Selected Physical and Spectroscopic Properties of TPS Moldings Enriched with Durum Wheat Bran
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. The Extrusion Cooking of TPS Granulates
2.3. The Injection Molding Process
2.4. Moisture Content Stability
2.5. Structural Analysis of TPS Moldings
2.6. Mechanical Properties of TPS Granulates and Moldings
2.7. Measurement of Moldings Color
2.8. Infrared Spectra Measurements
2.9. Statistical Analysis
3. Results and Discussion
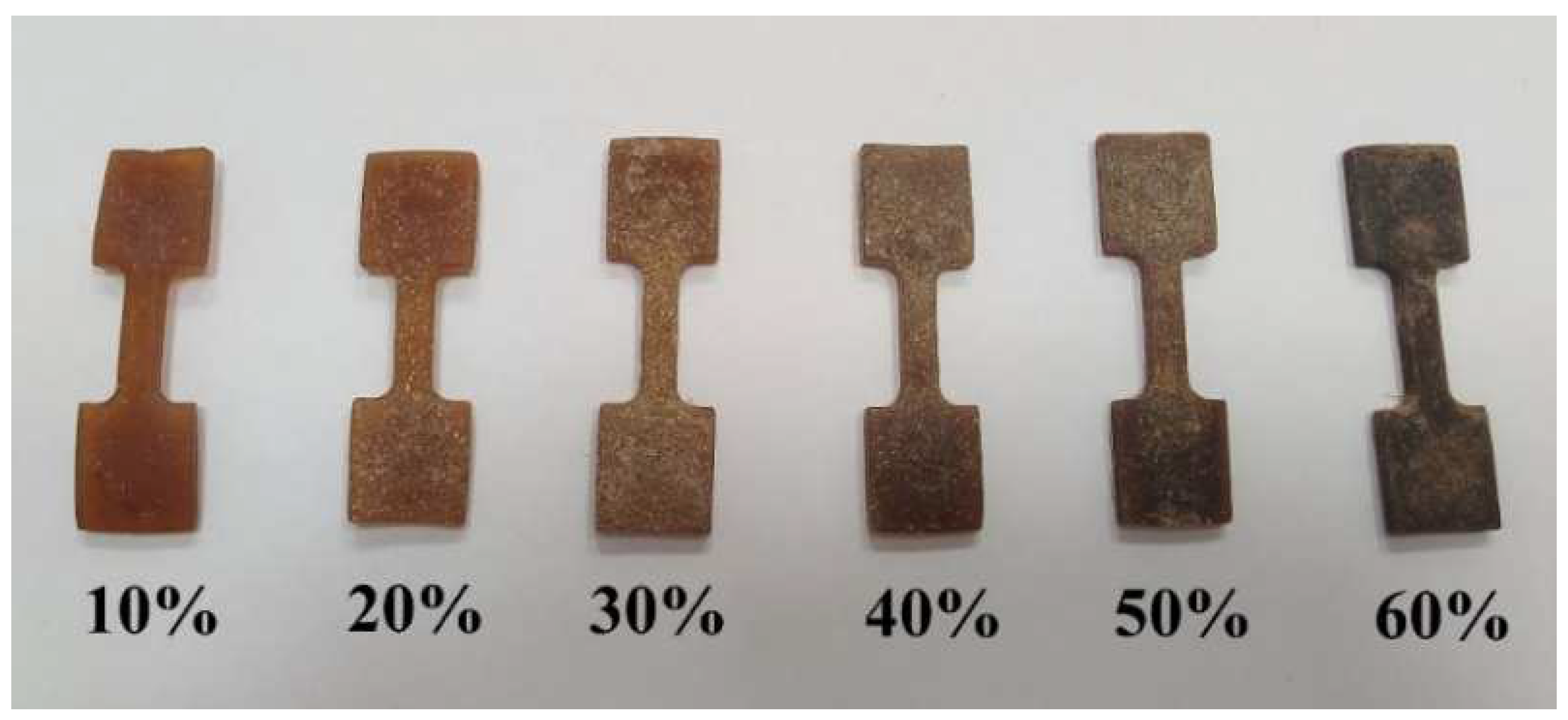
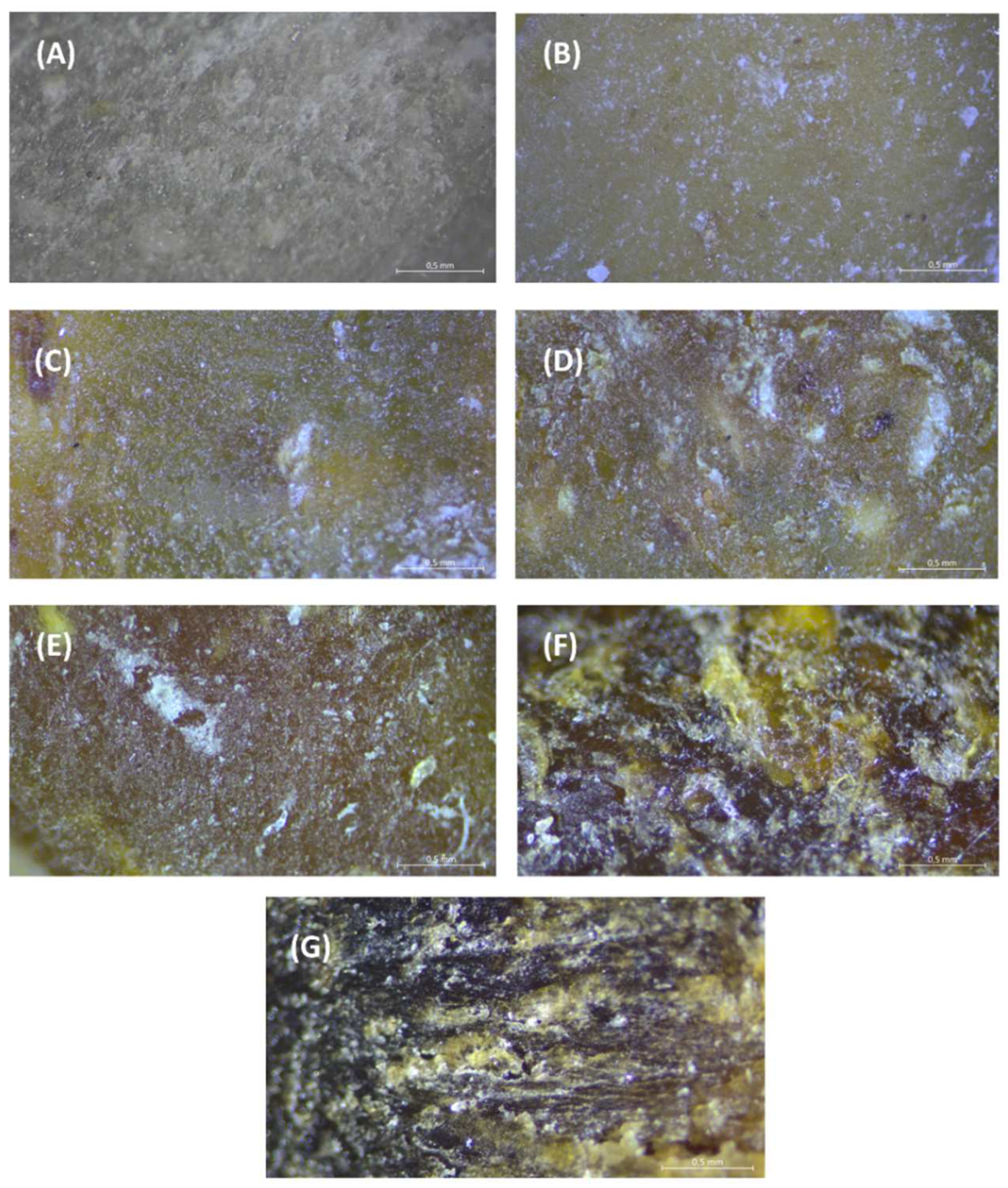
3.1. Structure of TPS Moldings
3.2. Selected Mechanical Properties of TPS Moldings
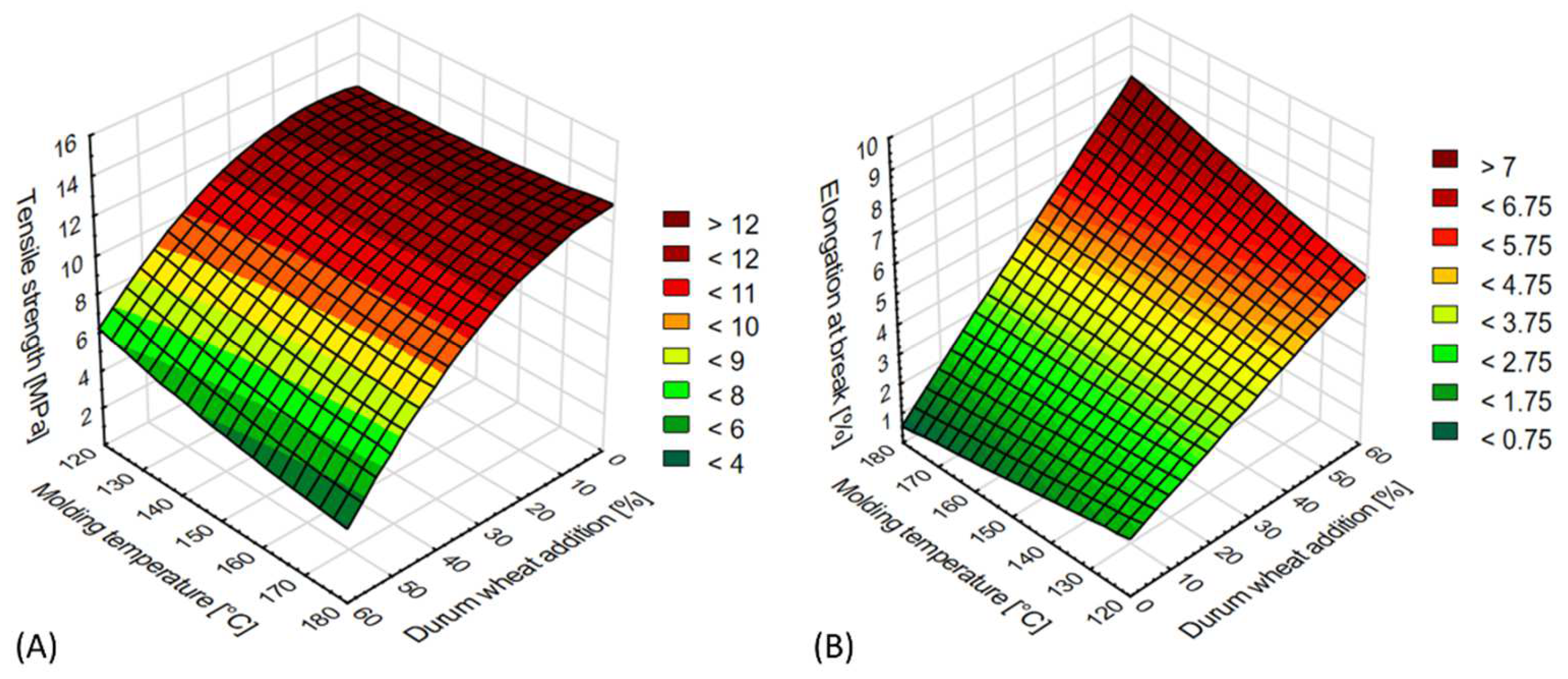
3.2.1. Tensile Tests
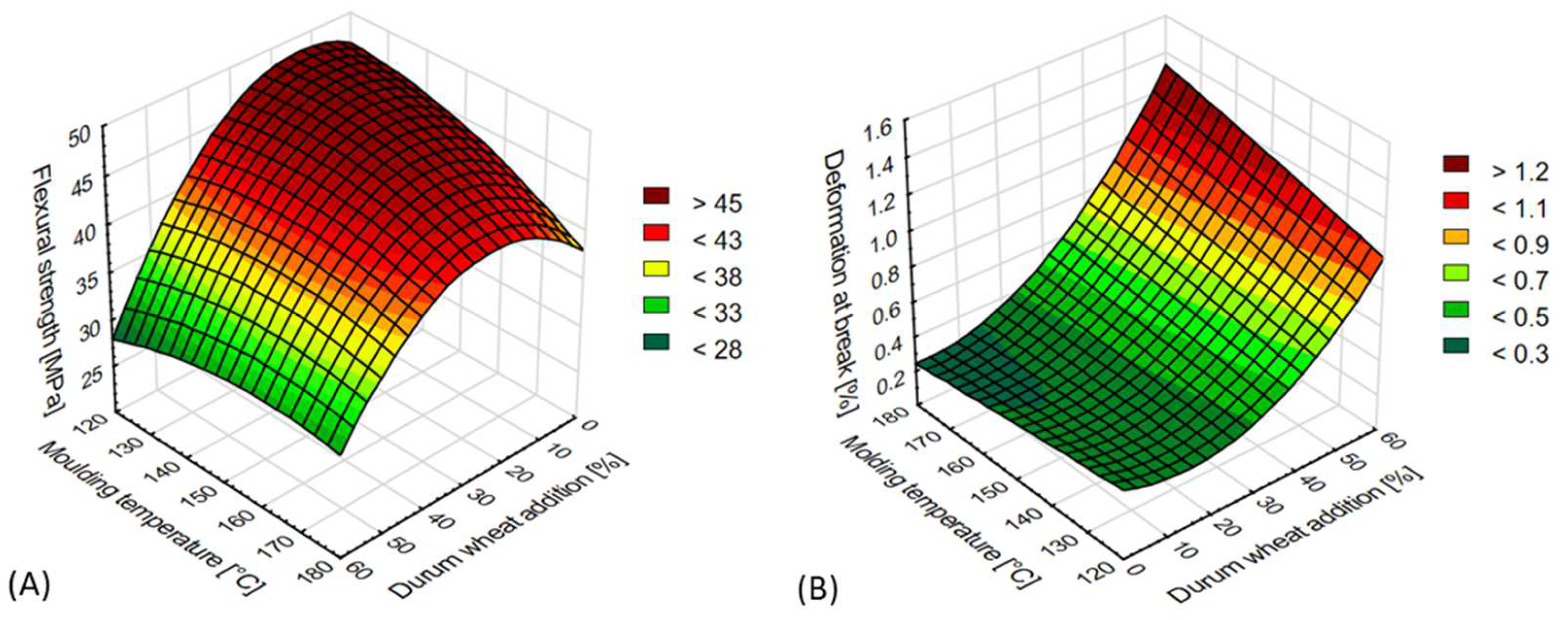
3.2.2. Bending Test
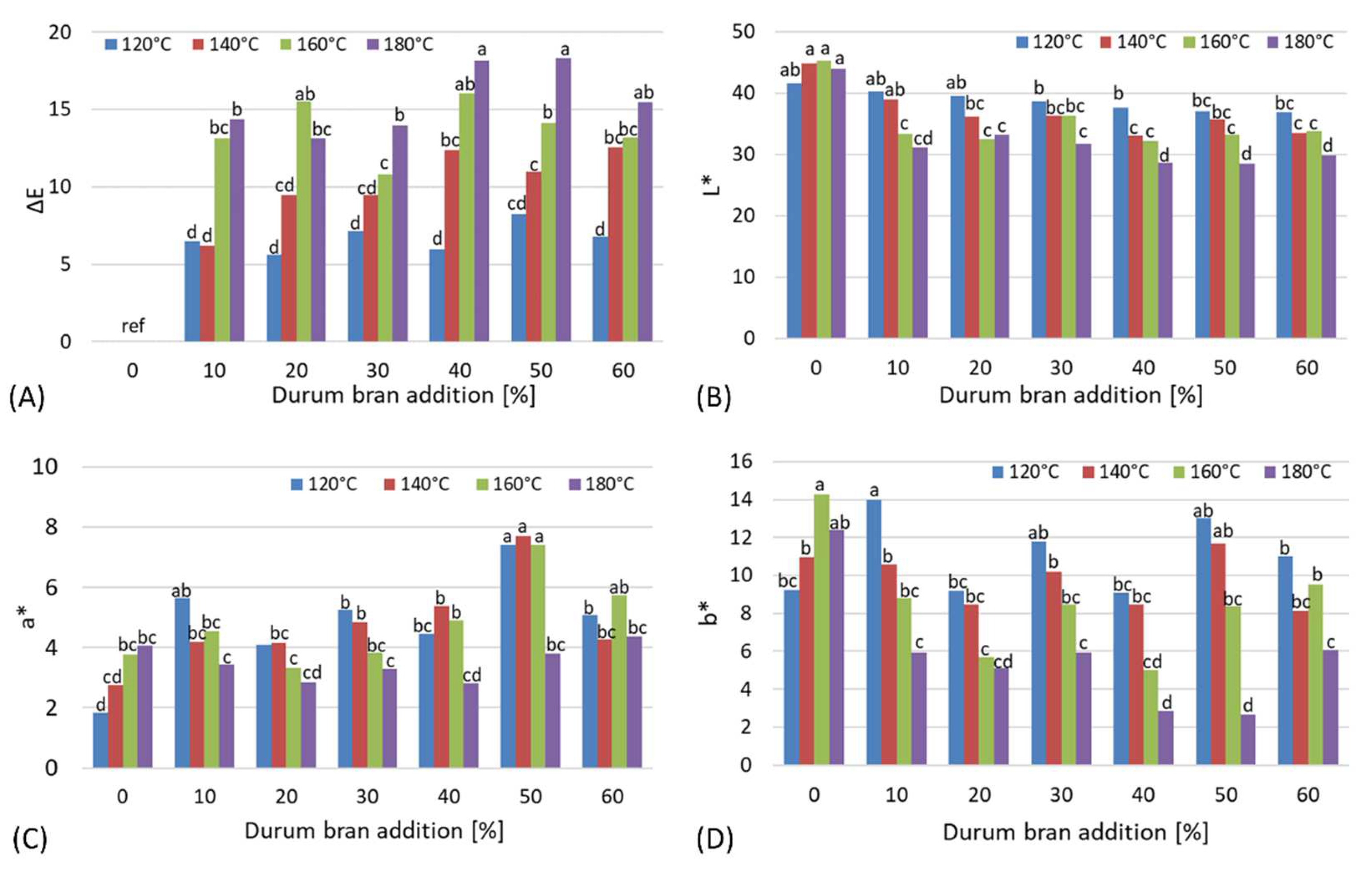
3.3. Color of Starch-Based Moldings
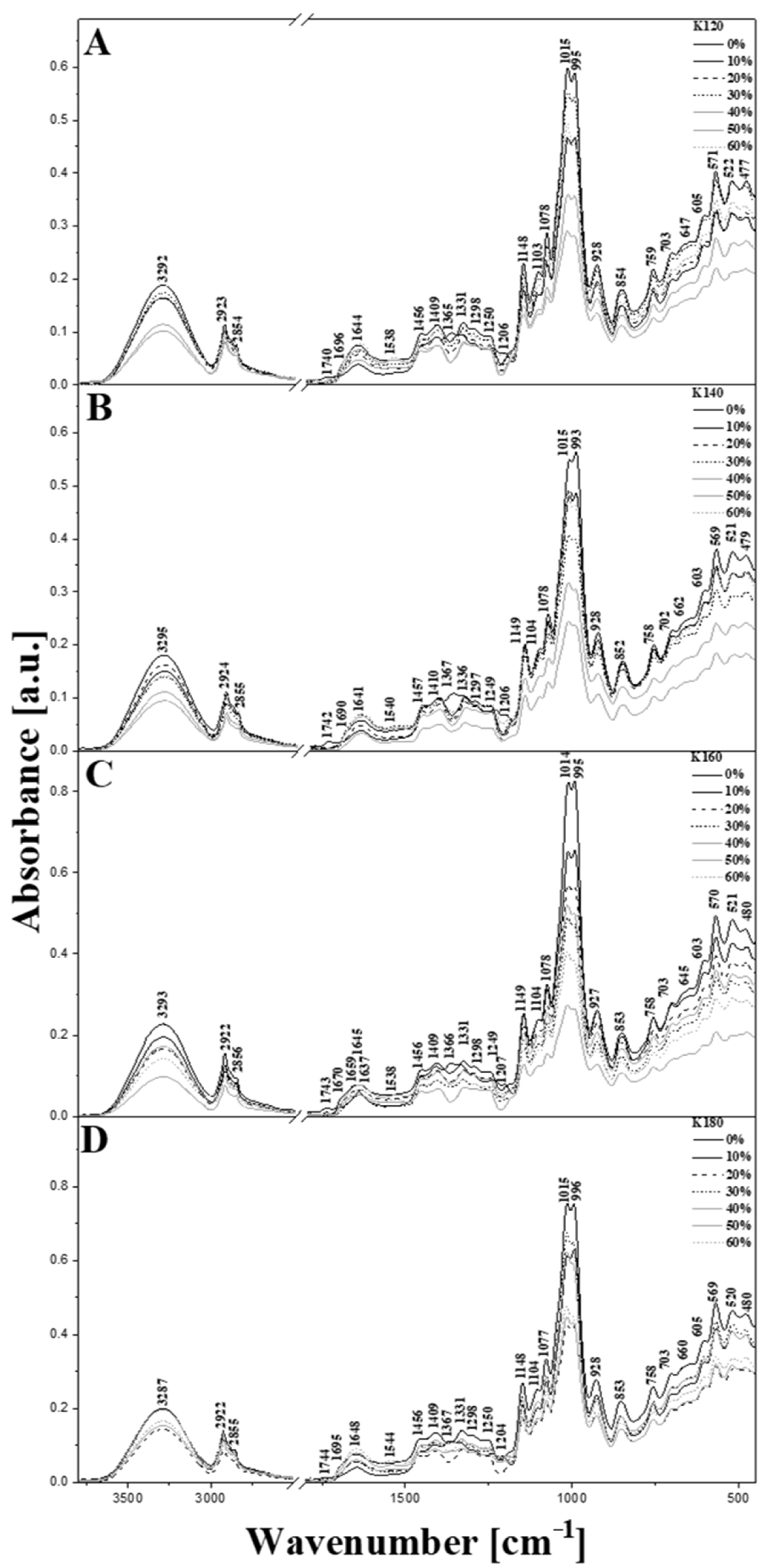
3.4. Analysis and Characteristics of Starch-Based Biopolymer Samples Using ART/FTIR Infrared Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Combrzyński, M.; Özmen, Ö. Foamed Bioplastics: A Review. Int. Agrophys. 2021, 35, 375–388. [Google Scholar] [CrossRef]
- Oniszczuk, T.; Wójtowicz, A.; Oniszczuk, A.; Mitrus, M.; Combrzyński, M.; Kręcisz, M.; Mościcki, L. Effect of Processing Conditions on Selected Properties of Starch-Based Biopolymers. Agric. Agric. Sci. Procedia 2015, 7, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Mitrus, M.; Combrzyński, M.; Kupryaniuk, K.; Wójtowicz, A.; Oniszczuk, T.; Kręcisz, M.; Matysiak, A.; Smurzyńska, A.; Mościcki, L. A study of the solubility of biodegradable foams of thermoplastic starch. J. Ecol. Eng. 2016, 17, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Bergel, B.F.; Araujo, L.L.; Santana, R.M.C. Effects of the Addition of Cotton Fibers and Cotton Microfibers on the Structure and Mechanical Properties of Starch Foams Made from Potato Starch. Carbohydr. Polym. Technol. Appl. 2021, 2, 100167. [Google Scholar] [CrossRef]
- De Graaf, R.A.; Janssen, L.P.B.M. The Production of a New Partially Biodegradable Starch Plastic by Reactive Extrusion. Polym. Eng. Sci. 2000, 40, 2086–2094. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-Based Biodegradable Materials: Challenges and Opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Janssen, L.P.B.M.; Mościcki, L. Thermoplastic Starch; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar] [CrossRef]
- Abinader, G.; Lacoste, C.; Baillif, M.L.; Erre, D.; Copinet, A. Effect of the Formulation of Starch-Based Foam Cushions on the Morphology and Mechanical Properties. J. Cell. Plast. 2015, 51, 31–44. [Google Scholar] [CrossRef]
- Gamarano, D.d.S.; Pereira, I.M.; da Silva, M.C.; Mottin, A.C.; Ayres, E. Crystal Structure Transformations in Extruded Starch Plasticized with Glycerol and Urea. Polym. Bull. 2020, 77, 4971–4992. [Google Scholar] [CrossRef]
- Guan, J.; Eskridge, K.M.; Hanna, M.A. Acetylated Starch-Polylactic Acid Loose-Fill Packaging Materials. Ind. Crop. Prod. 2005, 22, 109–123. [Google Scholar] [CrossRef]
- Bae, S.O.; Lim, S.T. Physical Properties of Extruded Strands of Hydroxypropylated Normal and High-Amylose Corn Starch. Cereal Chem. 1998, 75, 449–454. [Google Scholar] [CrossRef]
- Chaudhary, A.L.; Miler, M.; Torley, P.J.; Sopade, P.A.; Halley, P.J. Amylose Content and Chemical Modification Effects on the Extrusion of Thermoplastic Starch from Maize. Carbohydr. Polym. 2008, 74, 907–913. [Google Scholar] [CrossRef]
- Morrison, W.R.; Laignelet, B. An Improved Colorimetric Procedure for Determining Apparent and Total Amylose in Cereal and Other Starches. J. Cereal Sci. 1983, 1, 9–20. [Google Scholar] [CrossRef]
- Fama, L.; Bittante, A.M.B.Q.; Sobral, P.J.A.; Goyanes, S.; Gerschenson, L.N. Garlic powder and wheat bran as fillers: Their effect on the physicochemical properties of edible biocomposites. Mater. Sci. Eng. C 2010, 30, 853–859. [Google Scholar] [CrossRef]
- Robin, F.; Dubois, C.; Curti, D.; Schuchmann, H.P.; Palzer, S. Effect of wheat bran on the mechanical properties of extruded starchy foams. Food Res. Int. 2011, 44, 2880–2888. [Google Scholar] [CrossRef]
- Combrzyński, M.; Oniszczuk, T.; Kupryaniuk, K.; Wójtowicz, A.; Mitrus, M.; Milanowski, M.; Soja, J.; Budziak-Wieczorek, I.; Karcz, D.; Kamiński, D.; et al. Physical Properties, Spectroscopic, Microscopic, X-ray, and Chemometric Analysis of Starch Films Enriched with Selected Functional Additives. Materials 2021, 14, 2673. [Google Scholar] [CrossRef] [PubMed]
- Anuar, H.; Zuraida, A.; Kovacs, J.G.; Tabi, T. Improvement of Mechanical Properties of Injection-Molded Polylactic Acid–Kenaf Fiber Biocomposite. J. Thermoplast. Compos. Mater 2012, 25, 153–164. [Google Scholar] [CrossRef]
- PN-EN ISO 178:2019-06; Plastics—Determination of Bending Properties. Polish Committee for Standardization: Warsaw, Poland, 2019.
- Bouasla, A.; Wójtowicz, A.; Zidoune, M.N. Gluten-free precooked rice pasta enriched with legumes flours: Physical properties, texture, sensory attributes and microstructure. LWT 2017, 75, 569–577. [Google Scholar] [CrossRef]
- Berti, S.; Jagus, R.; Flores, S. Effect of rice bran addition on physical properties of antimicrobial biocomposite films based on starch. Food Bioproc. Technol. 2021, 14, 1700–1711. [Google Scholar] [CrossRef]
- Kuciel, S.; Liber-Knec, A. Biocomposites on the base of thermoplastic starch filled by wood and kenaf fiber. J. Biobased Mater. Bioenergy 2009, 3, 269–274. [Google Scholar] [CrossRef]
- Lu, Y.; Weng, L.; Cao, X. Morphological, thermal and mechanical properties of ramie crystallites—reinforced plasticized starch biocomposites. Carbohyd. Polym. 2006, 63, 198–204. [Google Scholar] [CrossRef]
- Gutiérrez, T.; Alvarez, V. Cellulosic materials as natural fillers in starch-containing matrix-based films: A review. Polym. Bull. 2017, 74, 2401–2430. [Google Scholar] [CrossRef]
- Ayse, A.; Mohini, S. Biocomposites from wheat straw nanofibers: Morphology, thermal and mechanical properties. Com. Sci. Technol. 2008, 68, 557–565. [Google Scholar] [CrossRef]
- Cinelli, P.; Chiellini, E.; Lawton, J.W.; Imam, S.H. Foamed articles based on potato starch, corn fibers and poly(vinyl alcohol). Polym. Degrad. Stab. 2006, 91, 1147–1155. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt, A. Improving properties of thermoplastic starch films by incorporating active extracts and cellulose fibres isolated from rice or coffee husk. Food Packag. Shelf Life 2019, 22, 100383. [Google Scholar] [CrossRef]
- Jumaidin, R.; Khiruddin, M.A.A.; Asyul Sutan Saidi, Z.; Salit, M.S.; Ilyas, R.A. Effect of cogon grass fibre on the thermal, mechanical and biodegradation properties of thermoplastic cassava starch biocomposite. Int. J. Biol. Macromol. 2020, 146, 746–755. [Google Scholar] [CrossRef]
- Khan, Z.I.; Habib, U.; Mohamad, Z.B.; Bin Rahmat, A.R.; Sahirah Binti Abdullah, N.A. Mechanical and thermal properties of sepolite strengthened thermoplastic polymer nanocomposites: A comprehensive review. Alex. Eng. J. 2022, 61, 975–990. [Google Scholar] [CrossRef]
- Wójtowicz, A. Precooked Pasta. In Extrusion-Cooking Techniques: Applications, Theory and Sustainability; Mościcki, L., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 99–117. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Suan, M.S.M. Thermoplastic starch/beeswax blend: Characterization on thermal mechanical and moisture absorption properties. Int. J. Biol. Macromol. 2021, 190, 224–232. [Google Scholar] [CrossRef]
- Girončs, J.; López, J.P.; Mutjé, P.; Carvalho, A.J.F.; Curvelo, A.A.S.; Vilaseca, F. Natural fiber-reinforced thermoplastic starch composites obtained by melt processing. Compos. Sci. Technol. 2012, 72, 858–863. [Google Scholar] [CrossRef]
- Wollerdorfer, M.; Bader, H. Influence of natural fibres on the mechanical properties of biodegradable polymers. Ind. Crop. Prod. 1998, 8, 105–112. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Kennedy, J.F. Studies on the properties of natural fibers-reinforced thermoplastic starch composites. Carb. Polym. 2005, 62, 19–24. [Google Scholar] [CrossRef]
- Espinach, F.X.; Delgado-Aguilar, M.; Puig, J.; Boufi, S.; Mutjé, P. Flexural properties of fully biodegradable alpha-grass fibers reinforced starch-based thermoplastics. Compos. Part B 2015, 81, 98–106. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of seaweed on mechanical, thermal, and biodegradation properties of thermoplastic sugar palm starch/agar composites. Int. J. Biol. Macromol. 2017, 99, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Wakeling, L.; Gamlath, S. Retention of Essential Amino Acids during Extrusion of Protein and Reducing Sugars. J. Agric. Food Chem. 2007, 55, 8779–8786. [Google Scholar] [CrossRef]
- Mercier, C.; Linko, P.; Harper, J.M. Extrusion Cooking; American Association of Cereal Chemists. Inc.: Saint Paul, MN, USA, 1987. [Google Scholar] [CrossRef]
- Kesre, C.; Masatcioglu, M.T. Physical characteristics of corn extrudates supplemented with red lentil bran. LWT 2022, 153, 112530. [Google Scholar] [CrossRef]
- Wójtowicz, A.; Zalewska-Korona, M.; Jabłońska-Ryś, E.; Skalicka-Woźniak, K.; Oniszczuk, A. Chemical characteristics and physical properties of functional snacks enriched with powdered tomato. Pol. J. Food Nutr. Sci. 2018, 68, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Combrzyński, M.; Matwijczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Karcz, D.; Szponar, J.; Niemczynowicz, A.; Bober, D.; Mitrus, M.; Kupryaniuk, K.; et al. Potato starch utilization in ecological loose-fill packaging materials—Sustainability and characterization. Materials 2020, 13, 1390. [Google Scholar] [CrossRef] [Green Version]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food. Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Oniszczuk, T.; Combrzyński, M.; Matwijczuk, A.; Oniszczuk, A.; Gładyszewska, B.; Podleśny, J.; Czernel, G.; Karcz, D.; Niemczynowicz, A.; Wójtowicz, A. Physical assessment, spectroscopic and chemometric analysis of starch-based foils with selected functional additives. PLoS ONE 2019, 14, e0212070. [Google Scholar] [CrossRef]
- Samborska, K.; Wiktor, A.; Jedlińska, A.; Matwijczuk, A.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Tułodziecki, M.; Błażowski, Ł.; Witrowa-Rajchert, D. Development and characterization of physical properties of honey-rich powder. Food Bioprod. Process. 2019, 115, 78–86. [Google Scholar] [CrossRef]
- Wójcik, M.; Różyło, R.; Schönlechner, R.; Matwijczuk, A.; Dziki, D. Low-Carbohydrate, High-Protein, and Gluten-Free Bread Supplemented with Poppy Seed Flour: Physicochemical, Sensory, and Spectroscopic Properties. Molecules 2022, 27, 1574. [Google Scholar] [CrossRef] [PubMed]
- Polat, S.; Uslu, M.K.; Aygün, A.; Certel, M. The effects of the addition of corn husk fibre, kaolin and beeswax on cross-linked corn starch foam. J. Food Eng. 2013, 116, 267–276. [Google Scholar] [CrossRef]
- Dai, H.; Chang, P.R.; Geng, F.; Yu, J.; Ma, X. Preparation and properties of thermoplastic starch/montmorillonite nanocomposite using N-(2-hydroxyethyl) formamide as a new additive. J. Polym. Environ. 2009, 17, 225–232. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Y.; Chen, Q.; Chen, D.; Lin, J.; Bai, X. Preparation of hydrophobic carboxymethyl starches and analysis of their properties as fluid loss additives in drilling fluids. Starch-Stärke 2017, 69, 1600153. [Google Scholar] [CrossRef]
- Kachel-Jakubowska, M.; Matwijczuk, A.; Gagoś, M. Analysis of the physicochemical properties of post-manufacturing waste derived from production of methyl esters from rapeseed oil. Int. Agrophys. 2017, 31, 175. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Matwijczuk, A.; Florek, M.; Barłowska, J.; Wolanciuk, A.; Matwijczuk, A.; Chruściel, E.; Walkowiak, R.; Karcz, D.; Gładyszewska, B. Application of FTIR spectroscopy for analysis of the quality of honey. In: BIO Web of Conferences. EDP Sci. 2018, 10, 02008. [Google Scholar] [CrossRef] [Green Version]
- Matwijczuk, A.; Oniszczuk, T.; Matwijczuk, A.; Chruściel, E.; Kocira, A.; Niemczynowicz, A.; Wójtowicz, A.; Combrzyński, M.; Wiącek, D. Use of FTIR spectroscopy and chemometrics with respect to storage conditions of Moldavian Dragonhead Oil. Sustainability 2019, 11, 6414. [Google Scholar] [CrossRef] [Green Version]
- Odiongenyi, A.O.; Essien, N.B.; Ukpe, R.A. Corn starch as a substitute for commercial food starch: FT-IR and rheological characterization. Int. J. Sci. Eng. Res. 2016, 3, 494–501. [Google Scholar]
- Basilio-Cortés, U.A.; González-Cruz, L.; Velazquez, G.; Teniente-Martínez, G.; Gómez-Aldapa, C.A.; Castro-Rosas, J.; Bernardino-Nicanor, B. Effect of dual modification on the spectroscopic, calorimetric, viscosimetric and morphological characteristics of corn starch. Polymers 2019, 11, 333. [Google Scholar] [CrossRef] [Green Version]
- Dankar, I.; Haddarah, A.; Omar, F.E.; Pujolà, M.; Sepulcre, F. Characterization of food additive-potato starch complexes by FTIR and X-ray diffraction. Food Chem. 2018, 260, 7–12. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Wu, X.; Chen, Q.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Effect of acetylation on the properties of corn starch. Food Chem. 2008, 106, 923–928. [Google Scholar] [CrossRef]
- Flores-Silva, P.C.; Roland-Cruz, C.A.; Chavez-Esquivel, G.; Vernon-Carter, E.J.; Bello-Perez, L.A.; Alvarez-Ramirez, J. In vitro digestibility of ultrasound-treated corn starch. Starch-Stärke 2017, 69, 1700040. [Google Scholar] [CrossRef]
- Pi-Xin, W.; Xiu-Li, W.; Xue, D.; Xu, K.; Tan, Y.; Du, X.; Li, W. Preparation and characterization of cationic corn starch with a high degree of substitution in dioxane–THF–water media. Carbohydr. Res. 2009, 344, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Sivam, A.S.; Sun-Waterhouse, D.; Perera, C.O.; Waterhouse, G.I.N. Exploring the interactions between blackcurrant polyphenols, pectin and wheat biopolymers in model breads; A FTIR and HPLC investigation. Food Chem. 2012, 131, 802–810. [Google Scholar] [CrossRef]
| Property | Fit Function for the Response Surface Model |
|---|---|
| Tensile strength (MPa) | 17.9994 + 0.14x − 0.0864y − 0.0024x2 − 0.0008xy + 0.0003y2 |
| Elongation at break (%) | 4.967 − 0.057x − 0.0283y − 6.9685 × 10−5x2 + 0.001xy + 2.1464 × 10−5y2 |
| Property | Fit Function for the Response Surface Model |
|---|---|
| Flexural strength (MPa) | 20.0532 − 0.1552x + 0.474y − 0.0093x2 + 0.0033xy − 0.0021y2 |
| Deformation at break (%) | 0.5338 − 0.026x − 0.0003y + 0.0003x2 + 0.0001xy − 7.1429 × 10−6y2 |
| Maximum Position (cm−1) | Types and Origin of Vibrations | |||
|---|---|---|---|---|
| K120 | K140 | K160 | K180 | |
| 3292 | 3295 | 3293 | 3287 | νst.(-OH) in the structure of cellulose and with H2O |
| 2923 | 2924 | 2922 | 2922 | νs+as (C-H) in CH2 |
| 2854 | 2855 | 2856 | 2855 | |
| 1740 | 1742 | 1743 | 1744 | νm (C=O) |
| 1644 | 1641 | 1645 | 1648 | ν (C=O) and δm (O-H) in cellulose or adsorbed H2O |
| 1538 | 1540 | 1538 | 1544 | νm (C-C) and δ(CH2) |
| 1456 | 1457 | 1456 | 1456 | |
| 1409 | 1410 | 1409 | 1409 | δ(CH2) |
| 1365 | 1367 | 1366 | 1367 | ν (C-H) + δ(C-OH) + ν (C-C) |
| 1331 | 1336 | 1331 | 1331 | |
| 1298 | 1297 | 1298 | 1298 | |
| 1250 | 1249 | 1249 | 1250 | ν (CH2) |
| 1206 | 1206 | 1207 | 1204 | |
| 1148 | 1149 | 1149 | 1148 | ν (C-O-C) entire ring in the structure of starch + ν (C-C) |
| 1103 | 1104 | 1104 | 1104 | ν (C-O-H) |
| 1078 | 1078 | 1078 | 1077 | |
| 1015 | 1015 | 1014 | 1015 | νst. (C-O-H) and νst. (C-O) |
| 995 | 993 | 995 | 996 | |
| 928 | 928 | 927 | 928 | ν (C-O) and νw (C-C) in the starch ring and CH2 in the ring and C-OH out of plane bending β-linkage of cellulose ν (C-O-C) + δ (C-O-C) |
| 854 | 852 | 853 | 853 | |
| 759 | 758 | 758 | 758 | |
| 703 | 702 | 703 | 703 | |
| 647 | 662 | 645 | 660 | ring breathing |
| 605 | 603 | 603 | 605 | |
| 571 | 569 | 570 | 569 | |
| 522 | 521 | 521 | 520 | |
| 477 | 479 | 480 | 480 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Combrzyński, M.; Wójtowicz, A.; Oniszczuk, A.; Karcz, D.; Szponar, J.; Matwijczuk, A.P. Selected Physical and Spectroscopic Properties of TPS Moldings Enriched with Durum Wheat Bran. Materials 2022, 15, 5061. https://doi.org/10.3390/ma15145061
Combrzyński M, Wójtowicz A, Oniszczuk A, Karcz D, Szponar J, Matwijczuk AP. Selected Physical and Spectroscopic Properties of TPS Moldings Enriched with Durum Wheat Bran. Materials. 2022; 15(14):5061. https://doi.org/10.3390/ma15145061
Chicago/Turabian StyleCombrzyński, Maciej, Agnieszka Wójtowicz, Anna Oniszczuk, Dariusz Karcz, Jarosław Szponar, and Arkadiusz P. Matwijczuk. 2022. "Selected Physical and Spectroscopic Properties of TPS Moldings Enriched with Durum Wheat Bran" Materials 15, no. 14: 5061. https://doi.org/10.3390/ma15145061






