RETRACTED: Bi and Sn Doping Improved the Structural, Optical and Photovoltaic Properties of MAPbI3-Based Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimentation
3. Results and Discussion
3.1. XRD Analysis
Grain Size (D) and Dislocation Line Density (δ)
3.2. Optical Properties
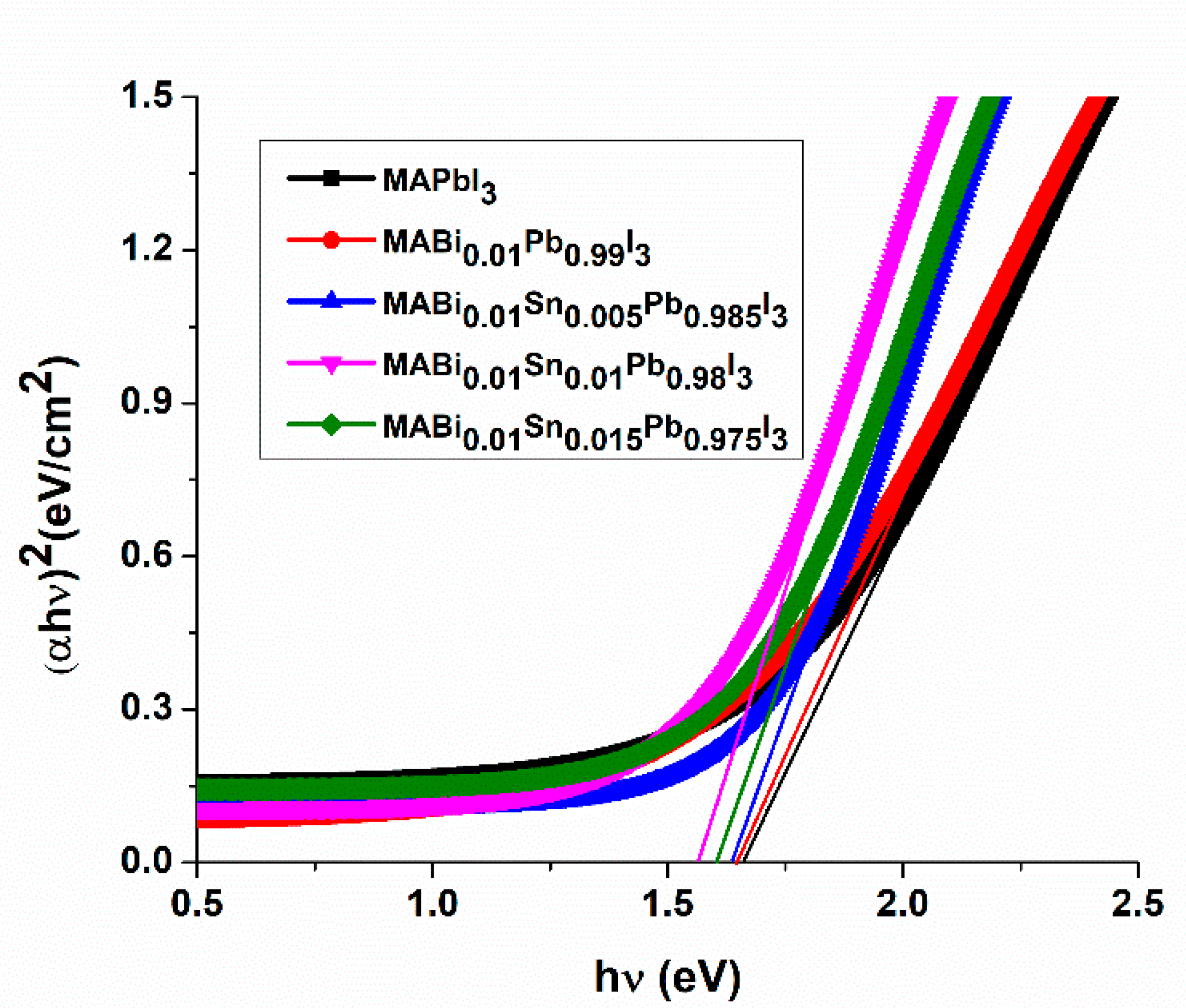
3.2.1. Band Gap Energy (Eg)
3.2.2. Refractive Index
3.2.3. Extinction Coefficient (k)
3.2.4. Dielectric Constants (εr, εi)
3.3. J–V Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zulkifili, A.N.B.; Kento, T.; Daiki, M.; Fujiki, A. The basic research on the dye-sensitized solar cells (DSSC). J. Clean Energy Technol. 2015, 3, 382–387. [Google Scholar] [CrossRef]
- Gheorghe, I.F.; Ion, B. The Effects of Air Pollutants on Vegetation and the Role of Vegetation in Reducing Atmospheric Pollution; The Impact of Air Pollution on Health, Economy, Environment and Agricultural Sources; IntechOpen: London, UK, 2011; pp. 241–280. [Google Scholar]
- Grätzel, M. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef]
- Gérenton, F.; Eymard, J.; Harrison, S.; Clerc, R.; Munoz, D. Analysis of edge losses on silicon heterojunction half solar cells. Sol. Energy Mater. Sol. Cells 2020, 204, 110213. [Google Scholar] [CrossRef]
- Lewis, N.S. Toward cost-effective solar energy use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lim, K.-G.; Lee, T.-W. Planar heterojunction organometal halide perovskite solar cells: Roles of interfacial layers. Energy Environ. Sci. 2016, 9, 12–30. [Google Scholar] [CrossRef]
- Xu, W.; Ma, X.; Son, J.H.; Jeong, S.Y.; Niu, L.; Xu, C.; Zhang, S.; Zhou, Z.; Gao, J.; Woo, H.Y. Smart Ternary Strategy in Promoting the Performance of Polymer Solar Cells Based on Bulk-Heterojunction or Layer-By-Layer Structure. Small 2022, 18, 2104215. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Jeong, S.Y.; Son, J.H.; Zhou, Z.; Jiang, Q.; Woo, H.Y.; Wu, Q.; Zhu, X.; Ma, X. Achieving 17.5% efficiency for polymer solar cells via a donor and acceptor layered optimization strategy. J. Mater. Chem. C 2022, 10, 5489–5496. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, X.; Xu, C.; Xu, W.; Jeong, S.Y.; Woo, H.Y.; Zhou, Z.; Zhang, X.; Zhang, F. Boosted efficiency over 18.1% of polymer solar cells by employing large extinction coefficients material as the third component. Macromol. Rapid Commun. 2022, 2200345. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Jiang, Y.; Soufiani, A.M.; Ho-Baillie, A. Optical properties of photovoltaic organic–inorganic lead halide perovskites. J. Phys. Chem. Lett. 2015, 6, 4774–4785. [Google Scholar] [CrossRef]
- Li, F.; Ma, C.; Wang, H.; Hu, W.; Yu, W.; Sheikh, A.D.; Wu, T. Ambipolar solution-processed hybrid perovskite phototransistors. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths> 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- Galkowski, K.; Mitioglu, A.; Miyata, A.; Plochocka, P.; Portugall, O.; Eperon, G.E.; Wang, J.T.-W.; Stergiopoulos, T.; Stranks, S.D.; Snaith, H.J. Determination of the exciton binding energy and effective masses for methylammonium and formamidinium lead tri-halide perovskite semiconductors. Energy Environ. Sci. 2016, 9, 962–970. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Baikie, T.; Boix, P.P.; Yantara, N.; Mathews, N.; Mhaisalkar, S. Band-gap tuning of lead halide perovskites using a sequential deposition process. J. Mater. Chem. A 2014, 2, 9221–9225. [Google Scholar] [CrossRef]
- Quarti, C.; Mosconi, E.; Ball, J.M.; D’Innocenzo, V.; Tao, C.; Pathak, S.; Snaith, H.J.; Petrozza, A.; De Angelis, F. Structural and optical properties of methylammonium lead iodide across the tetragonal to cubic phase transition: Implications for perovskite solar cells. Energy Environ. Sci. 2016, 9, 155–163. [Google Scholar] [CrossRef]
- Babayigit, A.; Thanh, D.D.; Ethirajan, A.; Manca, J.; Muller, M.; Boyen, H.-G.; Conings, B. Assessing the toxicity of Pb-and Sn-based perovskite solar cells in model organism Danio rerio. Sci. Rep. 2016, 6, 18721. [Google Scholar] [CrossRef]
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 15, 247–251. [Google Scholar] [CrossRef]
- Yi, C.; Luo, J.; Meloni, S.; Boziki, A.; Ashari-Astani, N.; Grätzel, C.; Zakeeruddin, S.M.; Röthlisberger, U.; Grätzel, M. Entropic stabilization of mixed A-cation ABX 3 metal halide perovskites for high performance perovskite solar cells. Energy Environ. Sci. 2016, 9, 656–662. [Google Scholar] [CrossRef]
- Liu, C.; Fan, J.; Li, H.; Zhang, C.; Mai, Y. Highly efficient perovskite solar cells with substantial reduction of lead content. Sci. Rep. 2016, 6, 35705. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological control for high performance, solution-processed planar heterojunction perovskite solar cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Tang, Z.-K.; Xu, Z.-F.; Zhang, D.-Y.; Hu, S.-X.; Lau, W.-M.; Liu, L.-M. Enhanced optical absorption via cation doping hybrid lead iodine perovskites. Sci. Rep. 2017, 7, 7843. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Bai, F.; Liu, X.; Ji, Q.; Miao, X.; Qiu, T.; Zhang, S. Bismuth incorporation stabilized α-CsPbI3 for fully inorganic perovskite solar cells. ACS Energy Lett. 2017, 2, 2219–2227. [Google Scholar] [CrossRef]
- Nayak, P.K.; Sendner, M.; Wenger, B.; Wang, Z.; Sharma, K.; Ramadan, A.J.; Lovrinčić, R.; Pucci, A.; Madhu, P.; Snaith, H.J. Impact of Bi3+ heterovalent doping in organic–inorganic metal halide perovskite crystals. J. Am. Chem. Soc. 2018, 140, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, E.; Merabet, B.; Meggiolaro, D.; Zaoui, A.; De Angelis, F. First-principles modeling of bismuth doping in the MAPbI3 perovskite. J. Phys. Chem. C 2018, 122, 14107–14112. [Google Scholar] [CrossRef]
- Han, L.; Wu, L.; Liu, C.; Zhang, J. Doping-Enhanced Visible-Light Absorption of CH3NH3PbBr3 by the Bi3+-Induced Impurity Band without Sacrificing a Band gap. J. Phys. Chem. C 2019, 123, 8578–8587. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Sarkar, P.; Srivastava, A.; Tripathy, S.; Baishnab, K.; Lenka, T.; Menon, P.; Lin, F.; Aberle, A. Impact of Sn doping on methylammonium lead chloride perovskite: An experimental study. J. Appl. Phys. 2020, 127, 125110. [Google Scholar] [CrossRef]
- Hoefler, S.F.; Trimmel, G.; Rath, T. Progress on lead-free metal halide perovskites for photovoltaic applications: A review. Mon. Für Chem.-Chem. Mon. 2017, 148, 795–826. [Google Scholar] [CrossRef]
- Oku, T.; Ohishi, Y.; Suzuki, A. Effects of SbBr3 addition to CH3NH3PbI3 solar cells. Proc. AIP Conf. Proc. 2017, 1807, 020007. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, T.; Tian, Y.; Zhu, X.; Tu, Y. Perovskite-Based Solar Cells: Materials, Methods, and Future Perspectives. J. Nanomater. 2018, 2018, 8148072. [Google Scholar] [CrossRef]
- Khan, M.; Bhatti, K.; Qindeel, R.; Althobaiti, H.S.; Alonizan, N. Structural, electrical and optical properties of multilayer TiO2 thin films deposited by sol–gel spin coating. Results Phys. 2017, 7, 1437–1439. [Google Scholar] [CrossRef]
- Jassim, S.M.; Bakr, N.A.; Mustafa, F.I. Synthesis and characterization of MAPbI 3 thin film and its application in C-Si/perovskite tandem solar cell. J. Mater. Sci. Mater. Electron. 2020, 31, 16199–16207. [Google Scholar] [CrossRef]
- Bartolomé, J.; Climent-Pascual, E.; Redondo-Obispo, C.; Zaldo, C.; Álvarez, A.n.L.; de Andrés, A.; Coya, C. Huge Photostability Enhancement in Bismuth-Doped Methylammonium Lead Iodide Hybrid Perovskites by Light-Induced Transformation. Chem. Mater. 2019, 31, 3662–3671. [Google Scholar] [CrossRef]
- Tang, M.-C.; Barrit, D.; Munir, R.; Li, R.; Barbé, J.M.; Smilgies, D.-M.; Del Gobbo, S.; Anthopoulos, T.D.; Amassian, A. Bismuth-Based Perovskite-Inspired Solar Cells: In Situ Diagnostics Reveal Similarities and Differences in the Film Formation of Bismuth-and Lead-Based Films. Sol. RRL 2019, 3, 1800305. [Google Scholar] [CrossRef]
- Parrey, K.A.; Aziz, A.; Ansari, S.; Mir, S.H.; Khosla, A.; Niazi, A. Synthesis and characterization of an efficient hole-conductor free halide perovskite CH3NH3PbI3 semiconductor absorber based photovoltaic device for IOT. J. Electrochem. Soc. 2018, 165, B3023. [Google Scholar] [CrossRef]
- Ahmed, M.; Bakry, A.; Shaaban, E.R.; Dalir, H. Structural, electrical, and optical properties of ITO thin films and their influence on performance of CdS/CdTe thin-film solar cells. J. Mater. Sci. Mater. Electron. 2021, 32, 11107–11118. [Google Scholar] [CrossRef]
- Mehmood, B.; Khan, M.; Iqbal, M.; Mahmood, A.; Al-Masry, W. Structural and optical properties of Ti and Cu co-doped ZnO thin films for photovoltaic applications of dye sensitized solar cells. Int. J. Energy Res. 2021, 45, 2445–2459. [Google Scholar] [CrossRef]
- Zhang, C.; Luan, W.; Yin, Y. High efficient Planar-heterojunction perovskite solar cell based on two-step deposition process. Energy Procedia 2017, 105, 793–798. [Google Scholar] [CrossRef]
- Albrecht, S.; Saliba, M.; Baena, J.P.C.; Lang, F.; Kegelmann, L.; Mews, M.; Steier, L.; Abate, A.; Rappich, J.; Korte, L. Monolithic perovskite/silicon-heterojunction tandem solar cells processed at low temperature. Energy Environ. Sci. 2016, 9, 81–88. [Google Scholar] [CrossRef]
- Jain, S.M.; Philippe, B.; Johansson, E.M.; Park, B.; Rensmo, H.; Edvinsson, T.; Boschloo, G. Vapor phase conversion of PbI 2 to CH 3 NH 3 PbI 3: Spectroscopic evidence for formation of an intermediate phase. J. Mater. Chem. A 2016, 4, 2630–2642. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, R.; Buyruk, A.; Chen, W.; Xiao, T.; Yin, S.; Jiang, X.; Kreuzer, L.P.; Mu, C.; Ameri, T. Sodium Dodecylbenzene Sulfonate Interface Modification of Methylammonium Lead Iodide for Surface Passivation of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 52643–52651. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, A.; Ravindra, N.M. Energy gap-refractive index relations in perovskites. Materials 2020, 13, 1917. [Google Scholar] [CrossRef]
- Bahadur, A.; Mishra, M. Correlation between refractive index and electronegativity difference for ANB8-N type binary semiconductors. Acta Phys. Pol. A 2013, 123, 737–740. [Google Scholar] [CrossRef]
- Herve, P.; Vandamme, L. General relation between refractive index and energy gap in semiconductors. Infrared Phys. Technol. 1994, 35, 609–615. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E.; Bhatia, A. Principles of Optics, 7th (expanded) ed.; Cambridge U. Press: Cambridge, UK, 1999. [Google Scholar]
- Papadimitrakopoulos, F.; Wisniecki, P.; Bhagwagar, D.E. Mechanically attrited silicon for high refractive index nanocomposites. Chem. Mater. 1997, 9, 2928–2933. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, C. Unusual photon tunneling in the presence of a layer with a negative refractive index. Appl. Phys. Lett. 2002, 80, 1097–1099. [Google Scholar] [CrossRef]
- Mosconi, E.; Umari, P.; De Angelis, F. Electronic and optical properties of MAPbX 3 perovskites (X= I, Br, Cl): A unified DFT and GW theoretical analysis. Phys. Chem. Chem. Phys. 2016, 18, 27158–27164. [Google Scholar] [CrossRef]
- Pauling, L. Die Natur der Chemischen Bindung; Verlag Chemie: Weinheim, Germany, 1962. [Google Scholar]
- Wang, S.; Zhao, K.; Shao, Y.; Xu, L.; Huang, Y.-P.; Li, W. Evolutions of optical constants, interband electron transitions, and bandgap of Sn-doped CH3NH3PbI3 perovskite films. Appl. Phys. Lett. 2020, 116, 261902. [Google Scholar] [CrossRef]
- Park, N.-G. Methodologies for high efficiency perovskite solar cells. Nano Converg. 2016, 3, 15. [Google Scholar] [CrossRef]
- Lin, Q.; Armin, A.; Nagiri, R.C.R.; Burn, P.L.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photonics 2015, 9, 106–112. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Lim, S.S.; Yantara, N.; Liu, X.; Sabba, D.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat. Mater. 2014, 13, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Aydin, C. Synthesis of Pd: ZnO nanofibers and their optical characterization dependent on modified morphological properties. J. Alloy. Compd. 2019, 777, 145–151. [Google Scholar] [CrossRef]
- Caglar, M.; Ilican, S.; Caglar, Y.; Yakuphanoglu, F. Electrical conductivity and optical properties of ZnO nanostructured thin film. Appl. Surf. Sci. 2009, 255, 4491–4496. [Google Scholar] [CrossRef]
- Wemple, S.; DiDomenico, M., Jr. Behavior of the electronic dielectric constant in covalent and ionic materials. Phys. Rev. B 1971, 3, 1338. [Google Scholar] [CrossRef]
- Chauhan, R.; Srivastava, A.K.; Mishra, M.; Srivastava, K. Effect of UV exposure on some optical properties of As-Se based chalcogenide glasses. Integr. Ferroelectr. 2010, 119, 22–32. [Google Scholar] [CrossRef]
- Kumar, P.; Gaur, A. Model for the JV characteristics of degraded polymer solar cells. J. Appl. Phys. 2013, 113, 094505. [Google Scholar] [CrossRef]
- Yue, G.; Tan, F.; Li, F.; Chen, C.; Zhang, W.; Wu, J.; Li, Q. Enhanced performance of flexible dye-sensitized solar cell based on nickel sulfide/polyaniline/titanium counter electrode. Electrochim. Acta 2014, 149, 117–125. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Ngqoloda, S.; Pescetelli, S.; Di Carlo, A.; Dinu, M.; Vladescu, A.; Parau, A.C.; Agresti, A.; Braic, M.; Arendse, C.J. Spin coating immobilisation of CN-TiO2 Co-doped nano catalyst on glass and application for photocatalysis or as electron transporting layer for perovskite solar cells. Coatings 2020, 10, 1029. [Google Scholar] [CrossRef]
- Khan, M.I.; Mukhtar, A.; Alwadai, N.; Irfan, M.; Haq, I.-u.; Albalawi, H.; Almuqrin, A.H.; Almoneef, M.M.; Iqbal, M. Improving the Structural, Optical and Photovoltaic Properties of Sb-and Bi-Co-Doped MAPbBr3 Perovskite Solar Cell. Coatings 2022, 12, 386. [Google Scholar] [CrossRef]
- Kakiage, K.; Tokutome, T.; Iwamoto, S.; Kyomen, T.; Hanaya, M. Fabrication of a dye-sensitized solar cell containing a Mg-doped TiO2 electrode and a Br 3−/Br− redox mediator with a high open-circuit photovoltage of 1.21 V. Chem. Commun. 2013, 49, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.-C. Hybrid Lead Halide Perovskite and Bismuth-Based Perovskite-Inspired Photovoltaics: An In Situ Investigation. Ph.D. Thesis, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia, 2019. [Google Scholar]
- Wang, Q.; Shao, S.; Xu, B.; Duim, H.; Dong, J.; Adjokatse, S.; Portale, G.; Hou, J.; Saba, M.; Loi, M.A. Impact of the Hole Transport Layer on the Charge Extraction of Ruddlesden–Popper Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 29505–29512. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, T.; Liu, Y.; Bahrami, B.; Guo, D.; Fang, Y.; Shao, Y.; Chowdhury, A.H.; Wang, Q.; Deng, Y. Perovskite solar cells with embedded homojunction via nonuniform metal ion doping. Cell Rep. Phys. Sci. 2021, 2, 100415. [Google Scholar] [CrossRef]

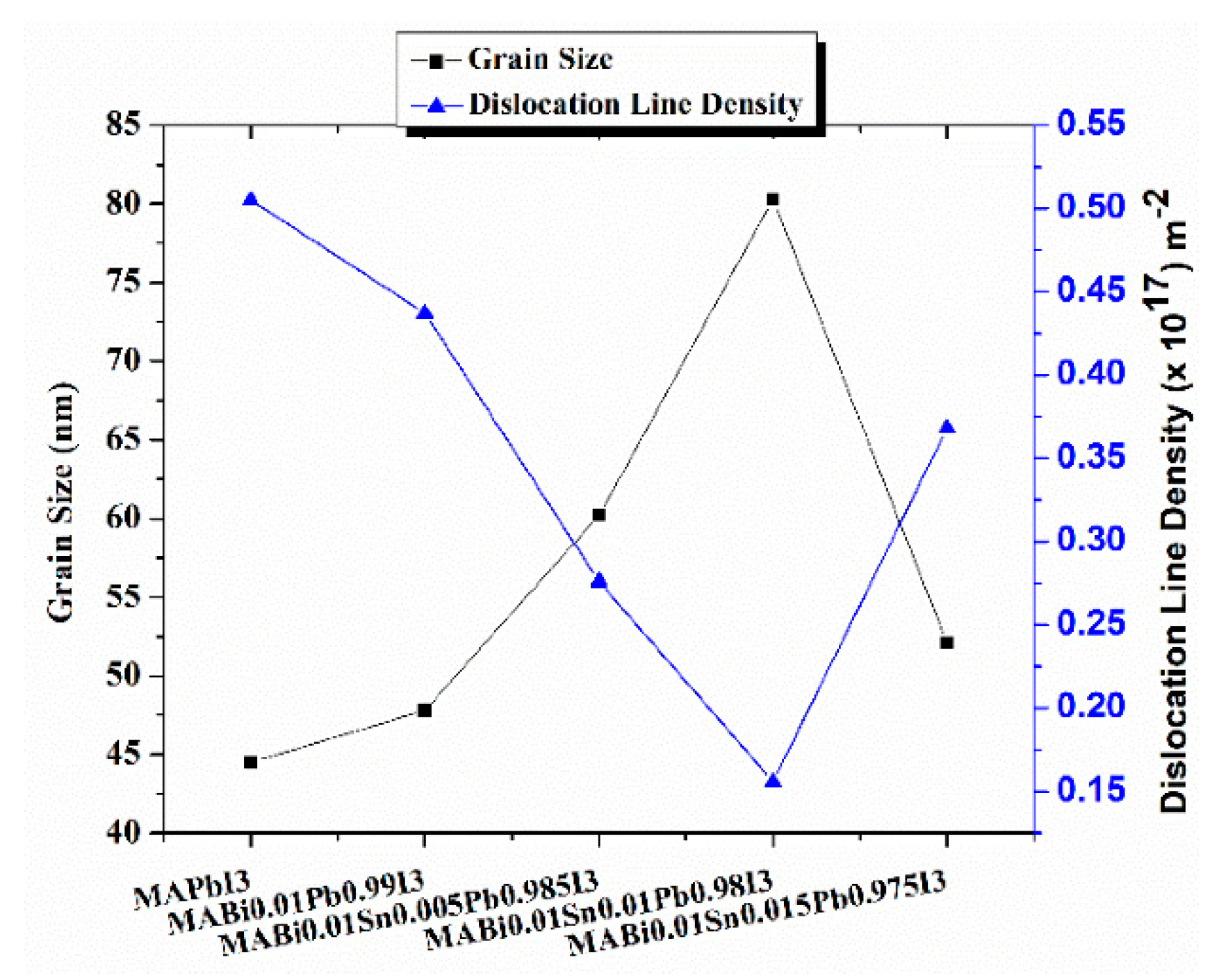

| Films | Grain Size(D) (nm) | Dislocation Line Density (DLD) (m−2) | d-Spacing(A°) |
|---|---|---|---|
| MAPbI3 | 44.5 | 0.505 × 1017 | 6.31 |
| 1%Bi-MAPbI3 | 47.8 | 0.437 × 1017 | 6.30 |
| (1% Bi + 0.5% Sn)-MAPbI3 | 60.2 | 0.276 × 1017 | 6.28 |
| (1% Bi + 1% Sn)-MAPbI3 | 80.3 | 0.156 × 1017 | 6.25 |
| (1% Bi + 1.5% Sn)-MAPbI3 | 52.1 | 0.368 × 1017 | 6.24 |
| Films | Eg (eV) | (εr) | (εi) |
|---|---|---|---|
| MAPbI3 | 1.66 | 3.308 | 12.69 |
| 1% Bi-MAPbI3 | 1.64 | 3.3085 | 12.74 |
| (1% Bi and 0.5% Sn)-MAPbI3 | 1.63 | 3.67 | 12.38 |
| (1% Bi and 1% Sn)-MAPbI3 | 1.56 | 3.63 | 12.80 |
| (1%Bi and 1.5%Sn)- MAPbI3 | 1.60 | 3.46 | 12.77 |
| Solar Cells | Jsc (mA/cm2) | FF | Voc (V) | η% |
|---|---|---|---|---|
| MAPbI3 | 9.69 | 0.587 | 1.08 | 6.14 ± 2% |
| 1%Bi-MAPbI3 | 10.41 | 0.577 | 1.079 | 6.48 ± 2% |
| (1% Bi and 0.5% Sn%)-MAPbI3 | 11.53 | 0.586 | 1.085 | 7.33 ± 2% |
| (1% Bi and 1% Sn)-MAPbI3 | 12.9 | 0.591 | 1.08 | 8.83 ± 2% |
| (1%Bi and 1.5%Sn)- MAPbI3 | 12.09 | 0.629 | 1.09 | 8.23 ± 2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.I.; Yasmin, S.; Alwadai, N.; Irfan, M.; Ikram-ul-Haq; Albalawi, H.; Almuqrin, A.H.; Almoneef, M.M.; Iqbal, M. RETRACTED: Bi and Sn Doping Improved the Structural, Optical and Photovoltaic Properties of MAPbI3-Based Perovskite Solar Cells. Materials 2022, 15, 5216. https://doi.org/10.3390/ma15155216
Khan MI, Yasmin S, Alwadai N, Irfan M, Ikram-ul-Haq, Albalawi H, Almuqrin AH, Almoneef MM, Iqbal M. RETRACTED: Bi and Sn Doping Improved the Structural, Optical and Photovoltaic Properties of MAPbI3-Based Perovskite Solar Cells. Materials. 2022; 15(15):5216. https://doi.org/10.3390/ma15155216
Chicago/Turabian StyleKhan, Muhammad I., Sumra Yasmin, Norah Alwadai, Muhammad Irfan, Ikram-ul-Haq, Hind Albalawi, Aljawhara H. Almuqrin, Maha M. Almoneef, and Munawar Iqbal. 2022. "RETRACTED: Bi and Sn Doping Improved the Structural, Optical and Photovoltaic Properties of MAPbI3-Based Perovskite Solar Cells" Materials 15, no. 15: 5216. https://doi.org/10.3390/ma15155216
APA StyleKhan, M. I., Yasmin, S., Alwadai, N., Irfan, M., Ikram-ul-Haq, Albalawi, H., Almuqrin, A. H., Almoneef, M. M., & Iqbal, M. (2022). RETRACTED: Bi and Sn Doping Improved the Structural, Optical and Photovoltaic Properties of MAPbI3-Based Perovskite Solar Cells. Materials, 15(15), 5216. https://doi.org/10.3390/ma15155216







