Tensile Property and Corrosion Performance of Ag Microalloying of Al-Cu Alloys for Positive Electrode Current Collectors of Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
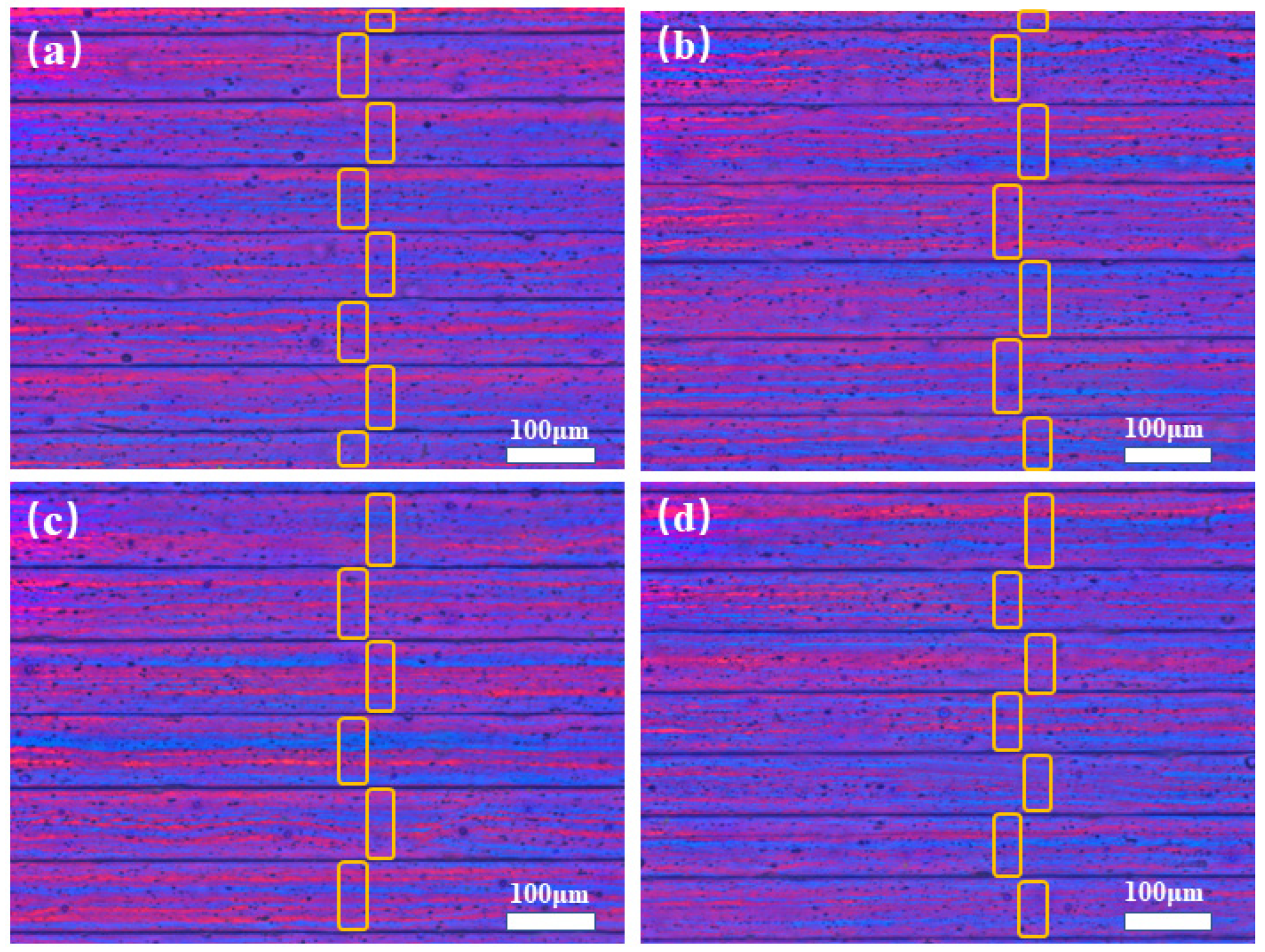
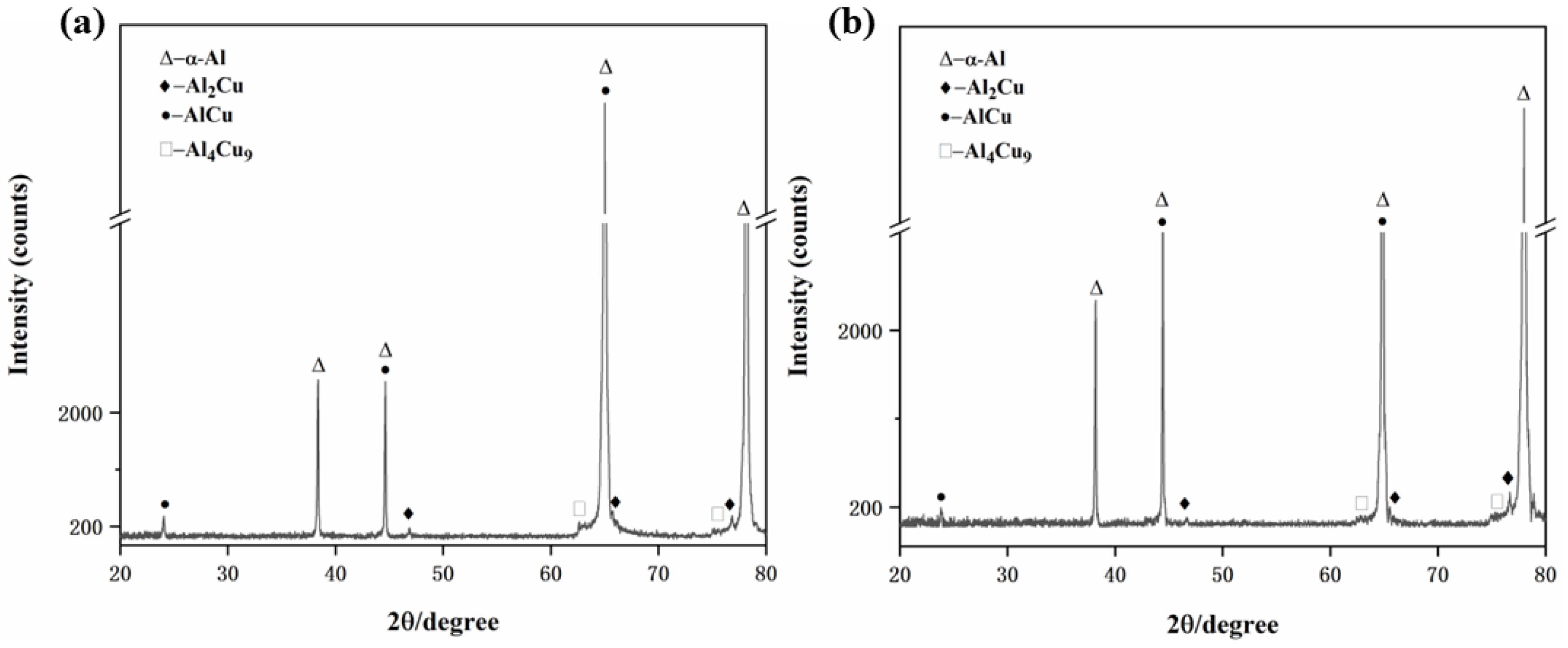
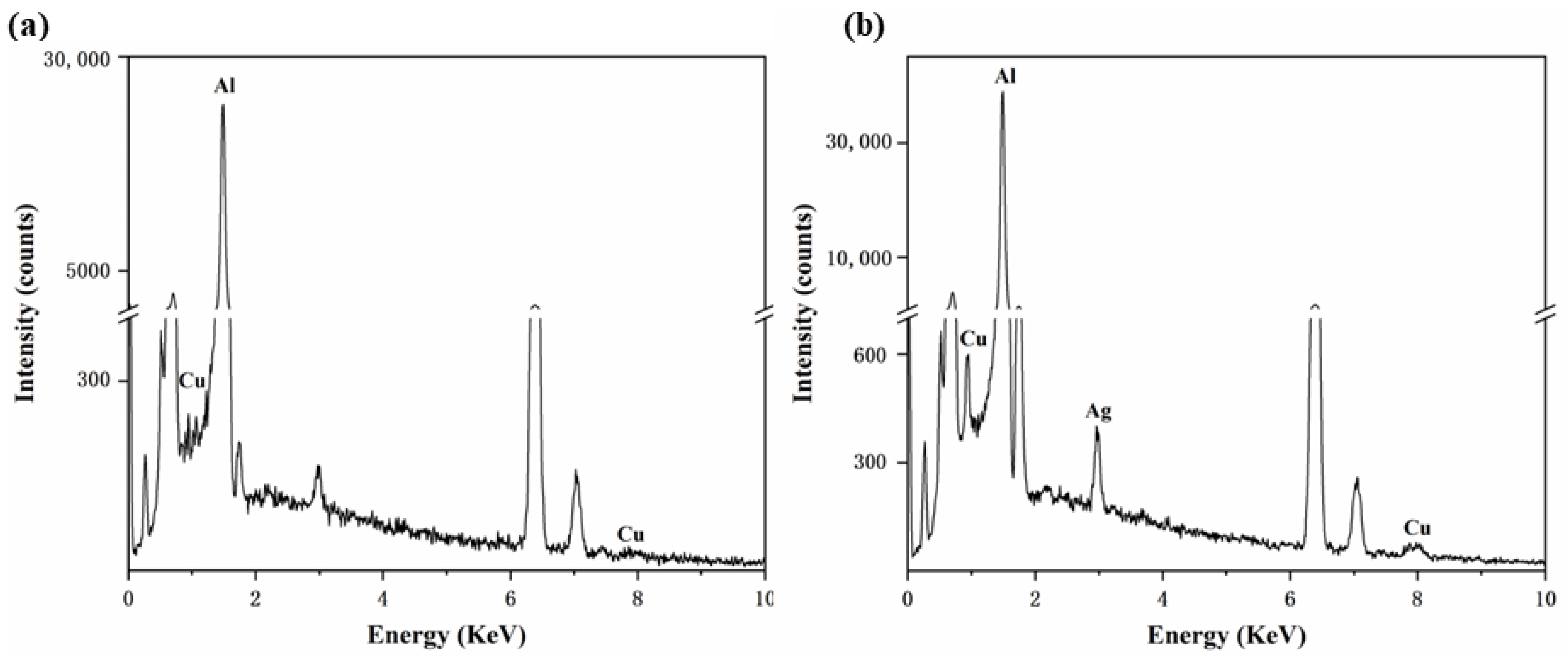
3.1. Microstructure
3.2. Mechanical Properties
3.3. Electrical Conductivity
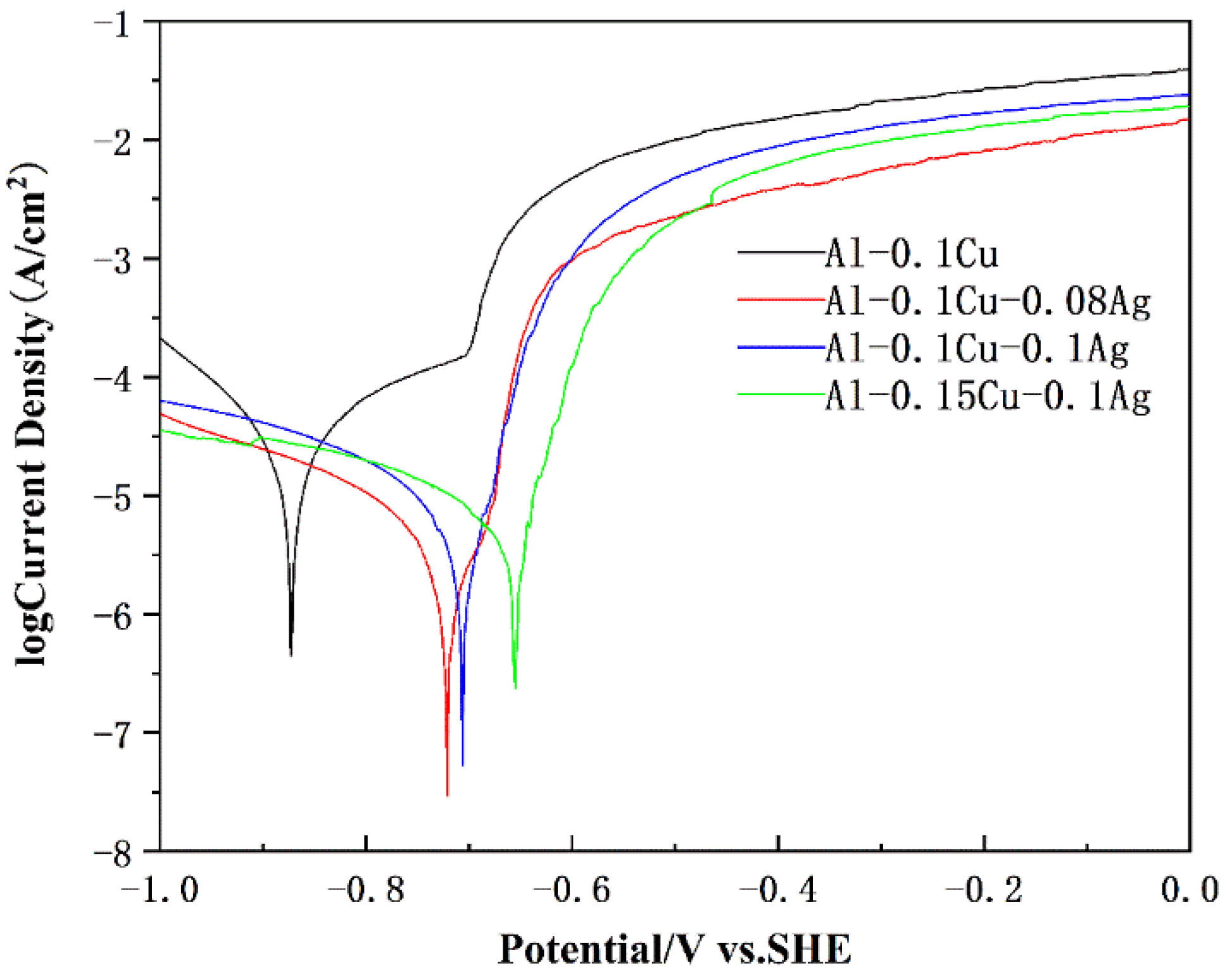
3.4. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrero, E.; Alessamdrini, S.; Balanzino, A. Impact of the electric vehicles on the air pollution from a highway. Appl. Energy 2016, 169, 450–459. [Google Scholar] [CrossRef]
- Armand, M.; Axmann, P.; Bresser, D. Lithium-ion batteries-Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Doughty, D.; Roth, E.P. A general discussion of Li-ion battery safety. Electrochem. Soc. Interface 2012, 21, 29–36. [Google Scholar]
- Huang, Y.; Tang, Y.; Yuan, W. Challenges and recent progress in thermal management with heat pipes for lithium-ion power batteries in electric vehicles. Sci. China Technol. Sci. 2021, 64, 919–956. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Tang, Z.; Guan, D.; Na, Z. Research on safety characteristics of high power lithium-ion batteries. J. Chem. Ind. Eng. 2005, 24, 1098–1102. [Google Scholar]
- Kai, L.; Liu, Y.Y.; Lin, D.C. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar]
- Chiang, H.L.; Hwu, C.S.; Chen, S.Y. Emission factors and characteristics of criteria pollutants and volatile organic compounds (VOCs) in a freeway tunnel study. Sci. Total Environ. 2007, 381, 200–211. [Google Scholar]
- Zhou, S.; Liu, G.; Ding, N.W. Improved performances of lithium-ion batteries by graphite-like carbon modified current collectors. Surf. Coat. Technol. 2020, 399, 126150. [Google Scholar] [CrossRef]
- Wang, C.C.; Lin, Y.C.; Chui, K.F. Advanced Carbon Cloth as Current Collector for Enhanced Electrochemical Performance of Lithium-Rich Layered Oxide Cathodes. Chem. Sel. 2017, 2, 4419–4427. [Google Scholar] [CrossRef]
- Myung, S.T.; Sasaki, Y.; Sakura Da, S. Electrochemical behavior and passivation of current collectors in lithium-ion batteries. J. Mater. Chem. A 2011, 21, 9891–9911. [Google Scholar] [CrossRef]
- Xu, Y.W.; Peng, Z.X.; Ding, D.Y.; Zhang, W.L.; Gao, Y.J.; Chen, G.Z.; Xie, Y.L.; Liao, Y.Q. Effect of La addition on microstructure and properties of Al-0.2Fe-0.06Cu alloy. Metals 2022, 12, 211. [Google Scholar] [CrossRef]
- Zhu, J.K.; Ding, D.Y.; Zhang, W.L.; Gao, Y.J.; Wu, Z.L.; Chen, G.Z.; Chen, R.Z.; Huang, Y.W.; Tang, J.S. Effect of La addition on the microstructure and properties of Al-Fe-Mn alloys for lithium battery current collectors. J. Electron. Mater. 2021, 50, 1032–1043. [Google Scholar] [CrossRef]
- Cassada, W.; Liu, J.; Staley, J. Aluminum alloys for aircraft structures. Adv. Mater. Processes 2022, 160, 27–29. [Google Scholar]
- Chen, Z.W.; Zhao, Y.N.; Zhang, Z. Theoretical and experimental study of precipitation and coarsening kinetics of θ’ phase in Al–Cu alloy. Vacuum 2021, 189, 110263. [Google Scholar] [CrossRef]
- Heinz, A.; Haszler, A.; Keidel, C.; Moldenhauer, S.; Benedictus, R.; Miller, W.S. Recent development in aluminium alloys for aerospace applications. Mater. Sci. Eng. A 2000, 280, 102–107. [Google Scholar] [CrossRef]
- Gao, Y.H.; Guan, P.F.; Su, R.; Chen, H.W.; Yang, C.; He, C.; Cao, L.F.; Song, H. Segregation-sandwiched stable interface suffocates nanoprecipitate coarsening to elevate creep resistance. Mater. Res. Lett. 2018, 8, 446–453. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y.C.; Zhang, X.C.; Jiang, Y.Q. Effects of two-stage creep-aging on precipitates of an Al–Cu–Mg alloy. Mater. Sci. Eng. A 2014, 614, 45–53. [Google Scholar] [CrossRef]
- Zhu, X.H.; Lin, Y.C.; Wu, Q.; Jiang, Y.Q. Effects of aging on precipitation behavior and mechanical properties of a tensile deformed Al–Cu alloy. J. Alloys Compd. 2020, 843, 155975. [Google Scholar] [CrossRef]
- Gao, Y.J.; Mo, Q.F.; Luo, Z.R.; Zhang, L.N.; Huang, C.G. Atomic bonding and properties of Al-Cu alloy with θ (Al2Cu). J. Electron. Mater. 2006, 35, 1801–1805. [Google Scholar] [CrossRef]
- He, Q.; Hui, Y.; Chen, L.; Fan, X.; Zhang, L. Study on the mechanical alloying process for preparing Ag/LSCO electrical contact material. Procedia Eng. 2014, 94, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Polmear, I.J. A trace element effect in alloys based on the aluminium-zinc-magnesium system. Nature 1960, 186, 303–304. [Google Scholar] [CrossRef]
- Jafarian, H.R.; Zeng, Y.; Mousavi, S.H.A.; Eivani, A.R. The effect of accumulative roll bonding on the precipitation behavior of a single step artificially aged Al-Ag-Sc alloy. Mater. Sci. Eng. A 2021, 823, 141769. [Google Scholar] [CrossRef]
- Senninger, O.; Peters, M.; Voorhees, P.W. Two-phase eutectic growth in Al-Cu and Al-Cu-Ag. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 1692–1707. [Google Scholar] [CrossRef]
- Genau, A.L.; Ratke, L. Crystal orientation and morphology in Al-Ag-Cu ternary eutectic. Mater. Sci. Eng. A 2012, 27, 012032. [Google Scholar] [CrossRef] [Green Version]
- Hecht, U.; Witusiewicz, V.; Drevermann, A.; Böttger, B.; Rex, S. Eutectic solidification of ternary Al-Cu-Ag alloys: Coupled growth of α(Al) and Al2Cu in univariant reaction. Mater. Sci. Forum. 2006, 508, 57–62. [Google Scholar] [CrossRef]
- Zhou, B.; Froyen, L. Microstructures of Al–Cu–Ag ternary eutectics under near-isothermal conditions. Trans. Indian Inst. Met. 2014, 67, 57–65. [Google Scholar] [CrossRef]
- Westphal, B.; Bockholt, H.; Günther, T.; Haselrieder, W.; Kwade, A. Influence of convective drying parameters on electrode performance and physical electrode properties. ECS Transcations 2015, 64, 57–68. [Google Scholar] [CrossRef]
- Han, L.N.; Sui, Y.D.; Wang, Q.D.; Wang, K.; Jiang, Y.H. Effects of Nd on microstructure and mechanical properties of cast Al-Si-Cu-Ni-Mg piston alloys. J. Alloys Compd. 2017, 695, 1566–1572. [Google Scholar] [CrossRef]
- Rosalie, J.M.; Bourgeois, L.; Muddle, B.C. Precipitation of the θ′(Al2Cu) phase in Al-Cu-Ag alloys. In Light Metals; Springer International Publishing: Berlin, Germany, 2012; pp. 307–312. [Google Scholar]
- Rosalie, J.M.; Bourgeois, L. Silver segregation to θ′ (Al2Cu)–Al interfaces in Al–Cu–Ag alloys. Acta Mater. 2012, 60, 6033–6041. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Wang, S.; Wu, Z.; Gao, W. Effect of trace silver on precipitation behavior of strengthening phases and mechanical properties of aluminum-copper alloys. JOM 2020, 72, 2957–2964. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, X.; Li, Y.; Zhou, Y. Influence of minor Ag addition on the microstructure and properties of powder metallurgy Cu-10 wt% Fe alloy. J. Alloys Compd. 2022, 904, 163983. [Google Scholar] [CrossRef]
- Miyake, J.; Fine, M.E. Electrical conductivity versus strength in a precipitation hardened alloy. Acta Metall. Mater. 1992, 40, 733–741. [Google Scholar] [CrossRef]
- Kino, T.; Endo, T.; Kawata, S. Deviations from matthiessen’s rule of the electrical resistivity of dislocations in aluminum. J. Phys. Soc. Jpn. 1974, 36, 698–705. [Google Scholar] [CrossRef]
- Sauvage, X.; Bobruk, E.V.; Murashkin, M.Y.; Nasedkina, Y.N.; Enikeev, A.R.; Valiev, Z. Optimization of electrical conductivity and strength combination by structure design at the nanoscale in Al-Mg-Si alloys. Acta Mater. 2015, 98, 355–366. [Google Scholar] [CrossRef]
- Wen, F.; Chen, J.Q.; Zhong, S.B.; Zhou, Z.X.; Han, S. Effect of crystal orientations and precipitates on the corrosion behavior of the Al-Cu alloy using single crystals. J. Alloys Compd. 2021, 890, 161858. [Google Scholar] [CrossRef]
- Yang, X.T.; Li, X.Q.; Yang, B.; Wei, H.L.; Fu, X.Y. Effects of WC on microstructure and corrosion resistance of directional structure Ni60 coatings. Surf. Coat. Technol. 2020, 385, 125359. [Google Scholar] [CrossRef]
- Zahavi, J.; Yahalom, J. Exfoliation corrosion of AlMgSi alloys in water. J. Electrochem. Soc. 1982, 129, 1181–1185. [Google Scholar] [CrossRef]

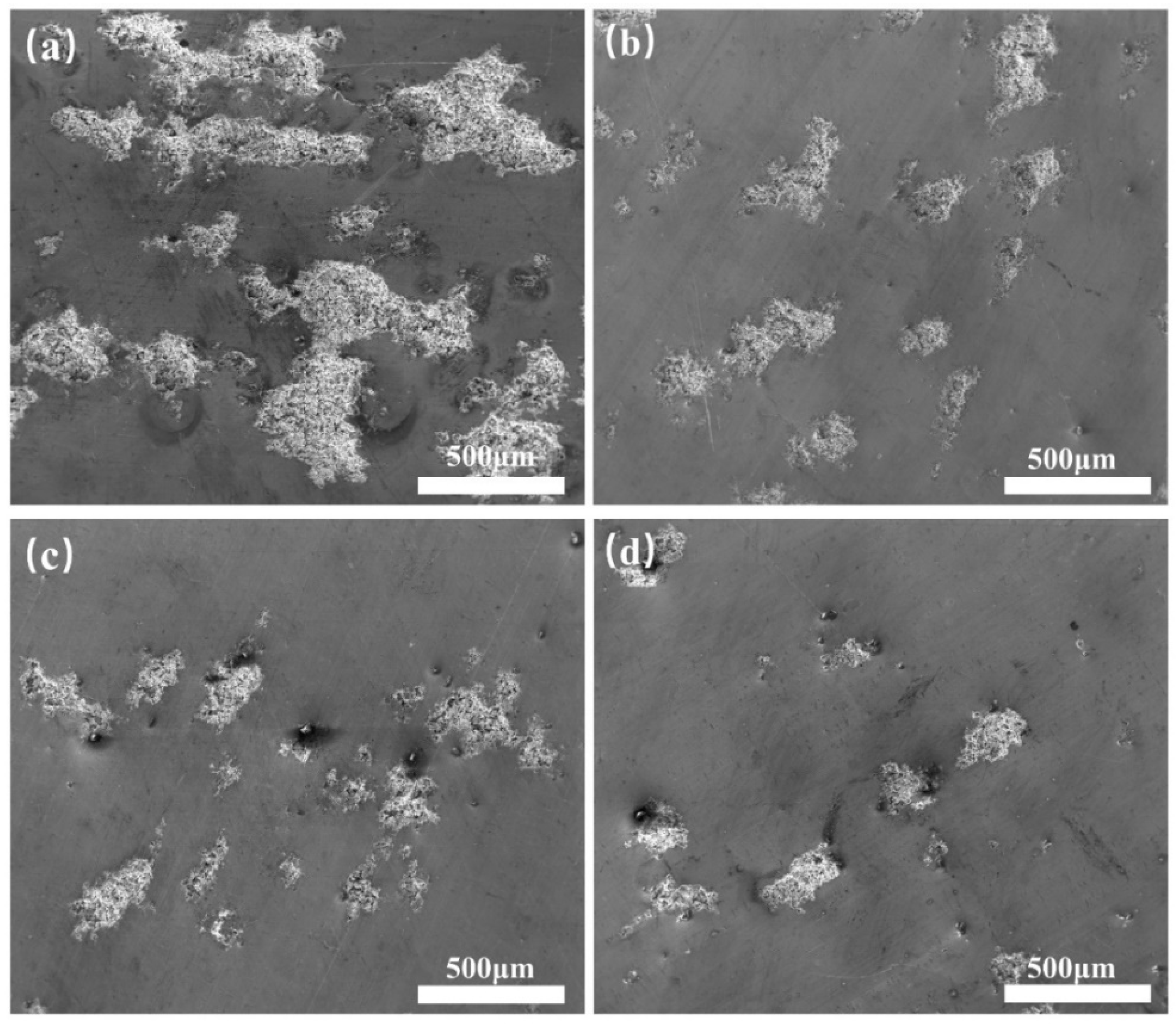
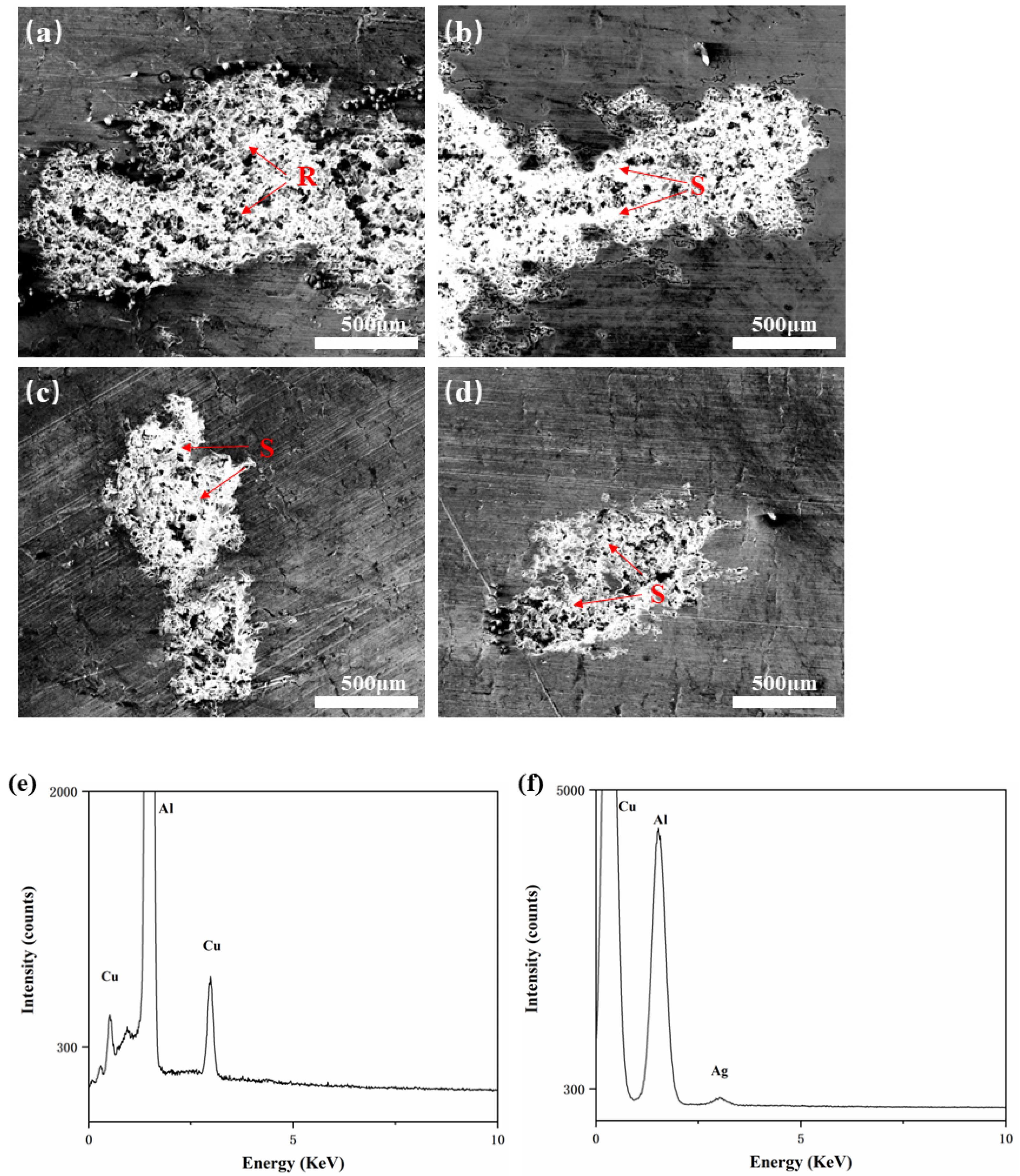
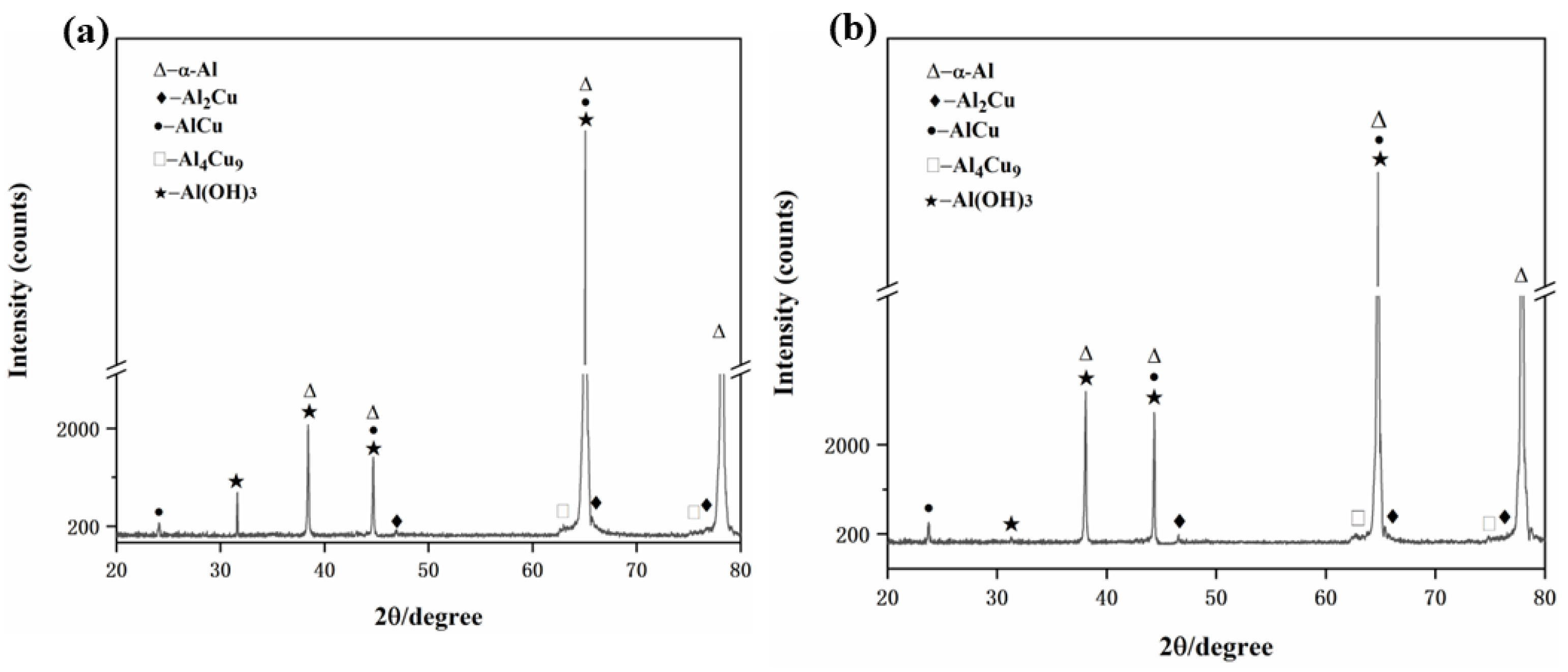
| Temperature | Alloy Composition |
σ0.2 (MPa) | σb (MPa) | δ (%) | |
|---|---|---|---|---|---|
| Base | Ag Addition | ||||
| 25 °C | Al-0.1Cu | 0 | 175 ± 4 | 189 ± 3 | 1.6 ± 0.1 |
| 0.08 | 170 ± 5 | 184 ± 5 | 1.4 ± 0.1 | ||
| 0.1 | 179 ± 3 | 196 ± 1 | 1.5 ± 0.1 | ||
| Al-0.15Cu | 0.1 | 185 ± 4 | 205 ± 4 | 1.3 ± 0.1 | |
| 60 °C | Al-0.1Cu | 0 | 156 ± 2 | 176 ± 3 | 1.7 ± 0.1 |
| 0.08 | 154 ± 2 | 172 ± 1 | 1.6 ± 0.1 | ||
| 0.1 | 158 ± 2 | 180 ± 2 | 1.6 ± 0.1 | ||
| Al-0.15Cu | 0.1 | 165 ± 3 | 179 ± 3 | 1.6 ± 0.1 | |
| Alloy | Electrical Conductivity (%IACS) |
|---|---|
| Al-0.1Cu | 58.43 ± 0.21 |
| Al-0.1Cu-0.08Ag | 58.12 ± 0.17 |
| Al-0.1Cu-0.1Ag | 57.94 ± 0.12 |
| Al-0.15Cu-0.1Ag | 58.41 ± 0.19 |
| Alloys Composition (wt.%) | Ecorr (mV vs. SCE) | Icorr (μA/cm2) |
|---|---|---|
| Al-0.1Cu | −873 ± 7 | 37.12 |
| Al-0.1Cu-0.08Ag | −721 ± 5 | 2.62 |
| Al-0.1Cu-0.1Ag | −706 ± 5 | 5.49 |
| Al-0.15Cu-0.1Ag | −655 ± 6 | 5.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Ding, D.; Zhang, W.; Gao, Y.; Chen, G.; Xie, Y.; Liao, Y. Tensile Property and Corrosion Performance of Ag Microalloying of Al-Cu Alloys for Positive Electrode Current Collectors of Li-Ion Batteries. Materials 2022, 15, 5126. https://doi.org/10.3390/ma15155126
Peng Z, Ding D, Zhang W, Gao Y, Chen G, Xie Y, Liao Y. Tensile Property and Corrosion Performance of Ag Microalloying of Al-Cu Alloys for Positive Electrode Current Collectors of Li-Ion Batteries. Materials. 2022; 15(15):5126. https://doi.org/10.3390/ma15155126
Chicago/Turabian StylePeng, Zixuan, Dongyan Ding, Wenlong Zhang, Yongjin Gao, Guozhen Chen, Yonglin Xie, and Yongqi Liao. 2022. "Tensile Property and Corrosion Performance of Ag Microalloying of Al-Cu Alloys for Positive Electrode Current Collectors of Li-Ion Batteries" Materials 15, no. 15: 5126. https://doi.org/10.3390/ma15155126
APA StylePeng, Z., Ding, D., Zhang, W., Gao, Y., Chen, G., Xie, Y., & Liao, Y. (2022). Tensile Property and Corrosion Performance of Ag Microalloying of Al-Cu Alloys for Positive Electrode Current Collectors of Li-Ion Batteries. Materials, 15(15), 5126. https://doi.org/10.3390/ma15155126






