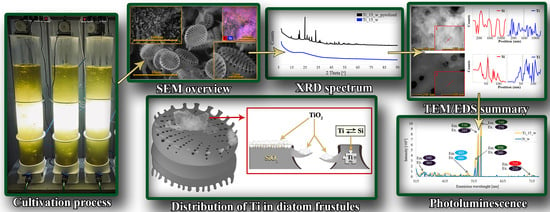
Metabolically Doping of 3D Diatomaceous Biosilica with Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diatom Cultivation Process
2.2. Purification of Diatom Frustules
2.3. Instrumental Methods
3. Results and Discussion
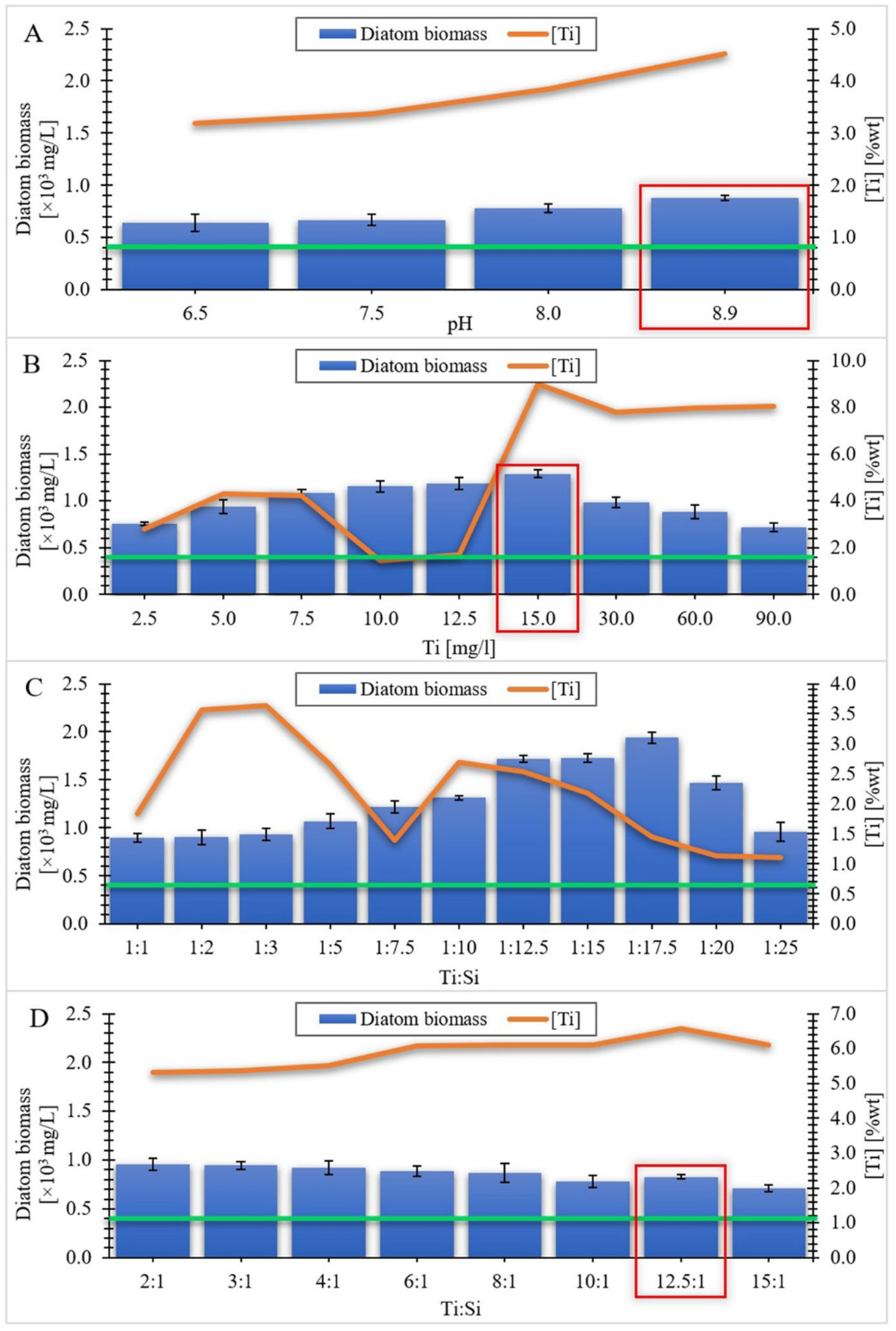
3.1. Equilibrium Study of the Titanium Uptake by Diatom Cells. Effect of pH Value, Initial Ti Concentration and Ti:Si Ratio in the Culture Medium
3.1.1. Series I
3.1.2. Series II
3.1.3. Series III
3.1.4. Series IV
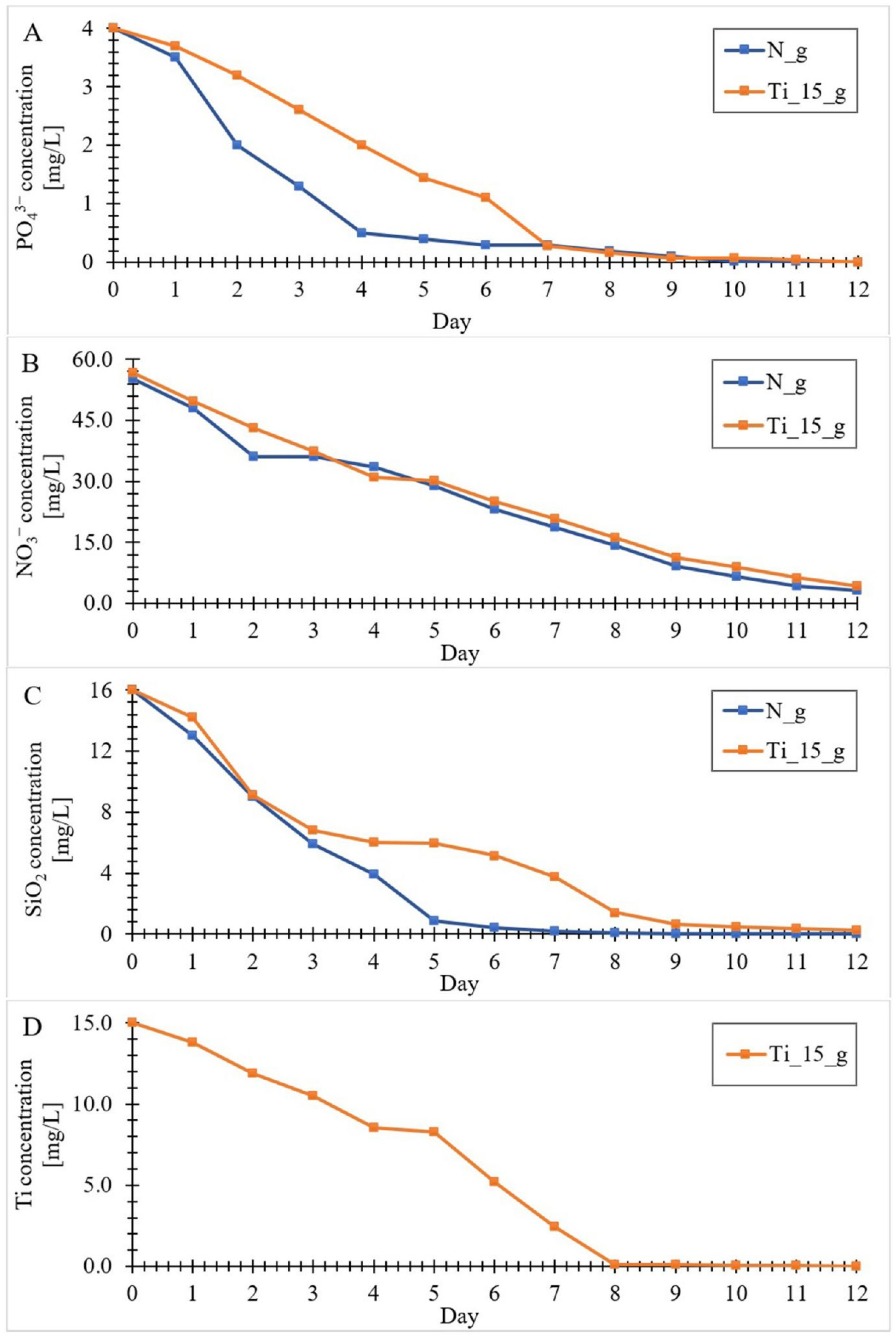
3.2. Kinetics of the Components (Si, N, P and Ti) Uptake by Diatom Cells from the Culture Medium
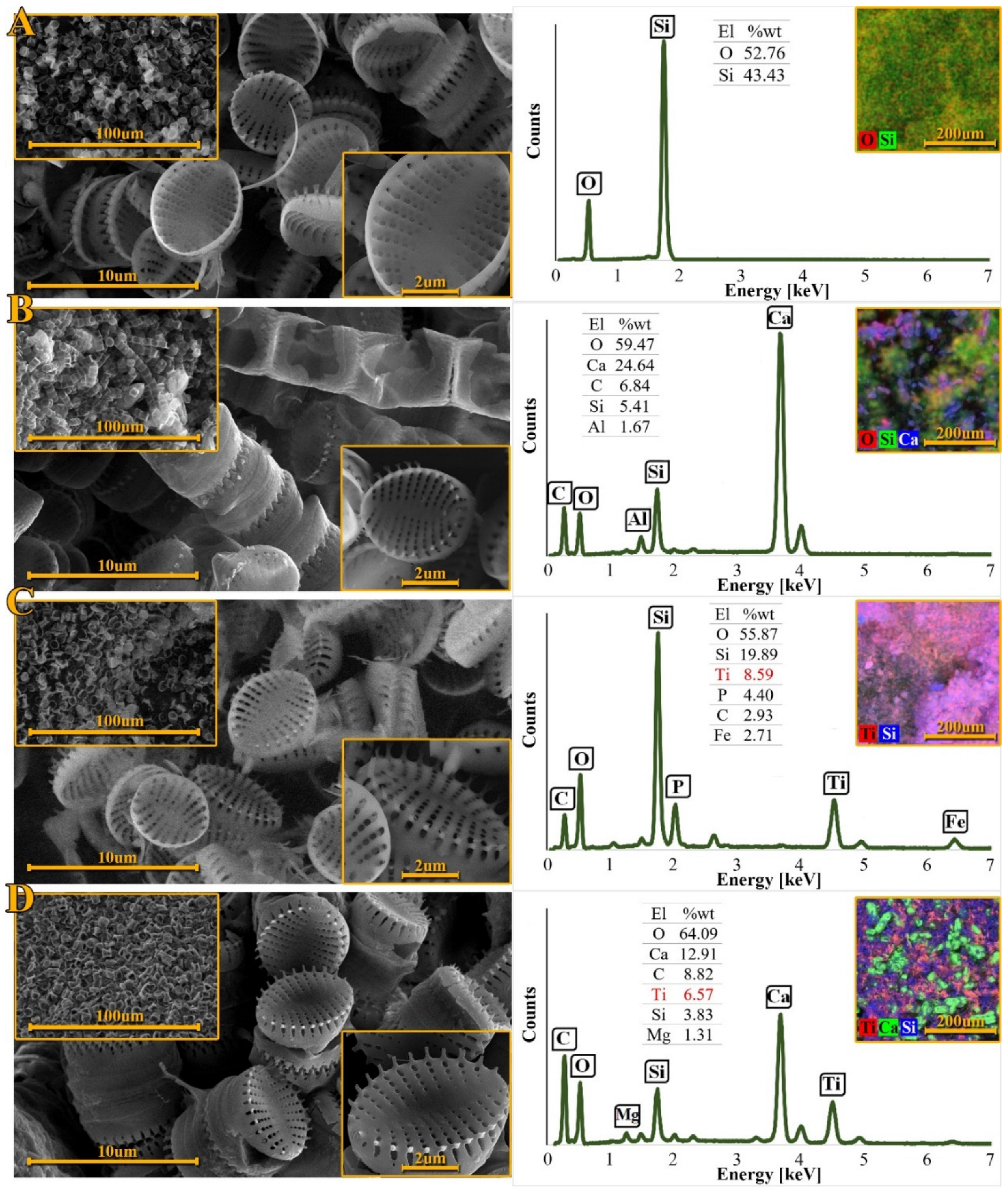
3.3. Scanning Electron Microscopy Analysis
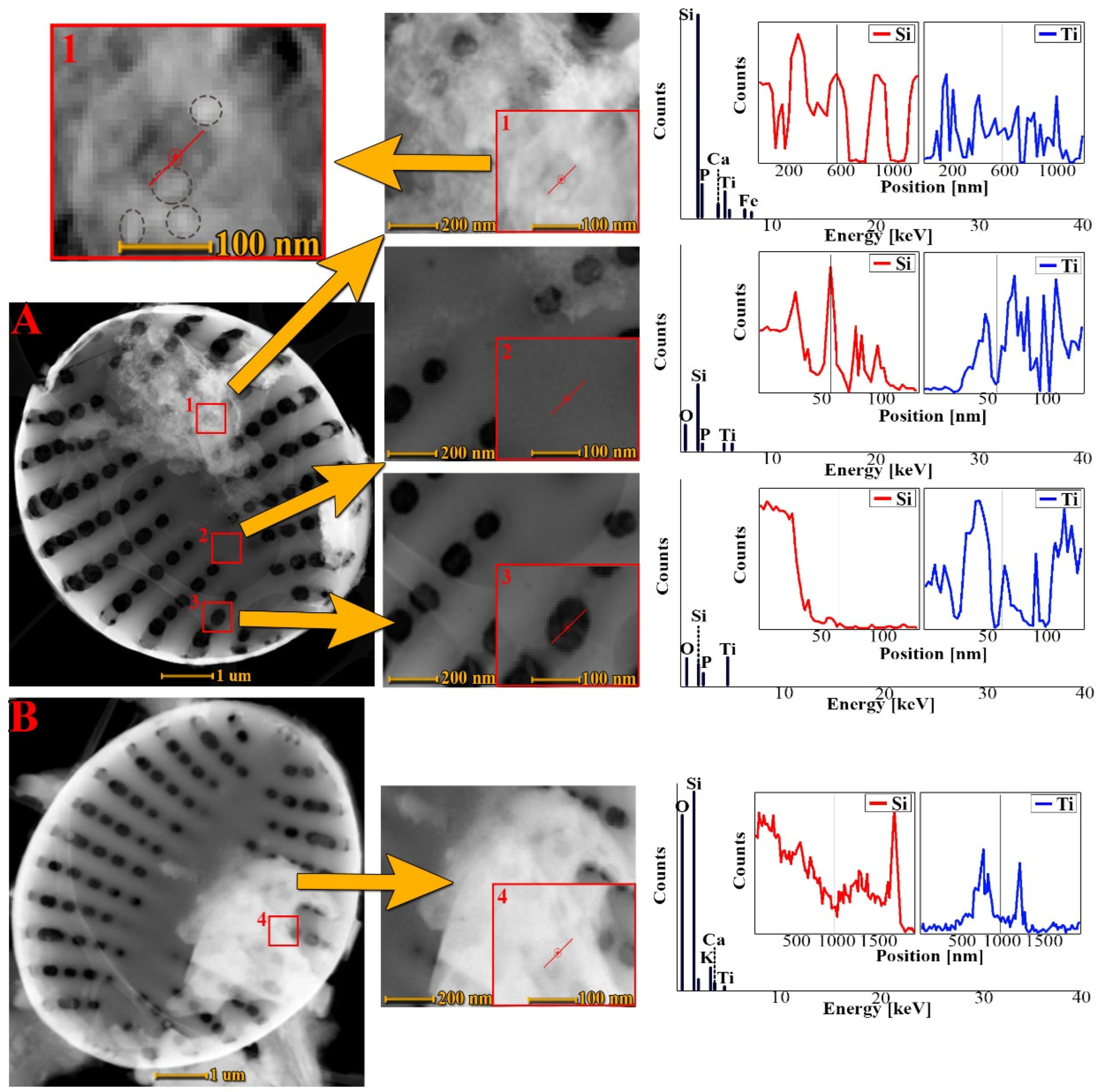
3.4. Transmission Electron Microscopy Analysis
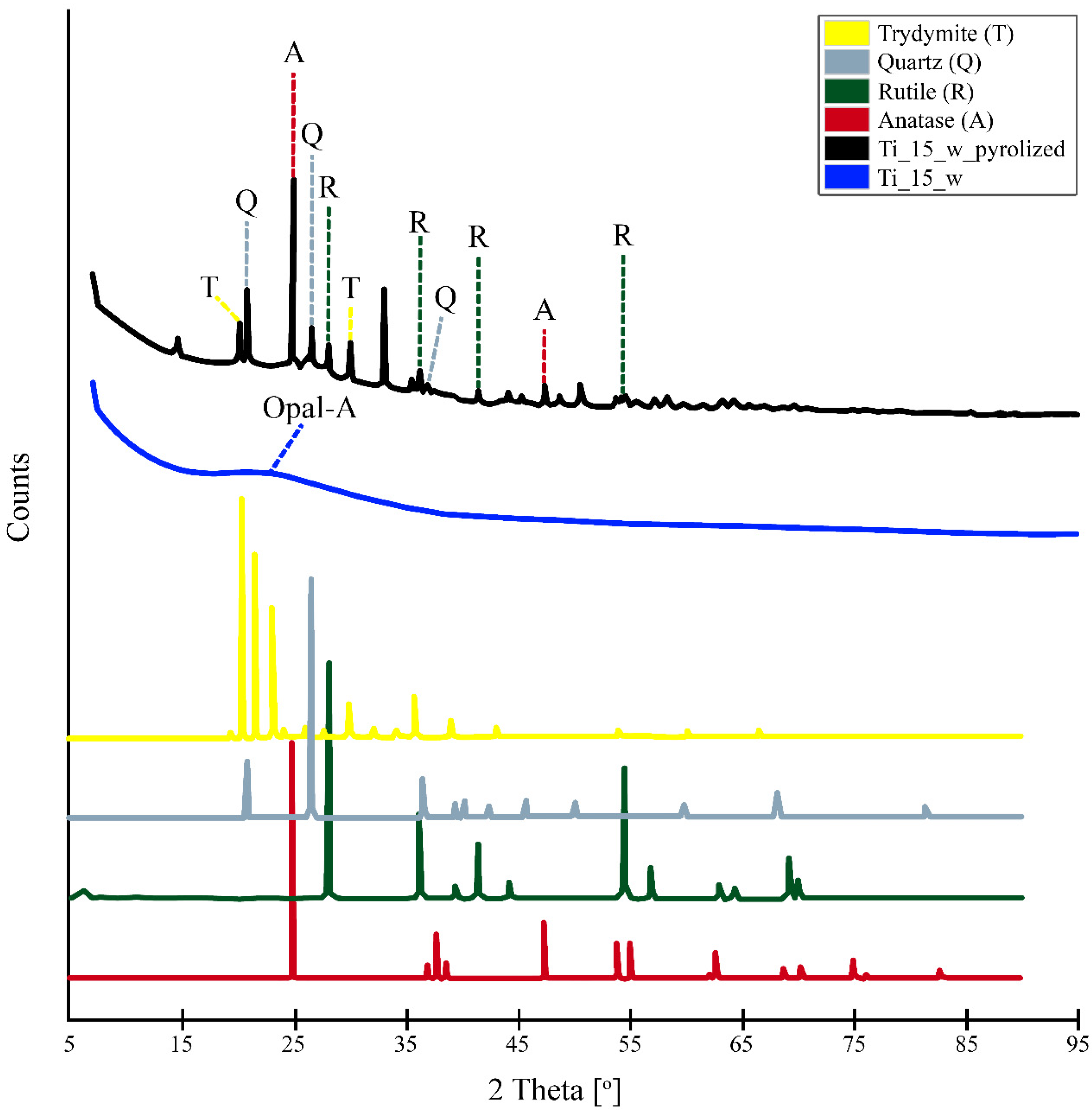
3.5. X-ray Powder Diffraction Patterns
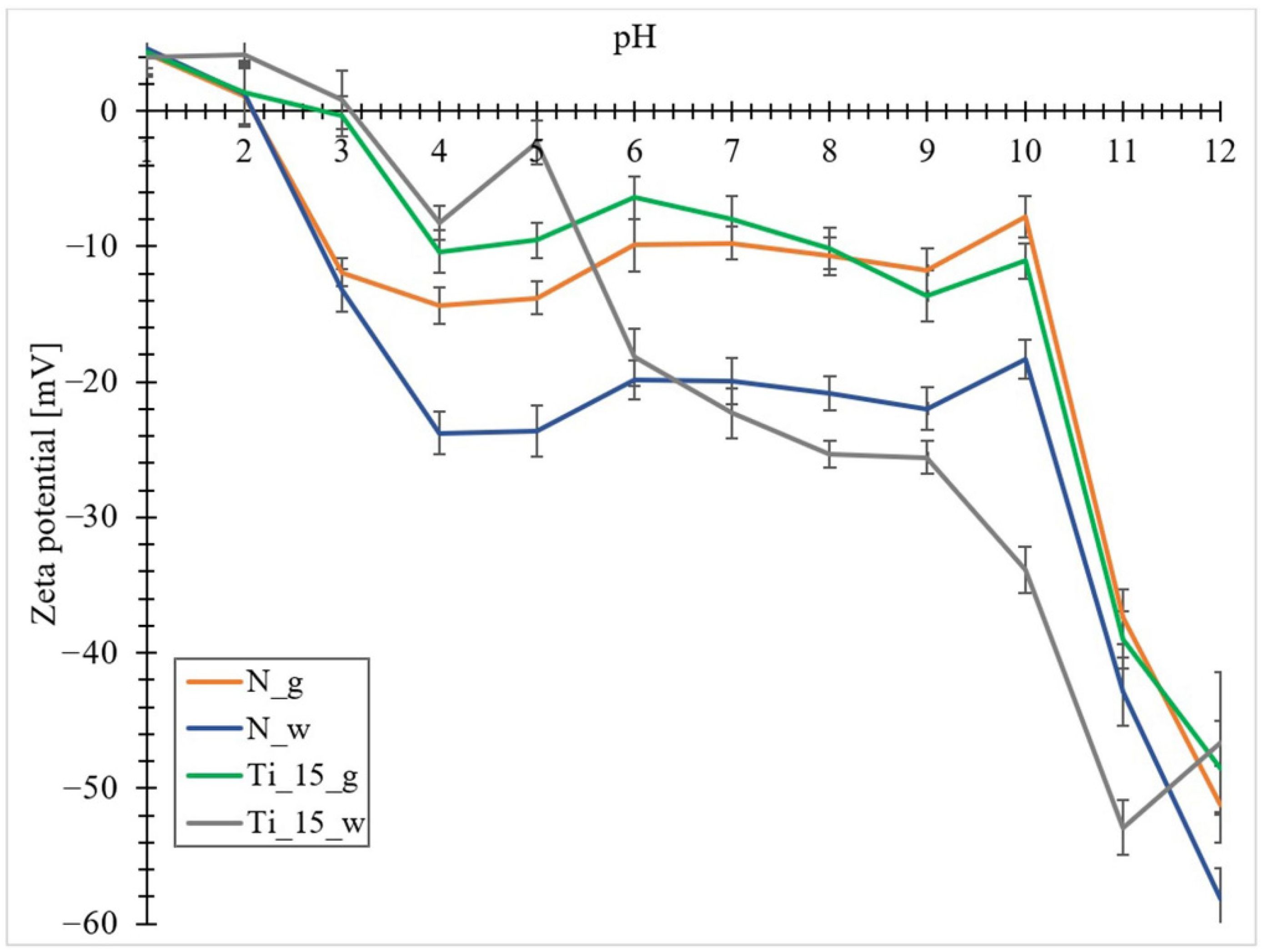
3.6. Zeta Potential Measurements
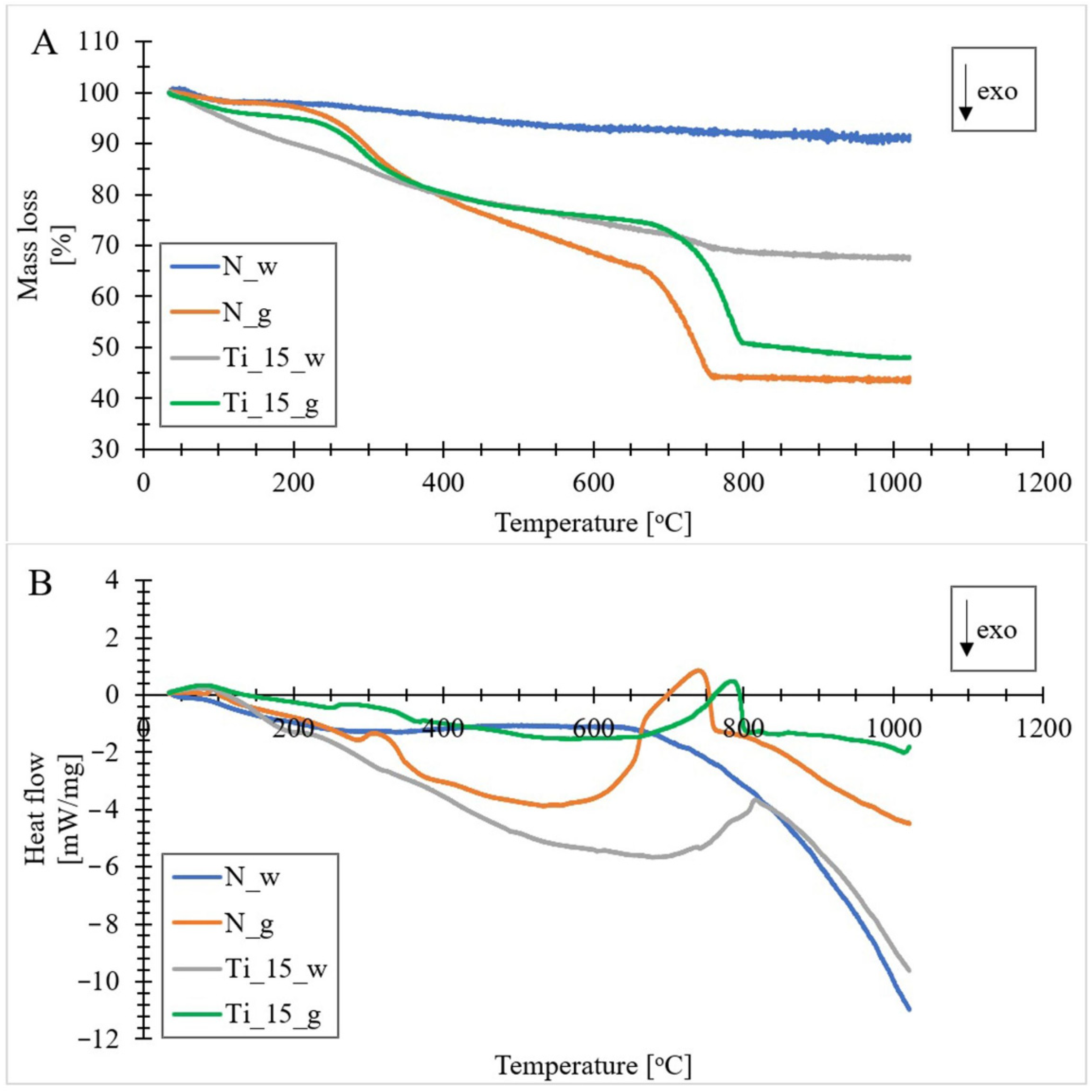
3.7. Thermogravimetric Analysis
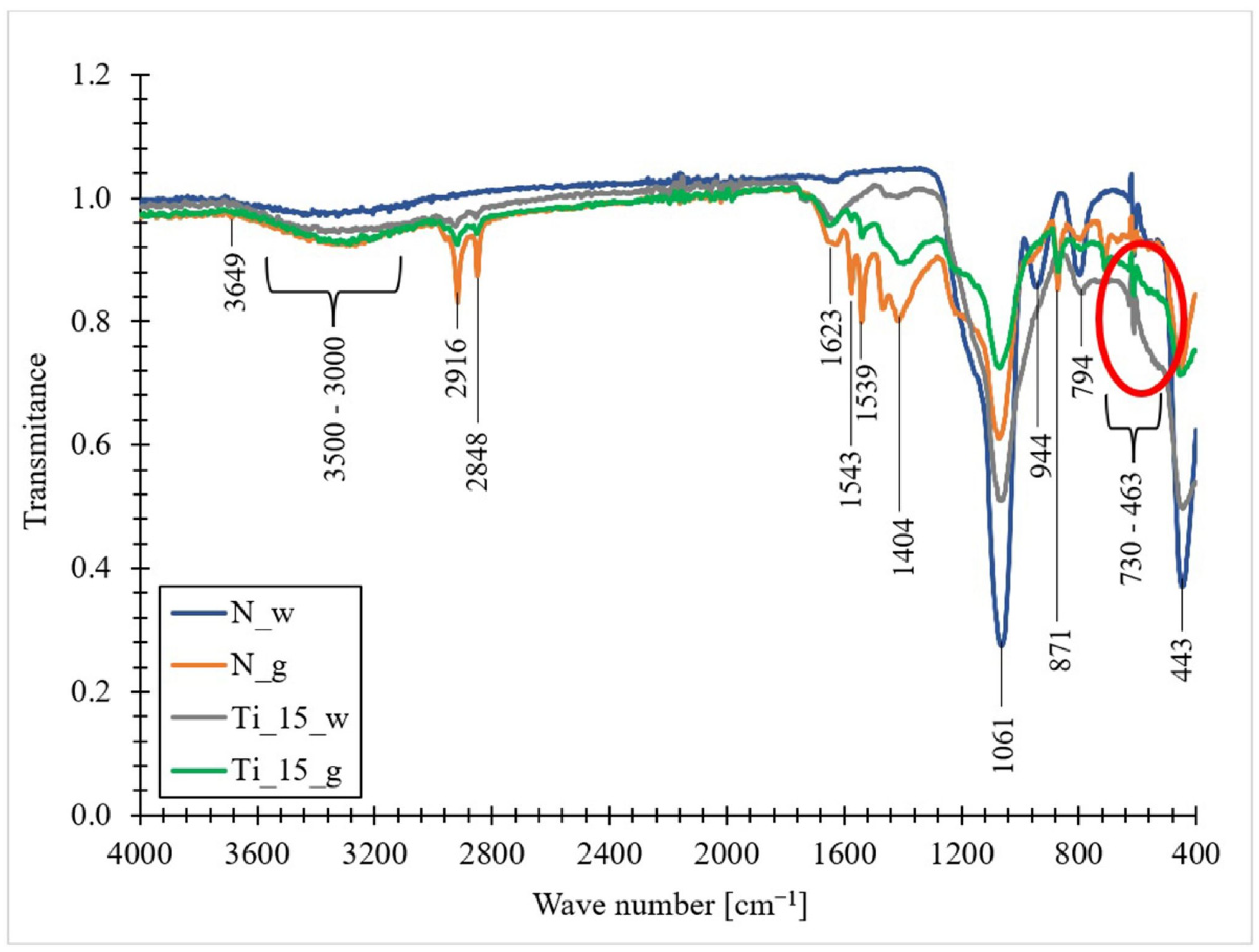
3.8. The FT-IR Spectra
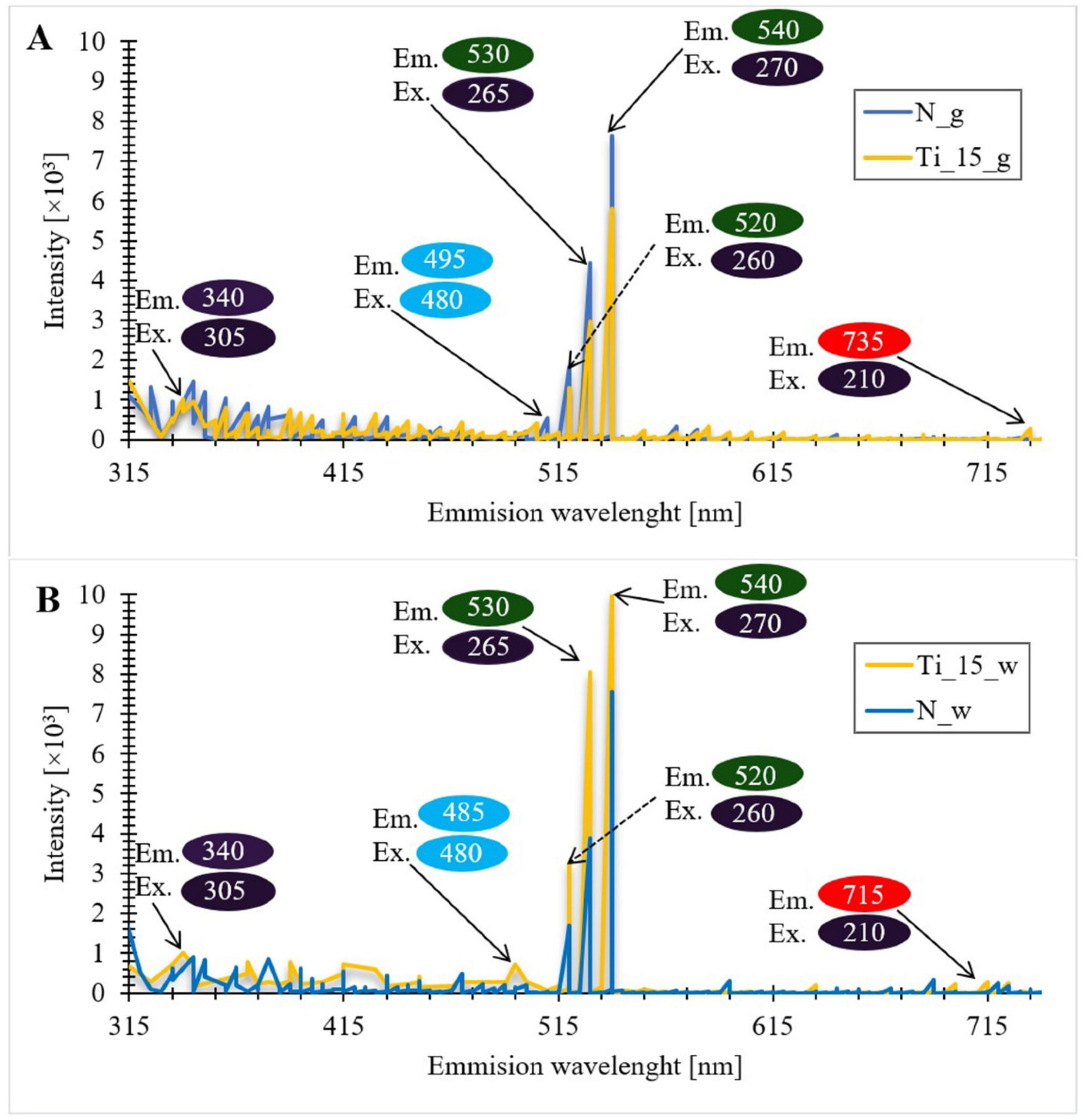
3.9. Photoluminescence Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diab, M.; Mokari, T. Bioinspired Hierarchical Porous Structures for Engineering Advanced Functional Inorganic Materials. Adv. Mater. 2018, 30, 1706349. [Google Scholar] [CrossRef] [PubMed]
- Vukusic, P.; Sambles, J.R. Photonic structures in biology. Nature 2003, 424, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.; Losic, D.; Tiffany, M.A.; Nagy, S.S.; Sterrenburg, F.A.S. The Glass Menagerie: Diatoms for novel applications in nanotechnology. Trends Biotechnol. 2009, 27, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Nassif, N.; Livage, J. From diatoms to silica-based biohybrids. Chem. Soc. Rev. 2011, 40, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Basharina, T.N.; Danilovtseva, E.N.; Zelinskiy, S.N.; Klimenkov, I.V.; Likhoshway, Y.V.; Annenkov, V.V. The Effect of Titanium, Zirconium and Tin on the Growth of Diatom Synedra Acus and Morphology of Its Silica Valves. Silicon 2012, 4, 239–249. [Google Scholar] [CrossRef]
- Dai, Z.; Tong, J.; Ren, L. Researches and developments of biomimetics in tribology. Chin. Sci. Bull. 2006, 51, 2681–2689. [Google Scholar]
- Zhang, D.Y.; Wang, Y.; Cai, J.; Pan, J.F.; Jiang, X.G.; Jiang, Y.G. Bio-manufacturing technology based on diatom micro- and nanostructure. Chin. Sci. Bull. 2012, 57, 3836–3849. [Google Scholar] [CrossRef] [Green Version]
- Gordon, R.; Aguda, B.D. Diatom morphogenesis: Natural fractal fabrication of a complex microstructure. IEEE/Eng. Med. Biol. Soc. Annu. Conf. 1988, 10, 273–274. [Google Scholar] [CrossRef]
- Drum, R.W.; Gordon, R. Star Trek replicators and diatom nanotechnology. Trends Biotechnol. 2003, 21, 325–328. [Google Scholar]
- Naik, R.R.; Stone, M.O. Integrating biomimetics. Mater. Today 2005, 8, 18–26. [Google Scholar] [CrossRef]
- Lopez, P.J.; Desclés, J.; Allen, A.E.; Bowler, C. Prospects in diatom research. Curr. Opin. Biotechnol. 2005, 16, 180–186. [Google Scholar] [CrossRef]
- Parker, A.R.; Townley, H.E. Biomimetics of photonic nanostructures. Nat. Nanotechnol. 2007, 2, 347–353. [Google Scholar] [CrossRef]
- Gebeshuber, I.C. Biotribology inspires new technologies. Nano Today 2007, 2, 30–37. [Google Scholar] [CrossRef]
- Halas, N.J. Nanoscience under Glass: The Versatile Chemistry of Silica Nanostructures. ACS Nano 2008, 2, 179–183. [Google Scholar] [CrossRef]
- Losic, D.; Mitchell, J.G.; Voelcker, N.H. Diatomaceous lessons in nanotechnology and advanced materials. Adv. Mater. 2009, 21, 2947–2958. [Google Scholar] [CrossRef]
- Mishra, M.; Arukha, A.P.; Bashir, T.; Yadav, D.; Prasad, G.B.K.S. All New Faces of Diatoms: Potential Source of Nanomaterials and Beyond. Front. Microbiol. 2017, 8, 1239. [Google Scholar] [CrossRef] [Green Version]
- Sterrenburg, F.A.S. Crystal palaces-Diatoms for engineers. J. Nanosci. Nanotechnol. 2005, 5, 100–107. [Google Scholar] [CrossRef]
- De Tommasi, E.; Gielis, J.; Rogato, A. Diatom Frustule Morphogenesis and Function: A Multidisciplinary Survey. Mar. Genom. 2017, 35, 1–18. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Brahmkhatri, V.; Sriram, G.; Jung, H.Y.; Yu, J.; Kurkuri, N.; Aminabhavi, T.M.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Nature engineered diatom biosilica as drug delivery systems. J. Control. Release 2018, 281, 70–83. [Google Scholar] [CrossRef]
- Nowak, A.P.; Sprynskyy, M.; Brzozowska, W.; Lisowska-Oleksiak, A. Electrochemical behavior of a composite material containing 3D-structured diatom biosilica. Algal Res. 2019, 41, 101538. [Google Scholar] [CrossRef]
- Fuhrmann, T.; Landwehr, S.; El Rharbl-Kucki, M.; Sumper, M. Diatoms as living photonic crystals. Appl. Phys. B Lasers Opt. 2004, 78, 257–260. [Google Scholar] [CrossRef]
- Soleimani, M.; Rutten, L.; Maddala, S.P.; Wu, H.; Eren, E.D.; Mezari, B.; Schreur-Piet, I.; Friedrich, H.; van Benthem, R.A.T.M. Modifying the thickness, pore size, and composition of diatom frustule in Pinnularia sp. with Al3+ ions. Sci. Rep. 2020, 10, 19498. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, W.; Sprynskyy, M.; Wojtczak, I.; Dabek, P.; Witkowski, A.; Buszewski, B. “Outsourcing” diatoms in fabrication of metal-doped 3D biosilica. Materials 2020, 13, 2576. [Google Scholar] [CrossRef] [PubMed]
- Chauton, M.S.; Skolem, L.M.B.; Olsen, L.M.; Vullum, P.E.; Walmsley, J.; Vadstein, O. Titanium uptake and incorporation into silica nanostructures by the diatom Pinnularia sp. (Bacillariophyceae). J. Appl. Phycol. 2015, 27, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Maeda, Y.; Niwa, Y.; Tang, H.; Kisailus, D.; Yoshino, T.; Tanaka, T. Development of Titania-Integrated Silica Cell Walls of the Titanium-Resistant Diatom, Fistulifera solaris. ACS Appl. Bio Mater. 2018, 1, 2021–2029. [Google Scholar] [CrossRef]
- Skolem, L.M.B. Biosynthesis and Characterization of Ti- Doped Silica-Based Nanostructures formed by the Diatoms Pinnularia sp. and Coscinodiscus wailesii; Norwegian University of Science and Technology: Trondheim, Norway, 2011; Volume 801. [Google Scholar]
- Kulacki, K.J.; Cardinale, B.J. Effects of Nano-Titanium Dioxide on Freshwater Algal Population Dynamics. PLoS ONE 2012, 7, e47130. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.L.; Kuang, M.; Li, F.; Liu, X.Y.; Zhang, Y.X.; Dong, F.; Losic, D. Engineering of three dimensional (3-D) diatom@TiO2@MnO2 composites with enhanced supercapacitor performance. Electrochim. Acta 2016, 190, 159–167. [Google Scholar] [CrossRef]
- He, J.; Chen, D.; Li, Y.; Shao, J.; Xie, J.; Sun, Y.; Yan, Z.; Wang, J. Diatom-templated TiO2 with enhanced photocatalytic activity: Biomimetics of photonic crystals. Appl. Phys. A Mater. Sci. Process. 2013, 113, 327–332. [Google Scholar] [CrossRef]
- Lang, Y.; Monte, F.D.; Rodriguez, B.J.; Dockery, P.; Finn, D.P.; Pandit, A. Integration of TiO2 into the diatom Thalassiosira weissflogii during frustule synthesis. Sci. Rep. 2013, 3, 3205. [Google Scholar] [CrossRef] [Green Version]
- Jeffryes, C.; Gutu, T.; Jiao, J.; Rorrer, G.L. Metabolic Insertion of Nanostructured TiO2 into the Patterned Biosilica of the Diatom Pinnularia sp. by a Two-Stage Bioreactor Cultivation Process. ACS Nano 2008, 2, 2103–2112. [Google Scholar] [CrossRef]
- Li, A.; Wang, J.; Zhang, W.; McNaughton, R.; Anderson, S.; Zhang, X. Sol-gel based TiO2 thin film deposition on frustules towards facile and scalable manufacturing. J. Phys. Conf. Ser. 2016, 773, 012112. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Luckey, H.; Kubli, F. Titanium Alloys in Surgical Implants; ASTM: West Conshohocken, PA, USA, 1983. [Google Scholar]
- Charlton, G.; Howes, P.B.; Nicklin, C.L.; Steadman, P.; Taylor, J.S.G.; Muryn, C.A.; Harte, S.P.; Mercer, J.; McGrath, R.; Norman, D.; et al. Relaxation of TiO2 (110)-(1×1) using surface X-ray diffraction. Phys. Rev. Lett. 1997, 78, 495. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Kijima, T. Inorganic and Metallic Nanotubular Materials: Recent Technologies and Applications. Top. Appl. Phys. 2010, 117. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Golberg, D.; Bando, Y.; Zhi, C.; Tang, C.; Mitome, M.; Kurashima, K. Deformation-driven electrical transport of individual boron nitride nanotubes. Nano Lett. 2007, 7, 632–637. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Tang, C.; Zni, C. Boron Nitride Nanotubes. Adv. Mater. 2007, 19, 2413–2432. [Google Scholar] [CrossRef]
- Golberg, D.; Mitome, M.; Kurashima, K.; Zhi, C.Y.; Tang, C.C.; Bando, Y.; Lourie, O. In situ electrical probing and bias-mediated manipulation of dielectric nanotubes in a high-resolution transmission electron microscope. Appl. Phys. Lett. 2006, 88, 123101. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.L. New Developments in Transmission Electron Microscopy for Nanotechnology. Adv. Mater. 2003, 15, 1497–1514. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Saravanan, P.; Ganapathy, M.; Charles, A.; Tamilselvan, S.; Jeyasekaran, R.; Vimalan, M. Electrical properties of green synthesized TiO2 nanoparticles. Pelagia Res. Libr. Adv. Appl. Sci. Res. 2016, 7, 158–168. [Google Scholar]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, M.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Hashmi, S.S.; Ahmad, W.; Zahir, A. The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem. Lett. Rev. 2018, 11, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.K.; Seibert, M. Prospects for commercial production of diatoms. Biotechnol. Biofuels 2017, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Van Eynde, E.; Tytgat, T.; Smits, M.; Verbruggen, S.W.; Hauchecorne, B.; Lenaerts, S. Biotemplated diatom silica-titania materials for air purification. Photochem. Photobiol. Sci. 2013, 12, 690–695. [Google Scholar] [CrossRef]
- Ouwehand, J.; Van Eynde, E.; De Canck, E.; Lenaerts, S.; Verberckmoes, A.; Van Der Voort, P. Titania-functionalized diatom frustules as photocatalyst for indoor air purification. Appl. Catal. B Environ. 2018, 226, 303–310. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Pal, S.; Ul Haq, E.; Licciulli, A. Nanocrystalline TiO2–diatomite composite catalysts: Effect of crystallization on the photocatalytic degradation of rhodamine B. Appl. Catal. A Gen. 2014, 485, 157–162. [Google Scholar] [CrossRef]
- Mao, L.; Liu, J.; Zhu, S.; Zhang, D.; Chen, Z.; Chen, C. Sonochemical fabrication of mesoporous TiO2 inside diatom frustules for photocatalyst. Ultrason. Sonochem. 2014, 21, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Van Eynde, E.; Hu, Z.Y.; Tytgat, T.; Verbruggen, S.W.; Watté, J.; Van Tendeloo, G.; Van Driessche, I.; Blust, R.; Lenaerts, S. Diatom silica–titania photocatalysts for air purification by bio-accumulation of different titanium sources. Environ. Sci. Nano 2016, 3, 1052–1061. [Google Scholar] [CrossRef]
- Monkonsit, S.; Powtongsook, S.; Pavasant, P. Comparison between Airlift Photobioreactor and Bubble Column for Skeletonema Costatum Cultivation. Eng. J. 2011, 15, 53–64. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Pomastowski, P.; Hornowska, M.; Król, A.; Rafińska, K.; Buszewski, B. Naturally organic functionalized 3D biosilica from diatom microalgae. Mater. Des. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- Sen, D.; Das, A.; Bahadur, J. Dissolution of amorphous SiO2 nanoparticles at high alkaline pH: Real time SAXS investigation. AIP Conf. Proc. 2019, 2115, 030035. [Google Scholar] [CrossRef]
- Lebeau, T.; Robert, J.M. Diatom cultivation and biotechnologically relevant products. Part I: Cultivation at various scales. Appl. Microbiol. Biotechnol. 2003, 60, 612–623. [Google Scholar] [CrossRef]
- Jeffryes, C.; Gutu, T.; Jiao, J.; Rorrer, G.L. Two-stage photobioreactor process for the metabolic insertion of nanostructured germanium into the silica microstructure of the diatom Pinnularia sp. Mater. Sci. Eng. C 2008, 28, 107–118. [Google Scholar] [CrossRef]
- Maumus, F.; Allen, A.E.; Mhiri, C.; Hu, H.; Jabbari, K.; Vardi, A.; Grandbastien, M.A.; Bowler, C. Potential impact of stress activated retrotransposons on genome evolution in a marine diatom. BMC Genom. 2009, 10, 624. [Google Scholar] [CrossRef] [Green Version]
- Martin-Jézéquel, V.; Hildebrand, M.; Brzezinski, M.A. Silicon metabolism in diatoms: Implications for growth. J. Phycol. 2000, 36, 821–840. [Google Scholar] [CrossRef]
- Thamatrakoln, K.; Hildebrand, M. Silicon Uptake in Diatoms Revisited: A Model for Saturable and Nonsaturable Uptake Kinetics and the Role of Silicon Transporters. Plant Physiol. 2008, 146, 1397–1407. [Google Scholar] [CrossRef] [Green Version]
- Morales, E.A.; Edlund, M.B.; Spaulding, S.A. Description and ultrastructure of araphid diatom species (Bacillariophyceae) morphologically similar to Pseudostaurosira elliptica (Schumann) Edlund et al. Phycol. Res. 2010, 58, 97–107. [Google Scholar] [CrossRef]
- Ketheesan, B.; Nirmalakhandan, N. Modeling microalgal growth in an airlift-driven raceway reactor. Bioresour. Technol. 2013, 136, 689–696. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; p. 719. [Google Scholar]
- Vaccari, D.A.; Strom, P.F.; Alleman, J.E. Environmental Biology for Engineers and Scientists. Environ. Biol. Eng. Sci. 2005, 1–931. [Google Scholar] [CrossRef]
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth kinetic models for microalgae cultivation: A review. Algal Res. 2015, 12, 497–512. [Google Scholar] [CrossRef]
- Pujo-Pay, M.; Raimbault, P.; Conan, P. Sous-estimation des teneurs en azote particulaire en milieu oceanique par l’utilisation des filtres GF/F (Underestimation of particulate nitrogen concentrations in open ocean by the use of GF/F filters). Oceanogr. Lit. Rev. 1997, 11, 1251. [Google Scholar]
- Flynn, K.J.; Butler, I. Nitrogen sources for the growth of marine microalgae: Role of dissolved free amino acids. Mar. Ecol. Ser. Mar. Ecol. Prog. Ser 1986, 34, 281304. [Google Scholar] [CrossRef]
- Hellebust, J.A.; Ahmad, I. Biological Oceanography Regulation of Nitrogen Assimilation in Green Microalgae. Biol. Oceanogr. 1988, 6, 241–255. [Google Scholar] [CrossRef]
- Morales, E.A. Morphological studies in selected fragilarioid diatoms (Bacillariophyceae) from Connecticut waters (USA). Proc. Acad. Nat. Sci. Phila. 2001, 151, 105–120. [Google Scholar] [CrossRef]
- Poulsen, N.; Sumper, M.; Kröger, N. Biosilica formation in diatoms: Characterization of native silaffin-2 and its role in silica morphogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 12075–12080. [Google Scholar] [CrossRef] [Green Version]
- Uthappa, U.T.; Kigga, M.; Sriram, G.; Ajeya, K.V.; Jung, H.Y.; Neelgund, G.M.; Kurkuri, M.D. Facile green synthetic approach of bio inspired polydopamine coated diatoms as a drug vehicle for controlled drug release and active catalyst for dye degradation. Microporous Mesoporous Mater. 2019, 288, 109572. [Google Scholar] [CrossRef]
- Aw, M.S.; Bariana, M.; Yu, Y.; Addai-Mensah, J.; Losic, D. Surface-functionalized diatom microcapsules for drug delivery of water-insoluble drugs. J. Biomater. Appl. 2013, 28, 163–174. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Munz, R.J.; Rossi, J.; Zekri, A.Y. Plasma heating of carbonate formations. Pet. Sci. Technol. 2007, 25, 1143–1161. [Google Scholar] [CrossRef]
- Nowak, A.P.; Sprynskyy, M.; Wojtczak, I.; Trzciński, K.; Wysocka, J.; Szkoda, M.; Buszewski, B.; Lisowska-Oleksiak, A. Diatoms Biomass as a Joint Source of Biosilica and Carbon for Lithium-Ion Battery Anodes. Materials 2020, 13, 1673. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, C.; Hu, Z. Selective removal of pharmaceuticals and personal care products from water by titanium incorporated hierarchical diatoms in the presence of natural organic matter. Water Res. 2021, 189, 116628. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Lyman, C.E.; Lifshin, E.; Sawyer, L.; Michael, J.R. Scanning Electron Microscopy and X-ray Microanalysis; Springer: Berlin/Heidelberg, Germany, 2003; pp. 391–451. [Google Scholar] [CrossRef]
- Newbury, D.E. Quantitative electron probe microanalysis of rough targets: Testing the peak-to-local background method. Scanning 2004, 26, 103–114. [Google Scholar] [CrossRef]
- Pannico, M.; Rea, I.; Chandrasekaran, S.; Musto, P.; Voelcker, N.H.; De Stefano, L. Electroless Gold-Modified Diatoms as Surface-Enhanced Raman Scattering Supports. Nanoscale Res. Lett. 2016, 11, 315. [Google Scholar] [CrossRef] [Green Version]
- DeMaster, D.J. The Diagenesis of Biogenic Silica: Chemical Transformations Occurring in the Water Column, Seabed, and Crust. Treatise Geochem. 2003, 7–9, 87–98. [Google Scholar] [CrossRef]
- Wang, Z.; Saxena, S.K.; Pischedda, V.; Liermann, H.P.; Zha, C.S. X-ray diffraction study on pressure-induced phase transformationsin nanocrystalline anatase/rutile (TiO2). J. Phys. Condens. Matter 2001, 13, 8317. [Google Scholar] [CrossRef]
- Cole, K.E.; Ortiz, A.N.; Schoonen, M.A.; Valentine, A.M. Peptide- And long-chain potyamine- induced synthesis of micro- and nanostructured titanium phosphate and protein encapsulation. Chem. Mater. 2006, 18, 4592–4599. [Google Scholar] [CrossRef]
- Unocic, R.R.; Zalar, F.M.; Sarosi, P.M.; Cai, Y.; Sandhage, K.H. Anatase assemblies from algae: Coupling biological self-assembly of 3-D nanoparticle structures with synthetic reaction chemistry. Chem. Commun. 2004, 4, 796–797. [Google Scholar] [CrossRef]
- Sumerel, J.L.; Yang, W.; Kisailus, D.; Weaver, J.C.; Choi, J.H.; Morse, D.E. Biocatalytically Templated Synthesis of Titanium Dioxide. Chem. Mater. 2003, 15, 4804–4809. [Google Scholar] [CrossRef]
- Kröger, N.; Dickerson, M.B.; Ahmad, G.; Cai, Y.; Haluska, M.S.; Sandhage, K.H.; Poulsen, N.; Sheppard, V.C. Bioenabled Synthesis of Rutile (TiO2) at Ambient Temperature and Neutral pH. Angew. Chemie Int. Ed. 2006, 45, 7239–7243. [Google Scholar] [CrossRef]
- Dickerson, M.B.; Jones, S.E.; Cai, Y.; Ahmad, G.; Naik, R.R.; Kröger, N.; Sandhage, K.H. Identification and design of peptides for the rapid, high-yield formation of nanoparticulate TiO2 from aqueous solutions at room temperature. Chem. Mater. 2008, 20, 1578–1584. [Google Scholar] [CrossRef]
- Bansal, V.; Rautaray, D.; Bharde, A.; Ahire, K.; Sanyal, A.; Ahmad, A.; Sastry, M. Fungus-mediated biosynthesis of silica and titania particles. J. Mater. Chem. 2005, 15, 2583–2589. [Google Scholar] [CrossRef]
- Bachheimer, J.P. An anomaly in the β phase near the α-β transition of quartz. J. Phys. Lett. 1980, 41, 559–561. [Google Scholar] [CrossRef]
- Kosmulski, M.; Matijević, E. Zeta potential of anatase (TiO2) in mixed solvents. Colloids Surf. 1992, 64, 57–65. [Google Scholar] [CrossRef]
- Henderson, R.K.; Baker, A.; Parsons, S.A.; Jefferson, B. Characterisation of algogenic organic matter extracted from cyanobacteria, green algae and diatoms. Water Res. 2008, 42, 3435–3445. [Google Scholar] [CrossRef]
- Wang, L.; Pan, K.; Zhang, L.; Zhou, C.; Li, Y.; Zhu, B.; Han, J. Tentative identification of key factors determining the hemostatic efficiency of diatom frustule. Biomater. Sci. 2021, 9, 2162–2173. [Google Scholar] [CrossRef]
- Galletti, A.; Seo, S.; Joo, S.H.; Su, C.; Blackwelder, P. Effects of titanium dioxide nanoparticles derived from consumer products on the marine diatom Thalassiosira pseudonana. Environ. Sci. Pollut. Res. 2016, 23, 21113–21122. [Google Scholar] [CrossRef]
- Konno, H. Settling and Coagulation of Slender Type Diatoms. Water Sci. Technol. 1993, 27, 231–240. [Google Scholar] [CrossRef]
- Franks, G.V. Zeta Potentials and Yield Stresses of Silica Suspensions in Concentrated Monovalent Electrolytes: Isoelectric Point Shift and Additional Attraction. J. Colloid Interface Sci. 2002, 249, 44–51. [Google Scholar] [CrossRef]
- Lebrette, S.; Pagnoux, C.; Abélard, P. Stability of aqueous TiO2 suspensions: Influence of ethanol. J. Colloid Interface Sci. 2004, 280, 400–408. [Google Scholar] [CrossRef]
- Liao, D.L.; Wu, G.S.; Liao, B.Q. Zeta potential of shape-controlled TiO2 nanoparticles with surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 270–275. [Google Scholar] [CrossRef]
- Kosmulski, M.; Prochniak, P.; Rosenholm, J. Letter: The IEP of Carbonate-Free Neodymium(III) Oxide. J. Dispers. Sci. Technol. 2009, 30, 589–591. [Google Scholar] [CrossRef]
- Uskoković, V. Dynamic Light Scattering Based Microelectrophoresis: Main Prospects and Limitations. J. Dispers. Sci. Technol. 2012, 33, 1762–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coats, A.W.; Redfern, J.P. Thermogravimetric analysis. A review. Analyst 1963, 88, 906–924. [Google Scholar] [CrossRef]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Kadam, D.M.; Thunga, M.; Wang, S.; Kessler, M.R.; Grewell, D.; Lamsal, B.; Yu, C. Preparation and characterization of whey protein isolate films reinforced with porous silica coated titania nanoparticles. J. Food Eng. 2013, 117, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Safonova, T.A.; Annenkov, V.V.; Chebykin, E.P.; Danilovtseva, E.N.; Likhoshway, Y.V.; Grachev, M.A. Aberration of morphogenesis of siliceous frustule elements of the diatom Synedra acus in the presence of germanic acid. Biochemistry 2007, 72, 1261–1269. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, Q.; Xi, T.; Wang, J.; Liu, Y. Thermal analysis on nanosized TiO2 prepared by hydrolysis. Thermochim. Acta 2002, 381, 45–48. [Google Scholar] [CrossRef]
- Ibrahim, S.; Selim, A.Q. Heat treatment of natural diatomite. Physicochem. Probl. Miner. Process 2012, 48, 413–424. [Google Scholar]
- Eckert, J.; Gourdon, O.; Jacob, D.E.; Meral, C.; Monteiro, P.J.M.; Vogel, S.C.; Wirth, R.; Wenk, H.-R. Ordering of water in opals with different microstructures. Eur. J. Mineral. 2015, 27, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Heredia, A.; Figueira, E.; Rodrigues, C.T.; Rodríguez-Galván, A.; Basiuk, V.A.; Vrieling, E.G.; Almeida, S.F.P. Cd2+ affects the growth, hierarchical structure and peptide composition of the biosilica of the freshwater diatom Nitzschia palea (Kützing) W. Smith. Phycol. Res. 2012, 60, 229–240. [Google Scholar] [CrossRef]
- Kröger, N.; Deutzmann, R.; Sumper, M. Silica-precipitating Peptides from Diatoms: The chemical structure of silaffin-1a from Cylindrotheca fusiformis. J. Biol. Chem. 2001, 276, 26066–26070. [Google Scholar] [CrossRef] [Green Version]
- Della Rosa, G.; Vona, D.; Aloisi, A.; Ragni, R.; Di Corato, R.; Lo Presti, M.; Cicco, S.R.; Altamura, E.; Taurino, A.; Catalano, M.; et al. Luminescent Silica-Based Nanostructures from in vivo Iridium-Doped Diatoms Microalgae. ACS Sustain. Chem. Eng. 2019, 7, 2207–2215. [Google Scholar] [CrossRef]
- Boyatzis, S.C.; Velivasaki, G.; Malea, E. A study of the deterioration of aged parchment marked with laboratory iron gall inks using FTIR-ATR spectroscopy and micro hot table. Herit. Sci. 2016, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Kong, R.; Pan, X.; Xu, H.; Xia, D.; Shan, H.; Lu, J.R. Role of ovalbumin in the stabilization of metastable vaterite in calcium carbonate biomineralization. J. Phys. Chem. B 2009, 113, 8975–8982. [Google Scholar] [CrossRef]
- Rickert, D.; Schlüter, M.; Wallmann, K. Dissolution kinetics of biogenic silica from the water column to the sediments. Geochim. Cosmochim. Acta 2002, 66, 439–455. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectra of Minerals. Infrared Spectra Miner. 1974, 4. [Google Scholar] [CrossRef]
- Hernández-Ortiz, M.; Hernández-Ortiz, M.; Hernández-Padrón, G.; Bernal, R.; Cruz-Vázquez, C.; Castano, V. Nanocrystalline mimetic opals: Synthesis and comparative characterization vs. natural stones. Int. J. Basic Appl. Sci. 2015, 4, 238–243. [Google Scholar] [CrossRef]
- Adamo, I.; Ghisoli, C.; Caucia, F. A contribution to the study of FTIR spectra of opals. Neues Jahrb. Für Mineral.-Abh. 2010, 187, 63–68. [Google Scholar] [CrossRef]
- Lu, H.B.; Zhou, Y.Z.; Vongehr, S.; Tang, S.C.; Meng, X.K. Effects of hydrothermal temperature on formation and decoloration characteristics of anatase TiO2 nanoparticles. Sci. China Technol. Sci. 2012, 55, 894–902. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, L.; Peng, S.; Shi, J.; Liu, Z.; Wen, W. Wettability of urea-doped TiO2 nanoparticles and their high electrorheological effects. J. Sol-Gel Sci. Technol. 2008, 47, 311–315. [Google Scholar] [CrossRef]
- Camargo, E.; Jaime, P.C.J.; Lin, C.-F.; Lin, M.-S.; Yu, T.-Y.; Wu, M.-C.; Lai, S.-Y.; Wang, M.-Y. Chemical and optical characterization of Psammodictyon panduriforme (Gregory) Mann comb nov (Bacillariophyta) frustules. Opt. Mater. Express 2016, 6, 1436. [Google Scholar] [CrossRef]
- Kammer, M.; Hedrich, R.; Ehrlich, H.; Popp, J.; Brunner, E.; Krafft, C. Spatially resolved determination of the structure and composition of diatom cell walls by Raman and FTIR imaging. Anal. Bioanal. Chem. 2010, 398, 509–517. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, E. Light Manipulation by Single Cells: The Case of Diatoms. J. Spectrosc. 2016, 2016, 2490128. [Google Scholar] [CrossRef]
- Tang, H.; Berger, H.; Schmid, P.E.; Lévy, F.; Burri, G. Photoluminescence in TiO2 anatase single crystals. Solid State Commun. 1993, 87, 847–850. [Google Scholar] [CrossRef]
- Saraf, L.V.; Patil, S.I.; Ogale, S.B.; Sainkar, S.R.; Kshirsager, S.T. Synthesis of Nanophase TiO2 by Ion Beam Sputtering and Cold Condensation Technique. Int. J. Mod. Phys. B 2012, 12, 2635–2647. [Google Scholar] [CrossRef]
- Gale, D.K.; Jeffryes, C.; Gutu, T.; Jiao, J.; Chang, C.H.; Rorrer, G.L. Thermal annealing activates amplified photoluminescence of germanium metabolically doped in diatom biosilica. J. Mater. Chem. 2011, 21, 10658–10665. [Google Scholar] [CrossRef]
- Itoh, C.; Tanimura, K.; Itoh, N. Optical studies of self-trapped excitons in SiO2. J. Phys. C Solid State Phys. 1988, 21, 4693. [Google Scholar] [CrossRef]
- Pacchioni, G.; Skuja, L.; Griscom, D.L. Defects in SiO2 and Related Dielectrics: Science and Technology; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2000; Volume 2, pp. 161–197. [Google Scholar] [CrossRef]
- Qin, T.; Gutu, T.; Jiao, J.; Chang, C.H.; Rorrer, G.L. Photoluminescence of Silica Nanostructures from Bioreactor Culture of Marine Diatom Nitzschia frustulum. J. Nanosci. Nanotechnol. 2008, 8, 2392–2398. [Google Scholar] [CrossRef]
- Gallis, S.; Nikas, V.; Suhag, H.; Huang, M.; Kaloyeros, A.E. White light emission from amorphous silicon oxycarbide (a-SiCxOy) thin films: Role of composition and postdeposition annealing. Appl. Phys. Lett. 2010, 97, 081905. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, L.D. Fabrication, characterization, and photoluminescence properties of highly ordered TiO2 nanowire arrays. J. Mater. Res. 2001, 16, 1138–1144. [Google Scholar] [CrossRef]
- Garcia, L.M.P.; Tavares, M.T.S.; Andrade Neto, N.F.; Nascimento, R.M.; Paskocimas, C.A.; Longo, E.; Bomio, M.R.D.; Motta, F.V. Photocatalytic activity and photoluminescence properties of TiO2, In2O3, TiO2/In2O3 thin films multilayer. J. Mater. Sci. Mater. Electron. 2018, 29, 6530–6542. [Google Scholar] [CrossRef] [Green Version]
- Serpone, N.; Lawless, D.; Khairutdinov, R. Size Effects on the Photophysical Properties of Colloidal Anatase TiO2 Particles: Size Quantization versus Direct Transitions in This Indirect Semiconductor? J. Phys. Chem. 2002, 99, 16646–16654. [Google Scholar] [CrossRef]
- Armini, S.; Whelan, C.M.; Moinpour, M.; Veksler, H.; Fishman, S.; Wang, Y.; Man, Z.-X.; Zhang, Y.-J.; Setaro, A.; Lettieri, S.; et al. Nanograined anatase titania-based optochemical gas detection. New J. Phys. 2008, 10, 053030. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Kumar Prasad, A.; Benoy, T.; Rakesh, P.P.; Hari, M.; Libish, T.M.; Radhakrishnan, P.; Nampoori, V.P.N.; Vallabhan, C.P.G. UV-visible photoluminescence of TiO2 nanoparticles prepared by hydrothermal method. J. Fluoresc. 2012, 22, 1563–1569. [Google Scholar] [CrossRef]
| Series (I–IV) | |
|---|---|
| Temperature [°C] | 25 |
| Light intensity [lux] | 1500 |
| Ilumination (fotoperiod) [h:h] | 12:12 |
| Photobioreactor volume [L] | 25 |
| Culture medium | Guilard’s f/2 |
| Si precursor | Na2SiO3 × 5H2O |
| Ti precursor | C16H36O4Ti in 37% HClaq |
| Culture period [days] | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzozowska, W.; Sprynskyy, M.; Wojtczak, I.; Dąbek, P.; Markuszewski, M.J.; Witkowski, A.; Buszewski, B. Metabolically Doping of 3D Diatomaceous Biosilica with Titanium. Materials 2022, 15, 5210. https://doi.org/10.3390/ma15155210
Brzozowska W, Sprynskyy M, Wojtczak I, Dąbek P, Markuszewski MJ, Witkowski A, Buszewski B. Metabolically Doping of 3D Diatomaceous Biosilica with Titanium. Materials. 2022; 15(15):5210. https://doi.org/10.3390/ma15155210
Chicago/Turabian StyleBrzozowska, Weronika, Myroslav Sprynskyy, Izabela Wojtczak, Przemysław Dąbek, Michał J. Markuszewski, Andrzej Witkowski, and Bogusław Buszewski. 2022. "Metabolically Doping of 3D Diatomaceous Biosilica with Titanium" Materials 15, no. 15: 5210. https://doi.org/10.3390/ma15155210
APA StyleBrzozowska, W., Sprynskyy, M., Wojtczak, I., Dąbek, P., Markuszewski, M. J., Witkowski, A., & Buszewski, B. (2022). Metabolically Doping of 3D Diatomaceous Biosilica with Titanium. Materials, 15(15), 5210. https://doi.org/10.3390/ma15155210