First-Principles Study on the Adsorption Behavior of O2 on the Surface of Plutonium Gallium System
Abstract
:1. Introduction
2. Calculation Methods and Models
2.1. Calculation Method
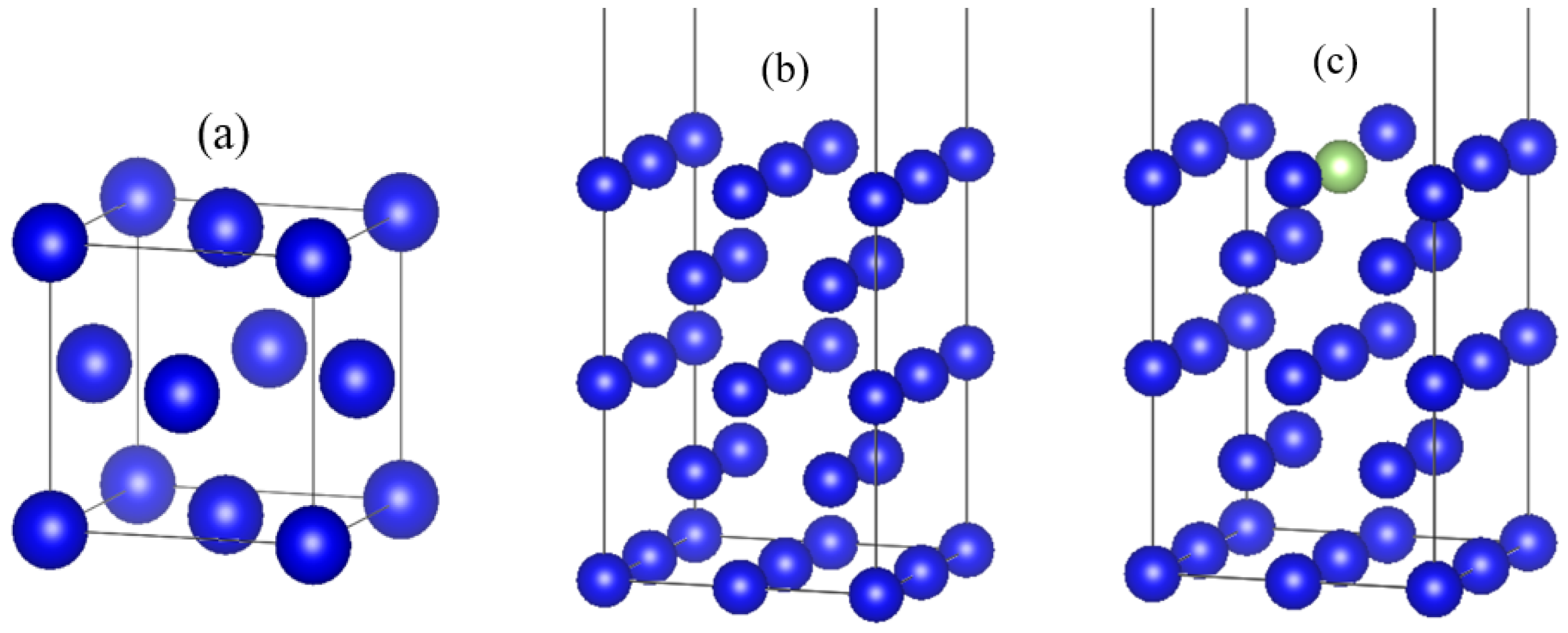
2.2. Computational Models
3. Results and Discussion
3.1. Adsorption Conformation and Adsorption Energy
3.2. Bader Charge Analysis
3.3. Electronic Structure Analysis
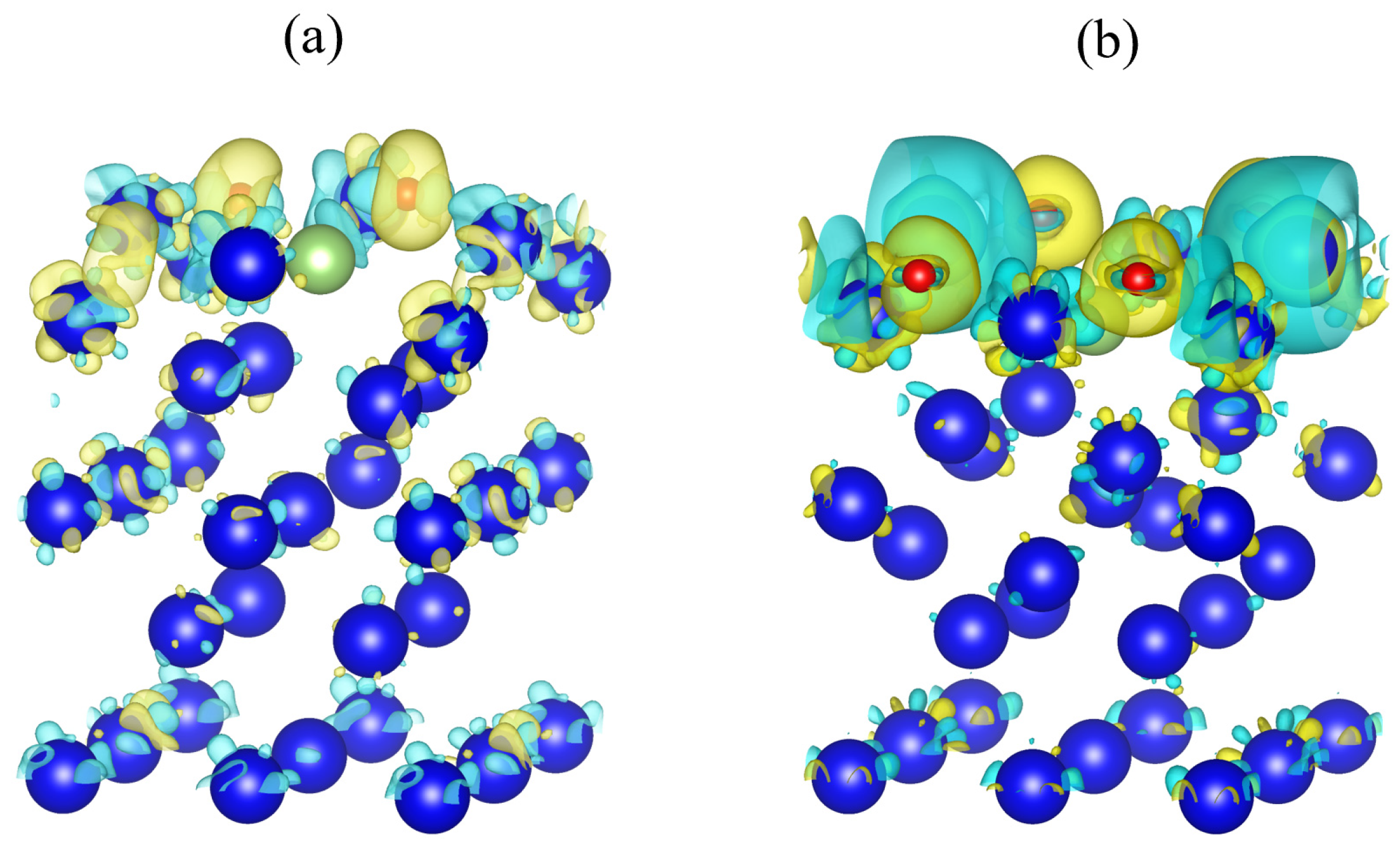
3.3.1. Differential Charge Density Analysis
3.3.2. Electron Density of States Analysis
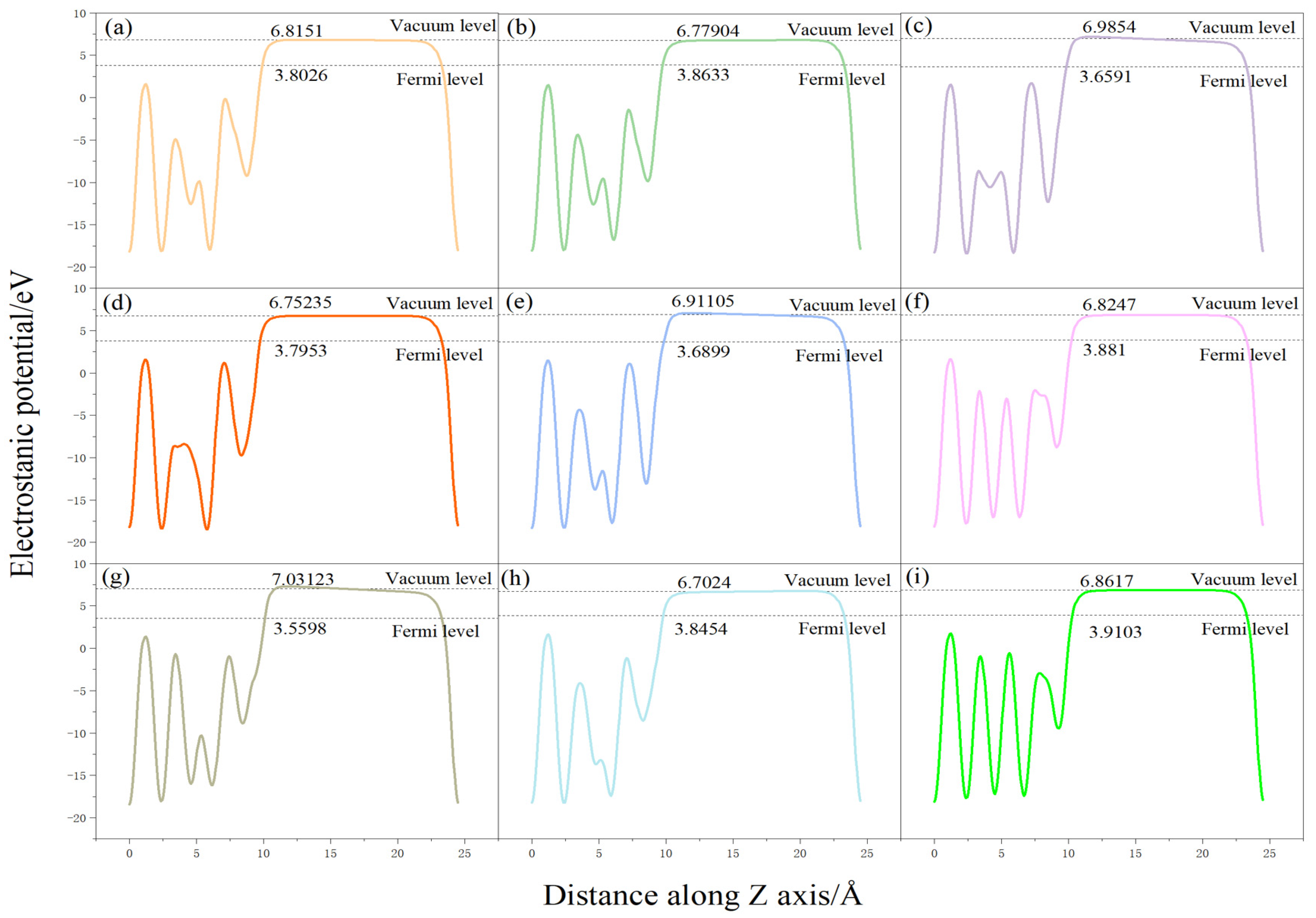
3.4. Surface Power Function Analysis
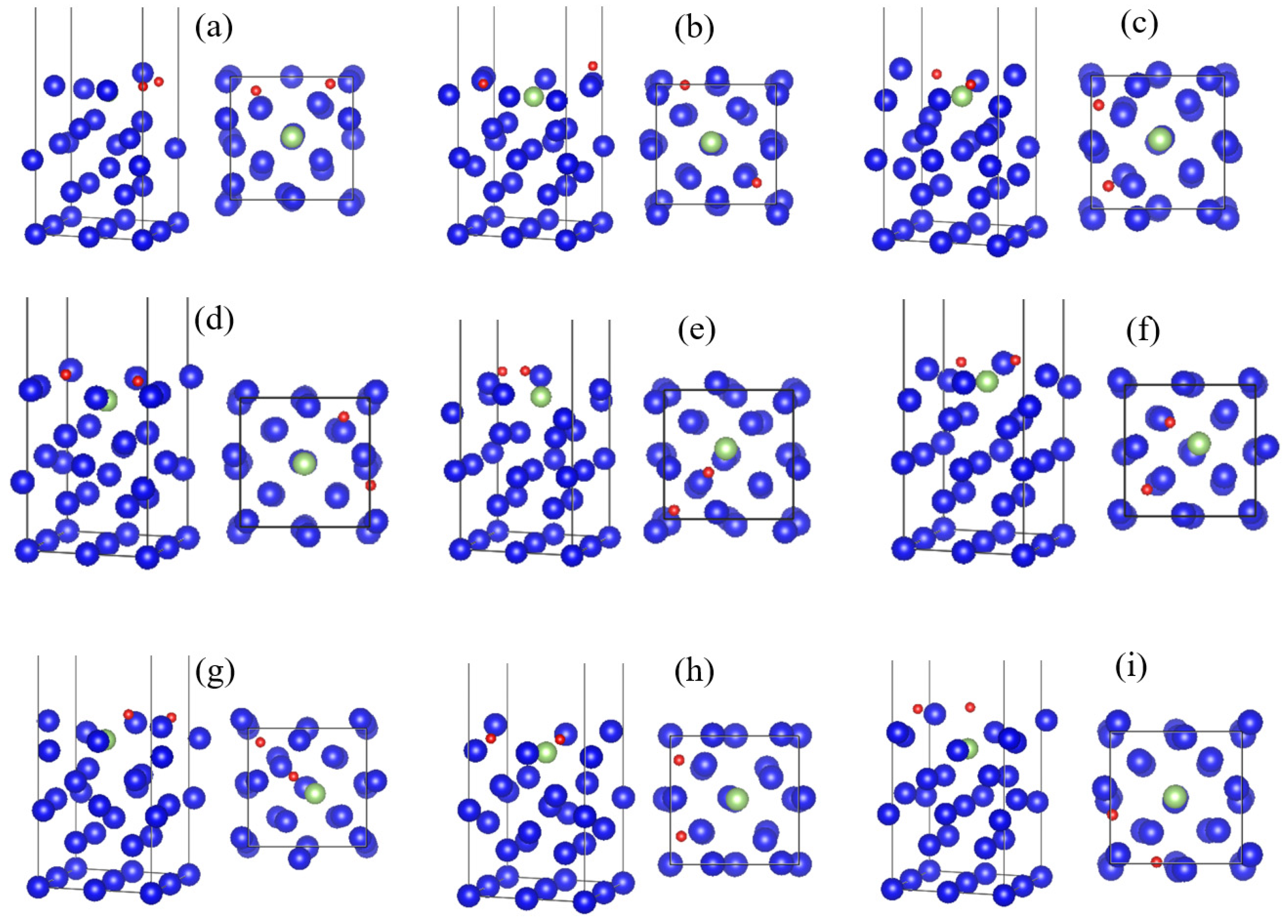
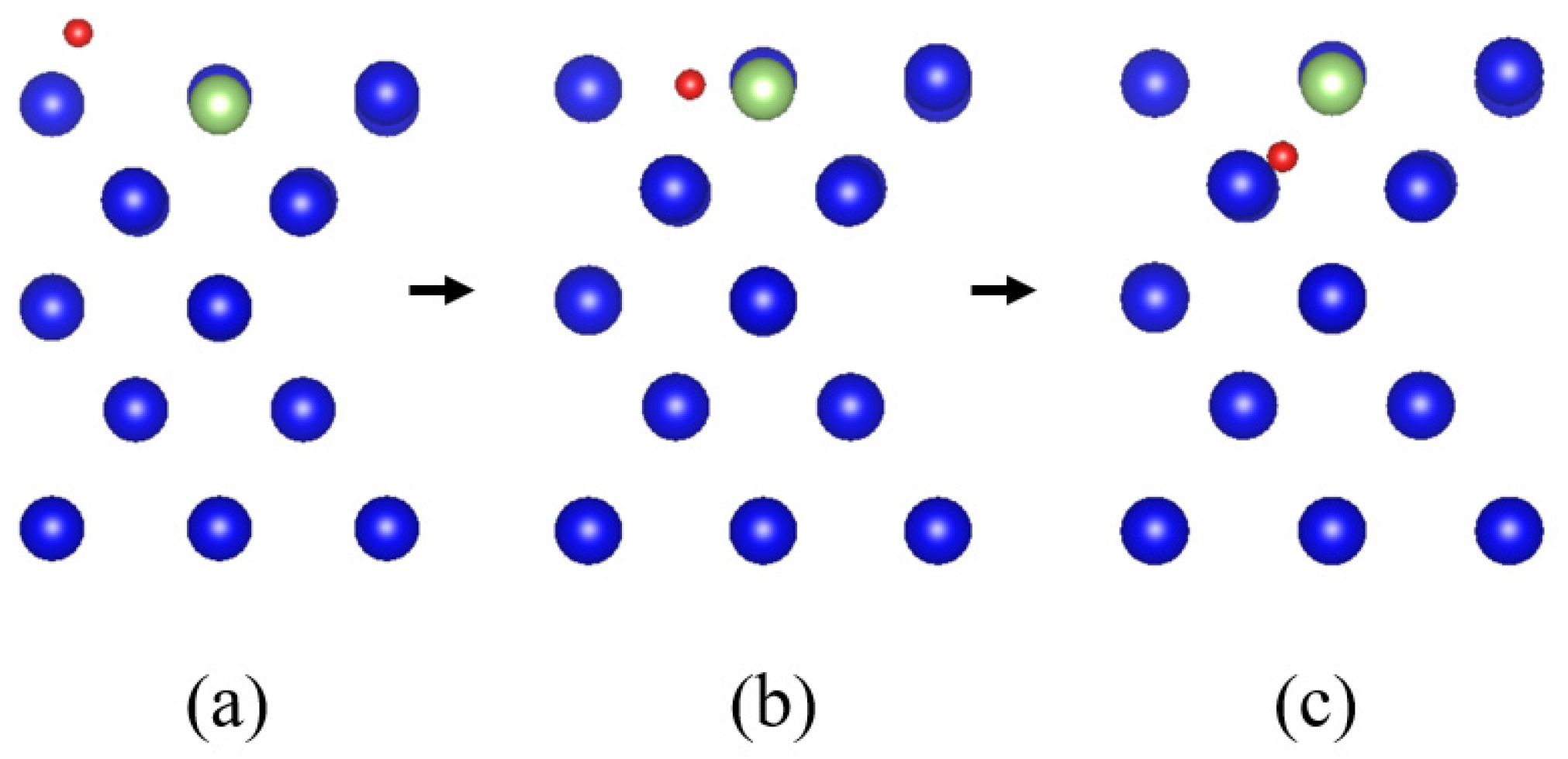
3.5. Diffusion of Oxygen into the Interior of the Plutonium Gallium System
4. Conclusions
- (1)
- Oxygen molecules dissociate and adsorb on the surface of the plutonium gallium system without overcoming the energy barrier. The O-O bond in the oxygen molecule breaks and dissociates into two oxygen atoms attached to the surface. The best adsorption position was vertical adsorption in the hole, and the adsorption energy was 10.7 eV. The order of oxygen adsorption stability on the surface of plutonium gallium system is hole position > bridge position > top position.
- (2)
- Bader charge calculation results show that oxygen molecules gain charge (2.2618 e–2.4617 e) during the adsorption process, mainly by interacting with the first layer of atoms on the surface. Electron structure analysis shows that the reaction mechanism between oxygen molecules and surface atoms of plutonium gallium system is that new chemical bonds are generated by hybridization of 2p orbital of oxygen atom with 6s, 7s, 6d orbital of plutonium atom and 3d orbital of gallium atom.
- (3)
- After oxygen molecules are dissociated and chemically adsorbed on the surface, they can diffuse to the sub-surface atomic layer on the surface of the plutonium gallium system after surmounting the energy barrier of 16.7 eV, and form a stable structure. This indicates that after oxygen molecules are dissociated and adsorbed, they can continue to diffuse inward under certain energy, which changes the surface structure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boring, A.M.; Smith, J.L. Plutonium condensed-matter physics—A survey of theory and experiment. Los Alamos Sci. 2000, 26, 90–127. [Google Scholar]
- Ek, J.V.; Sterne, P.A.; Gonis, A. Phase stability of plutonium. Phys. Rev. B Condens. Matter 1993, 48, 16280. [Google Scholar] [PubMed]
- Hecker, S.S. Plutonium—An Element Never at Equilibrium. Metall. Mater. Trans. A 2008, 39, 1585–1592. [Google Scholar] [CrossRef]
- Shim, J.H.; Haule, K.; Kotliar, G. Fluctuating valence in a correlated solid and the anomalous properties of δ-plutonium. Nature 2007, 446, 513–516. [Google Scholar] [CrossRef] [Green Version]
- Cooper, N.G. Challenges in plutonium science. Los Alamos Sci. Los Alamos Sci. Lab. 2000, I, 26. [Google Scholar]
- Schwartz, A.J.; Wall, M.A.; Zocco, T.G.; Wolfer, W.G. Characterization and modelling of helium bubbles in self-irradiated plutonium alloys. Philos. Mag. 2005, 85, 479–488. [Google Scholar] [CrossRef]
- Sadigh, B.; Wolfer, W.G. Gallium stabilization of δ-Pu: Density-functional calculations. Phys. Rev. B 2005, 72, 205122. [Google Scholar] [CrossRef]
- Luo, W.; Meng, D.; Li, G.; Huchi, C. Electronic Structure and Formation Heat of Pu3M and PuM3 (M = Ga, In, Sn and Ge) Compounds. Acta Phys. Chim. Sin. 2008, 24, 388–392. [Google Scholar] [CrossRef]
- Huda, M.N.; Ray, A.K. Electronic Structures and Bonding of Oxygen on Plutonium Layers. Eur. Phys. J. B-Condens. Matter Complex Syst. 2004, 40, 337–346. [Google Scholar] [CrossRef]
- Huda, M.N.; Ray, A.K. Density functional study of O2 adsorption on (100) surface of γ-uranium. Int. J. Quantum Chem. 2005, 102, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Song, H.; Xiong, X.; Wang, G.; Hu, R.; Luo, S. Adsorption structure and electronic states of Oxygen atoms on δ-PU (111) surface. Comput. Appl. Chem. 2009, 4, 913–916. [Google Scholar]
- Guo, J.; Liu, G.; Wei, H. First-principles study on ADSORPTION behavior of O2 on δ-Pu (100) Surface. Comput. Appl. Chem. 2013, 30, 4. [Google Scholar]
- Hernandez, S.C.; Venhaus, T.J.; Huda, M.N. Atomic oxygen adsorption on 3.125 at.% Ga stabilized δ-Pu (1 1 1) surface. J. Alloy. Compd. 2015, 643, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B Cover. Condens. Matter Mater. Phys. 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Kresse, G.G.; Furthmüller, J.J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Review. B Condens. Matter 1996, 54, 11169. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Physical Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Review. B Condens. Matter 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Bottin, F.; Jomard, G.; Geneste, G. Water adsorption and dissociation on the PuO2(110) surface. J. Nucl. Mater. Mater. Asp. Fission Fusion 2014, 451, 28–34. [Google Scholar]
- Hao, Y.G.; Eriksson, O.; Fernando, G.W.; Cooper, B.R. Surface electronic structure of gamma -uranium. Phys. Rev. B Condens. Matter 1993, 47, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Hum, R.; Xiong, X.; Wang, G.; Song, H.; Luo, S. Study on the first principle of H2 adsorption on δ-Pu (100) surface. Chin. J. Mol. Sci. 2010, 26, 37–41. [Google Scholar]
- Atta-Fynn, R.; Ray, A.K. A first principles study of the adsorption and dissociation of CO2 on the δ-Pu (111) surface. Eur. Phys. J. B Condens. Matter Complex Syst. 2009, 70, 171–184. [Google Scholar] [CrossRef]
- Henkelman, G.; Jόnsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef] [Green Version]
- Henkelman, G.; Uberuaga, B.P.; Jόnsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
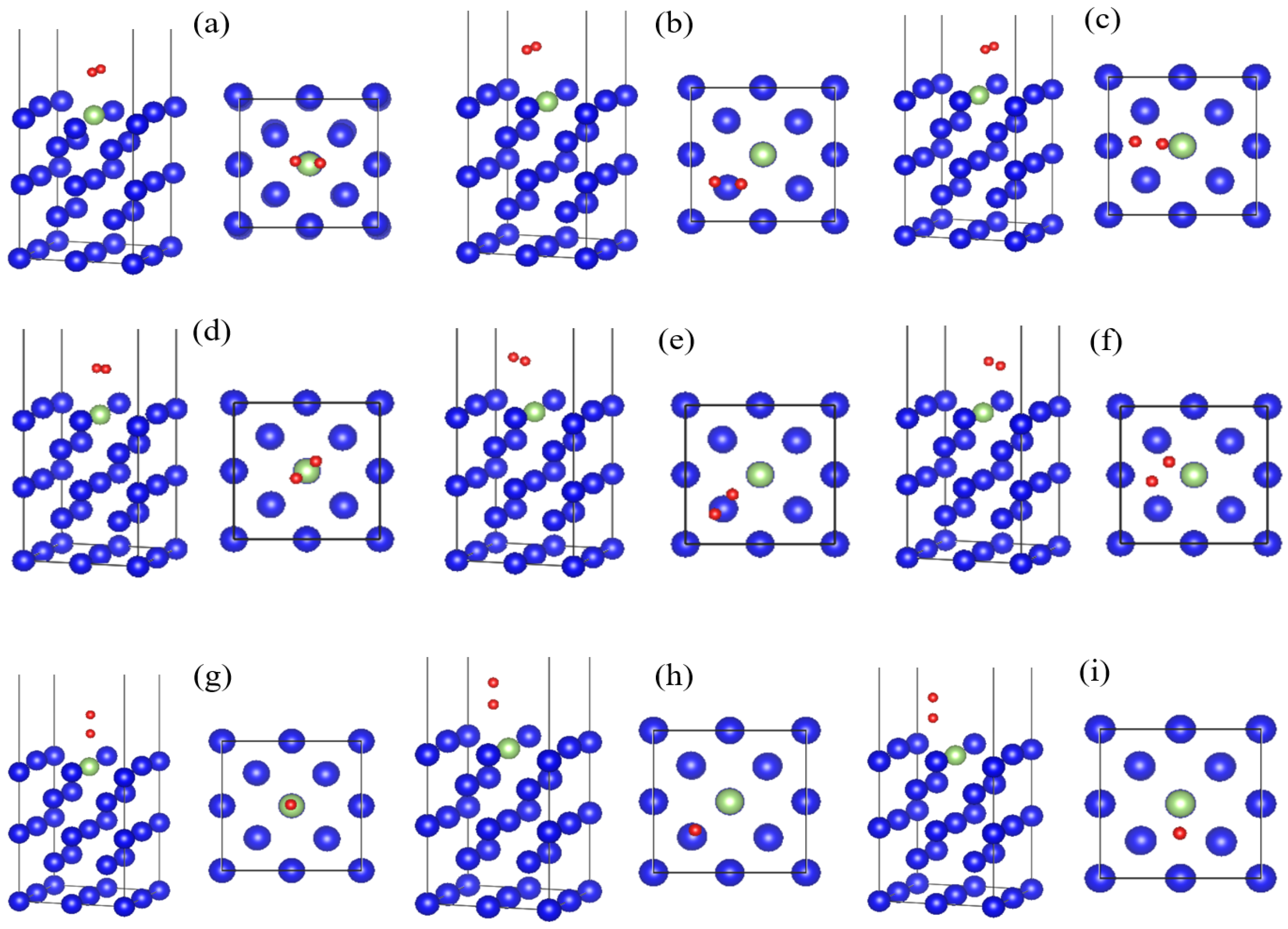


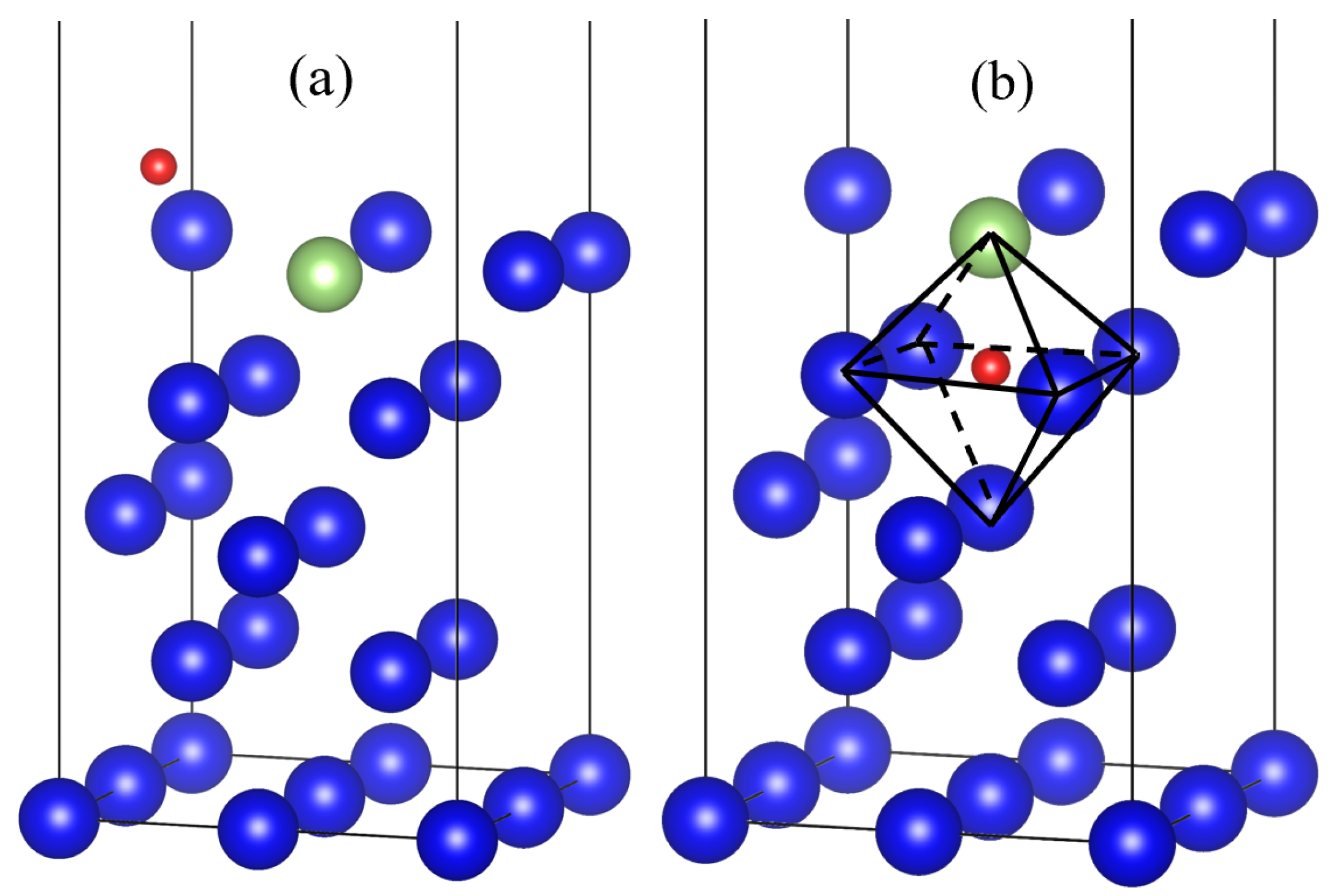

| Adsorption Conformation | Adsorption Energy Eads (eV) | Oxygen Atomic Spacing RO-O (Å) | Oxygen Atom to Surface Nearest Atom Distance hO-Pu/Ga (Å) | Nearest Distance between Oxygen Atom and Surface hO-Surface (Å) | |||
|---|---|---|---|---|---|---|---|
| O-t | hor1 | −6.0406 | 4.1317 | 2.1900 | 3.2502 | 0.6085 | 0.2281 |
| hor2 | −8.5106 | 3.8721 | 2.1365 | 2.0500 | 0.4880 | 0.8873 | |
| ver | −7.3165 | 2.7324 | 2.0540 | 2.2354 | 0.5599 | 0.1831 | |
| O-h | hor1 | −9.6203 | 6.8671 | 2.1552 | 2.0726 | 0.9253 | 0.2106 |
| hor2 | −9.4771 | 2.7048 | 2.1309 | 2.0189 | 0.1997 | 0.6141 | |
| ver | −10.7000 | 4.0031 | 2.1887 | 2.1961 | 0.1108 | 0.2480 | |
| O-b | hor1 | −10.3804 | 4.1748 | 2.3221 | 2.2002 | 1.0132 | 0.3981 |
| hor2 | −6.9156 | 3.6822 | 2.0930 | 2.2135 | 0.0400 | 0.5042 | |
| ver | −9.5051 | 3.3041 | 2.0941 | 2.1112 | 0.5968 | 0.4603 | |
| Configuration | Adsorption of Atoms in Molecules | Total Number of Charges in Each Layer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Qtotal | Q1st | Q2nd | Q3rd | Q4th | Q5th | ||
| O2 | 0.0091 | −0.0091 | 0.0000 | -- | -- | -- | -- | -- | |
| Surface | -- | -- | -- | −0.0050 | −0.0377 | 0.1573 | 0.1915 | −0.3062 | |
| H-t | hor1 | 1.2222 | 1.2258 | 2.4480 | −2.4791 | −0.0787 | 0.4194 | 0.0237 | −0.3332 |
| hor2 | 1.1506 | 1.2245 | 2.3752 | −2.4181 | −0.3527 | 0.7470 | 0.0853 | −0.4366 | |
| ver | 1.1762 | 1.2392 | 2.4154 | −2.5363 | −0.0398 | 0.4997 | −0.0729 | −0.2661 | |
| H-h | hor1 | 1.1303 | 1.2193 | 2.3496 | −2.4283 | −0.0315 | 0.4687 | −0.0445 | −0.3141 |
| hor2 | 1.2252 | 1.1877 | 2.4129 | −2.3895 | −0.1105 | 0.2109 | 0.1688 | −0.2926 | |
| ver | 1.2271 | 1.2152 | 2.4424 | −2.5452 | −0.2250 | 0.8239 | −0.0852 | −0.4108 | |
| H-b | hor1 | 1.2509 | 1.2108 | 2.4617 | −2.4698 | −0.1225 | 0.5288 | −0.1380 | −0.2602 |
| hor2 | 1.2156 | 1.2373 | 2.4529 | −2.1872 | −0.3107 | 0.1202 | 0.1613 | −0.2364 | |
| ver | 1.1336 | 1.1281 | 2.2618 | −2.3769 | 0.1120 | 0.2726 | −0.1081 | −0.1614 | |
| Adsorption Conformation | Vacuum Energy Level/eV | Fermi Energy Level/eV | Power Letter/eV | ∆Φ/eV |
|---|---|---|---|---|
| O-t-hor1 | 6.7774 | 3.8633 | 2.9141 | −0.0755 |
| O-t-hor2 | 6.9854 | 3.6591 | 3.3263 | 0.3368 |
| O-t-ver | 6.8152 | 3.8026 | 3.0126 | 0.0230 |
| O-h-hor1 | 6.9134 | 3.6899 | 3.2235 | 0.2340 |
| O-h-hor2 | 6.8247 | 3.8810 | 2.9437 | −0.0458 |
| O-h-ver | 6.7524 | 3.7953 | 2.9571 | −0.0325 |
| O-b-hor1 | 6.7025 | 3.8454 | 2.8571 | −0.1325 |
| O-b-hor2 | 6.8617 | 3.9103 | 2.9514 | −0.0381 |
| O-b-ver | 7.0312 | 3.5598 | 3.4714 | 0.4819 |
| Surface | 6.7817 | 3.7921 | 2.9896 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhu, M.; Zheng, G.; Li, Y.; Yang, Y.; Liu, Y.; Su, H. First-Principles Study on the Adsorption Behavior of O2 on the Surface of Plutonium Gallium System. Materials 2022, 15, 5035. https://doi.org/10.3390/ma15145035
Li L, Zhu M, Zheng G, Li Y, Yang Y, Liu Y, Su H. First-Principles Study on the Adsorption Behavior of O2 on the Surface of Plutonium Gallium System. Materials. 2022; 15(14):5035. https://doi.org/10.3390/ma15145035
Chicago/Turabian StyleLi, Longxian, Min Zhu, Guikai Zheng, Yan Li, Yang Yang, Yilong Liu, and Huan Su. 2022. "First-Principles Study on the Adsorption Behavior of O2 on the Surface of Plutonium Gallium System" Materials 15, no. 14: 5035. https://doi.org/10.3390/ma15145035





