Evaluation of the Biocompatibility and Osteogenic Properties of Metal Oxide Coatings Applied by Magnetron Sputtering as Potential Biofunctional Surface Modifications for Orthopedic Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metal Oxide Coatings Deposition
2.2. Metal Oxide Coatings Characterization
2.3. Cells
2.4. Cell Attachment and Viability of BM-MSC on the Metal Oxide Coatings
2.5. Proliferation of BM-MSC on the Metal Oxide Coatings
2.6. Evaluation of the Osteogenic Properties of the Metal Oxide Coatings
2.7. Statistical Analysis
3. Results
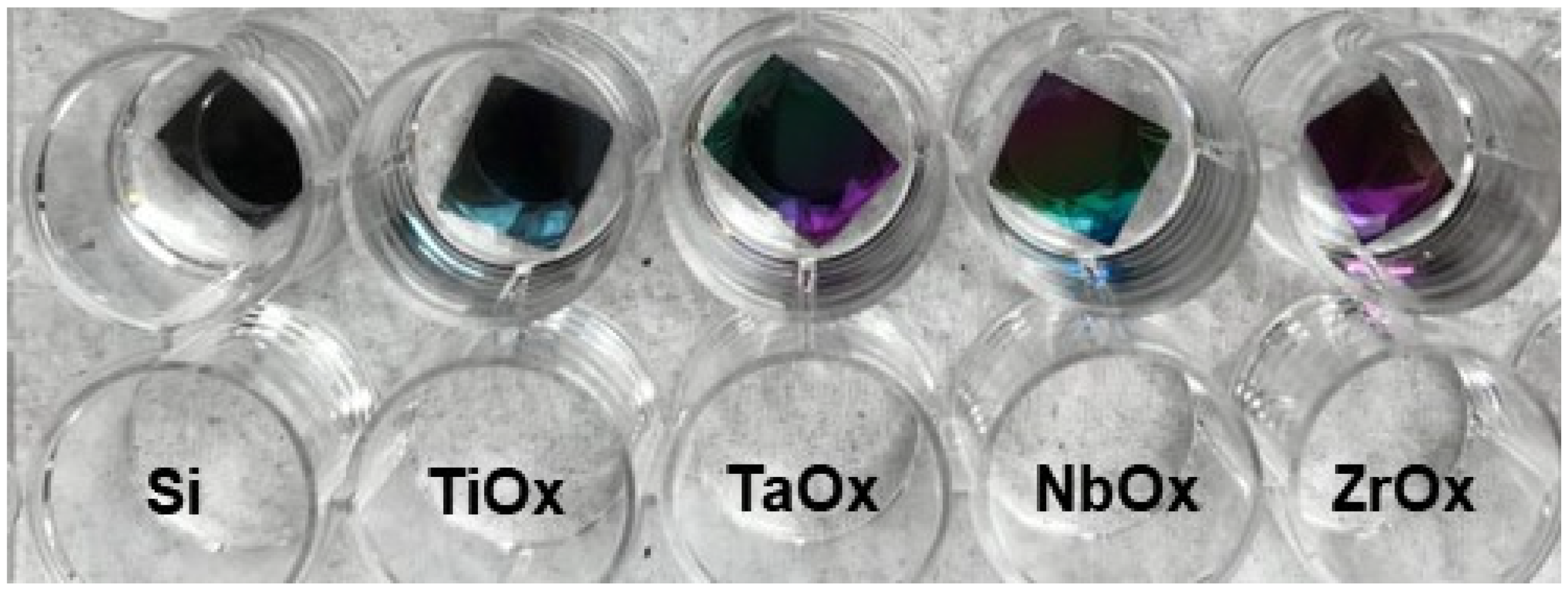
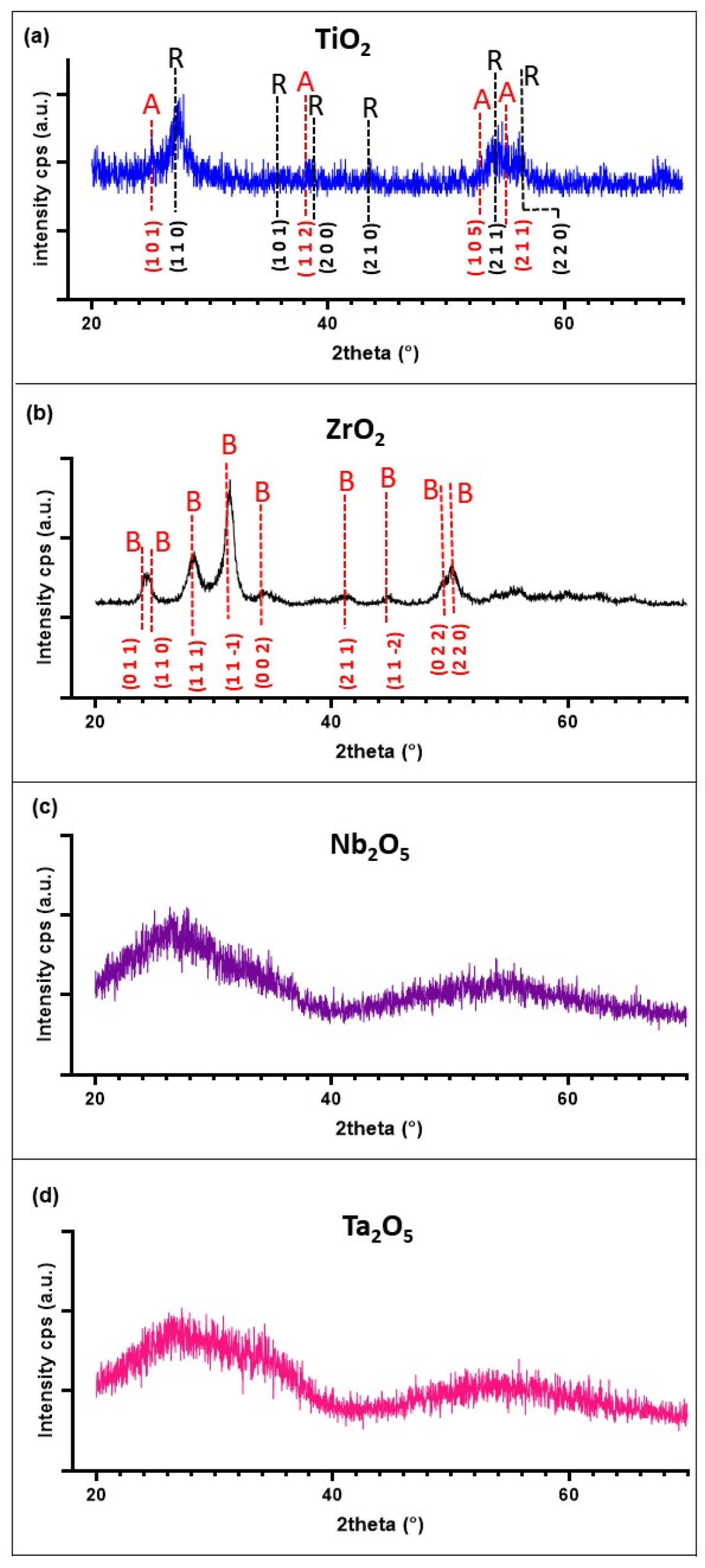
3.1. Metal Oxides Coatings Characterization
3.2. BM-MSC Characterization
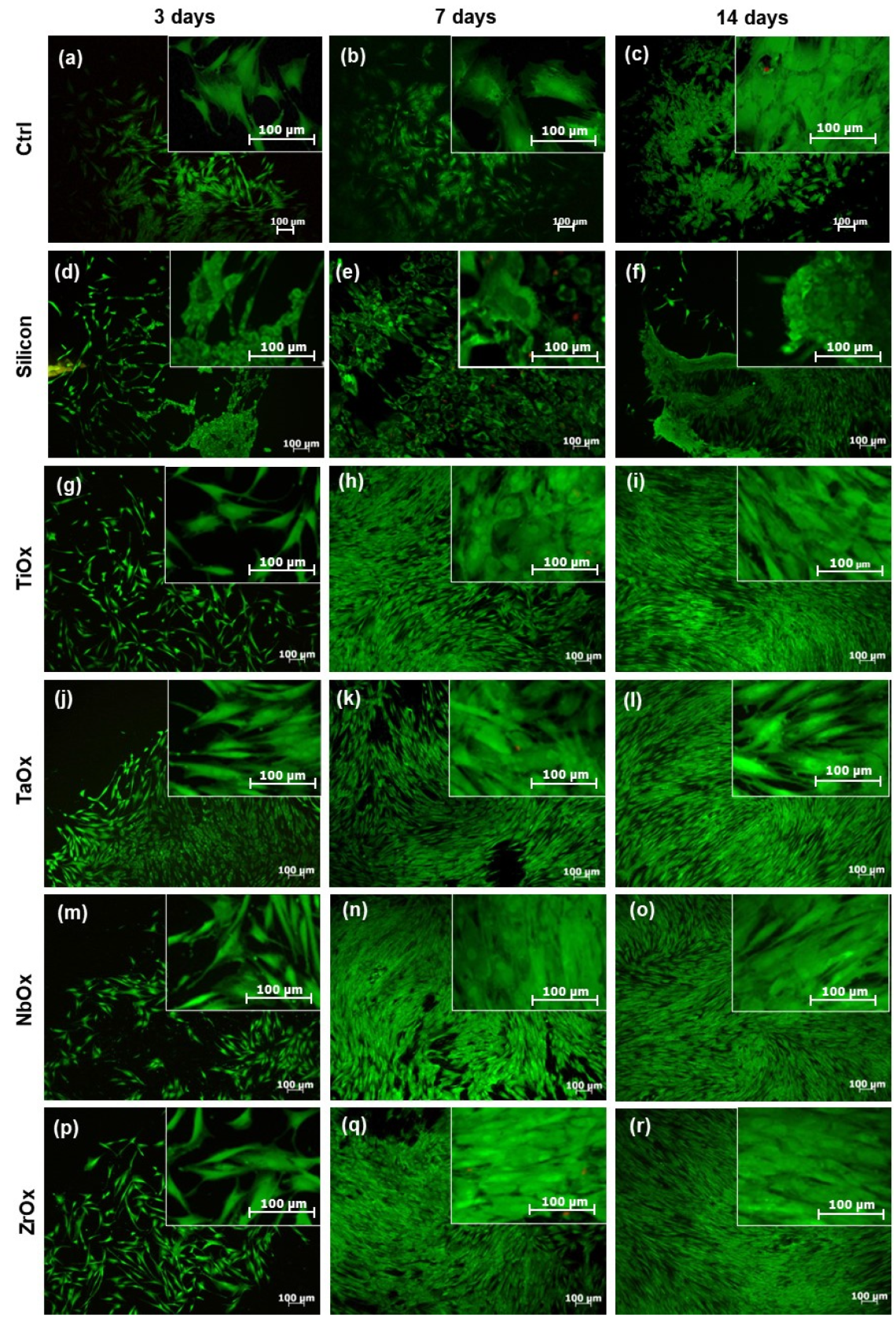
3.3. Viability of BM-MSC Cultured on the Surface of the Coatings
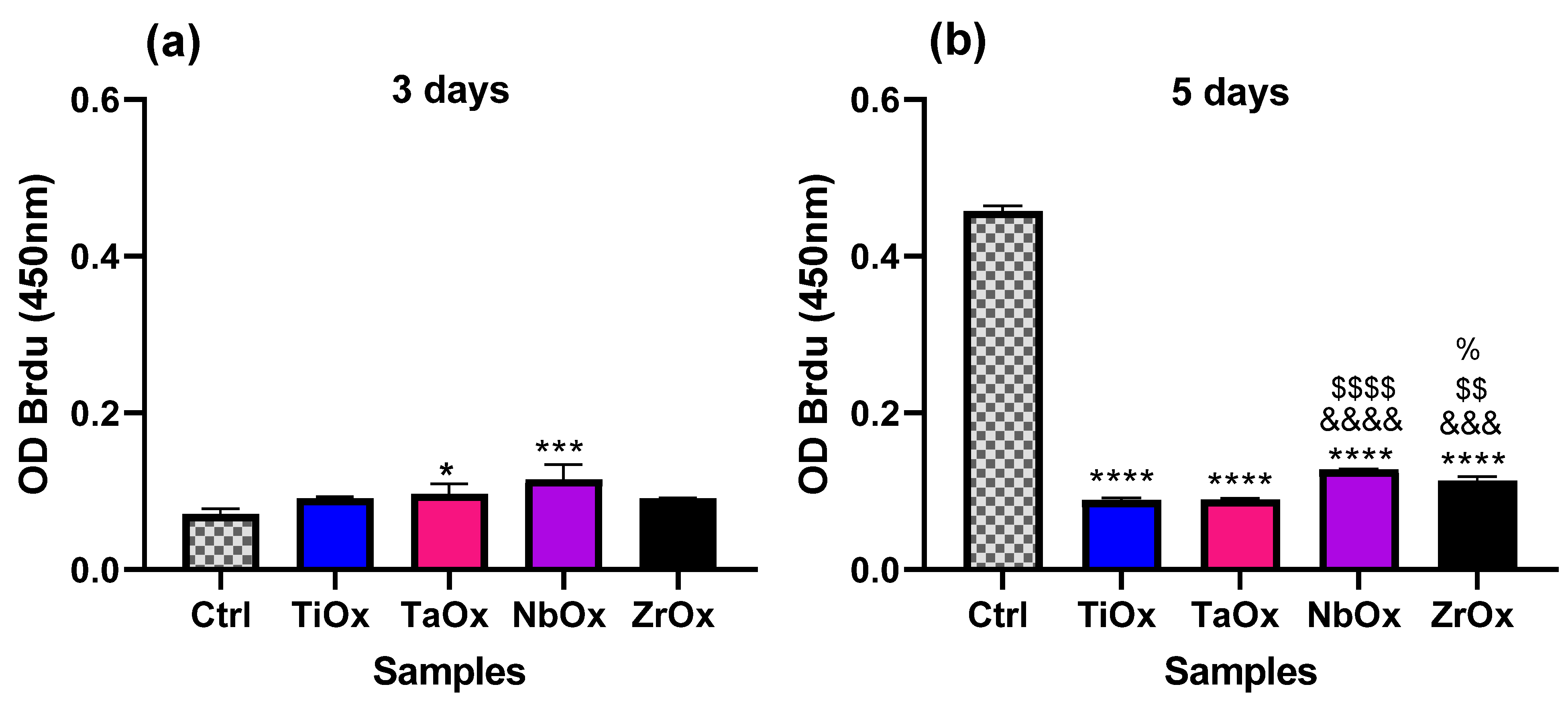
3.4. Proliferation of BM-MSC Cultured on the Coatings
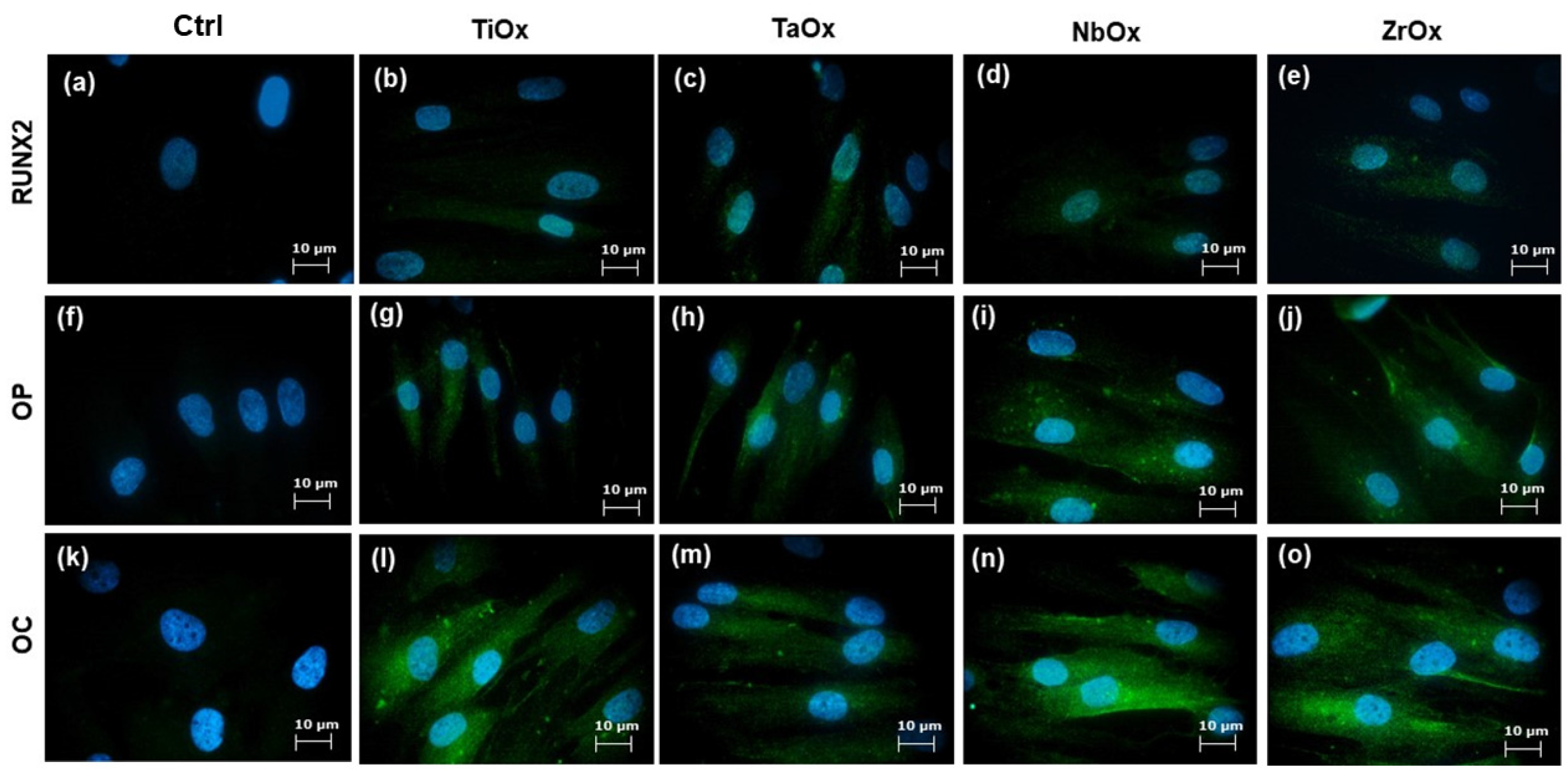
3.5. Osteogenic Properties of the Coatings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grün, N.G.; Holweg, P.L.; Donohue, N.; Klestil, T.; Weinberg, A.-M. Resorbable Implants in Pediatric Fracture Treatment. Innov. Surg. Sci. 2018, 3, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G.; Pandey, A. Design Approaches and Challenges for Biodegradable Bone Implants: A Review. Expert Rev. Med. Devices 2021, 18, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Siti Nur Hazwani, M.R.; Lim, L.X.; Lockman, Z.; Zuhailawati, H. Fabrication of Titanium-Based Alloys with Bioactive Surface Oxide Layer as Biomedical Implants: Opportunity and Challenges. Trans. Nonferrous Met. Soc. China Engl. Ed. 2022, 32, 1–44. [Google Scholar] [CrossRef]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of Implants Surface Modification in Osseointegration: A Systematic Review. J. Biomed. Mater. Res. Part A 2020, 108, 470–484. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, P. A Review on the Latest Advancements in the Non-Invasive Evaluation/Monitoring of Dental and Trans-Femoral Implants. Biomed. Eng. Lett. 2020, 10, 83–102. [Google Scholar] [CrossRef]
- Silva, R.C.S.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.S.; Santos, L.R.L.; Vasconcelos, N.F.; Machado, G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials 2022, 15, 3150. [Google Scholar] [CrossRef]
- Yadav, R.; Meena, A.; Patnaik, A. Biomaterials for Dental Composite Applications: A Comprehensive Review of Physical, Chemical, Mechanical, Thermal, Tribological, and Biological Properties. Polym. Adv. Technol. 2022, 33, 1762–1781. [Google Scholar] [CrossRef]
- Wang, L.; Ding, X.; Feng, W.; Gao, Y.; Zhao, S.; Fan, Y. Biomechanical Study on Implantable and Interventional Medical Devices. Acta Mech. Sin. Xuebao 2021, 37, 875–894. [Google Scholar] [CrossRef]
- Tipan, N.; Pandey, A.; Mishra, P. Selection and Preparation Strategies of Mg-Alloys and Other Biodegradable Materials for Orthopaedic Applications: A Review. Mater. Today Commun. 2022, 31, 103658. [Google Scholar] [CrossRef]
- Yoshinari, M. Future Prospects of Zirconia for Oral Implants—A Review. Dent. Mater. J. 2020, 39, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Johansson, C. Osteoinduction, Osteoconduction and Osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [Green Version]
- Melo-Fonseca, F.; Gasik, M.; Madeira, S.; Silva, F.S.; Miranda, G. Surface Characterization of Titanium-Based Substrates for Orthopaedic Applications. Mater. Charact. 2021, 177, 111161. [Google Scholar] [CrossRef]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial Coating of Implants in Orthopaedics and Trauma: A Classification Proposal in an Evolving Panorama. J. Orthop. Surg. Res. 2015, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sultana, A.; Zare, M.; Luo, H.; Ramakrishna, S. Surface Engineering Strategies to Enhance the in Situ Performance of Medical Devices Including Atomic Scale Engineering. Int. J. Mol. Sci. 2021, 22, 11788. [Google Scholar] [CrossRef]
- Stepanovska, J.; Matejka, R.; Rosina, J.; Bacakova, L.; Kolarova, H. Treatments for Enhancing the Biocompatibility of Titanium Implants. Biomed. Pap. 2020, 164, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Dehghanghadikolaei, A.; Fotovvati, B. Coating Techniques for Functional Enhancement of Metal Implants for Bone Replacement: A Review. Materials 2019, 12, 1795. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Kumar, R.; Kumar, S. Coatings on Orthopedic Implants to Overcome Present Problems and Challenges: A Focused Review. Mater. Today Proc. 2021, 45, 5269–5276. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Ramakrishna, S. Surface Engineering of Biomaterials in Orthopedic and Dental Implants: Strategies to Improve Osteointegration, Bacteriostatic and Bactericidal Activities. Biotechnol. J. 2021, 16, 2000116. [Google Scholar] [CrossRef]
- Blendinger, F.; Seitz, D.; Ottenschläger, A.; Fleischer, M.; Bucher, V. Atomic Layer Deposition of Bioactive TiO2Thin Films on Polyetheretherketone for Orthopedic Implants. ACS Appl. Mater. Interfaces 2021, 13, 3536–3546. [Google Scholar] [CrossRef]
- Chen, C.S.; Chang, J.H.; Srimaneepong, V.; Wen, J.Y.; Tung, O.H.; Yang, C.H.; Lin, H.C.; Lee, T.H.; Han, Y.; Huang, H.H. Improving the in Vitro Cell Differentiation and in Vivo Osseointegration of Titanium Dental Implant through Oxygen Plasma Immersion Ion Implantation Treatment. Surf. Coatings Technol. 2020, 399, 126125. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Surmeneva, M.A.; Ignatov, V.P.; Peltek, O.O.; Goncharenko, A.A.; Muslimov, A.R.; Timin, A.S.; Tyurin, A.I.; Ivanov, Y.F.; Grandini, C.R.; et al. Comprehensive Characterization of Titania Nanotubes Fabricated on Ti-Nb Alloys: Surface Topography, Structure, Physicomechanical Behavior, and a Cell Culture Assay. ACS Biomater. Sci. Eng. 2020, 6, 1487–1499. [Google Scholar] [CrossRef]
- Gomez Sanchez, A.; Katunar, M.R.; Pastore, J.I.; Tano de la Hoz, M.F.; Ceré, S. Evaluation of Annealed Titanium Oxide Nanotubes on Titanium: From Surface Characterization to in Vivo Assays. J. Biomed. Mater. Res. Part A 2021, 109, 1088–1100. [Google Scholar] [CrossRef]
- İzmir, M.; Ercan, B. Anodization of Titanium Alloys for Orthopedic Applications. Front. Chem. Sci. Eng. 2019, 13, 28–45. [Google Scholar] [CrossRef]
- Mathai, S.; Shaji, P.S. Different Coating Methods of Titanium Dioxide on Metal Substrates for Orthopedic and Dental Applications: A Review. Asian J. Chem. 2022, 34, 9–17. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Surface Modification of Titanium and Titanium Alloy by Plasma Electrolytic Oxidation Process for Biomedical Applications: A Review. Mater. Today Proc. 2021, 46, 594–602. [Google Scholar] [CrossRef]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review. Mater. Today Proc. 2020, 39, 84–90. [Google Scholar] [CrossRef]
- Prestat, M.; Thierry, D. Corrosion of Titanium under Simulated Inflammation Conditions: Clinical Context and in Vitro Investigations. Acta Biomater. 2021, 136, 72–87. [Google Scholar] [CrossRef]
- Burstein, G.T.; Souto, R.M. Observations of Localised Instability of Passive Titanium in Chloride Solution. Electrochim. Acta 1995, 40, 1881–1888. [Google Scholar] [CrossRef]
- Vrchovecká, K.; Weiser, A.; Přibyl, J.; Kuta, J.; Holzer, J.; Pávková-Goldbergová, M.; Sobola, D.; Dlouhý, A. A Release of Ti-Ions from Nanostructured Titanium Oxide Surfaces. Surf. Interfaces 2022, 29, 101699. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General Review of Titanium Toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Comino-Garayoa, R.; Brinkmann, J.C.B.; Peláez, J.; López-Suárez, C.; Martínez-González, J.M.; Suárez, M.J. Allergies to Titanium Dental Implants: What Do We Really Know about Them? A Scoping Review. Biology 2020, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Alhamad, M.; Ampadi Ramachandran, R.; Shukla, A.; Barão, V.A.; Sukotjo, C.; Mathew, M.T. Peri-Implantitis in Relation to Titanium Corrosion: Current Status and Future Perspectives. J. Bio-Tribo-Corros. 2022, 8, 46. [Google Scholar] [CrossRef]
- Ding, Z.; Tang, Y.; Liu, L.; Ding, Z.; Tan, Y.; He, Q. Improving the Adhesive, Mechanical, Tribological Properties and Corrosion Resistance of Reactive Sputtered Tantalum Oxide Coating on Ti6Al4V Alloy via Introducing Multiple Interlayers. Ceram. Int. 2022, 48, 5983–5994. [Google Scholar] [CrossRef]
- Chen, Y.W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in Biomedical Applications. Expert Rev. Med. Devices 2016, 13, 945–963. [Google Scholar] [CrossRef]
- Mani, G.; Porter, D.; Grove, K.; Collins, S.; Ornberg, A.; Shulfer, R. A Comprehensive Review of Biological and Materials Properties of Tantalum and Its Alloys. J. Biomed. Mater. Res. Part A 2022, 110, 1291–1306. [Google Scholar] [CrossRef]
- Grobelny, M.; Kalisz, M.; Mazur, M.; Wojcieszak, D.; Kaczmarek, D.; Domaradzki, J.; Świniarski, M.; Mazur, P. Functional Nb2O5 Film and Nb2O5 + CuO, Nb2O5 + Graphene, Nb2O5 + CuO + Graphene Composite Films to Modify the Properties of Ti6Al4V Titanium Alloy. Thin Solid Films 2016, 616, 64–72. [Google Scholar] [CrossRef]
- Fomina, M.; Koshuro, V.; Shumilin, A.; Voyko, A.; Zakharevich, A.; Skaptsov, A.; Steinhauer, A.; Fomin, A. Functionally Graded “Ti-Base + (Ta, Ta2O5)-Coatings” Structure and Its Production Using Induction Heat Treatment. Compos. Struct. 2020, 234, 111688. [Google Scholar] [CrossRef]
- Sarraf, M.; Razak, B.A.; Nasiri-Tabrizi, B.; Dabbagh, A.; Kasim, N.H.A.; Basirun, W.J.; Bin Sulaiman, E. Nanomechanical Properties, Wear Resistance and in-Vitro Characterization of Ta2O5 Nanotubes Coating on Biomedical Grade Ti–6Al–4V. J. Mech. Behav. Biomed. Mater. 2017, 66, 159–171. [Google Scholar] [CrossRef]
- Pereira, B.L.; da Luz, A.R.; Lepienski, C.M.; Mazzaro, I.; Kuromoto, N.K. Niobium Treated by Plasma Electrolytic Oxidation with Calcium and Phosphorus Electrolytes. J. Mech. Behav. Biomed. Mater. 2018, 77, 347–352. [Google Scholar] [CrossRef]
- Pieralli, S.; Kohal, R.J.; Lopez Hernandez, E.; Doerken, S.; Spies, B.C. Osseointegration of Zirconia Dental Implants in Animal Investigations: A Systematic Review and Meta-Analysis. Dent. Mater. 2018, 34, 171–182. [Google Scholar] [CrossRef]
- de Almeida Bino, M.C.; Eurídice, W.A.; Gelamo, R.V.; Leite, N.B.; da Silva, M.V.; de Siervo, A.; Pinto, M.R.; de Almeida Buranello, P.A.; Moreto, J.A. Structural and Morphological Characterization of Ti6Al4V Alloy Surface Functionalization Based on Nb2O5 Thin Film for Biomedical Applications. Appl. Surf. Sci. 2021, 55, 149739. [Google Scholar] [CrossRef]
- Beline, T.; da Silva, J.H.D.; Matos, A.O.; Azevedo Neto, N.F.; de Almeida, A.B.; Nociti Júnior, F.H.; Leite, D.M.G.; Rangel, E.C.; Barão, V.A.R. Tailoring the Synthesis of Tantalum-Based Thin Films for Biomedical Application: Characterization and Biological Response. Mater. Sci. Eng. C 2019, 101, 111–119. [Google Scholar] [CrossRef]
- Wang, X.; Ning, B.; Pei, X. Tantalum and Its Derivatives in Orthopedic and Dental Implants: Osteogenesis and Antibacterial Properties. Colloids Surf. B Biointerfaces 2021, 208, 112055. [Google Scholar] [CrossRef]
- Almeida Alves, C.F.; Fialho, L.; Marques, S.M.; Pires, S.; Rico, P.; Palacio, C.; Carvalho, S. MC3T3-E1 Cell Response to Microporous Tantalum Oxide Surfaces Enriched with Ca, P and Mg. Mater. Sci. Eng. C 2021, 124, 112008. [Google Scholar] [CrossRef]
- Antonini, L.M.; Menezes, T.L.; dos Santos, A.G.; Takimi, A.S.; Villarinho, D.J.; dos Santos, B.P.; Camassola, M.; Marcuzzo, J.S.; de Fraga Malfatti, C. Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells on Anodized Niobium Surface. J. Mater. Sci. Mater. Med. 2019, 30, 2–13. [Google Scholar] [CrossRef]
- Fialho, L.; Grenho, L.; Fernandes, M.H.; Carvalho, S. Porous Tantalum Oxide with Osteoconductive Elements and Antibacterial Core-Shell Nanoparticles: A New Generation of Materials for Dental Implants. Mater. Sci. Eng. C 2021, 120, 111761. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Zhang, S.; Liu, H. Tantalum Coated on Titanium Dioxide Nanotubes by Plasma Spraying Enhances Cytocompatibility for Dental Implants. Surf. Coatings Technol. 2020, 382, 125161. [Google Scholar] [CrossRef]
- Horandghadim, N.; Khalil-Allafi, J.; Urgen, M. Effect of Ta2O5 Content on the Osseointegration and Cytotoxicity Behaviors in Hydroxyapatite-Ta2O5 Coatings Applied by EPD on Superelastic NiTi Alloys. Mater. Sci. Eng. C 2019, 102, 683–695. [Google Scholar] [CrossRef]
- Huang, H.L.; Tsai, M.T.; Chang, Y.Y.; Lin, Y.J.; Hsu, J.T. Fabrication of a Novel Ta(Zn)O Thin Film on Titanium by Magnetron Sputtering and Plasma Electrolytic Oxidation for Cell Biocompatibilities and Antibacterial Applications. Metals 2020, 10, 649. [Google Scholar] [CrossRef]
- Vitti, R.P.; Catelan, A.; Amaral, M.; Pacheco, R.R. Zirconium in dentistry. Adv. Dent. Biomater. 2019, 317–345. [Google Scholar] [CrossRef]
- Saleem, S.; Ahmad, R.; Ayub, R.; Ikhlaq, U.; Jin, W.; Chu, P.K. Investigation of Nano-Structured Zirconium Oxide Film on Ti6Al4V Substrate to Improve Tribological Properties Prepared by PIII&D. Appl. Surf. Sci. 2017, 394, 586–597. [Google Scholar] [CrossRef]
- Maminskas, J.; Pilipavicius, J.; Staisiunas, E.; Baranovas, G.; Alksne, M.; Daugela, P.; Juodzbalys, G. Novel Yttria-Stabilized Zirconium Oxide and Lithium Disilicate Coatings on Titanium Alloy Substrate for Implant Abutments and Biomedical Application. Materials 2020, 13, 2070. [Google Scholar] [CrossRef]
- Peron, M.; Bertolini, R.; Cogo, S. On the Corrosion, Stress Corrosion and Cytocompatibility Performances of ALD TiO2 and ZrO2 Coated Magnesium Alloys. J. Mech. Behav. Biomed. Mater. 2022, 125, 104945. [Google Scholar] [CrossRef]
- Peron, M.; Cogo, S.; Bjelland, M.; Bin Afif, A.; Dadlani, A.; Greggio, E.; Berto, F.; Torgersen, J. On the Evaluation of ALD TiO2, ZrO2 and HfO2 Coatings on Corrosion and Cytotoxicity Performances. J. Magnes. Alloy. 2021, 9, 1806–1819. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Visai, L.; Khalil-Allafi, J. Progress in Niobium Oxide-Containing Coatings for Biomedical Applications: A Critical Review. ACS Omega 2022, 7, 9088–9107. [Google Scholar] [CrossRef]
- Senocak, T.C.; Ezirmik, K.V.; Aysin, F.; Simsek Ozek, N.; Cengiz, S. Niobium-Oxynitride Coatings for Biomedical Applications: Its Antibacterial Effects and in-Vitro Cytotoxicity. Mater. Sci. Eng. C 2021, 120, 111662. [Google Scholar] [CrossRef]
- Almaguer-Flores, A.; Silva-Bermudez, P.; Galicia, R.; Rodil, S.E. Bacterial Adhesion on Amorphous and Crystalline Metal Oxide Coatings. Mater. Sci. Eng. C 2015, 57. [Google Scholar] [CrossRef]
- Silva-Bermudez, P.; Almaguer-Flores, A.; Garcia, V.I.; Olivares-Navarrete, R.; Rodil, S.E. Enhancing the Osteoblastic Differentiation through Nanoscale Surface Modifications. J. Biomed. Mater. Res. Part A 2017, 105, 498–509. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Huang, H.-L.; Chen, H.-J.; Lai, C.-H.; Wen, C.-Y. Antibacterial Properties and Cytocompatibility of Tantalum Oxide Coatings. Surf. Coatings Technol. 2014, 259, 193–198. [Google Scholar] [CrossRef]
- Donkov, N.; Zykova, A.; Safonov, V.; Yakovin, S.; Dudin, S.; Melnikova, G.; Petrovskaya, A.; Kuznetsova, T.; Chizhik, S.A. Antibacterial Properties of Ta-Based Ceramic Coatings Deposited by Magnetron Sputtering. J. Phys. Conf. Ser. 2021, 1859, 012062. [Google Scholar] [CrossRef]
- Huang, H.L.; Tsai, M.T.; Lin, Y.J.; Chang, Y.Y. Antibacterial and Biological Characteristics of Tantalum Oxide Coated Titanium Pretreated by Plasma Electrolytic Oxidation. Thin Solid Films 2019, 688, 137268. [Google Scholar] [CrossRef]
- Chen, X.; Fan, H.; Deng, X.; Wu, L.; Yi, T.; Gu, L.; Zhou, C.; Fan, Y.; Zhang, X. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarakinos, K.; Alami, J.; Konstantinidis, S. High Power Pulsed Magnetron Sputtering: A Review on Scientific and Engineering State of the Art. Surf. Coat. Technol. 2010, 204, 1661–1684. [Google Scholar] [CrossRef]
- Greene, J.E. Tracing the Recorded History of Thin-Film Sputter Deposition: From the 1800s to 2017. J. Vac. Sci. Technol. A Vac. Surf. Film. 2017, 35, 05C204. [Google Scholar] [CrossRef] [Green Version]
- Gudmundsson, J.T. Physics and Technology of Magnetron Sputtering Discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Lin, J.; Sproul, W.D.; Moore, J.J.; Wu, Z.; Lee, S.; Chistyakov, R.; Abraham, B. Recent Advances in Modulated Pulsed Power Magnetron Sputtering for Surface Engineering. JOM 2011, 63, 48–58. [Google Scholar] [CrossRef]
- Vetter, J.; Eriksson, A.O.; Reiter, A.; Derflinger, V.; Kalss, W. Quo Vadis: Alcr-based Coatings in Industrial Applications. Coatings 2021, 11, 344. [Google Scholar] [CrossRef]
- Larhlimi, H.; Ghailane, A.; Makha, M.; Alami, J. Magnetron Sputtered Titanium Carbide-Based Coatings: A Review of Science and Technology. Vacuum 2022, 197, 110853. [Google Scholar] [CrossRef]
- Behera, A.; Rajak, D.K.; Kolahchi, R.; Scutaru, M.L.; Pruncu, C.I. Current Global Scenario of Sputter Deposited NiTi Smart Systems. J. Mater. Res. Technol. 2020, 9, 14582–14598. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, W.; Li, B.; Wang, C.; Kuang, T.; Li, Y. Physical Vapor Deposition Technology for Coated Cutting Tools: A Review. Ceram. Int. 2020, 46, 18373–18390. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering Physical Vapour Deposition (PVD) Coatings: A Critical Review on Process Improvement Andmarket Trend Demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef] [Green Version]
- Olivares-Navarrete, R.; Hyzy, S.L.; Berg, M.E.; Schneider, J.M.; Hotchkiss, K.; Schwartz, Z.; Boyan, B.D. Osteoblast Lineage Cells Can Discriminate Microscale Topographic Features on Titanium–Aluminum–Vanadium Surfaces. Ann. Biomed. Eng. 2014, 42, 2551–2561. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.B.; Slosar, P.; Schwartz, Z.; Cohen, D.J.; Goodman, S.B.; Anderson, P.A.; Boyan, B.D. A Review of Biomimetic Topographies and Their Role in Promoting Bone Formation and Osseointegration: Implications for Clinical Use. Biomimetics 2022, 7, 7020046. [Google Scholar] [CrossRef]
- Anselme, K.; Ploux, L.; Ponche, A. Cell/Material Interfaces: Influence of Surface Chemistry and Surface Topography on Cell Adhesion. J. Adhes. Sci. Technol. 2010, 24, 831–852. [Google Scholar] [CrossRef]
- Luo, J.; Walker, M.; Xiao, Y.; Donnelly, H.; Dalby, M.J.; Salmeron-Sanchez, M. The Influence of Nanotopography on Cell Behaviour through Interactions with the Extracellular Matrix—A Review. Bioact. Mater. 2022, 15, 145–159. [Google Scholar] [CrossRef]
- Silva-Bermudez, P.; Muhl, S.; Rodil, S.E. A Comparative Study of Fibrinogen Adsorption onto Metal Oxide Thin Films. Appl. Surf. Sci. 2013, 282, 351–362. [Google Scholar] [CrossRef]
- Silva-Bermudez, P.; Rodil, S.E. An Overview of Protein Adsorption on Metal Oxide Coatings for Biomedical Implants. Surf. Coat. Technol. 2013, 233, 147–158. [Google Scholar] [CrossRef]
- Akterian, S. Evaluating the Vapour Evaporation from the Surface of Pure Organic Solvents and Their Mixtures. Food Sci. Appl. Biotechnol. 2020, 3, 77. [Google Scholar] [CrossRef]
- Ansari, S.; Ito, K.; Hofmann, S. Alkaline Phosphatase Activity of Serum Affects Osteogenic Differentiation Cultures. ACS Omega 2022, 7, 12724–12733. [Google Scholar] [CrossRef]
- Nobles, K.P.; Janorkar, A.V.; Williamson, R.S. Surface Modifications to Enhance Osseointegration–Resulting Material Properties and Biological Responses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1909–1923. [Google Scholar] [CrossRef]
- Lincks, J.; Boyan, B.D.; Blanchard, C.R.; Lohmann, C.H.; Liu, Y.; Cochran, D.L.; Dean, D.D.; Schwartz, Z. Response of MG63 Osteoblast-like Cells to Titanium and Titanium Alloy Is Dependent on Surface Roughness and Composition. Biomater. Silver Jubil. Compend. 1998, 19, 147–160. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; McLachlan, T.; Cai, Y.; Hyzy, S.L.; Schneider, J.M.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. Differential Responses of Osteoblast Lineage Cells to Nanotopographically-Modified, Microroughened Titanium-Aluminum-Vanadium Alloy Surfaces. Biomaterials 2012, 33, 8986–8994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.M.; McLachlan, T.; Stephan, I.; Geis-Gerstorfer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The Roles of Titanium Surface Micro/Nanotopography and Wettability on the Differential Response of Human Osteoblast Lineage Cells. Acta Biomater. 2013, 9, 6268–6277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zareidoost, A.; Yousefpour, M.; Ghasemi, B.; Amanzadeh, A. The Relationship of Surface Roughness and Cell Response of Chemical Surface Modification of Titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Silva-Bermudez, P.; Rodil, S.E.; Muhl, S. Albumin Adsorption on Oxide Thin Films Studied by Spectroscopic Ellipsometry. Appl. Surf. Sci. 2011, 258, 1711–1718. [Google Scholar] [CrossRef]
- Al-Amin, M.; Abdul-Rani, A.M.; Danish, M.; Rubaiee, S.; Mahfouz, A.B.; Thompson, H.M.; Ali, S.; Unune, D.R.; Sulaiman, M.H. Investigation of Coatings, Corrosion and Wear Characteristics of Machined Biomaterials through Hydroxyapatite Mixed-Edm Process: A Review. Materials 2021, 14, 3597. [Google Scholar] [CrossRef]
- Torres-Sanchez, C.; Alabort, E.; Wang, J.; Norrito, M.; Conway, P.P. In-Silico Design and Experimental Validation of TiNbTaZrMoSn to Assess Accuracy of Mechanical and Biocompatibility Predictive Models. J. Mech. Behav. Biomed. Mater. 2021, 124, 104858. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Kang, Y.; Jeon, D.G.; Albrektsson, T. Bone Reactions to Oxidized Titanium Implants with Electrochemical Anion Sulphuric Acid and Phosphoric Acid Incorporation. Clin. Implant Dent. Relat. Res. 2002, 4, 78–87. [Google Scholar] [CrossRef]
- Shokuhfar, T.; Hamlekhan, A.; Chang, J.Y.; Choi, C.K.; Sukotjo, C.; Friedrich, C. Biophysical Evaluation of Cells on Nanotubular Surfaces: The Effects of Atomic Ordering and Chemistry. Int. J. Nanomed. 2014, 9, 3737–3748. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhou, W.; Zhou, X.; Zhong, X.; Zhang, X.; Wan, P.; Zhu, B.; Chen, W. The Anatase Phase of Nanotopography Titania Plays an Important Role on Osteoblast Cell Morphology and Proliferation. J. Mater. Sci. Mater. Med. 2008, 19, 3465–3472. [Google Scholar] [CrossRef]
- Jarmar, T.; Palmquist, A.; Brånemark, R.; Hermansson, L.; Engqvist, H.; Thomsen, P. Characterization of the Surface Properties of Commercially Available Dental Implants Using Scanning Electron Microscopy, Focused Ion Beam, and High-Resolution Transmission Electron Microscopy. Clin. Implant Dent. Relat. Res. 2008, 10, 11–22. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of Wettability and Surface Functional Groups on Protein Adsorption and Cell Adhesion Using Well-Defined Mixed Self-Assembled Monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Silva-Bermudez, P.; Muhl, S.; Rivera, M.; Rodil, S.E. A look into the interaction of metal oxide thin films with biological media: Albumin and Fibrinogen adsorption. MRS Proc. 2011, 1376, 45–52. [Google Scholar] [CrossRef]
- Ochsenbein, A.; Chai, F.; Winter, S.; Traisnel, M.; Breme, J.; Hildebrand, H.F. Osteoblast Responses to Different Oxide Coatings Produced by the Sol-Gel Process on Titanium Substrates. Acta Biomater. 2008, 4, 1506–1517. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of Surface Energy and Roughness on Cell Adhesion and Growth-Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Thiagarajan, L.; Abu-Awwad, H.A.D.M.; Dixon, J.E. Osteogenic Programming of Human Mesenchymal Stem Cells with Highly Efficient Intracellular Delivery of RUNX2. Stem Cells Transl. Med. 2017, 6, 2146–2159. [Google Scholar] [CrossRef]
- SIFFERT, R.S. The Role of Alkaline Phosphatase in Osteogenesis. J. Exp. Med. 1951, 93, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Giachelli, C.M.; Steitz, S. Osteopontin: A Versatile Regulator of Inflammation and Biomineralization. Matrix Biol. 2000, 19, 615–622. [Google Scholar] [CrossRef]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Hou, W.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, 1–9. [Google Scholar] [CrossRef]
- Makishi, S.; Yamazaki, T.; Ohshima, H. Osteopontin on the Dental Implant Surface Promotes Direct Osteogenesis in Osseointegration. Int. J. Mol. Sci. 2022, 23, 1039. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast Differentiation by RANKL and OPG Signaling Pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct Proliferative and Differentiated Stages of Murine MC3T3-E1 Cells in Culture: An in Vitro Model of Osteoblast Development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, J.; Xu, Y.; Qian, S.; Wang, B.; Liu, F.; Liu, X. Biological Behavior of Osteoblast-like Cells on Titania and Zirconia Films Deposited by Cathodic Arc Deposition. Biointerphases 2012, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.; Kim, Y.T.; Cho, H.; Ji, M.K.; Heo, J.; Lim, H.P. Atomic Layer Deposition of ZrO2 on Titanium Inhibits Bacterial Adhesion and Enhances Osteoblast Viability. Int. J. Nanomed. 2021, 16, 1509–1523. [Google Scholar] [CrossRef]
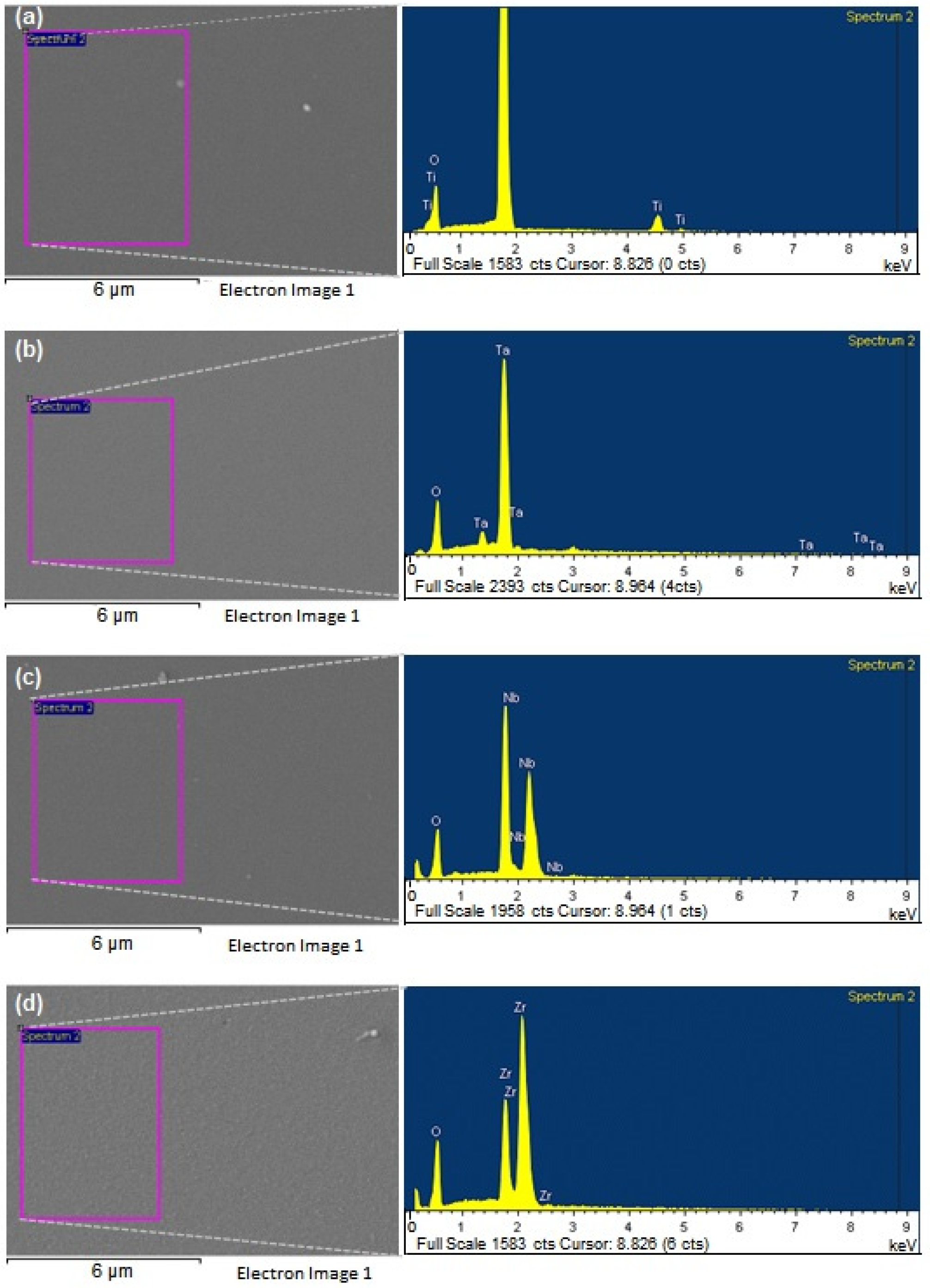




| Coating | Element | Weight % | Atomic % |
|---|---|---|---|
| TiOx | O | 57.8 ± 0.5 | 80.4 ± 0.3 |
| Ti | 42.2 ± 0.5 | 19.6 ± 0.3 | |
| TaOx | O | 17.5 ± 0.3 | 70.5 ± 0.5 |
| Ta | 82.6 ± 0.3 | 29.5 ± 0.5 | |
| NbOx | O | 31.7 ± 0.2 | 72.9 ± 0.2 |
| Nb | 68.3 ± 0.2 | 27.1 ± 0.2 | |
| ZrOx | O | 26.2 ± 0.2 | 66.9 ± 0.2 |
| Zr | 73.8 ± 0.2 | 33.1 ± 0.2 |
| Coating Property | TiOx | TaOx | NbOx | ZrOx |
|---|---|---|---|---|
| Sa * (nm) | 0.5 ± 0.1 | 0.4 ± 0.2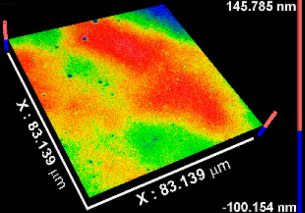 | 0.8 ± 0.1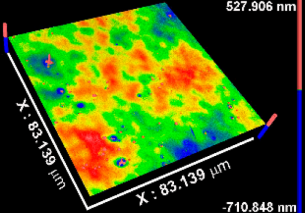 | 0.3 ± 0.1 |
| Thickness (nm) | 54.5 ± 7.3 | 346.5 ± 31.5 | 175.9 ± 15 | 227.7 ± 18.5 |
| Sample | Initial Measurement (0 min) | 1 min | 2 min | 3 min | 4 min | 5 min |
|---|---|---|---|---|---|---|
| Si |  |  |  |  |  |  |
| TiOx |  |  |  |  |  |  |
| TaOx |  |  |  |  |  |  |
| NbOx |  |  |  |  |  |  |
| ZrOx |  |  |  |  |  |  |
| Samples | WCA (°) | Surface Free Energy (mN/m) | γp/(γp +γd) (%) | ||
|---|---|---|---|---|---|
| γd/p | γd | γp | |||
| Si | 79.8 ± 0.9 | 30.0 ± 0.1 | 21.2 ± 0.4 | 8.8 ± 0.5 | 29.3 |
| TiOx | 87.6 ± 0.5 | 26.2 ± 0.1 | 21.2 ± 0.3 | 5.1 ± 0.2 | 19.4 |
| TaOx | 88.3 ± 0.9 | 22.6 ± 0.2 | 14.9 ± 0.3 | 7.7 ± 0.5 | 34.0 |
| NbOx | 86.3 ± 1.2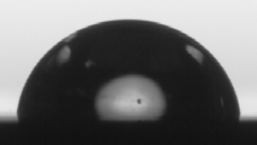 | 26.1 ± 0.1 | 19.5 ± 0.4 | 6.7 ± 0.5 | 25.7 |
| ZrOx | 89.2 ± 0.9 | 23.7 ± 0.1 | 18.0 ± 0.4 | 5.7 ± 0.4 | 24.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lizárraga, M.; García-López, J.; Rodil, S.E.; Ribas-Aparicio, R.M.; Silva-Bermudez, P. Evaluation of the Biocompatibility and Osteogenic Properties of Metal Oxide Coatings Applied by Magnetron Sputtering as Potential Biofunctional Surface Modifications for Orthopedic Implants. Materials 2022, 15, 5240. https://doi.org/10.3390/ma15155240
Fernández-Lizárraga M, García-López J, Rodil SE, Ribas-Aparicio RM, Silva-Bermudez P. Evaluation of the Biocompatibility and Osteogenic Properties of Metal Oxide Coatings Applied by Magnetron Sputtering as Potential Biofunctional Surface Modifications for Orthopedic Implants. Materials. 2022; 15(15):5240. https://doi.org/10.3390/ma15155240
Chicago/Turabian StyleFernández-Lizárraga, Mariana, Julieta García-López, Sandra E. Rodil, Rosa María Ribas-Aparicio, and Phaedra Silva-Bermudez. 2022. "Evaluation of the Biocompatibility and Osteogenic Properties of Metal Oxide Coatings Applied by Magnetron Sputtering as Potential Biofunctional Surface Modifications for Orthopedic Implants" Materials 15, no. 15: 5240. https://doi.org/10.3390/ma15155240
APA StyleFernández-Lizárraga, M., García-López, J., Rodil, S. E., Ribas-Aparicio, R. M., & Silva-Bermudez, P. (2022). Evaluation of the Biocompatibility and Osteogenic Properties of Metal Oxide Coatings Applied by Magnetron Sputtering as Potential Biofunctional Surface Modifications for Orthopedic Implants. Materials, 15(15), 5240. https://doi.org/10.3390/ma15155240







