Processing and Microstructure of As-Cast Ti-45Al-2W-xC Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Composition and Microstructure of the As-Cast Ingots
3.2. Segregation Behaviour of Alloying Elements
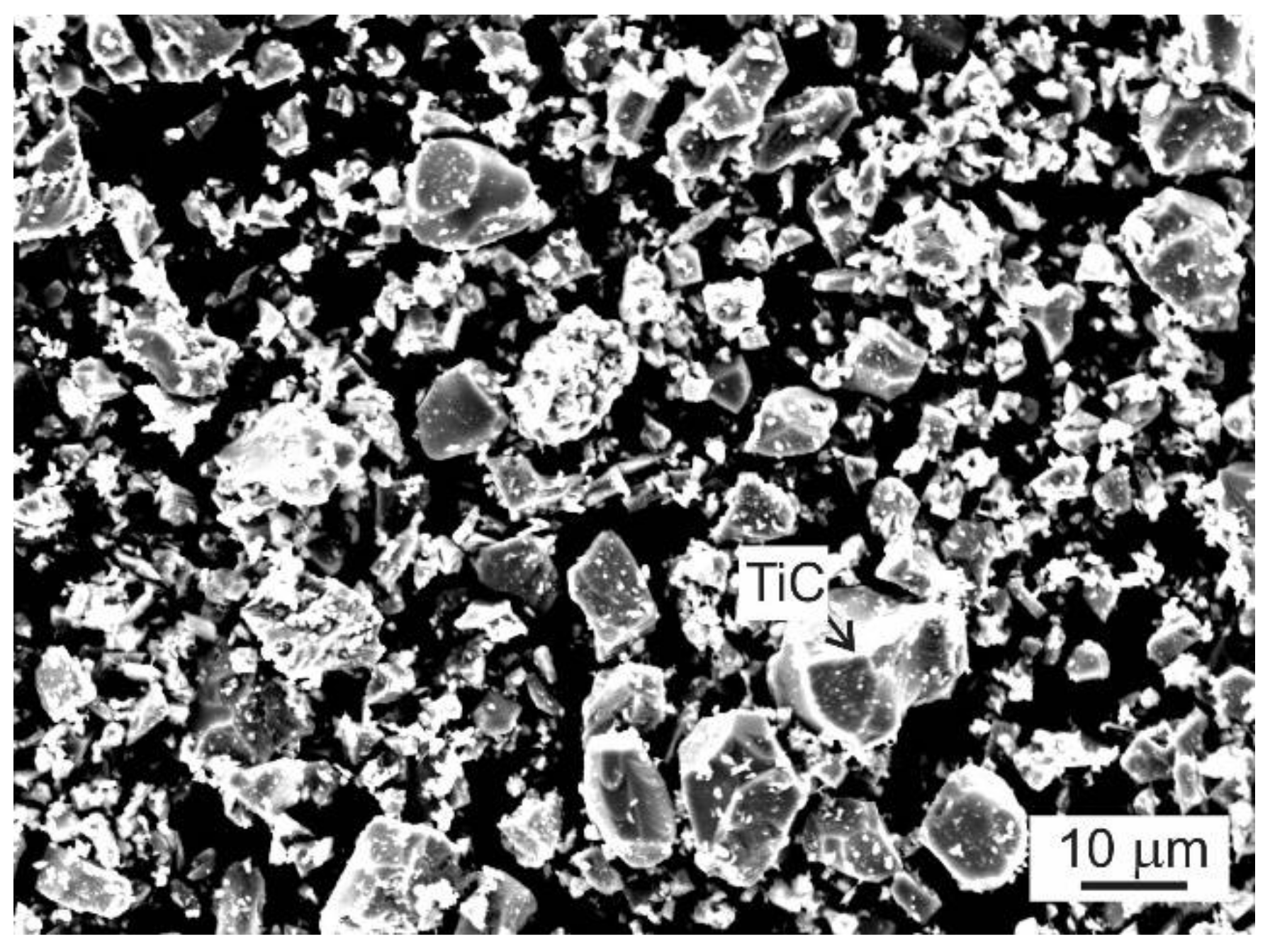
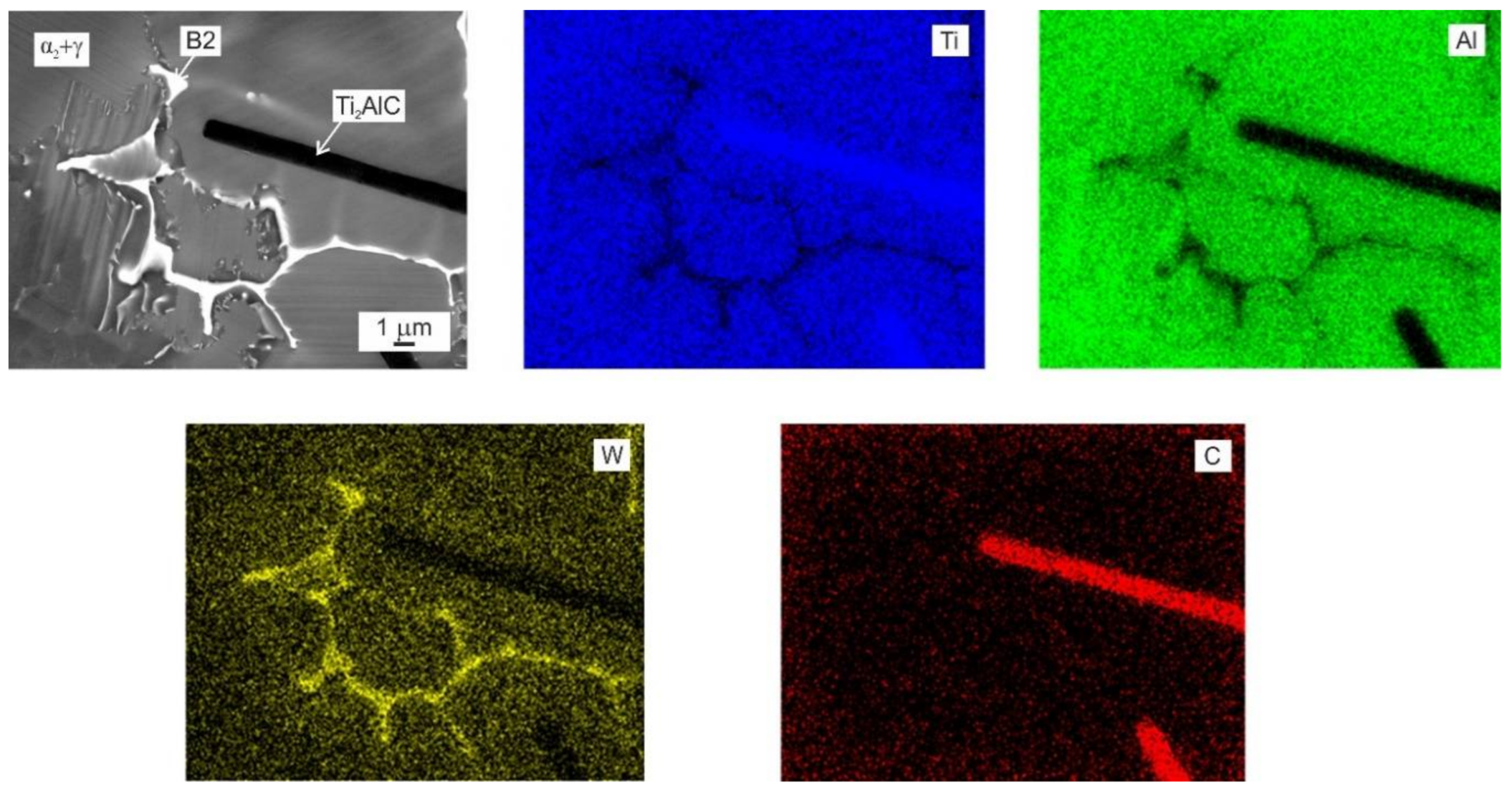
3.3. Formation of Primary Carbide Particles
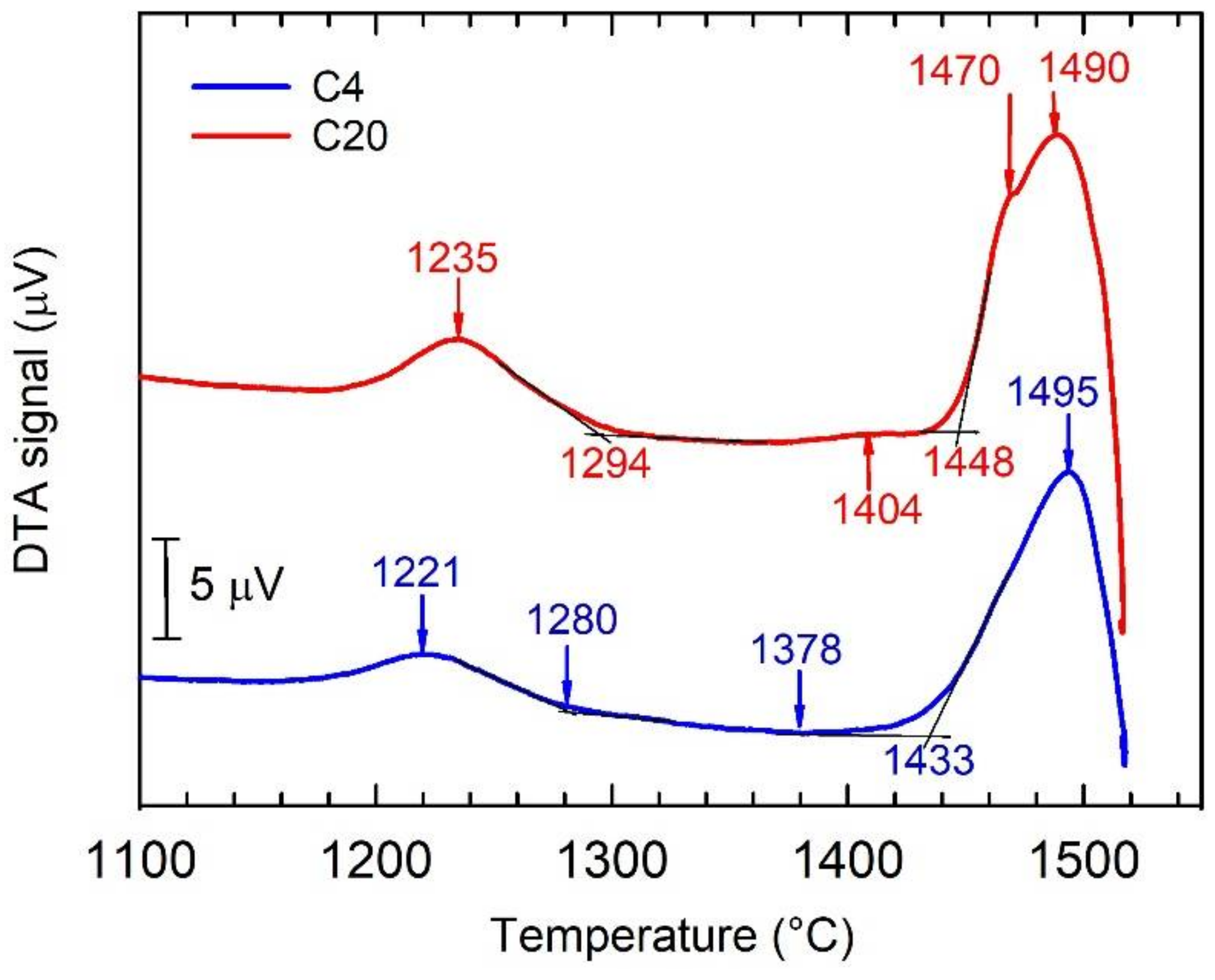
3.4. DTA Analysis of As-Cast Alloys
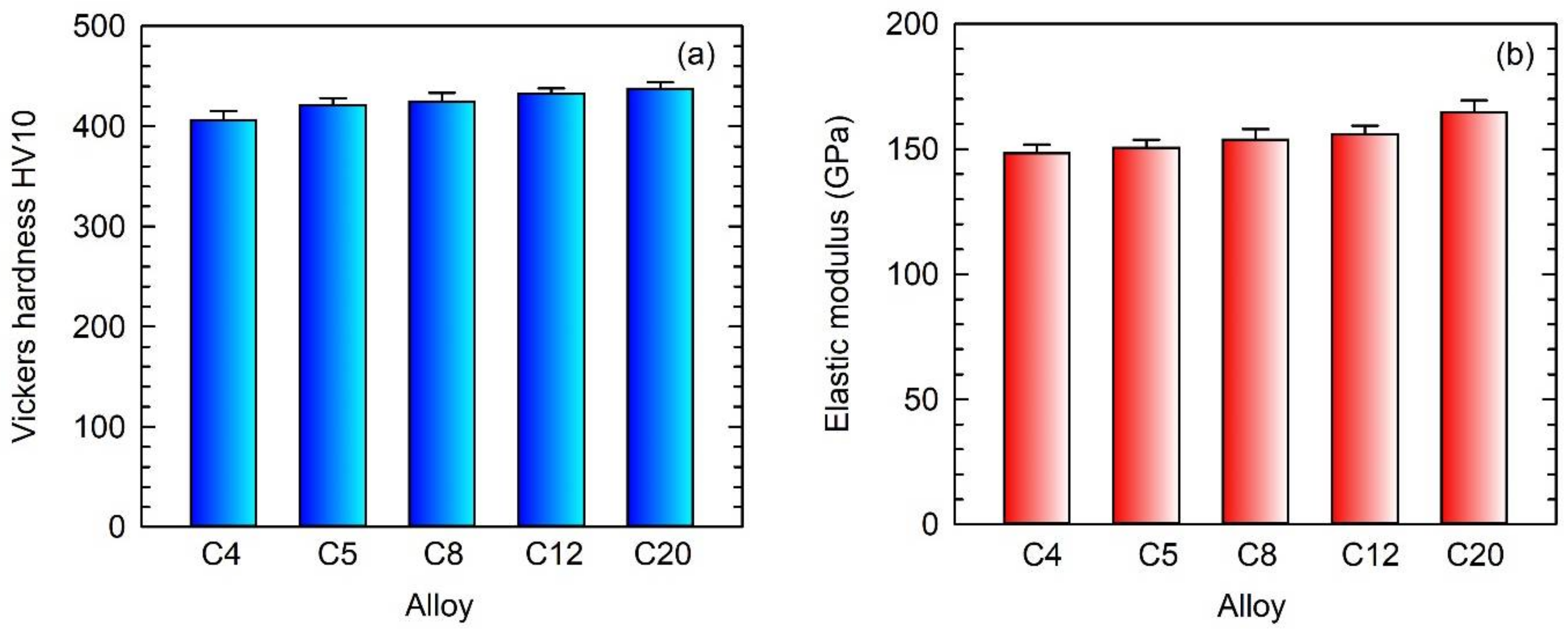
3.5. Hardness and Elastic Modulus
4. Conclusions
- The use of master 15W-85Al (at.%) alloy facilitated a fast dissolution of W in the melt and led to the metallurgical preparation of Ti-45Al-2W-(0.4–2) C alloys with designed and reproducible chemical composition, using VIM in graphite crucibles and centrifugal casting into a graphite mould.
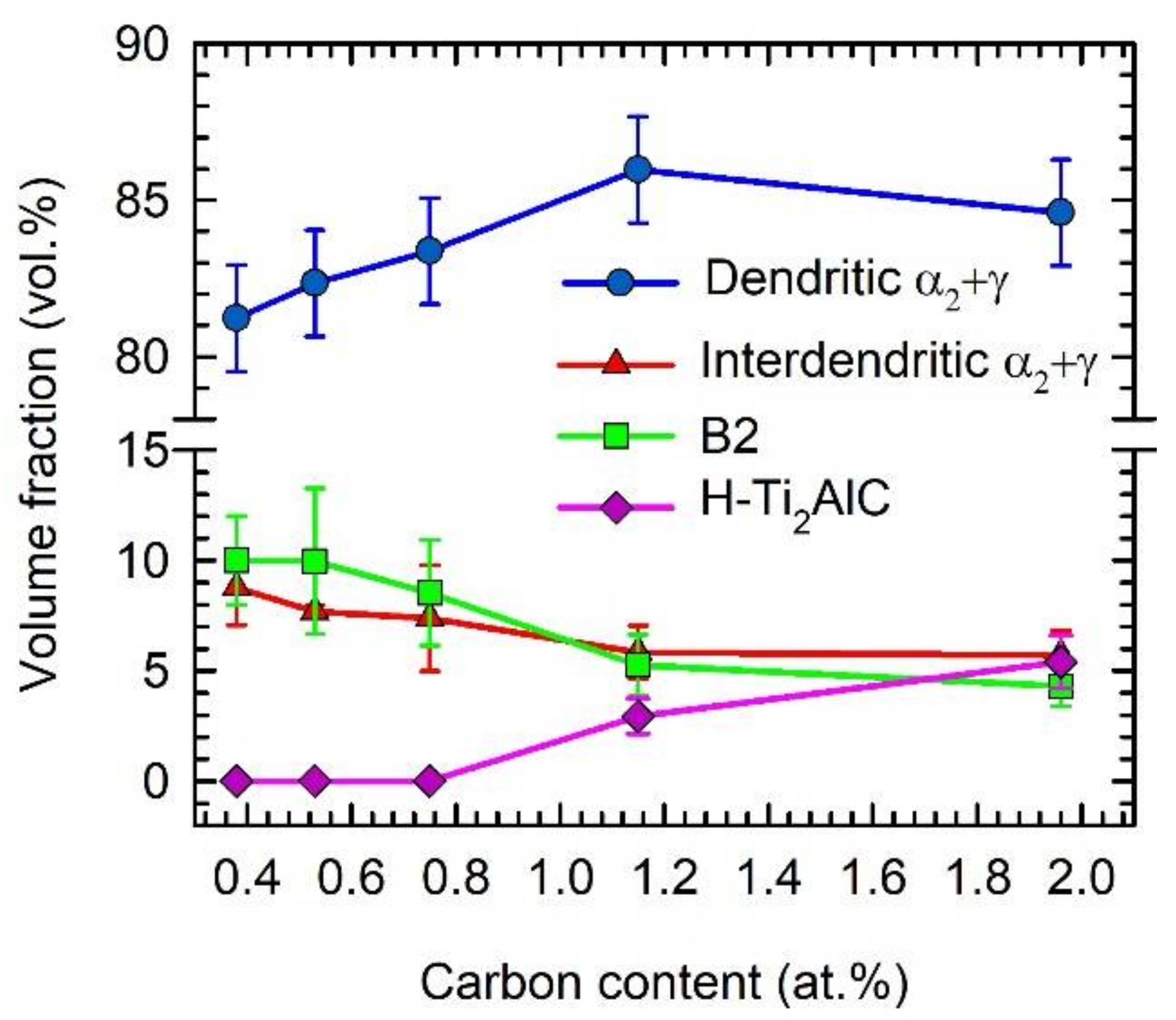
- The microstructure of the as-cast alloys was dendritic, with high segregation of W to the dendrites. Within the dendrites, a network of B2 phase surrounded by α phase was formed, which transformed into fine lamellar α2 and γ phases during cooling. The interdendritic regions mainly contained the γ phase and a small amount of α2 phase.
- The alloys with additions of 1.15 and 1.96 at.% C contained coarse lathe-shaped Ti2AlC particles. The solubility limit of C in the studied alloys was determined to be between 0.75 and 1.15 at.%. The Ti2AlC carbides dissolve only a low amount of W, which segregates preferentially into β dendrites during solidification.
- The Ti2AlC particles acted as heterogeneous nucleation sites during solidification, significantly refining the dendritic microstructure and reducing the grain size. The volume fraction of interdendritic α2 + γ regions and B2 phase decreased with increasing carbon content in the as-cast alloys.
- The DTA and microstructural analysis showed that the alloys with carbon content up to 0.75 at.% solidify through β primary phase. The alloys with the carbon content above 1.15 at.% solidify through Ti2AlC primary phase. The increase in carbon content up to 1.15 at.% changed significantly on the solidification path, from L → L + β to L + H → L + β + H.
- The Vickers hardness increases with the increasing content of C in the as-cast alloys. The elastic modulus of the C20 alloy with 5.4 vol.% of primary Ti2AlC particles is higher than that measured in the C4, C5, C8, and C12 alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.W. Ordered intermetallic alloys. 3. Gamma-titanium aluminides. J. Met. 1994, 46, 30–39. [Google Scholar] [CrossRef]
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Vojtěch, D.; Popela, T.; Hamáček, J.; Kützendörfer, J. The influence of tantalum on the high temperature characteristics of lamellar gamma + alpha 2 titanium aluminide. Mater. Sci. Eng. A 2011, 528, 8557–8564. [Google Scholar] [CrossRef]
- Daloz, D.; Hecht, U.; Zollinger, J.; Combeau, H.; Hazotte, A.; Zaloznik, M. Micro-segregation, macrosegregation and related phase transformation in TiAl alloys. Intermetallics 2011, 19, 749–756. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, L.; He, J.; Zhang, F.; Feng, X.; Nan, H.; Lin, J.; Kim, Y.W. As-cast microstructure characteristics dependent on solidification mode in TiAl-Nb alloys. J. Alloys Compd. 2019, 809, 151862. [Google Scholar] [CrossRef]
- Appel, F.; Paul, J.D.H.; Oehring, M. Gamma Titanium Aluminide Alloys: Science and Technology; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, S.L. Advances in gamma alloy materials–processes–application technology: Successes, dilemmas, and future. JOM 2018, 70, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Couret, A.; Monchoux, J.P.; Cailard, D. On the high creep strength of the W containing IRIS-TiAl alloy at 850 °C. Acta Mater. 2019, 181, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Lapin, J.; Pelachova, T. Microstructural stability of a cast Ti-45.2Al-2W-0.6Si-0.7B alloy at temperatures 973–1073 K. Intermetallics 2006, 14, 1175–1180. [Google Scholar] [CrossRef]
- Lapin, J.; Nazmy, M. Microstructure and creep properties of a cast intermetallic Ti-46Al-2W-0.5Si alloy for gas turbine applications. Mater. Sci. Eng. A 2004, 380, 298–307. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Lupinc, V.; Guo, J.T. Microstructural stability of the intermetallic Ti-45Al-2W-0.5Si-0.5B in the 800–980 °C temperature range. Mater. Sci. Eng. A 2003, 354, 97–105. [Google Scholar] [CrossRef]
- Seo, D.Y.; Beddoes, J.; Zhao, L. Primary creep behavior of Ti-48Al-2W as a function of stress and lamellar morphology. Metall. Mater. Trans. A 2003, 34, 2177–2190. [Google Scholar] [CrossRef]
- Beddoes, J.; Seo, D.Y.; Saari, H. Long term creep of TiAl + W + Si with polycrystalline and columnar grain structures. Scr. Mater. 2005, 52, 745–750. [Google Scholar] [CrossRef]
- Szkliniarz, W.; Szkliniarz, A. Microstructure and properties of TiAl-based alloys melted in graphite crucible. Metals 2021, 11, 669. [Google Scholar] [CrossRef]
- Appel, F.; Paul, J.D.H.; Oehring, M.; Fröbel, U.; Lorenz, U. Creep behavior of TiAl alloys with enhanced high-temperature capability. Metall. Mater. Trans. A 2003, 34, 2149–2164. [Google Scholar] [CrossRef]
- Schwaighofer, E.; Rashkova, B.; Clemens, H.; Stark, A.; Mayer, S. Effect of carbon addition on solidification behavior, phase evolution and creep properties of an intermetallic β-stabilized γ-TiAl based alloy. Intermetallics 2014, 46, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Lapin, J.; Kamyshnykova, K. Processing, microstructure and mechanical properties of in-situ Ti3Al + TiAl matrix composite reinforced with Ti2AlC particles prepared by centrifugal casting. Intermetallics 2018, 98, 34–44. [Google Scholar] [CrossRef]
- Scheu, C.; Sterger, E.; Schober, M.; Cha, L.; Clemens, H.; Bartels, A.; Schimansky, F.P.; Cerezo, A. High Carbon Solubility in a gamma-TiAl based Ti-45Al-5Nb-0.5C Alloy and its Effect on Hardening. Acta Mater. 2009, 57, 1504–1511. [Google Scholar] [CrossRef] [Green Version]
- Perdrix, F.; Trichet, M.F.; Bonnetien, J.L.; Cornet, M. Relationships between interstitial content, microstructure and mechanical properties in fully lamellar Ti–48Al alloys, with special reference to carbon. Intermetallics 2001, 9, 807–815. [Google Scholar] [CrossRef]
- Song, L.; Hu, X.; Wang, L.; Stark, A.; Lazurenko, D.; Lorenz, U.; Lin, J.; Pyczak, F.; Zhang, T. Microstructure evolution and enhanced creep property of a high Nb containing TiAl alloy with carbon addition. J. Alloys Compd. 2019, 807, 151649. [Google Scholar] [CrossRef]
- Gabrisch, H.; Stark, A.; Schimansky, F.P.; Wang, L.; Schell, N.; Lorenz, U.; Pyczak, F. Investigation of carbides in Ti-45Al-5Nb-xC alloys (0 ≤ x ≤ 1) by transmission electron microscopy and high energy-XRD. Intermetallics 2013, 33, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Oehring, M.; Lorenz, U.; Stark, A.; Pyczak, F. New insights into perovskite-Ti3AlC precipitate splitting in a Ti-45Al-5Nb-0.75C alloy by transmission electron microscopy. Intermetallics 2018, 100, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Lapin, J.; Klimová, A.; Gabalcová, Z.; Pelachová, T.; Bajana, O.; Štamborská, M. Microstructure and mechanical properties of cast in-situ TiAl matrix composites reinforced with (Ti,Nb)2AlC particles. Mater. Des. 2017, 133, 404–415. [Google Scholar] [CrossRef]
- Klimová, A.; Lapin, J. Effect of Al content on microstructure of Ti-Al-Nb-C-Mo composites reinforced with carbide particles. Kov. Mater. 2019, 57, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Lapin, J.; Kamyshnykova, K. Effect of Ta and W additions on microstructure and mechanical properties of tilt-cast Ti-45Al-5Nb-2C alloy. Metals 2021, 11, 2052. [Google Scholar] [CrossRef]
- Lapin, J.; Klimová, A. Vacuum induction melting and casting of TiAl-based matrix in-situ composites reinforced by carbide particles using graphite crucibles and moulds. Vacuum 2019, 169, 108930. [Google Scholar] [CrossRef]
- Chen, R.; Fang, H.; Chen, X.; Su, Y.; Ding, H.; Guo, J.; Fu, H. Formation of TiC/Ti2AlC and α2 + γ in in-situ TiAl composites with different solidification paths. Intermetallics 2017, 81, 9–15. [Google Scholar] [CrossRef]
- Lapin, J.; Kamyshnykova, K.; Klimova, A. Comparative study of microstructure and mechanical properties of two TiAl-based alloys reinforced with carbide particles. Molecules 2020, 25, 3423. [Google Scholar] [CrossRef]
- Cegan, T.; Cagala, M.; Kursa, M.; Kawulok, P.; Rusz, S.; Jurica, J.; Vontorova, J. Effect of Ti2AlC particles on the microstructure and elevated-temperature-deformation properties of γ-TiAl alloys. Mater. Technol. 2014, 48, 831–835. [Google Scholar]
- Aguilar, J.; Schievenbusch, A.; Kättlitz, O. Investment casting technology for production of TiAl low pressure turbine blades—Process engineering and parameter analysis. Intermetallics 2011, 19, 757–761. [Google Scholar] [CrossRef]
- Brotzu, A.; Felli, F.; Mondal, A.; Pilone, D. Production issues in the manufacturing of TiAl turbine blades by investment casting. Procedia Struct. Integr. 2020, 25, 79–87. [Google Scholar] [CrossRef]
- Ye, Q.C.; Xiao, K.Q.; Cao, R.X.; Wu, H.; Zhao, G.W.; Li, B. Microstructure evolution and microhardness of TiAl based alloy blade by vacuum suction casting. Vacuum 2019, 163, 186–193. [Google Scholar] [CrossRef]
- Jovanović, M.T.; Dimčić, B.; Bobić, I.; Zec, S.; Maksimović, V. Microstructure and mechanical properties of precision cast TiAl turbocharger wheel. J. Mater. Process. Technol. 2005, 167, 14–21. [Google Scholar] [CrossRef]
- Shouren, W.; Peiquan, G.; Liying, Y. Centrifugal precision cast TiAl turbocharger wheel using ceramic mold. J. Mater. Process. Technol. 2008, 204, 492–497. [Google Scholar] [CrossRef]
- Wang, M.S.; Liu, E.W.; Du, Y.L.; Liu, T.T.; Liao, W.H. Cracking mechanism and a novel strategy to eliminate cracks in TiAl alloy additively manufactured by selective laser melting. Scr. Mater. 2021, 204, 114151. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Cheng, X.; Zhang, S.; Wang, H. Study of microstructure evolution and preference growth direction in a fully laminated directional micro-columnar TiAl fabricated using laser additive manufacturing technique. Mater. Lett. 2019, 243, 62–65. [Google Scholar] [CrossRef]
- Huang, D.; Tan, Q.; Zhou, Y.; Yin, Y.; Wang, F.; Wu, T.; Yang, X.; Fan, Z.; Liu, Y.; Zhang, J.; et al. The significant impact of grain refiner on γ-TiAl intermetallic fabricated by laser-based additive manufacturing. Addit. Manuf. 2021, 46, 102172. [Google Scholar] [CrossRef]
- Güther, V.; Allen, M.; Klose, J.; Clemens, H. Metallurgical processing of titanium aluminides on industrial scale. Intermetallics 2018, 103, 12–22. [Google Scholar] [CrossRef]
- Chen, G.; Lan, B.; Xiong, F.; Gao, P.; Zhang, H.; Lu, X.; Li, C. Pilot-scale experimental evaluation of induction melting of Ti-46Al-8Nb alloy in the fused BaZrO3 crucible. Vacuum 2019, 159, 293–298. [Google Scholar] [CrossRef]
- Chen, G.; Yu, F.; Hou, X.; Yang, Y.; Duan, B.; Feng, Q.; Zou, X.; Wang, E.; Hou, X.; Lu, X.; et al. Performance of BaZrO3/Y2O3 dual-phase refractory applied to TiAl alloy melting. Ceram. Int. 2022, 48, 20158–20167. [Google Scholar] [CrossRef]
- Kuang, J.P.; Harding, R.A.; Campbell, J. Investigation into refractories as crucible and mould materials for melting and casting γ-TiAl alloys. Mater. Sci. Technol. 2000, 16, 1007–1016. [Google Scholar] [CrossRef]
- Gomez, F.; Barbosa, J.; Ribiero, C.S. Induction melting of γ-TiAl in CaO crucibles. Intermetallics 2008, 16, 1292–1297. [Google Scholar] [CrossRef]
- Lapin, J.; Ondrúš, L.; Nazmy, M. Directional solidification of intermetallic Ti-46Al-2W-0.5Si alloy in alumina moulds. Intermetallics 2002, 10, 1019–1031. [Google Scholar] [CrossRef]
- Szkliniarz, W.; Szkliniarz, A. The chemical composition and microstructure of Ti-47Al-2W-0.5Si alloy melted in ceramic crucibles. Solid State Phenom. 2012, 191, 211–220. [Google Scholar] [CrossRef]
- Szkliniarz, W.; Szkliniarz, A. The characteristics of TiAl-based alloys melted in graphite crucibles. Mater. Sci. Technol. 2019, 35, 297–305. [Google Scholar] [CrossRef]
- Lapin, J.; Gabalcová, Z.; Pelachová, T. Effect of Y2O3 crucible on contamination of directionally solidified intermetallic Ti-46Al-8Nb alloy. Intermetallics 2011, 19, 396–403. [Google Scholar] [CrossRef]
- Kamyshnykova, K.; Lapin, J. Vacuum induction melting and solidification of TiAl-based alloy in graphite crucibles. Vacuum 2018, 154, 218–226. [Google Scholar] [CrossRef]
- Jurica, J.; Cegan, T.; Skotnicova, K.; Petlak, D.; Smetana, B.; Matejka, V. Preparation and properties of master alloys Nb-Al and Ta-Al for melting and casting of γ-TiAl intermetallics. Mater. Tehnol. 2015, 49, 27–30. [Google Scholar]
- Harding, R.A. Recent developments in the induction skull melting and investment casting of titanium aluminides. Kov. Mater. 2004, 42, 225–241. [Google Scholar]
- Bojarevicz, V.; Harding, R.A.; Pericleous, K.; Wickins, M. The Development and Experimental Validation of a Numerical Model of an Induction Skull Melting Furnace. Metall. Mater. Trans. B 2004, 35, 785–803. [Google Scholar] [CrossRef]
- Kamyshnykova, K.; Lapin, J.; Pelachová, T.; Cegan, T.; Jurica, J.; Volodarskaja, A. Microstructure and mechanical properties of Ti–45Al–2W–xC alloys. Intermetallics 2022, 148, 107168. [Google Scholar] [CrossRef]
- Cegan, T.; Szurman, I.; Kursa, M.; Holesinsky, J.; Vontorova, J. Preparation of TiAl-based alloys by induction melting in graphite crucibles. Kov. Mater. 2015, 53, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Cegan, T.; Petlak, D.; Skotnicova, K.; Jurica, J.; Smetana, B.; Zla, S. Metallurgical Preparation of Nb–Al and W–Al Intermetallic Compounds and Characterization of Their Microstructure and Phase Transformations by DTA Technique. Molecules 2020, 25, 2001. [Google Scholar] [CrossRef] [PubMed]
- Lapin, J.; Kamyshnykova, K.; Pelachová, T.; Nagy, S. Effect of carbon addition and cooling rate on lamellar structure of peritectic TiAl-based alloy. Intermetallics 2021, 128, 107007. [Google Scholar] [CrossRef]
- Zollinger, J.; Gabalcová, Z.; Daloz, D.; Lapin, J.; Combeau, H. Microsegregation induced microstructures in intermetallic Ti-46Al-8Nb alloy. Kov. Mater. 2008, 46, 291–296. [Google Scholar]
- Gil, I.; Muñoz-Morris, M.A.; Morris, D.G. The effect of heat treatments on the microstructural stability of the intermetallic Ti–46.5Al– 2W–0.5Si. Intermetallics 2001, 9, 373–385. [Google Scholar] [CrossRef]
- Klimová, A.; Lapin, J. The effect of heat treatment on microstructure and hardness of in-situ Ti-38Al-7.5Nb-5C-0.9Mo composite. Kov. Mater. 2020, 58, 433–443. [Google Scholar] [CrossRef]
- Lapin, J.; Gabalcova, Z. Solidification behaviour of TiAl-based alloys studied by directional solidification technique. Intermetallics 2011, 19, 797–804. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Huang, L.; Huang, J.; Zhang, Y.; Huang, B. Effect of trace tungsten on the microstructure of TiAl alloys containing Nb and B. Rare Met. 2007, 26, 323–329. [Google Scholar] [CrossRef]
- Zollinger, J.; Lapin, J.; Daloz, D.; Combeau, H. Influence of oxygen on solidification behaviour of cast TiAl-based alloys. Intermetallics 2007, 15, 1343–1350. [Google Scholar] [CrossRef]
- Klimová, A.; Lapin, J. Microsegregation behaviour of the alloying elements in directionally solidified intermetallic Ti-44Al-5Nb-0.2B-0.2C alloy. In Proceedings of the METAL 2012—21st Anniversary International Conference on Metallurgy and Materials, Brno, Czech Republic, 23–25 May 2012. [Google Scholar]
- Witusiewicz, V.T.; Hallstedt, B.; Bondar, A.A.; Hecht, U.; Sleptsov, S.V.; Velikanova, T.Y. Thermodynamic description of the Al-C-Ti system. J. Alloys Compd. 2015, 623, 480–496. [Google Scholar] [CrossRef]
- Muñoz-Morris, M.A.; Gil, A.I.; Morris, D.G. Microstructural stability of γ-based TiAl intermetallics containing β phase. Intermetallics 2005, 13, 929–936. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, H.; Zhang, H.; Chen, R.; Guo, J.; Fu, H. Variations of microstructure and tensile property of γ-TiAl alloys with 0–0.5 at% C additives. Mater. Sci. Eng. A 2017, 700, 198–208. [Google Scholar] [CrossRef]
- Menand, A.; Huguet, A.; Nérac-Partaix, A. Interstitial solubility in γ and α2 phases of TiAl-based alloys. Acta Mater. 1996, 44, 4729–4737. [Google Scholar] [CrossRef]
- Klimová, A.; Lapin, J. Effects of C and N additions on primary MAX phase particles in intermetallic Ti-Al-Nb-Mo matrix in-situ composites prepared by vacuum induction melting. Kov. Mater. 2019, 57, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Lu, Y.; Zhang, X.L.; Hong Shao, X. The elastic behaviors and theoretical tensile strength of γ-TiAl alloy from the first principles calculations. Intermetallics 2018, 101, 1–7. [Google Scholar] [CrossRef]
- Tanaka, K.; Okamoto, K.; Inul, H.; Mlnonishl, Y.; Yamaguch, M.; Koiwa, M. Elastic constants and their temperature dependence for the intermetallic compound Ti3Al. Philos. Mag. A 1996, 73, 1475–1488. [Google Scholar] [CrossRef]






| Alloy | Al (at.%) | Ti (at.%) | W (at.%) | O (wt.ppm) | N (wt.ppm) | C (at.%) |
|---|---|---|---|---|---|---|
| C4 | 44.7 ± 0.4 | 52.9 ± 0.3 | 2.0 ± 0.2 | 1120 ± 50 | 500 ± 20 | 0.38 ± 0.03 |
| C5 | 45.2 ± 0.3 | 52.2 ± 0.3 | 2.1 ± 0.1 | 1130 ± 15 | 460 ± 25 | 0.53 ± 0.02 |
| C8 | 44.9 ± 0.3 | 52.3 ± 0.3 | 2.0 ± 0.1 | 910 ± 15 | 370 ± 10 | 0.75 ± 0.02 |
| C12 | 44.7 ± 0.4 | 52.1 ± 0.4 | 2.0 ± 0.1 | 1190 ± 75 | 480 ± 25 | 1.15 ± 0.02 |
| C20 | 44.5 ± 0.5 | 51.5 ± 0.5 | 2.0 ± 0.1 | 1010 ± 20 | 400 ± 25 | 1.96 ± 0.03 |
| Alloy | Phases | Al | Ti | W | C |
|---|---|---|---|---|---|
| C4 | B2 | 38.4 ± 0.4 | 55.2 ± 1.0 | 6.40 ± 0.08 | - |
| α2 + γ | 43.9 ± 0.3 | 53.0 ± 0.3 | 3.10 ± 0.03 | - | |
| γ | 50.3 ± 0.8 | 49.4 ± 0.6 | 0.30 ± 0.02 | - | |
| C5 | B2 | 39.1 ± 0.4 | 54.4 ± 1.3 | 6.60 ± 0.07 | - |
| α2 + γ | 43.9 ± 0.2 | 53.4 ± 0.2 | 2.70 ± 0.03 | - | |
| γ | 51.2 ± 0.6 | 48.2 ± 0.8 | 0.60 ± 0.02 | - | |
| C8 | B2 | 39.3 ± 0.9 | 53.5 ± 0.8 | 7.20 ± 0.09 | - |
| α2 + γ | 44.2 ± 0.3 | 53.3 ± 0.3 | 2.50 ± 0.04 | - | |
| γ | 51.7 ± 0.6 | 47.7 ± 0.6 | 0.60 ± 0.03 | - | |
| C12 | B2 | 39.4 ± 0.7 | 53.3 ± 0.9 | 7.30± 0.07 | - |
| α2 + γ | 43.7 ± 0.4 | 54.1 ± 0.5 | 2.20 ± 0.04 | - | |
| γ | 50.9 ± 0.4 | 48.7 ± 0.4 | 0.40 ± 0.05 | - | |
| H | 25.4± 0.6 | 47.2 ± 0.2 | 0.80 ± 0.02 | 26.6 ± 0.4 | |
| C20 | B2 | 38.9 ± 0.8 | 53.8 ± 0.9 | 7.40 ± 0.08 | |
| α2 + γ | 44.5 ± 0.3 | 52.8 ± 0.3 | 2.70 ± 0.03 | ||
| γ | 50.4 ± 0.4 | 49.0 ± 0.5 | 0.60 ± 0.03 | ||
| Ti2AlC | 25.6 ± 0.5 | 46.7 ± 0.2 | 0.80 ± 0.03 | 26.9 ± 0.4 |
| Sample | Phase Transformation | Temperature (°C) |
|---|---|---|
| C4 | β → β + α | 1495 |
| β + α → α | 1433 | |
| α → α + γ | 1378 | |
| α + γ → α + γ + β | 1280 | |
| α + γ + β → γ + B2 | 1221 | |
| C20 | β + L + H | 1490 |
| β + L + H → β + α + H | 1470 | |
| β + α + H → α + H | 1448 | |
| α + H → α + γ + H | 1404 | |
| α + γ + H → α + γ + β + H | 1294 | |
| α + γ + β + H → γ + B2 + H | 1235 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cegan, T.; Kamyshnykova, K.; Lapin, J.; Szurman, I.; Jurica, J.; Klimantova, V. Processing and Microstructure of As-Cast Ti-45Al-2W-xC Alloys. Materials 2022, 15, 5049. https://doi.org/10.3390/ma15145049
Cegan T, Kamyshnykova K, Lapin J, Szurman I, Jurica J, Klimantova V. Processing and Microstructure of As-Cast Ti-45Al-2W-xC Alloys. Materials. 2022; 15(14):5049. https://doi.org/10.3390/ma15145049
Chicago/Turabian StyleCegan, Tomas, Kateryna Kamyshnykova, Juraj Lapin, Ivo Szurman, Jan Jurica, and Vendula Klimantova. 2022. "Processing and Microstructure of As-Cast Ti-45Al-2W-xC Alloys" Materials 15, no. 14: 5049. https://doi.org/10.3390/ma15145049






