Osteogenesis Performance of Boronized Ti6Al4V/HA Composites Prepared by Microwave Sintering: In Vitro and In Vivo Studies
Abstract
:1. Introduction
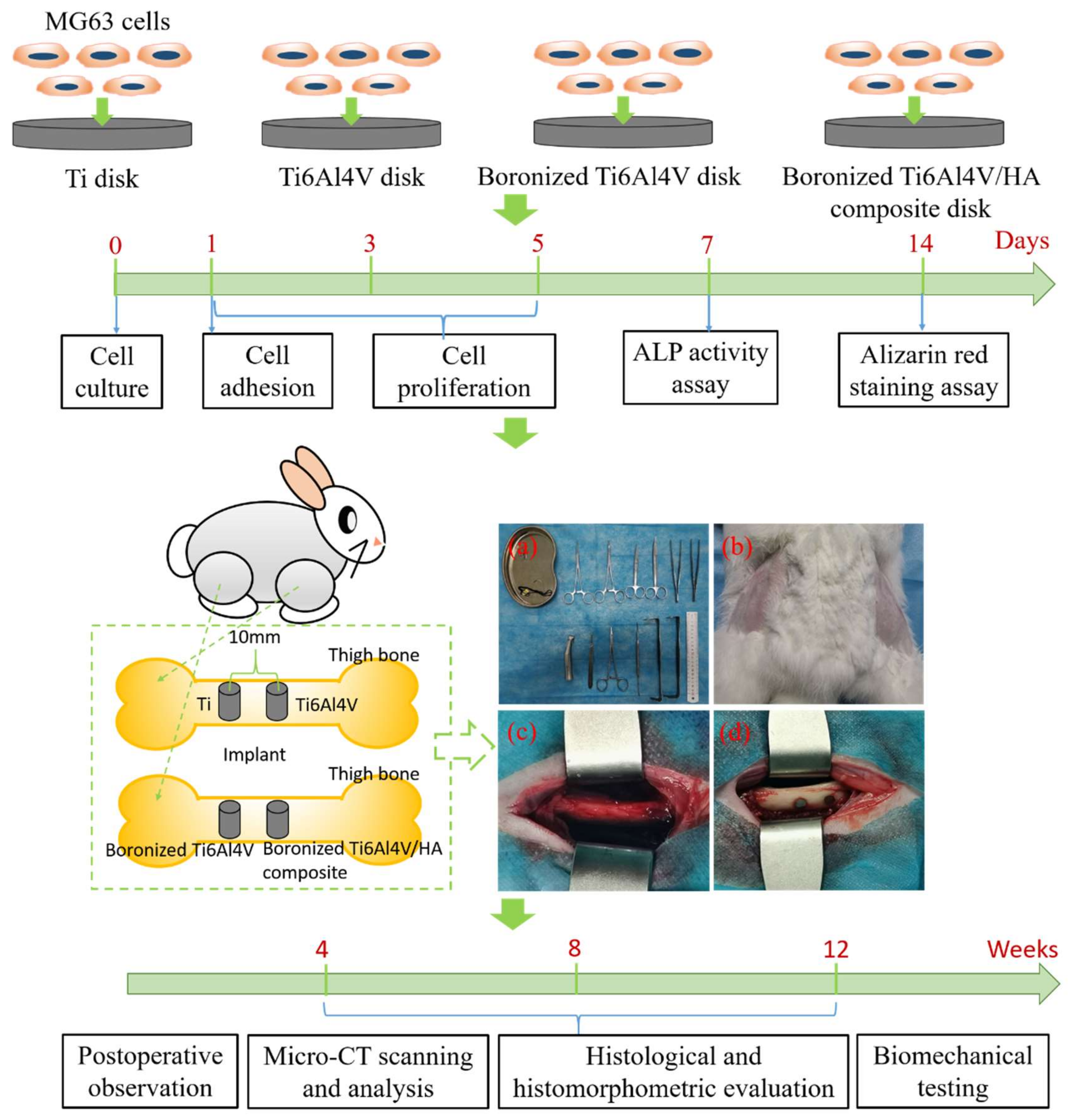
2. Materials and Methods
2.1. Samples and Animals
2.2. In Vitro Study
2.2.1. Cell Culture
2.2.2. Cell Adhesion
2.2.3. Cell Proliferation
2.2.4. ALP Activity Assay
2.2.5. Alizarin Red Staining Assay
2.3. In Vivo Study
2.3.1. Surgical Procedures
2.3.2. Postoperative Observation
2.3.3. Micro-CT Scanning and Analysis
2.3.4. Histological and Histomorphometric Evaluation
2.3.5. Biomechanical Testing
3. Results and Discussion
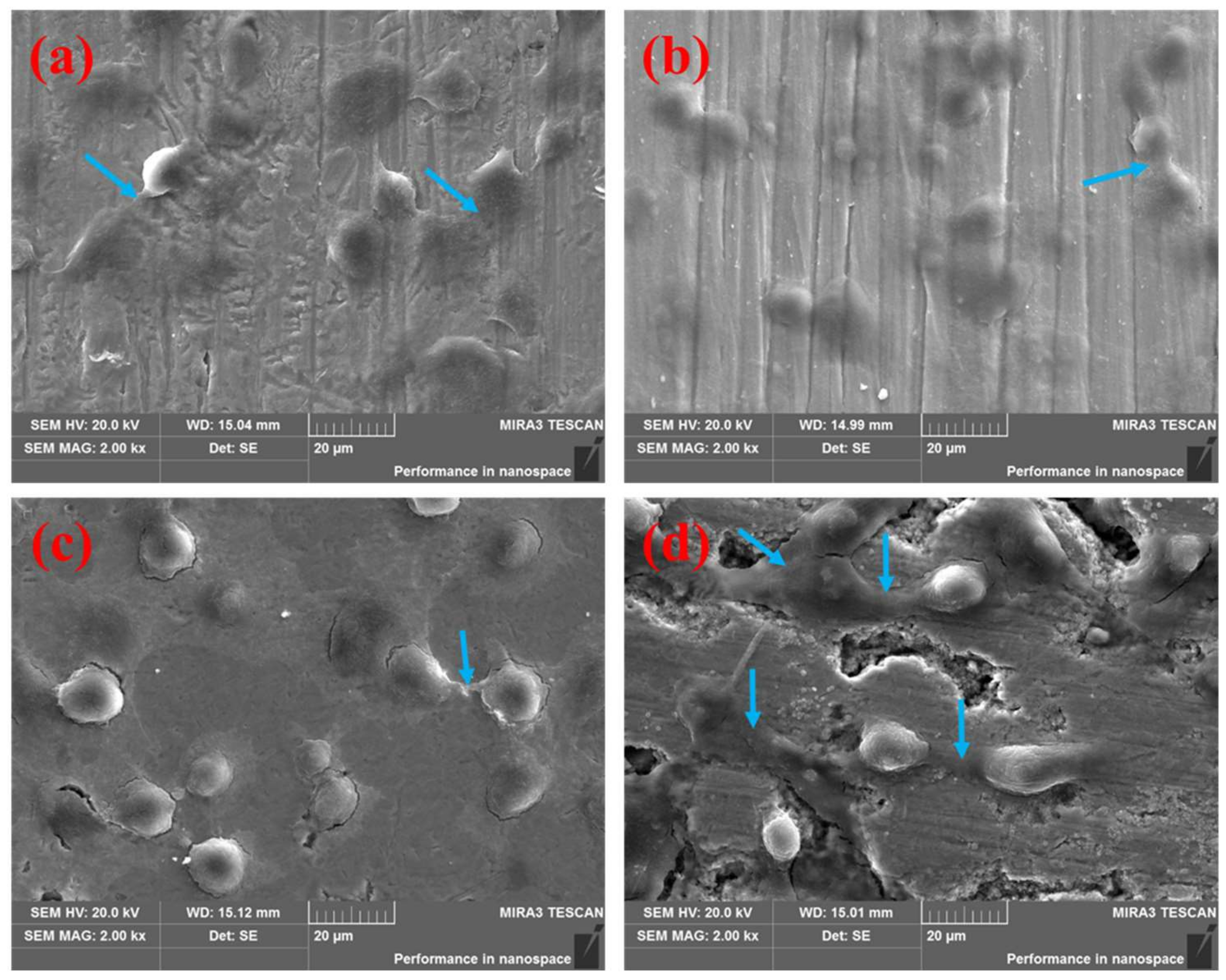
3.1. Cell Adhesion Behavior
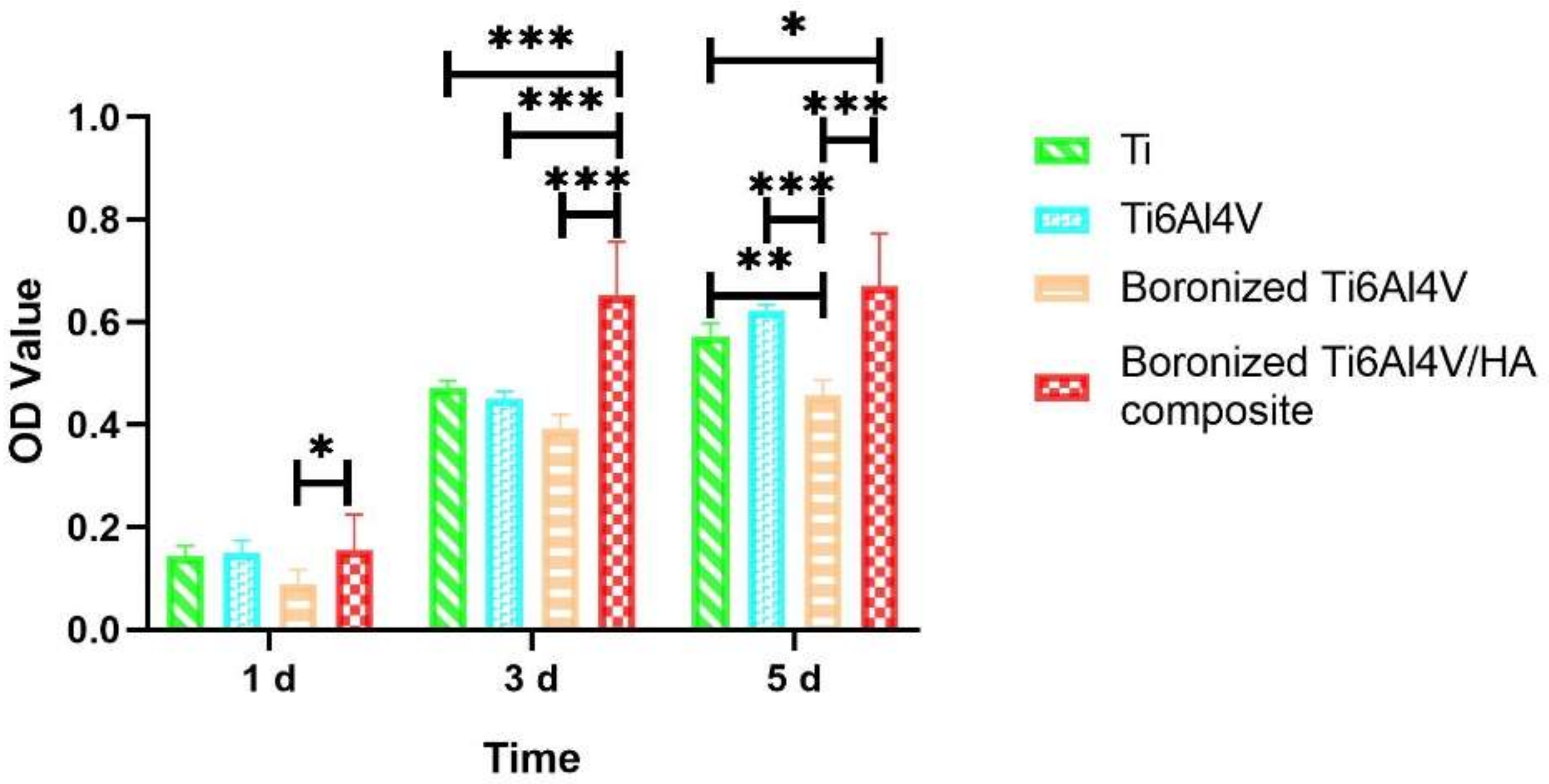
3.2. Cell Proliferation Behavior
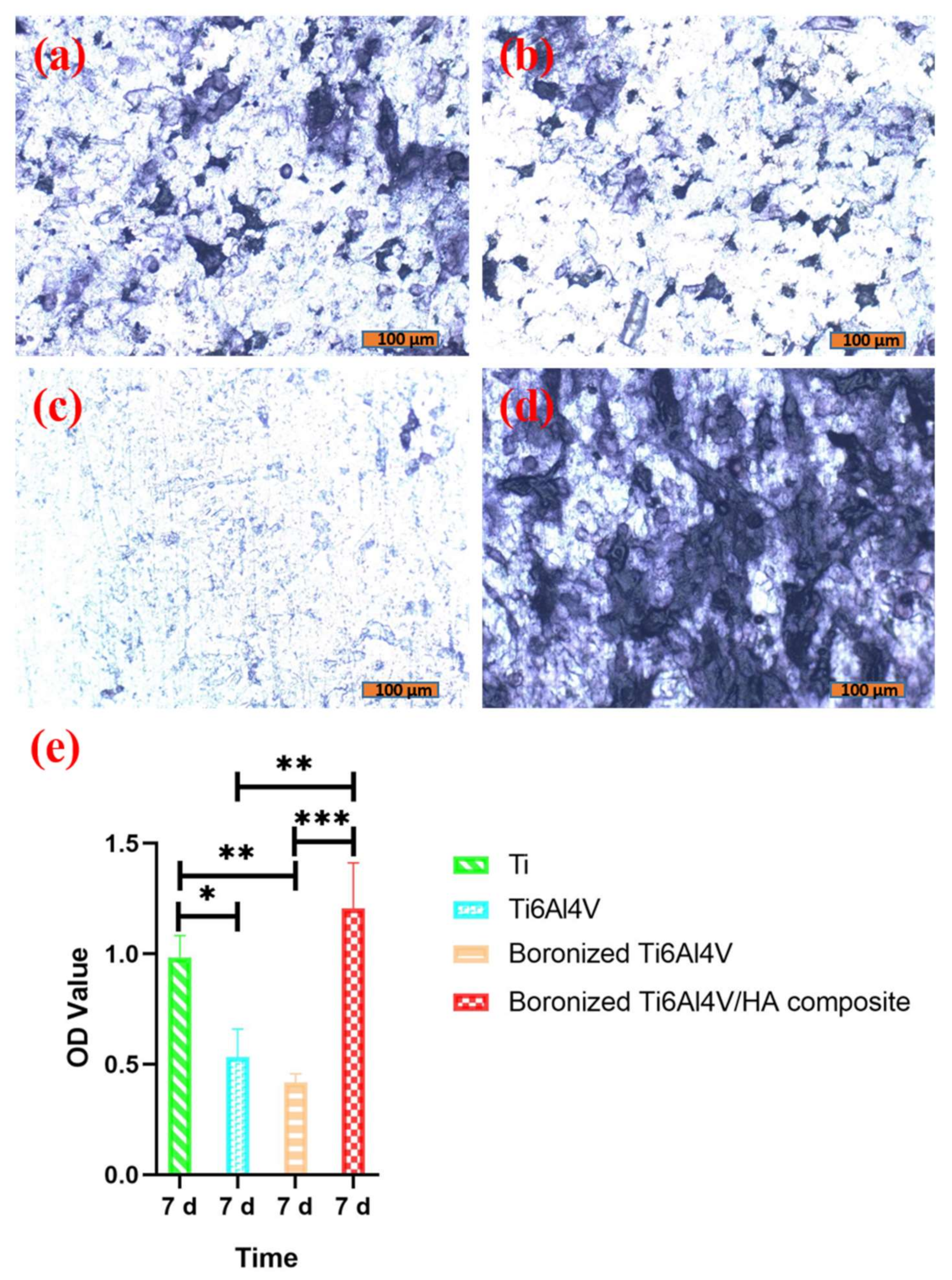
3.3. ALP Activity Analysis
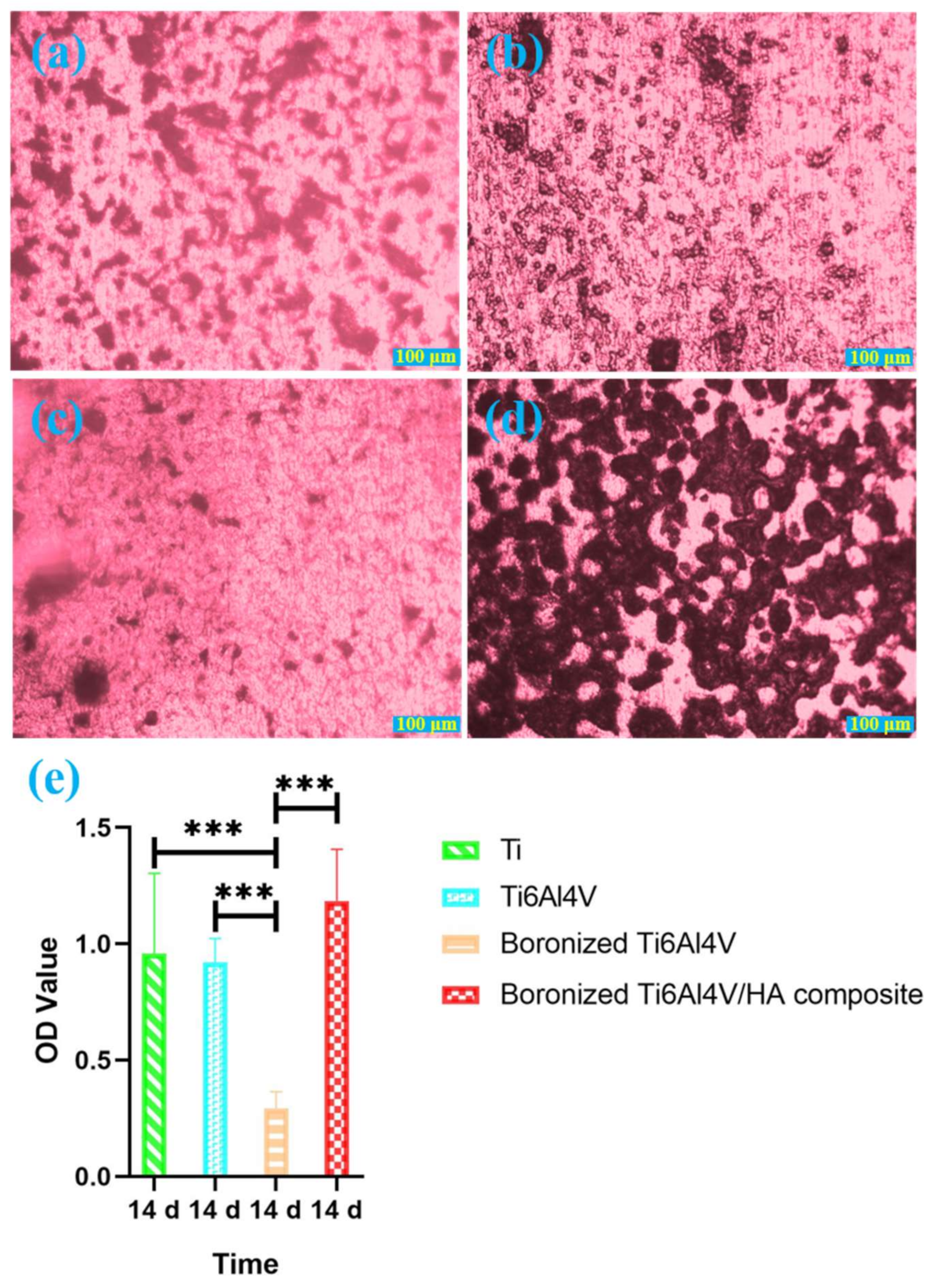
3.4. Alizarin Red Staining Analysis
3.5. Postoperative Observation Results
3.6. Micro-CT Scanning Analysis
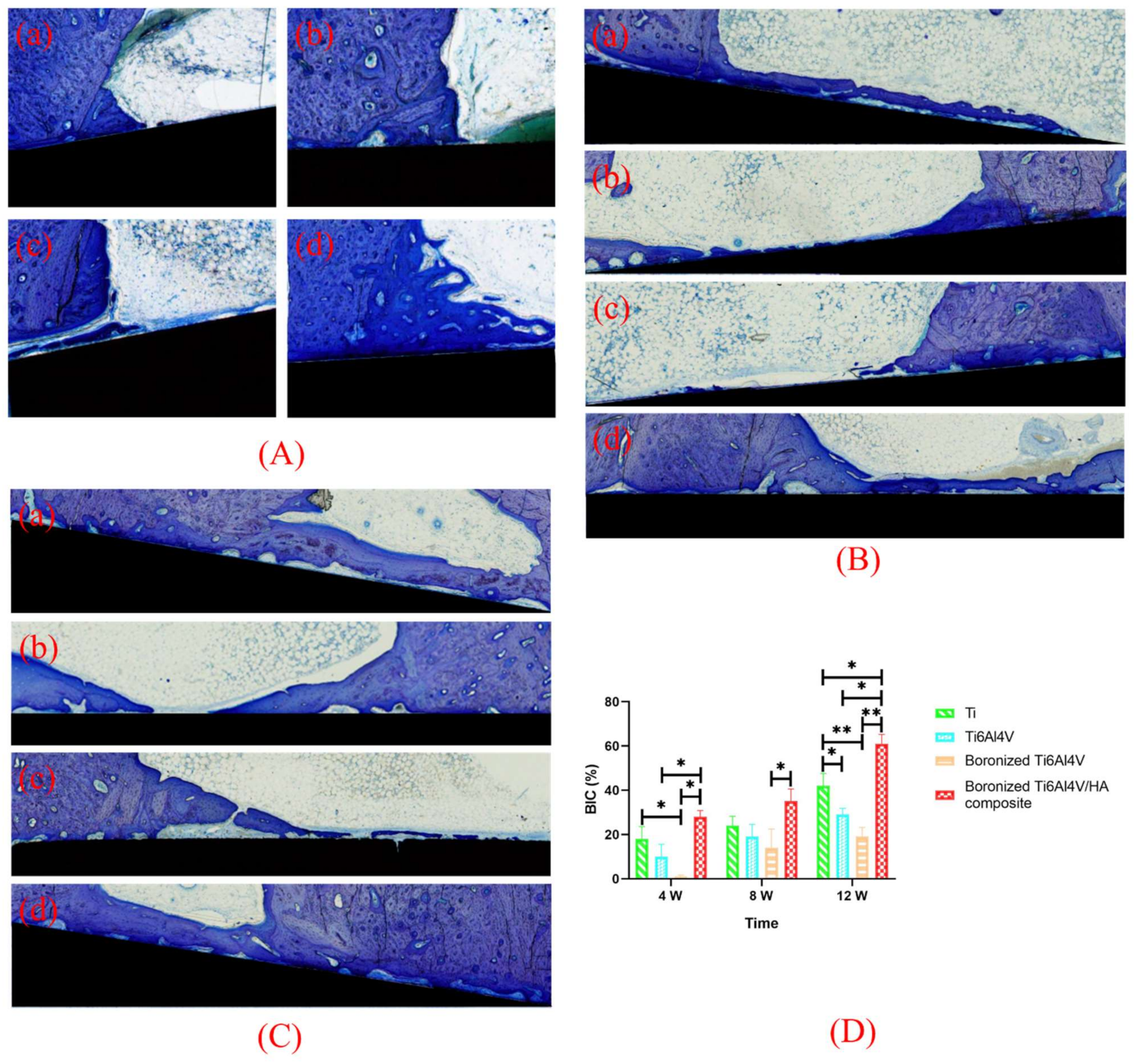
3.7. Toluidine Blue Staining and BIC Analysis
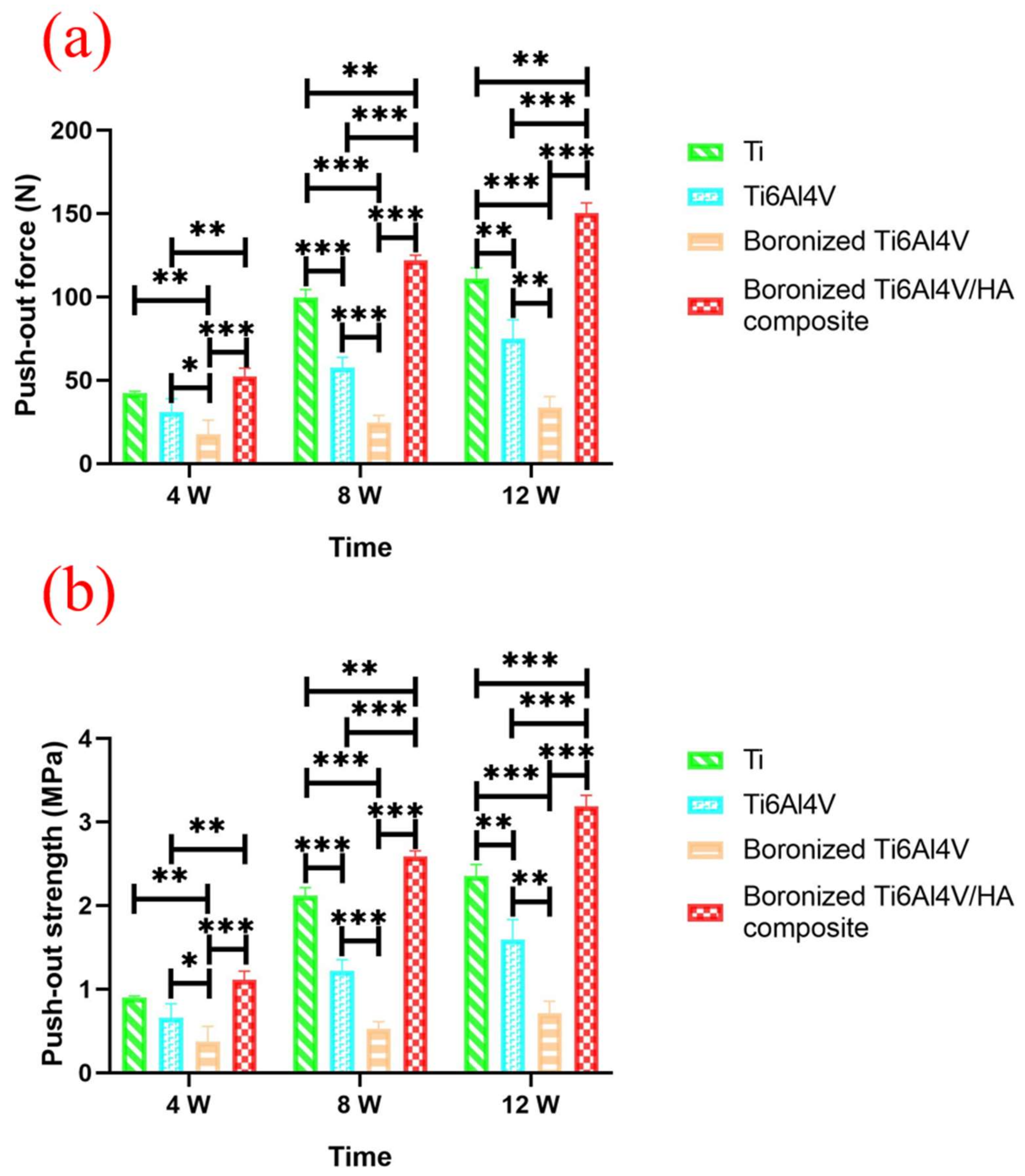
3.8. Push-Out Force Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duraccio, D.; Mussano, F.; Faga, M.G. Biomaterials for dental implants: Current and future trends. J. Mater. Sci. 2015, 50, 4779–4812. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, T.J.; Geng, T.Y.; Wang, X.D.; Lin, K.L.; Wang, P.L. Dental implants loaded with bioactive agents promote osseointegration in osteoporosis: A review. Front. Bioeng. Biotech. 2021, 9, 591796. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.D.; Stenberg, K.; Bose, S.; Bandyopadhyay, A. Hydroxyapatite reinforced Ti6Al4V composites for load-bearing implants. Acta Biomater. 2021, 123, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Majumdar, J.D. Studies on corrosion resistance and bio-activity of plasma spray deposited hydroxylapatite (HA) based TiO2, and ZrO2, dispersed composite coatings on titanium alloy (Ti-6Al-4V) and the same after post spray heat treatment. App. Surf. Sci. 2017, 420, 935–943. [Google Scholar] [CrossRef]
- Alem, S.A.A.; Latifi, R.; Angizi, S.; Hassanaghaei, F.; Aghaahmadi, M.; Ghasali, E.; Rajabi, M. Microwave sintering of ceramic reinforced metal matrix composites and their properties: A review. Mater. Manuf. Process. 2020, 35, 303–327. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A. Pivotal role of boron supplementation on bone health: A narrative review. J. Trace Elem. Med. Biol. 2020, 62, 126577. [Google Scholar] [CrossRef]
- Peng, Q.; Tang, Z.G.; Wang, Y.H.; Peng, Z.W. Mechanical performance and in-vitro biological behaviors of boronized Ti6Al4V/HA composites synthesized by microwave sintering. Ceram. Int. 2019, 45, 24684–24690. [Google Scholar] [CrossRef]
- Peng, Q.; Bin, X.; Xuxin, H.Y.; Wang, Y.H.; Peng, Z.W.; Tang, Z.G. Facile fabrication of boronized Ti6Al4V/HA composites for loadbearing applications. J. Alloy. Compd. 2020, 825, 153102. [Google Scholar] [CrossRef]
- Chen, S.; Lee, C.Y.; Li, R.W.; Smith, P.N.; Qin, Q.H. Modelling osteoblast adhesion on surface-engineered biomaterials: Optimisation of nanophase grain size. Comput. Method. Biomec. Biomed. Eng. 2017, 20, 905–914. [Google Scholar] [CrossRef]
- Huang, G.; Yu, H.; Wang, X.; Ning, B.; Gao, J.; Shi, Y.; Zhu, Y.; Duan, J. Highly porous and elastic aerogel based on ultralong hydroxyapatite nanowires for high-performance bone regeneration and neovascularization. J. Mater. Chem. B 2021, 9, 1277–1287. [Google Scholar] [CrossRef]
- Kukueva, E.V.; Putlyaev, V.I.; Tikhonov, A.A.; Safronova, T.V. Octacalcium phosphate as a precursor for the fabrication of composite bioceramics. Inorgan. Mater. 2017, 53, 212–219. [Google Scholar] [CrossRef]
- Barrak, F.N.; Li, S.; Muntane, A.M.; Jones, J.R. Particle release from implantoplasty of dental implants and impact on cells. Int. J. Implant Dent. 2020, 6, 50–60. [Google Scholar] [CrossRef]
- Dede, E.C.; Korkusuz, P.; Bilgiç, E.; Çetinkaya, M.A.; Korkusuz, F. Boron nano-hydroxyapatite composite increases the bone regeneration of ovariectomized rabbit femurs. Biol. Trace Elem. Res. 2022, 200, 183–196. [Google Scholar] [CrossRef]
- Shao, Y.; Qing, X.; Peng, Y.; Wang, H.; Shao, Z.; Zhang, K. Enhancement of mechanical and biological performance on hydroxyapatite/silk fibroin scaffolds facilitated by microwave-assisted mineralization strategy. Colloids Surf. B Biointer. 2021, 197, 111401. [Google Scholar] [CrossRef]
- Mohammadtaheri, M.; Bozorg, M.; Yazdani, A.; Majid, S. Fabrication of Ti-Al2O3-HA composites by spark plasma sintering and its properties for medical applications. J. Mater. Res. 2022, 27, 1–12. [Google Scholar] [CrossRef]
- Taubenberger, A.V.; Woodruff, M.A.; Bai, H.; Muller, D.J.; Hutmacher, D.W. The effect of unlocking RGD-motifs in collagen I on pre-osteoblast adhesion and differentiation. Biomaterials 2010, 31, 2827–2835. [Google Scholar] [CrossRef]
- Mariné, O.; María, E.G.D.; Antonio, A.P.M.; Amaury, P.G.; Christian, G.; Héctor, F. Evaluation of the osteoblast behavior to PGA textile functionalized with RGD as a scaffold for bone regeneration. J. Nanomater. 2017, 2017, 4852190. [Google Scholar]
- Li, J.; Ge, L.; Zhao, Y.; Zhai, Y.; Rao, N.; Yuan, X.; Yang, J.; Li, J.; Yu, S. TGF-β2 and TGF-β1 differentially regulate the odontogenic and osteogenic differentiation of mesenchymal stem cells. Arch Oral Biol. 2022, 135, 105357. [Google Scholar] [CrossRef]
- Harjumäki, R.; Zhang, X.; Nugroho, R.W.N.; Farooq, M.; Sterberg, M. AFM force spectroscopy reveals the role of integrins and their activation in cell-biomaterial interactions. ACS Appl. Bio Mater. 2020, 3, 1406–1417. [Google Scholar] [CrossRef]
- Clark, E.A.; Brugge, J.S. Integrins and signal transduction pathways: The road taken. Science 1995, 268, 233–239. [Google Scholar] [CrossRef]
- Assefa, F.; Kim, J.A.; Lim, J.; Nam, S.H.; Shin, H.I.; Park, E.K. The neuropeptide spexin promotes the osteoblast differentiation of MC3T3-E1 cells via the MEK/ERK pathway and bone regeneration in a mouse calvarial defect model. Tissue Eng. Regen. Med. 2022, 9, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Kong, Y.M.; Kim, H.W.; Kim, Y.W.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Han, Y. High concentrations of calcium suppress osteogenic differentiation of human periodontal ligament stem cells invitro. J. Dent. Sci. 2021, 16, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Ramenzoni, L.L.; Bsch, A.; Proksch, S.; Attin, T.; Schmidlin, P.R. Effect of high glucose levels and lipopolysaccharides induced inflammation on osteoblast mineralization over sandblasted/acid etched titanium surface. Clin. Implant Dent. Relat. Res. 2020, 22, 213–219. [Google Scholar] [CrossRef]
- Ye, J.; Huang, B.; Gong, P. Nerve growth factor-chondroitin sulfate/hydroxyapatite-coating composite implant induces early osseointegration and nerve regeneration of peri-implant tissues in Beagle dogs. J. Orthop. Surg. Res. 2021, 16, 51. [Google Scholar] [CrossRef]
- Ritwik, A.; Saju, K.K.; Vengellur, A.; Saipriya, P.P. Development of thin-film hydroxyapatite coatings with an intermediate shellac layer produced by dip-coating process on Ti6Al4V implant materials. J. Coat. Technol. Res. 2022, 19, 597–605. [Google Scholar] [CrossRef]
- Rattan, P.V.; Sidhu, T.S.; Mittal, M. An overview of hydroxyapatite coated titanium implants. AJEAT 2012, 2, 40–43. [Google Scholar]
- Pinto, T.S.; Martins, B.R.; Ferreira, M.R.; Bezerra, F. Nanohydroxyapatite-blasted bioactive surface drives shear-stressed endothelial cell growth and angiogenesis. BioMed Res. Inter. 2022, 2022, 1433221. [Google Scholar] [CrossRef]
- Tao, Z.S.; Wu, X.J.; Yang, M.; Xu, H.G. Local administration with silymarin could increase osseointegration of hydroxyapatite-coated titanium implants in ovariectomized rats. J. Biomater. Appl. 2019, 34, 664–672. [Google Scholar] [CrossRef]
- Wang, H.J.; Yang, G.G.; Zhang, J.M.; Li, S.M.; Muhammad, B. Hydroxyapatite nanoparticles/polyimi.de-coated platinum electrodes for improved heat-insulating and heavy metal ion diffusion properties. JNC 2022, 10, 1–12. [Google Scholar]
- Aval, F.S.; Arab, M.R.; Sargolzaei, N.; Noushadi, F.; Pour, A.H. Efficacy of octacalcium phosphate and octacalcium phosphate/gelatin composite on the repair of critical-sized calvarial defects in rats. J. Dent. 2018, 15, 86–96. [Google Scholar]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Swelum, A.A.; Perillo, A.; Losacco, C. The vital roles of boron in animal health and production: A comprehensive review. J. Trace Elem. Med. Bio. 2018, 50, 296–304. [Google Scholar] [CrossRef]
- Toker, H.; Ozdemir, H.; Balci Yuce, H.; Goze, F. The effect of boron on alveolar bone loss in osteoporotic rats. J. Dent. Sci. 2016, 11, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Xu, A.T.; Wang, D.D.; Lin, G.F.; Liu, T.; He, F.M. The effects of Sr-incorporated micro/nano rough titanium surface on rBMSC migration and osteogenic differentiation for rapid osteointegration. Biomater. Sci. 2018, 6, 1946–1961. [Google Scholar] [CrossRef]
- He, J.; Feng, W.; Zhao, B.H.; Zhang, W.; Lin, Z. In vivo effect of titanium implants with porous zinc-containing coatings prepared by plasma electrolytic oxidation method on osseointegration in rabbits. Int. J. Oral Maxillofac. Implant. 2018, 33, 298–310. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhang, C.N.; Gu, Y.X.; Shi, J.Y.; Mo, J.J.; Qian, S.J.; Qiao, S.C.; Lai, H.C. The responses of human gingival fibroblasts to magnesium-doped titanium. J. Biomed. Mater. Res. A 2019, 108, 267–278. [Google Scholar] [CrossRef]
- Huang, T.B.; Li, Y.Z.; Yu, K.; Yu, Z.; Wang, Y.; Jiang, Z.W.; Wang, H.M.; Yang, G.L. Effect of the Wnt signal-RANKL/OPG axis on the enhanced osteogenic integration of a lithium incorporated surface. Biomater. Sci. 2019, 7, 1101–1116. [Google Scholar] [CrossRef]
- Song, S.; Gao, P.; Sun, L.; Kang, D.; Kongsted, J.; Poongavanam, V.; Zhan, P.; Liu, X. Recent developments in the medicinal chemistry of single boron atom-containing compounds. Acta Pharm. Sin. B 2021, 11, 3035–3059. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Tavakol, S.; Nikpour, M.R.; Amani, A.; Soltani, M.; Rabiee, S.M.; Rezayat, S.M.; Chen, P.; Jahanshahi, M. Bone regeneration based on nano-hydroxyapatite and hydroxyapatite/chitosan nanocomposites: An in vitro and in vivo comparative study. J. Nanoparti. Res. 2013, 15, 1373. [Google Scholar] [CrossRef]
- Causey, G.C.; Picha, G.J.; Price, J.; Pelletier, M.H.; Wang, T.; Walsh, W.R. In-Vivo response to a novel pillared surface morphology for osseointegration in an ovine model. J. Mech. Behav. Biomed. Mater. 2021, 119, 104462. [Google Scholar] [CrossRef]
- Bruyn, D.H.; Véronique, C.; Doornewaard, R.; Jacobsson, M.; Cosyn, J.; Jacquet, W.; Vervaeke, S. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontology 2017, 73, 218–227. [Google Scholar] [CrossRef]
- Shanbhag, S.; Shanbhag, V.; Stavropoulos, A. Genomic analyses of early peri-implant bone healing in humans: A systematic review. Inter. J. Imp. Dent. 2015, 1, 5–10. [Google Scholar] [CrossRef] [Green Version]

| Time | Group | BS (mm2) | TV (mm3) | BV (mm3) | BS/BV (1/mm) | BS/TV (1/mm) | BV/TV (%) | Tb.N (1/mm) | Tb.Th (mm) | Tb.Sp (mm) | BMD (g/cm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 W | Ti | 113.86 | 24.20 | 9.40 | 12.11 | 4.70 | 38.84 | 2.35 | 0.17 | 0.26 | 677.80 |
| Ti6Al4V | 94.22 | 19.19 | 5.77 | 16.34 | 4.91 | 30.05 | 2.46 | 0.12 | 0.28 | 541.54 | |
| Boronized Ti6Al4V | 127.16 | 22.80 | 5.46 | 23.30 | 5.58 | 23.94 | 2.79 | 0.09 | 0.27 | 459.85 | |
| Boronized Ti6Al4V/HA composite | 145.64 | 24.14 | 9.48 | 15.36 | 6.03 | 39.27 | 3.02 | 0.13 | 0.20 | 634.29 | |
| 8 W | Ti | 113.93 | 23.06 | 9.29 | 12.26 | 4.94 | 40.30 | 2.47 | 0.16 | 0.24 | 634.35 |
| Ti6Al4V | 112.08 | 19.98 | 6.78 | 16.54 | 5.61 | 33.92 | 2.81 | 0.12 | 0.24 | 756.00 | |
| Boronized Ti6Al4V | 103.90 | 27.68 | 4.81 | 21.58 | 3.75 | 17.39 | 1.88 | 0.09 | 0.44 | 315.28 | |
| Boronized Ti6Al4V/HA composite | 113.42 | 18.49 | 7.66 | 14.80 | 6.14 | 41.45 | 3.07 | 0.14 | 0.19 | 800.13 | |
| 12 W | Ti | 111.67 | 21.61 | 8.98 | 12.44 | 5.17 | 41.55 | 2.58 | 0.16 | 0.23 | 779.14 |
| Ti6Al4V | 92.47 | 18.23 | 6.54 | 14.15 | 5.07 | 35.84 | 2.54 | 0.14 | 0.25 | 673.93 | |
| Boronized Ti6Al4V | 94.59 | 25.39 | 5.24 | 18.05 | 3.73 | 20.64 | 1.86 | 0.11 | 0.43 | 376.15 | |
| Boronized Ti6Al4V/HA composite | 138.93 | 24.14 | 11.74 | 11.83 | 5.76 | 48.64 | 2.88 | 0.17 | 0.18 | 934.36 |
| Index | Group | 4 W | 8 W | 12 W |
|---|---|---|---|---|
| Maximum push-out force (N) | Ti Group | 42.4 ± 0.90 | 99.8 ± 4.58 | 111.0 ± 6.44 |
| Ti6Al4V Group | 31.0 ± 7.92 | 57.6 ± 6.14 | 75.1 ± 11.15 | |
| Boronized Ti6Al4V | 17.5 ± 8.57 | 24.7 ± 4.17 | 33.6 ± 6.66 | |
| Boronized Ti6Al4V/HA composite | 52.4 ± 4.86 | 122.0 ± 3.16 | 150.3 ± 6.07 | |
| Push-out strength (MPa) | Ti Group | 0.90 ± 0.02 | 2.12 ± 0.10 | 2.36 ± 0.14 |
| Ti6Al4V | 0.66 ± 0.17 | 1.22 ± 0.13 | 1.59 ± 0.24 | |
| Boronized Ti6Al4V | 0.37 ± 0.18 | 0.52 ± 0.09 | 0.71 ± 0.14 | |
| Boronized Ti6Al4V/HA composite | 1.11 ± 0.10 | 2.59 ± 0.07 | 3.19 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Peng, Q.; Zuo, J.; Wang, Y.; Zhou, H.; Tang, Z. Osteogenesis Performance of Boronized Ti6Al4V/HA Composites Prepared by Microwave Sintering: In Vitro and In Vivo Studies. Materials 2022, 15, 4985. https://doi.org/10.3390/ma15144985
Ding Z, Peng Q, Zuo J, Wang Y, Zhou H, Tang Z. Osteogenesis Performance of Boronized Ti6Al4V/HA Composites Prepared by Microwave Sintering: In Vitro and In Vivo Studies. Materials. 2022; 15(14):4985. https://doi.org/10.3390/ma15144985
Chicago/Turabian StyleDing, Zhenyu, Qian Peng, Jun Zuo, Yuehong Wang, Hongbo Zhou, and Zhangui Tang. 2022. "Osteogenesis Performance of Boronized Ti6Al4V/HA Composites Prepared by Microwave Sintering: In Vitro and In Vivo Studies" Materials 15, no. 14: 4985. https://doi.org/10.3390/ma15144985
APA StyleDing, Z., Peng, Q., Zuo, J., Wang, Y., Zhou, H., & Tang, Z. (2022). Osteogenesis Performance of Boronized Ti6Al4V/HA Composites Prepared by Microwave Sintering: In Vitro and In Vivo Studies. Materials, 15(14), 4985. https://doi.org/10.3390/ma15144985






