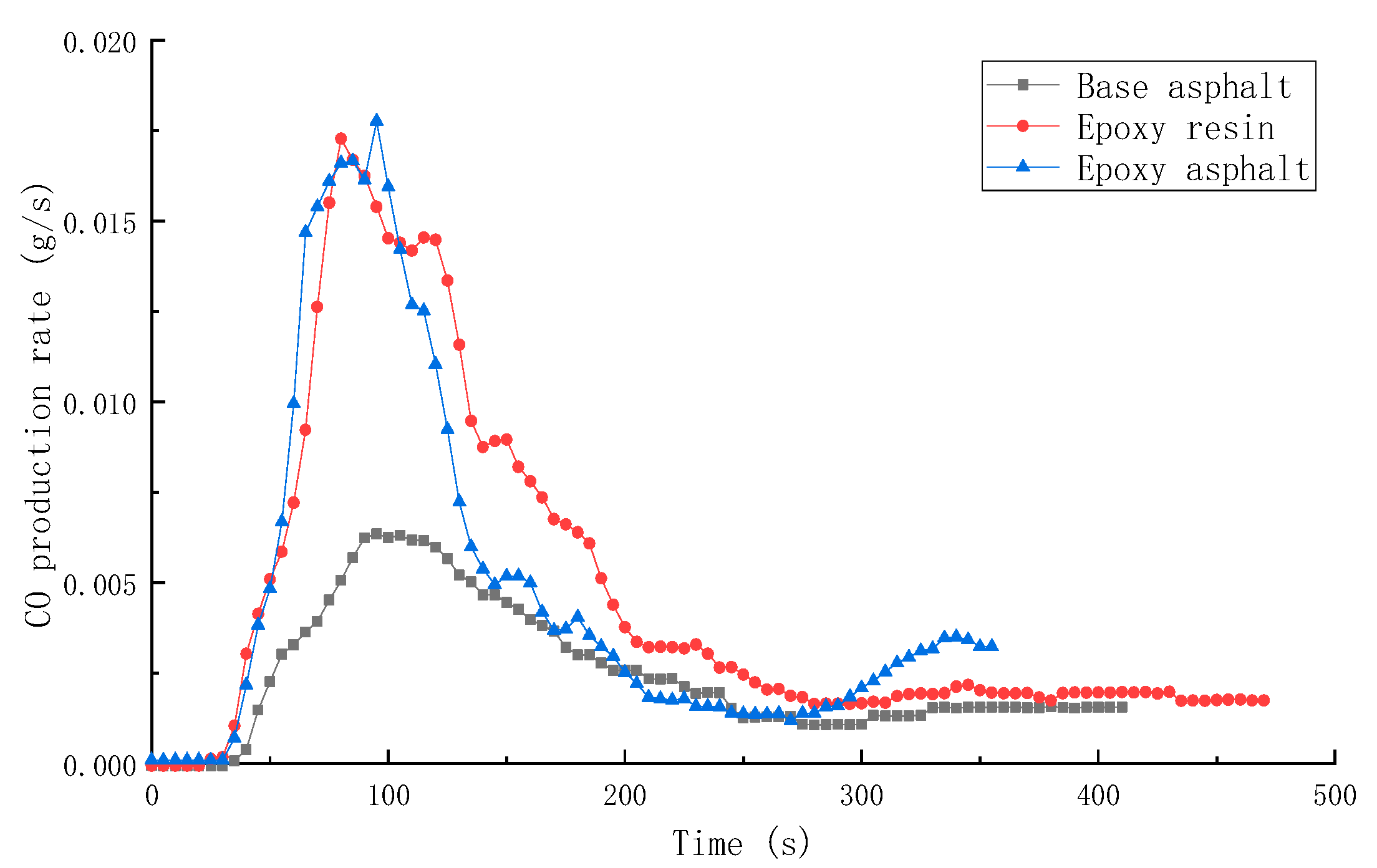
3.2.2. Combustion Smoke Release Performance of Different Materials
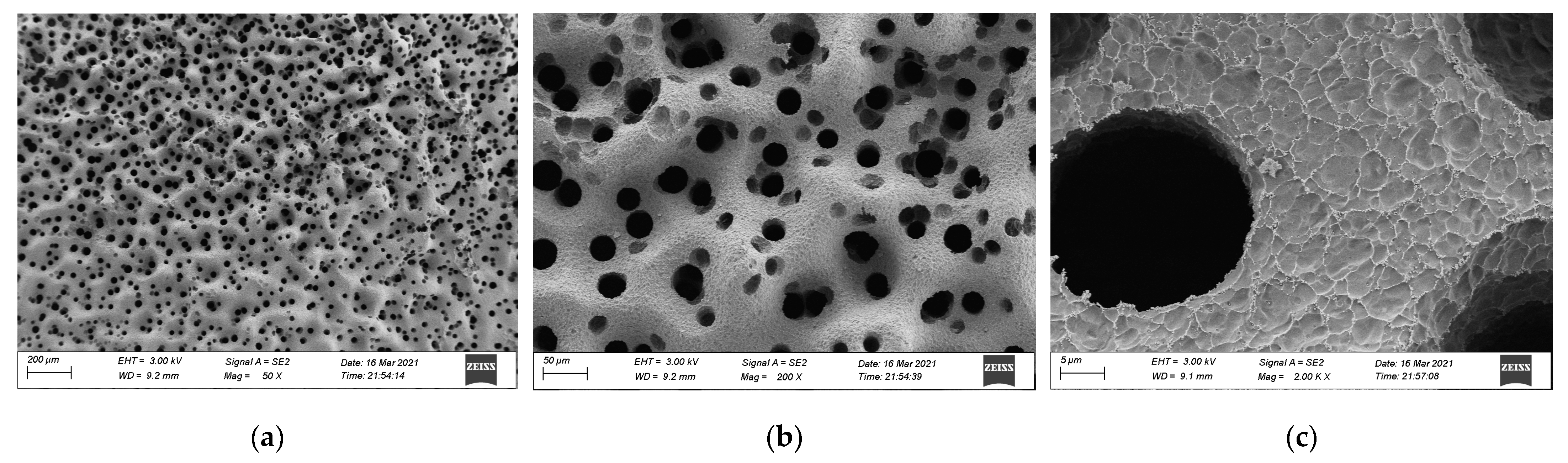
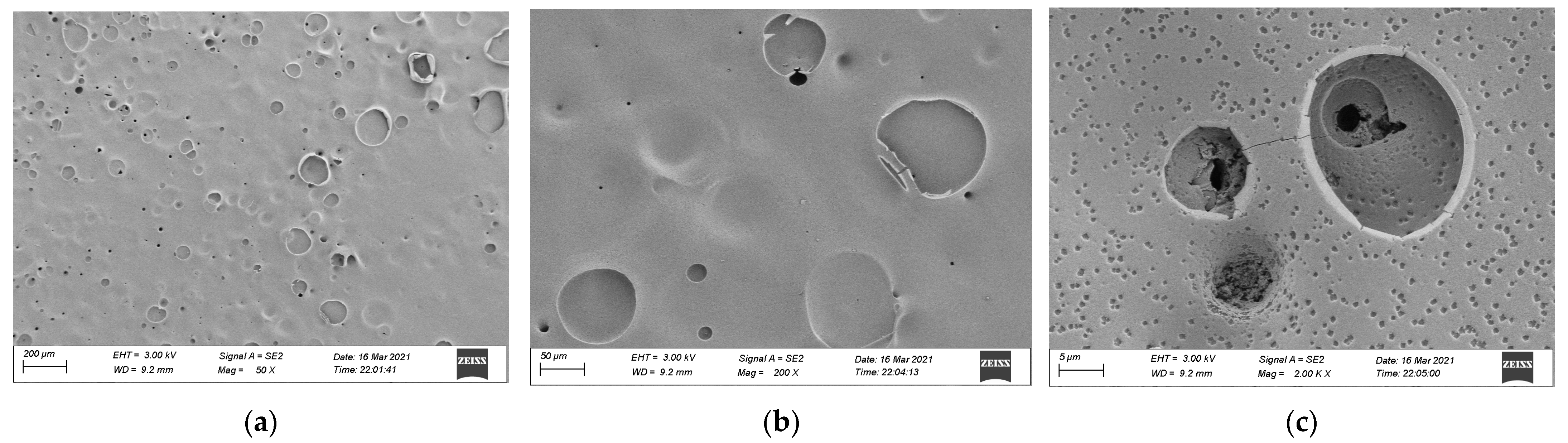
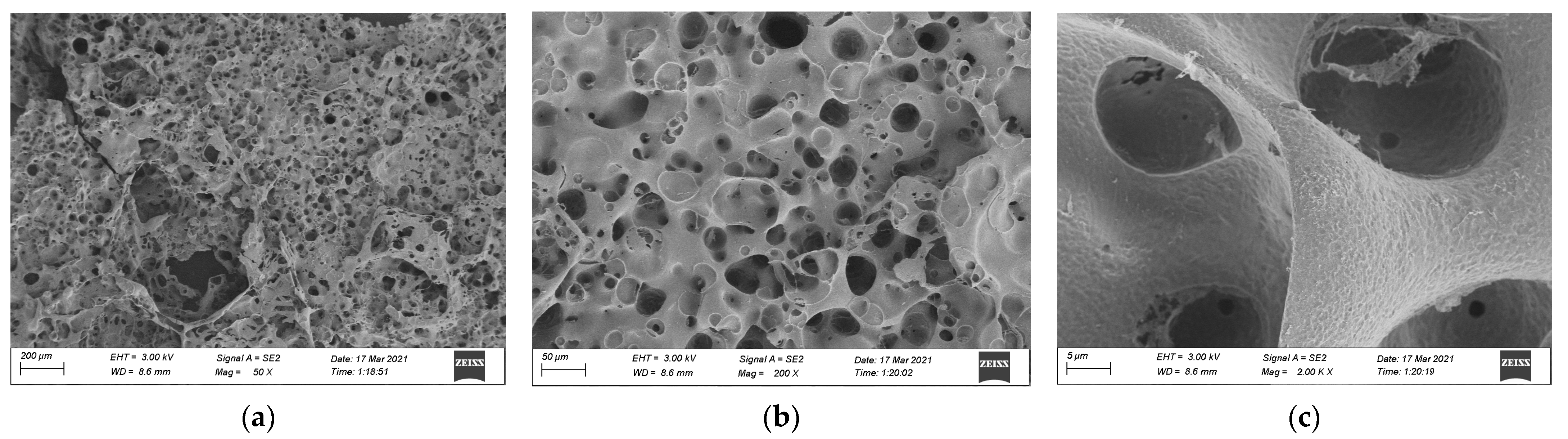
3.3. SEM Analysis
4. Conclusions
- (1)
- Epoxy asphalt exhibited different characteristics in pyrolysis and combustion stages. When it was close to the peak temperature of each stage, a peak value was observed in the ion flow curve, and the release of volatiles was the largest.
- (2)
- The peak HRR, THR, SPR, and TSR of epoxy resin were high, reaching 1015.7 kW/m2, 104.5 MJ/m2, 0.203m2/s, and 2995.2 m2/m2, respectively, and the combustion was intense. However, the smoke produced from epoxy resin reduced to a certain extent after the base asphalt was added in the epoxy resin in the form of an interpenetrating network. The SPR of epoxy asphalt was high in the early stage, up to 0.220 m2/s, then sharply decreased. Moreover, the TSR was close to that of base asphalt.
- (3)
- The residual carbon rate was low, and the volatile content was relatively small after the combustion of epoxy resin. Its combustion form is to deepen the combustion layer-by-layer at the pore-forming place. Therefore, the residual carbon surface was dense and flat with few through holes. However, the carbon residue surface of epoxy asphalt after combustion exhibited an irregular network structure, which was rough and loose, with some holes; nevertheless, most of them existed in the form of embedded nonpenetration, which is mainly caused by the impact of noncombustible gases such as N2 and CO2 released by polymers after combustion on the carbon layer.
- (4)
- The results of heat release and smoke release tests showed that epoxy asphalt is not a simple fusion of base asphalt and epoxy resin. Instead, they promote, interact with, and affect each other, and the influence of epoxy resin on the pyrolysis and combustion characteristics of epoxy asphalt was greater than that of base asphalt.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.M.; Zeng, G.D.; Xu, W.; Li, S.J.; Zhou, Z.G. Study of curing reaction mechanism and construction control performance of epoxy asphalt. J. Build. Mater. 2020, 23, 941–947. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Bo, Y.-Z.; Duan, S.-Y. Study on Low Temperature and High Temperature Performance of Epoxy Resin Particles Used for Asphalt Pavement. DEStech Trans. Mater. Sci. Eng. 2017, 448, 10861. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.C.; Yao, B.; Li, M.Z. Fracture characteristics and numerical simulation of epoxy resin mixture. J. Build. Mater. 2020, 23, 1160–1166,1176. [Google Scholar] [CrossRef]
- Xu, M.-J.; Xu, G.-R.; Leng, Y.; Li, B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stab. 2016, 123, 105–114. [Google Scholar] [CrossRef]
- Androjić, I.; Dimter, S. Laboratory evaluation of the physical properties of hot mix asphalt exposed to combustion. Constr. Build. Mater. 2022, 323, 126569. [Google Scholar] [CrossRef]
- Puente, E.; Lázaro, D.; Alvear, D. Study of tunnel pavements behaviour in fire by using coupled cone calorimeter—FTIR analysis. Fire Saf. J. 2016, 81, 1–7. [Google Scholar] [CrossRef]
- Zhu, K.; Huang, Z.Y.; Wu, K.; Wu, B.; Zhang, X.; Zhang, C. Hydrated lime modification of asphalt mixtures with improved fire performance. J. Zhejiang Univ. Sci. 2015, 49, 963–968. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer. 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Wang, J.; Qian, L.; Huang, Z.; Fang, Y.; Qiu, Y. Synergistic flame-retardant behavior and mechanisms of aluminum poly-hexamethylenephosphinate and phosphaphenanthrene in epoxy resin. Polym. Degrad. Stab. 2016, 130, 173–181. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Wang, J.; Zhang, B. Synergistic flame-retardant effect of expandable graphite and phosphorus-containing compounds for epoxy resin: Strong bonding of different carbon residues. Polym. Degrad. Stab. 2016, 128, 89–98. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, T.; Wang, X.; Wang, L.; Zhang, G. Review of the flame retardancy on highway tunnel asphalt pavement. Constr. Build. Mater. 2019, 195, 468–482. [Google Scholar] [CrossRef]
- Wu, K.; Zhu, K.; Huang, Z.Y.; Wang, J.C.; Yang, Q.M.; Liang, P. Research on the gas based on infrared spectral analysis. Spectrosc. Spectr. Anal. 2012, 32, 2089–2094. [Google Scholar] [CrossRef]
- Zhu, K.; Qin, X.; Wang, Y.; Lin, C.; Wang, Q.; Wu, K. Effect of the oxygen concentration on the combustion of asphalt binder. J. Anal. Appl. Pyrolysis. 2021, 160, 105370. [Google Scholar] [CrossRef]
- Shi, H.; Xu, T.; Jiang, R. Combustion mechanism of four components separated from asphalt binder. Fuel 2017, 192, 18–26. [Google Scholar] [CrossRef]
- Xia, W.; Xu, T.; Wang, H. Thermal behaviors and harmful volatile constituents released from asphalt components at high temperature. J. Hazard. Mater. 2019, 373, 741–752. [Google Scholar] [CrossRef]
- Xia, W.; Xu, T.; Wang, S.; Wang, H. Mass loss evolution of bituminous fractions at different heating rates and constituent conformation of emitted volatiles. Energy Sci. Eng. 2019, 7, 2782–2796. [Google Scholar] [CrossRef]
- Yang, D.; Yang, C.; Xie, J.; Wu, S.; Ye, Q.; Hu, R. Combusting behavior and pyrolysis products evaluation of SARA components separated from bitumen. Constr. Build. Mater. 2020, 244, 118401. [Google Scholar] [CrossRef]
- Huang, G.; He, Z.Y.; Huang, T. Laboratory measurement and analysis of effect factor on asphalt fume under the elevated temperature. J. Build. Mater. 2015, 18, 322–327. [Google Scholar] [CrossRef]
- Wang, W.Z.; Shen, A.Q.; Wang, L.S.; Liu, H.C. Measurements, emission characteristics and control methods of fire effluents from tunnel asphalt pavement during fire: A review. Res. Square. 2022, 480339. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Gibson, A.G. Fire Properties of Polymer Composite Materials; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Wen, H.; Zhang, X.; Xia, R.; Yang, Z.; Wu, Y. Thermal Decomposition Properties of Epoxy Resin in SF6/N2 Mixture. Materials 2018, 12, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhang, G.X.; Wang, W.B.; Wang, J.; Yang, Y.J.; Fan, H.J.; Tan, L. Research on the combustion performance of epoxy resin based on the cone calorimeter. Chem. Adhes. 2019, 41, 457–459,483. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Shen, R.; Parker, T.; Zhang, Y.; Wang, Z.; Wang, Q. Thermal behavior and kinetics study of carbon/epoxy resin composites. Polym. Compos. 2019, 40, 4530–4546. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, C.; Shen, R.; Wang, Z.; Wang, Q. Experimental investigation of thermal properties and fire behavior of carbon/epoxy laminate and its foam core sandwich composite. J. Therm. Anal. Calorim. 2019, 136, 1237–1247. [Google Scholar] [CrossRef]
- Zhong, Y.; Jing, X.; Wang, S.; Jia, Q.-X. Behavior investigation of phenolic hydroxyl groups during the pyrolysis of cured phenolic resin via molecular dynamics simulation. Polym. Degrad. Stab. 2016, 125, 97–104. [Google Scholar] [CrossRef]
- Ministry of Transport of the People’s Republic of China. Technical Specifications for Construction of Highway Asphalt Pavements; JTG F40–2014; Ministry of Transport of the People’s Republic of China: Beijing, China, 2014.
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. General Specifications of Epoxy Asphalt Materials for Paving Roads and Bridges; GB/T 30598–2014; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2014.
- Shi, H.; Xu, T.; Zhou, P.; Jiang, R. Combustion properties of saturates, aromatics, resins, and asphaltenes in asphalt binder. Constr. Build. Mater. 2017, 136, 515–523. [Google Scholar] [CrossRef]
- Xu, T.; Huang, X. Study on combustion mechanism of asphalt binder by using TG–FTIR technique. Fuel 2010, 89, 2185–2190. [Google Scholar] [CrossRef]


| Test Items | Pen. /0.1 mm | R&B/°C | Ductility (10 °C) /cm | RTFOT | ||
|---|---|---|---|---|---|---|
| Quality Change/% | Residual Penetration Ratio/% | Residual Ductility (10 °C)/cm | ||||
| Test value | 72 | 47.5 | 33 | 0.48 | 68.5 | 9 |
| Technical requirement | 60–80 | ≥45 | ≥15 | ≤±0.8 | ≥61 | ≥6 |
| Test Items | Viscosity (23 °C) /(Pa·s) | Epoxy Equivalent /(g·mol−1) | Water Content /% | Flash Point/°C | Density /(g·cm−3) | Appearance |
|---|---|---|---|---|---|---|
| Test value | 13.278 | 189 | 0.02 | 281 | 1.164 | Transparent |
| Technical requirement | 11–15 | 185–192 | ≤0.05 | ≥200 | 1.16–1.17 | Transparent |
| Test Items | Tensile Strength (23 °C)/MPa | Fracture Elongation (23 °C)/% | Thermoset (300 °C) | Residence Time /min |
|---|---|---|---|---|
| Test value | 4.3 | 221 | Un-melted | 98 |
| Technical requirement | ≥1.5 | ≥200 | 300°C Un-melted | ≥40 |
| Pyrolysis Combustion Stage | Temperature Range/°C | Thermal Weightlessness/% | Main Volatiles (Characteristic Products) |
|---|---|---|---|
| Phase I | 232.1–387.9 | 38.1 | H2(2), C ion fragment(12), NH3(17), H2O(18), CNH(27), N2(28), CO(28), C2H4(28), HS-ion fragment(33), H2S(34), H2O2(34), N3H(43), CH2O2(44), C3H8(44), CO2(44), N2O(44) |
| Phase II | 387.9–467.3 | 23.8 | H2(2), C ion fragment(12), NH3(17), H2O(18), CNH(27), N2(28), CO(28), C2H4(28), HS-ion fragment(33), H2S(34), H2O2(34), N3H(43), CO2(44), C2H4O(44), C3H8(44), N2O(44) |
| Phase III | 467.3–579.9 | 33.7 | H2(2), C ion fragment(12), NH3(17), H2O(18), CNH(27), N2(28), CO(28), C2H4(28), N3H(43), CO2(44), C2H4O(44), N2O(44), C3H8(44), C3H8O(60), C2H4O2(60), C2H8N2(60), C2H4S(60), SO2(64) |
| Sample | TTI (s) | HRR (kW/m2) | THR (MJ/m2) | SPR (m2/s) | TSR (m2/m2) | COP (g/s) | CO2P (g/s) |
|---|---|---|---|---|---|---|---|
| Base asphalt | 36 | 328.8 | 50.5 | 0.145 | 2413.9 | 0.00635 | 0.1639 |
| Epoxy resin | 30 | 1015.7 | 104.5 | 0.203 | 2995.2 | 0.01728 | 0.5845 |
| Epoxy asphalt | 31 | 910.5 | 85.2 | 0.220 | 2492.8 | 0.01776 | 0.4991 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Shen, J.; Ling, T.; Mei, Q. Pyrolysis Combustion Characteristics of Epoxy Asphalt Based on TG-MS and Cone Calorimeter Test. Materials 2022, 15, 4973. https://doi.org/10.3390/ma15144973
Li X, Shen J, Ling T, Mei Q. Pyrolysis Combustion Characteristics of Epoxy Asphalt Based on TG-MS and Cone Calorimeter Test. Materials. 2022; 15(14):4973. https://doi.org/10.3390/ma15144973
Chicago/Turabian StyleLi, Xiaolong, Junan Shen, Tianqing Ling, and Qingbin Mei. 2022. "Pyrolysis Combustion Characteristics of Epoxy Asphalt Based on TG-MS and Cone Calorimeter Test" Materials 15, no. 14: 4973. https://doi.org/10.3390/ma15144973
APA StyleLi, X., Shen, J., Ling, T., & Mei, Q. (2022). Pyrolysis Combustion Characteristics of Epoxy Asphalt Based on TG-MS and Cone Calorimeter Test. Materials, 15(14), 4973. https://doi.org/10.3390/ma15144973






