Ligand-Tuned Multi-Color Luminescence of Single Aluminum (III) Ion Atomic Centers and Their Selective Sensitivity to Different Metal Ions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis
2.3. Characterization
2.4. Smart Sensing to Different Metal Ions
2.5. Density Functional Theory (DFT) Calculations
3. Results and Discussion
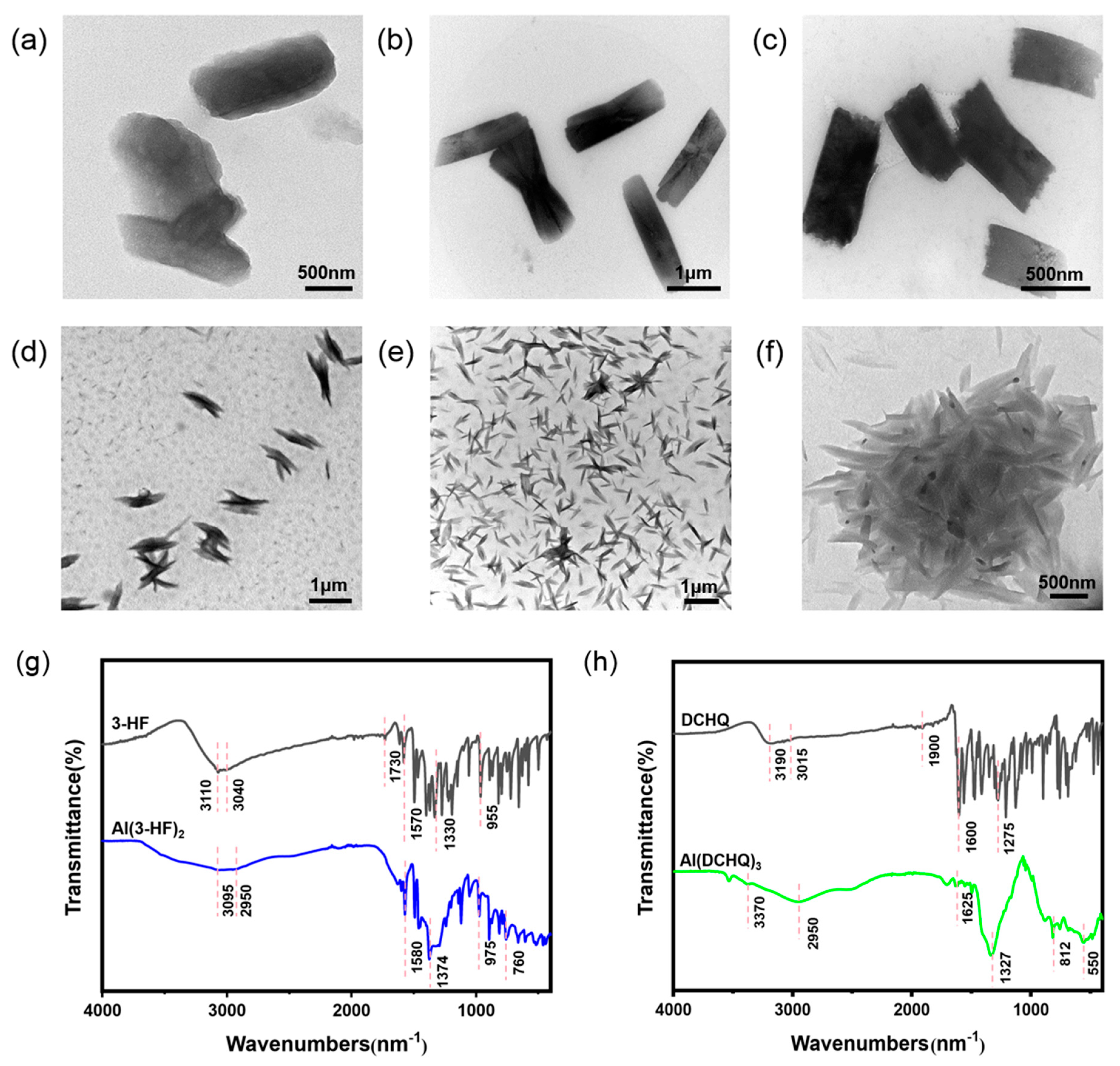
3.1. Complex Structure of Different Organic Ligands
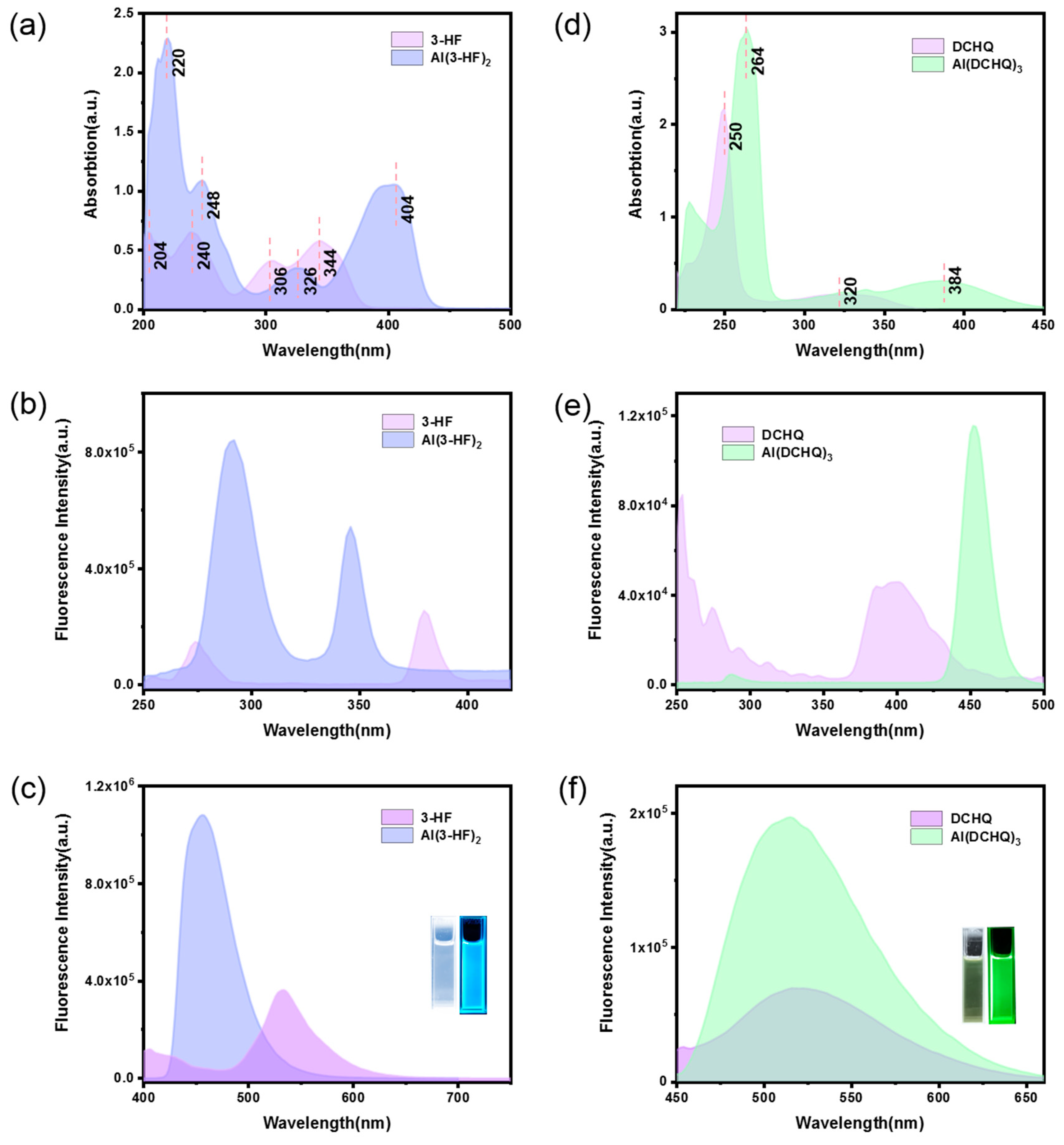
3.2. Photophysical Properties of Al(3-HF)2 and Al(DCHQ)3
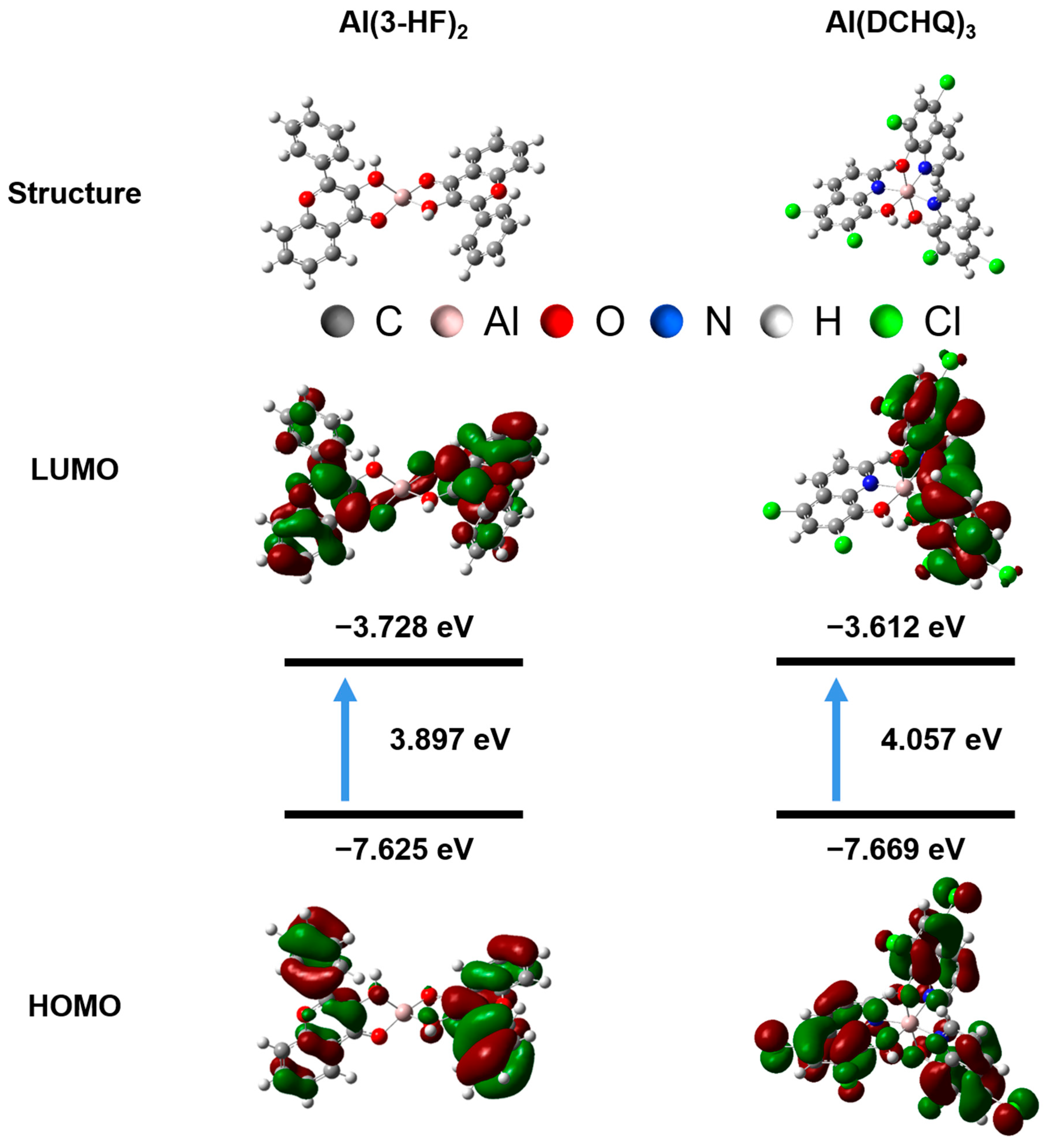
3.3. Density Functional Theory Calculation
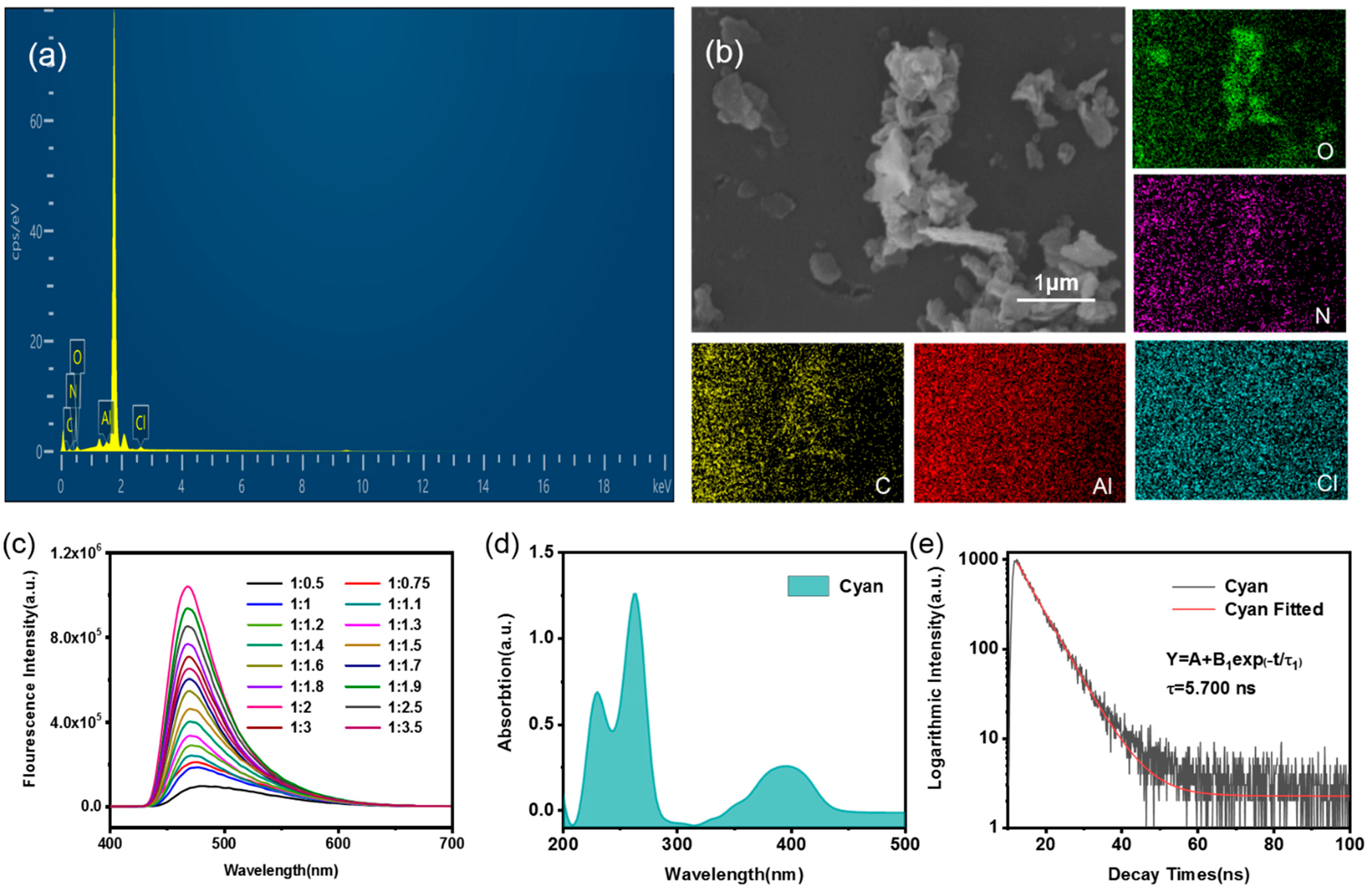
3.4. Cyan Emission Tuning
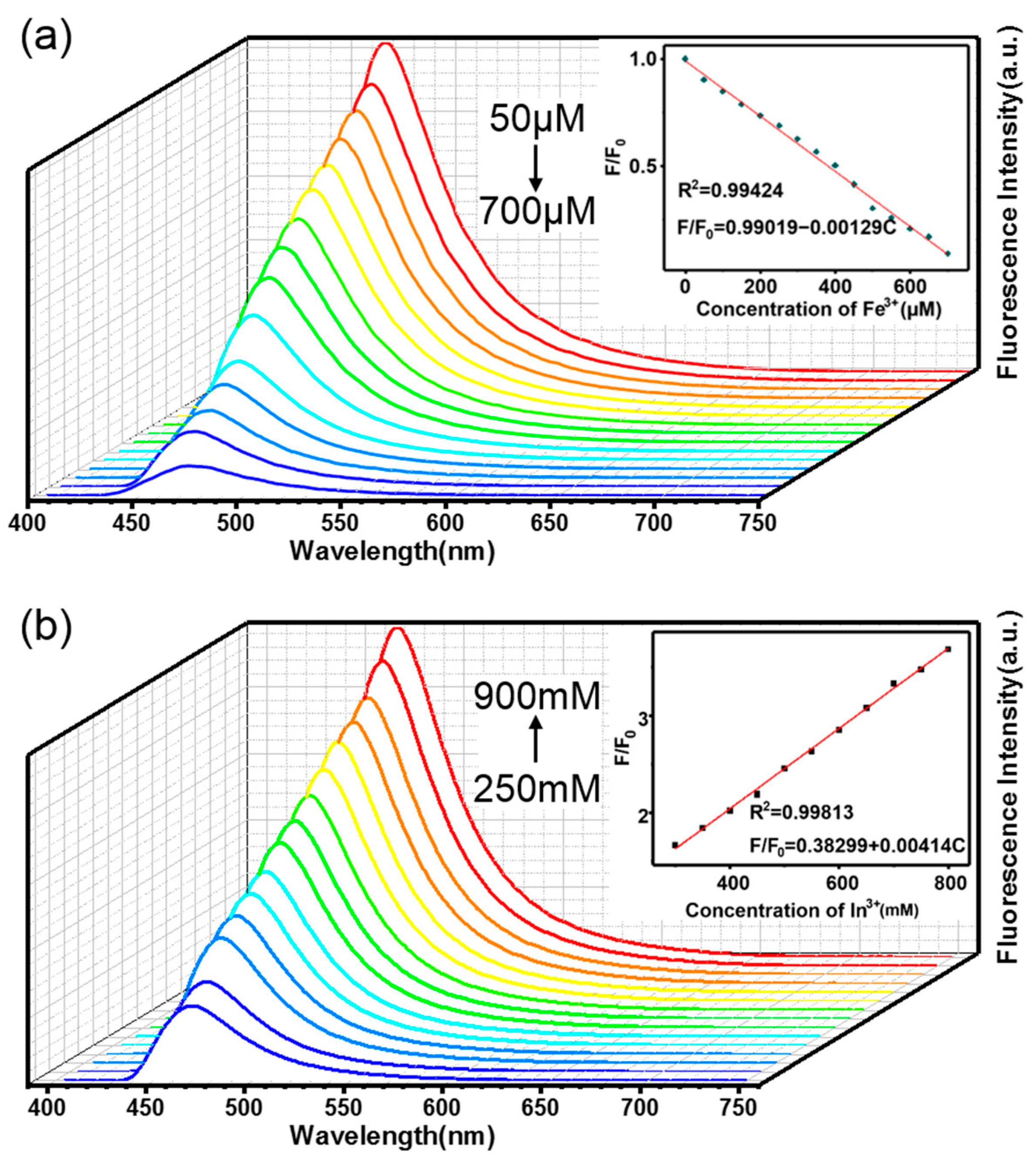
3.5. Selective Sensitivity to Different Metal Ions with Either Strong Enhancement or Quenching Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Wong, K.M.-C. Photophysical, ion-sensing and biological properties of rhodamine-containing transition metal complexes. Coord. Chem. Rev. 2020, 416, 213336. [Google Scholar] [CrossRef]
- Gupta, D.; Gaur, A.K.; Chauhan, D.; Thakur, S.K.; Jeyapalan, V.; Singh, S.; Rajaraman, G.; Venkataramani, S. Solid-state photochromic arylazopyrazole-based transition metal complexes. Inorg. Chem. Front. 2022, 9, 2315–2327. [Google Scholar] [CrossRef]
- Singh, D.; Bhagwan, S.; Saini, R.K.; Tanwar, V.; Nishal, V. Optoelectronic Properties of Color-Tunable Mixed Ligand-Based Light-Emitting Zinc Complexes. J. Electron. Mater. 2016, 45, 4865–4874. [Google Scholar] [CrossRef]
- Kim, H.S.; Angupillai, S.; Son, Y.A. A dual chemosensor for both Cu2+ and Al3+: A potential Cu2+ and Al3+ switched YES logic function with an INHIBIT logic gate and a novel solid sensor for detection and extraction of Al3+ ions from aqueous solution. Sens. Actuators B-Chem. 2016, 222, 447–458. [Google Scholar] [CrossRef]
- Ramos, M.L.; Justino, L.L.G.; Salvador, A.I.N.; de Sousa, A.R.E.; Abreu, P.E.; Fonseca, S.M.; Burrows, H.D. NMR, DFT and luminescence studies of the complexation of Al(iii) with 8-hydroxyquinoline-5-sulfonate. Dalton Trans. 2012, 41, 12478–12489. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Jiang, X.; Li, W.-W.; Shi, Y.; Lai, S.; Zhuang, J.; Yao, S.; Liu, Y.; Hu, J.; Gao, L.; et al. Proanthocyanidin–Aluminum Complexes Improve Aluminum Resistance and Detoxification ofCamellia sinensis. J. Agric. Food Chem. 2020, 68, 7861–7869. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Jiang, X.H.; Wang, B.D.; Yang, Z.Y. 4-(8’-Hydroxyquinolin-7’-yl)methyleneimino-l-phenyl-2,3-dimethyl-5-pyzole as a fluorescent chemosensor for aluminum ion in acid aqueous medium. Sens. Actuators B-Chem. 2014, 205, 249–254. [Google Scholar] [CrossRef]
- Song, H.; Liu, G.; Fan, C.; Pu, S. A novel fluorescent sensor for Al3+ and Zn2+ based on a new europium complex with a 1,10-phenanthroline ligand. J. Rare Earths 2021, 39, 460–468. [Google Scholar] [CrossRef]
- Myers, T.W.; Kazem, N.; Stoll, S.; Britt, R.D.; Shanmugam, M.; Berben, L.A. A Redox Series of Aluminum Complexes: Characterization of Four Oxidation States Including a Ligand Biradical State Stabilized via Exchange Coupling. J. Am. Chem. Soc. 2011, 133, 8662–8672. [Google Scholar] [CrossRef]
- Armstrong, A.; Reber, A.C.; Khanna, S.N. Multiple-Valence Aluminum and the Electronic and Geometric Structure of AlnOm Clusters. J. Phys. Chem. A 2019, 123, 5114–5121. [Google Scholar] [CrossRef]
- Petrova, P.K.; Tomova, R.L.; Stoycheva-Topalova, R.T.; Kaloyanova, S.S.; Deligeorgiev, T.G. Mixed-ligand Al complex—A new approach for more high efficient OLEDs. J. Lumin. 2012, 132, 495–501. [Google Scholar] [CrossRef]
- Jeong, Y.; Yoon, J. Recent progress on fluorescent chemosensors for metal ions. Inorg. Chim. Acta 2012, 381, 2–14. [Google Scholar] [CrossRef]
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Ramos, M.L.; Justino, L.L.G.; Barata, R.; Costa, T.; Burrows, H.D. Complexes of In(iii) with 8-hydroxyquinoline-5-sulfonate in solution: Structural studies and the effect of cationic surfactants on the photophysical behaviour. Dalton Trans. 2021, 50, 16970–16983. [Google Scholar] [CrossRef]
- Wang, T.; Yu, X.; Li, Z.; Wang, J.; Li, H. Multi-colored luminescent light-harvesting hybrids based on aminoclay and lanthanide complexes. Rsc Adv. 2015, 5, 11570–11576. [Google Scholar] [CrossRef]
- Iannazzo, D.; Espro, C.; Ferlazzo, A.; Celesti, C.; Branca, C.; Neri, G. Electrochemical and Fluorescent Properties of Crown Ether Functionalized Graphene Quantum Dots for Potassium and Sodium Ions Detection. Nanomaterials 2021, 11, 2897. [Google Scholar] [CrossRef]
- Kuai, Y.; Li, W.; Dong, Y.; Wong, W.-Y.; Yan, S.; Dai, Y.; Zhang, C. Multi-color electrochromism from coordination nanosheets based on a terpyridine-Fe(ii) complex. Dalton Trans. 2019, 48, 15121–15126. [Google Scholar] [CrossRef]
- Dou, H.J.; Niu, G.H.; Gu, M.M.; Masabni, J.G. Effects of Light Quality on Growth and Phytonutrient Accumulation of Herbs under Controlled Environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Devakumar, B.; Liang, J.; Wang, S.; Sun, Q.; Huang, X. A broadband cyan-emitting Ca2LuZr2(AlO4)3:Ce3+ garnet phosphor for near-ultraviolet-pumped warm-white light-emitting diodes with an improved color rendering index. J. Mater. Chem. C 2020, 8, 1095–1103. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, Z.; Molokeev, M.S.; Atuchin, V.V.; Liu, Q. Blue-shift of Eu2+ emission in (Ba,Sr)3Lu(PO4)3:Eu2+ eulytite solid-solution phosphors resulting from release of neighbouring-cation-induced stress. Dalton Trans. 2014, 43, 16800–16804. [Google Scholar] [CrossRef]
- Davila, Y.A.; Sancho, M.I.; Almandoz, M.C.; Blanco, S.E. Structural and spectroscopic study of Al(III)-3-hydroxyflavone complex: Determination of the stability constants in water-methanol mixtures. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2012, 95, 1–7. [Google Scholar] [CrossRef]
- Dimitrić Marković, J.M.; Marković, Z.S.; Veselinović, D.S.; Krstić, J.B.; Predojević Simović, J.D. Study on fisetin–aluminium(III) interaction in aqueous buffered solutions by spectroscopy and molecular modeling. J. Inorg. Biochem. 2009, 103, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhang, W.; Lei, Q.; Men, J. Preparation and characterization of 8-hydroxyquinoline-functionalized polysulfone and preliminary study on luminescence property of its complex with Al(III). Macromol. Res. 2012, 21, 599–607. [Google Scholar] [CrossRef]
- Shen, T.F.; Sun, Y.J. Preparation and Characterization of Novel Bipolar Copolymers Based on Quinoline Aluminum and Carbazole. Adv. Mater. Res. 2013, 785, 656–659. [Google Scholar] [CrossRef]
- Badiei, A.; Goldooz, H.; Ziarani, G.M. A novel method for preparation of 8-hydroxyquinoline functionalized mesoporous silica: Aluminum complexes and photoluminescence studies. Appl. Surf. Sci. 2011, 257, 4912–4918. [Google Scholar] [CrossRef]
- He, X.; Xiong, W.; Zhang, L.; Xu, C.; Fan, J.; Qian, Y.; Wen, J.; Ding, F.; Shen, J. ESIPT-based ratiometric fluorescent probe for highly selective and sensitive sensing and bioimaging of group IIIA ions in living cancer cells and zebrafish. Dye. Pigment. 2020, 174, 108059. [Google Scholar] [CrossRef]
- Coe, B.J.; Harris, J.A.; Brunschwig, B.S.; Asselberghs, I.; Clays, K.; Garín, J.; Orduna, J. Three-dimensional nonlinear optical chromophores based on metal-to-ligand charge-transfer from ruthenium (II) or iron (II) centers. J. Am. Chem. Soc. 2005, 127, 13399–13410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Kindy, S.M.Z.; Al-Hinai, K.H.; Suliman, F.E.O.; Al-Lawati, H.J.; Pillay, A. Development of a selective fluorimetric technique for rapid trace determination of zinc using 3-hydroxyflavone. Arab. J. Chem. 2011, 4, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.-Y.; Han, J.Y.; Im, W.B.; Cheong, S.H.; Jeon, D.Y. Novel Blue-Emitting NaxCa1-xAl2-xSi2+xO8:Eu2+ (x=0.34) Phosphor with High Luminescent Efficiency for UV-Pumped Light-Emitting Diodes. Inorg. Chem. 2012, 51, 10688–10694. [Google Scholar] [CrossRef]
- Yu, T.; Su, W.; Li, W.; Hong, Z.; Hua, R.; Li, M.; Chu, B.; Li, B.; Zhang, Z.; Hu, Z.Z. Synthesis, crystal structure and electroluminescent properties of a Schiff base zinc complex. Inorg. Chim. Acta 2006, 359, 2246–2251. [Google Scholar] [CrossRef]
- Wilson, C.A.; Gies, P.H.; Niven, B.E.; McLennan, A.; Bevin, N.K. The relationship between UV transmittance and color—Visual description and instrumental measurement. Text. Res. J. 2008, 78, 128–137. [Google Scholar] [CrossRef]
- Perez-Bolivar, C.; Montes, V.A.; Anzenbacher, P. True blue: Blue-emitting aluminum(III) quinolinolate complexes. Inorg. Chem. 2006, 45, 9610–9612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.E.; Wang, Y.; Yan, H.; Lu, J.; Liu, H.; Li, Y.; Wang, S.; Li, D.; Dou, J.; Yang, L.; et al. Multiresponsive Luminescent Sensitivities of a 3D Cd-CP with Visual Turn-on and Ratiometric Sensing toward Al3+ and Cr3+ as Well as Turn-off Sensing toward Fe3+. Inorg. Chem. 2020, 59, 3828–3837. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiao, X.; Hu, M.; Wang, Y.; Wang, Y.; Yan, X.; Huang, Z.; Servati, P.; Huang, L.; Tang, J. Mn, B, N co-doped graphene quantum dots for fluorescence sensing and biological imaging. Arab. J. Chem. 2022, 15, 103856. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Li, L.; Tang, Q.; Liu, J.; Wang, Y.; Wang, J.; Kipper, M.J.; Xie, H.; Belfiore, L.A.; Tang, J. Ligand-Tuned Multi-Color Luminescence of Single Aluminum (III) Ion Atomic Centers and Their Selective Sensitivity to Different Metal Ions. Materials 2022, 15, 5199. https://doi.org/10.3390/ma15155199
Wang Q, Li L, Tang Q, Liu J, Wang Y, Wang J, Kipper MJ, Xie H, Belfiore LA, Tang J. Ligand-Tuned Multi-Color Luminescence of Single Aluminum (III) Ion Atomic Centers and Their Selective Sensitivity to Different Metal Ions. Materials. 2022; 15(15):5199. https://doi.org/10.3390/ma15155199
Chicago/Turabian StyleWang, Qian, Longlong Li, Qinglin Tang, Jin Liu, Yao Wang, Jiuxing Wang, Matt J. Kipper, Haijiao Xie, Laurence A. Belfiore, and Jianguo Tang. 2022. "Ligand-Tuned Multi-Color Luminescence of Single Aluminum (III) Ion Atomic Centers and Their Selective Sensitivity to Different Metal Ions" Materials 15, no. 15: 5199. https://doi.org/10.3390/ma15155199





