Thermal Decomposition, Low Temperature Phase Transitions and Vapor Pressure of Less Common Ionic Liquids Based on the Bis(trifuoromethanesulfonyl)imide Anion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Samples
2.1.1. Synthesis of Halogenated ILs
2.1.2. Synthesis of 1-Decyl-1,4-diazabicyclo [2.2.2] Octan-1-ium Bromide [DABCO10+][Br−]
2.1.3. Synthesis of 3,3′-Dimethyl-1,1′-(1,3-phenylenedimethylene)-bis(1H-imidazolium) Dichloride [m-C6H4(CH2ImMe)+2][Cl−]2
2.1.4. Synthesis of 3,3’-Dibutyl-1,1’-(1,4-phenylenedimethylene)-bis (1H-imidazolium) Dibromide [p-C6H4 (CH2ImBu)+2][Br−]2
2.1.5. Synthesis of 1-Hexylpyridinium Bromide [C6Py+][Br−] IL
2.2. Synthesis of Fluorinated ILs
2.3. NMR Analysis
2.3.1. [DABCO10+][NTf2−]
2.3.2. [m-C6H4(CH2ImMe)2+][NTf2−]2
2.3.3. [p-C6H4(CH2ImBu)+2][NTf2−]2
2.3.4. [C6Py+][NTf2−]
2.4. Thermal Characterization of the Samples
3. Results and Discussion
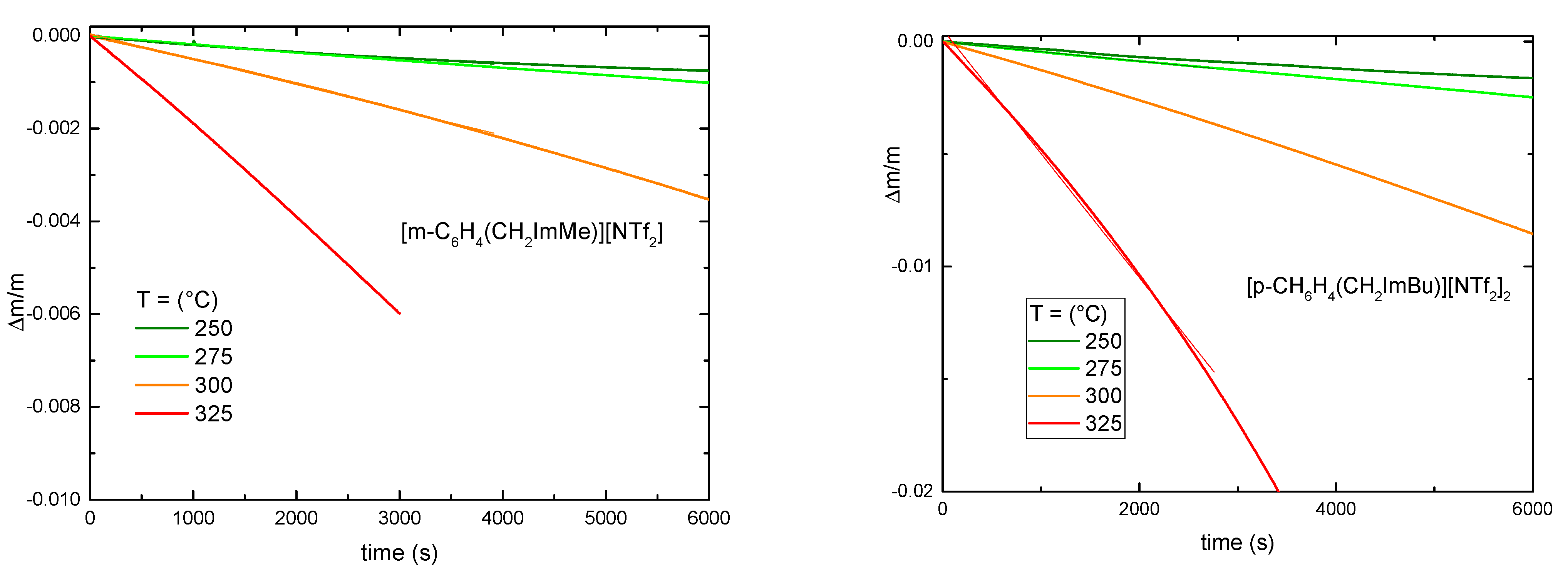
3.1. Thermal Decomposition
| Ionic Liquid | Td (°C) | Tg (°C) | Tm (°C) | |
|---|---|---|---|---|
| [EMI][NTf2] | 404 [24] | −14 [47] | ||
| [m- C6H4(CH2ImMe)][NTf2]2 | 398 [37] | −40 | 136 ± 18 | |
| [p-C6H4(CH2ImBu)][NTf2]2 | 384 | −42 | 147 ± 13 | |
| [N1113][NTf2] | 367 [24] | 13 [48] | ||
| [DABCO10][NTf2] | 354 | 38 | 147 ± 12 | |
| [C6Py][NTf2] | 353 | −75 | 1 | 180 ± 17 |
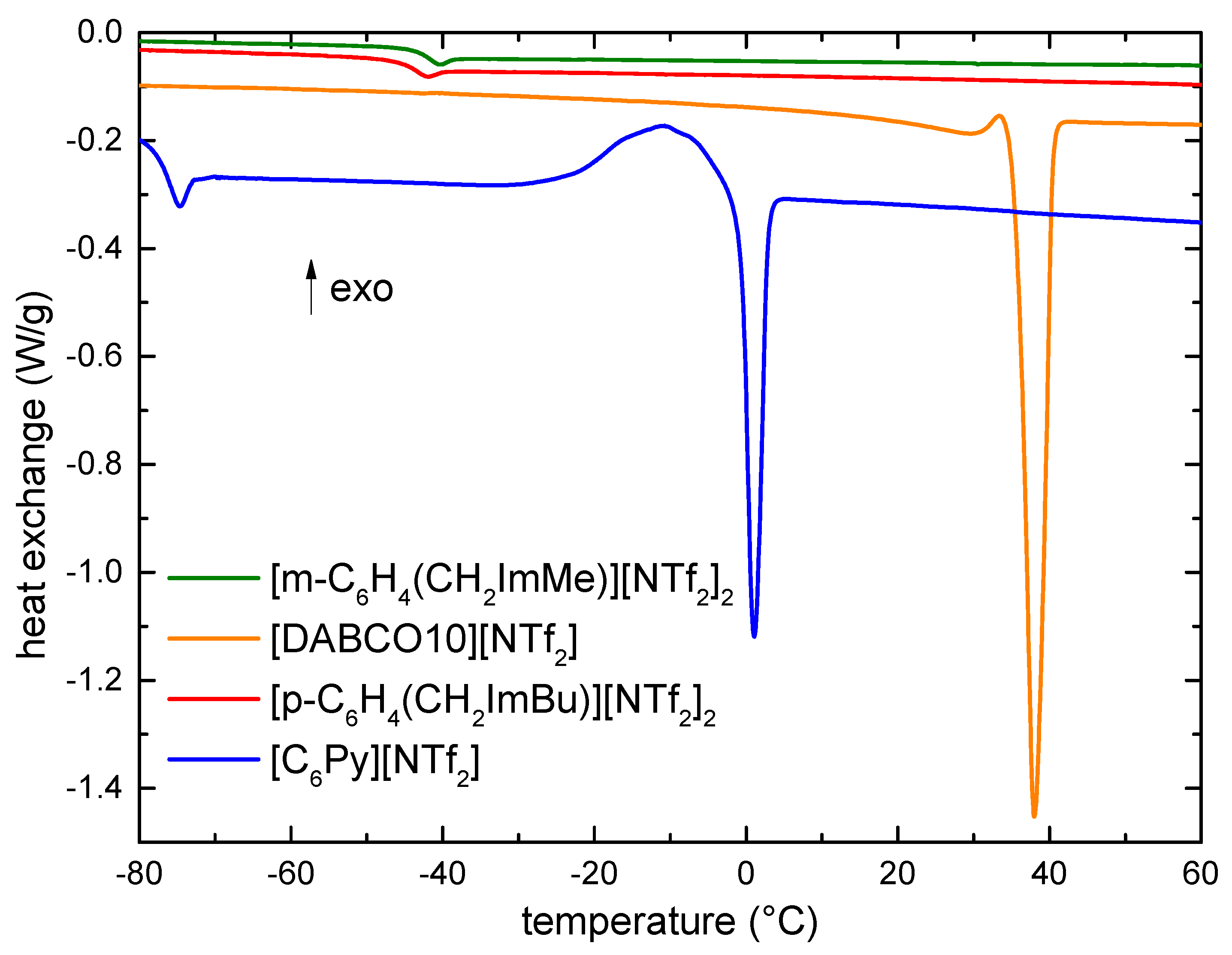
3.2. Low Temperature Phase Transitions
3.3. Vapor Pressure and Vaporization Enthalpy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paulechka, Y.U.; Zaitsau, D.H.; Kabo, G.J.; Strechan, A.A. Vapor pressure and thermal stability of ionic liquid 1-butyl-3-methylimidazolium bis(trifuoromethylsulfonyl)amide. Thermochim. Acta 2005, 439, 158–160. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Kabo, G.J.; Strechan, A.A.; Paulechka, Y.U.; Tschersich, A.; Verevkin, S.P.; Heintz, A. Experimental Vapor Pressures of 1-Alkyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imides and a Correlation Scheme for Estimation of Vaporization Enthalpies of Ionic Liquids. J. Phys. Chem. A 2006, 110, 7303–7306. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.A.A.; Lima, C.F.R.A.C.; Gomes, L.R.; Schröder, B.; Coutinho, J.A.P.; Marrucho, I.M.; Esperança, J.M.; Rebelo, L.P.N.; Shimizu, K.; Lopes, J.N.; et al. High-accuracy vapor pressure data of the extended [CnC1im][Ntf2] ionic liquid series: Trend changes and structural shifts. J. Phys. Chem. B 2011, 115, 10919–10926. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.A.A.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Vapor pressures of 1,3-dialkylimidazolium bis(trifluoromethylsulfonyl)imide ionic liquids with long alkyl chains. J. Chem. Phys. 2014, 141, 134502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, B.; Ciccioli, A.; Gigli, G.; Lapi, A.; Misceo, N.; Tanzi, L.; Ciprioti, S.V. Vaporization of the prototypical ionic liquid BMImNTf2 under equilibrium conditions: A multitechnique study. Phys. Chem. Chem. Phys. 2014, 16, 15653–15661. [Google Scholar] [CrossRef] [PubMed]
- Volpe, V.; Brunetti, B.; Gigli, G.; Lapi, A.; Ciprioti, S.V.; Ciccioli, A. Toward the Elucidation of the Competing Role of Evaporation and Thermal Decomposition in Ionic Liquids: A Multitechnique Study of the Vaporization Behavior of 1-Butyl-3-methylimidazolium Hexafluorophosphate under Effusion Conditions. J. Phys. Chem. B 2017, 121, 10382–10393. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Yermalayeu, A.V.; Emel’yanenko, V.N.; Butler, S.; Schubert, T.; Verevkin, S.P. Thermodynamics of imidazo-lium-based ionic liquids containing PF6 anions. J. Phys. Chem. B 2016, 120, 7949–7957. [Google Scholar] [CrossRef] [PubMed]
- Emel’Yanenko, V.N.; Verevkin, A.S.P.; Heintz, A. The Gaseous Enthalpy of Formation of the Ionic Liquid 1-Butyl-3-methylimidazolium Dicyanamide from Combustion Calorimetry, Vapor Pressure Measurements, and Ab Initio Calculations. J. Am. Chem. Soc. 2007, 129, 3930–3937. [Google Scholar] [CrossRef]
- Ravula, S.; Larm, N.E.; Mottaleb, M.A.; Heitz, M.P.; Baker, G.A. Vapour pressure mapping of ionic liquids and low-volatility fluids using graded isothermal thermogravimetric analysis. ChemEngineering 2019, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Rebelo, L.P.; Lopes, J.N.; Esperança, J.M.; Filipe, E. On the critical temperature, normal boiling point, and vapor pressure of ionic liquids. J. Phys. Chem. B 2005, 109, 6040–6043. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.P.; Hurst, C.; Jones, R.G.; Licence, P.; Lovelock, K.R.J.; Satterley, C.J.; Villar-Garcia, I.J. Vapourisation of ionic liquids. Phys. Chem. Chem. Phys. 2007, 9, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Verevkin, S.P. Predicting Enthalpy of Vaporization of Ionic Liquids: A Simple Rule for a Complex Property. Angew. Chem. Int. Ed. 2008, 47, 5071–5074. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Baker, G.; Dai, S. Isothermogravimetric Determination of the Enthalpies of Vaporization of 1-Alkyl-3-methylimidazolium Ionic Liquids. J. Phys. Chem. B 2008, 112, 10077–10081. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Emel’yanenko, V.N.; Heintz, A. A new method for the determination of vaporization enthalpies of ionic liquids at low temperatures. J. Phys. Chem. B 2011, 115, 12889–12895. [Google Scholar] [CrossRef]
- Rane, K.; Errington, J.R. Saturation Properties of 1-Alkyl-3-methylimidazolium Based Ionic Liquids. J. Phys. Chem. B 2014, 118, 8734–8743. [Google Scholar] [CrossRef] [PubMed]
- Zaitsau, D.; Plechkova, N.; Verevkin, S.P. Vaporization thermodynamics of ionic liquids with tetraalkylphosphonium cations. J. Chem. Thermodyn. 2019, 130, 204–212. [Google Scholar] [CrossRef]
- Schmitz, A.; Bülow, M.; Schmidt, D.; Zaitsau, D.H.; Junglas, F.; Knedel, T.-O.; Verevkin, S.P.; Held, C.; Janiak, T. Tetrahy-drothiophene-based ionic liquids: Synthesis and thermodynamic characterizations. ChemistryOpen 2021, 10, 153–163. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Gantman, M.; Schulz, P.; Wasserscheid, P.; Verevkin, S.P. Volatility of molten salts [Ph 4 P][NTf 2] and Cs[NTf 2]. ChemistryOpen 2021, 10, 199–204. [Google Scholar] [CrossRef]
- Wu, J.; Wu, W.; Liu, L.; Tong, J. Measurement of evaporation entropy, evaporation enthalpy, and Gibbs free energy for the [C4Dmim]Gly and [C4Dmim]Ala. J. Mol. Liq. 2022, 346, 117142. [Google Scholar] [CrossRef]
- Rodrigues, A.S.M.C.; Fernandes, A.M.; Dévemy, J.; Costa Gomes, M.C.; Santos, L.M.N.B.F. Fluorination effect in the volatility of imidazolium-based ionic liquids. J. Mol. Liq. 2019, 282, 385–391. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Verevkin, S.P. Imidazolium-based ionic liquids containing FAP anion: Thermodynamic study. J. Mol. Liq. 2019, 287, 110959. [Google Scholar] [CrossRef]
- Barulli, L.; Mezzetta, A.; Brunetti, B.; Guazzelli, L.; Vecchio Ciprioti, S.; Ciccioli, A. Evaporation thermodynamics of the tetraoctylphosphonium bis(trifluoromethansulfonyl)imide([P8888]NTf2) and tetraoctylphosphonium nonafluorobu-tane-1-sulfonate ([P8888]NFBS) ionic liquids. J. Mol. Liq. 2021, 333, 115892. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Verevkin, S.P. Vaporization thermodynamics of pyrrolidinium, pyridinium, and imidazolium-based ionic liquids bearing the bis(fluorosulfonyl)imide [FSI] and the bis(trifluoromethylsulfonyl)imide [NTf2] anions. Int. J. Thermophys. 2022, 43, 24. [Google Scholar] [CrossRef]
- Cimini, A.; Palumbo, O.; Simonetti, E.; De Francesco, M.; Appetecchi, G.B.; Fantini, S.; Lin, R.; Falgayrat, A.; Paolone, A. Decomposition temperatures and vapour pressures of selected ionic liquids for electrochemical applications. J. Therm. Anal. 2020, 142, 1791–1797. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Verevkin, S.P. Vaporization Thermodynamics of 1-Ethyl-3-Methylimidazolium Diethyl Phosphate. Russ. J. Inorg. Chem. 2020, 65, 699–702. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Yermalayeu, A.V.; Verevkin, S.P. Ionic liquids alkyl-imidazolium thiocyanates: Comprehensive thermochem-ical study. J. Mol. Liq. 2021, 321, 114284. [Google Scholar] [CrossRef]
- Królikowska, M.; Grzeszyk, K.; Skonieczny, M.; Królikowski, M.; Zawadzki, M. New Experimental Data on Thermodynamic Properties of the Aqueous Solution of N,N-Diethyl-N-methylammonium Bromide and N,N-Diethyl-N-methylammonium Methanesulfonate. J. Chem. Eng. Data 2021, 66, 2281–2294. [Google Scholar] [CrossRef]
- Królikowska, M.; Gos, N.; Skonieczny, M. Temperature and Composition Dependence of the Thermodynamic Properties of an Aqueous Solution of 1-Ethyl-3-methylimidazolium Formate and 1-Ethyl-3-methylimidazolium Acetate. J. Chem. Eng. Data 2021, 66, 3300–3314. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Siewert, R.; Pimerzin, A.A.; Bülow, M.; Held, C.; Loor, M.; Schulz, S.; Verevkin, S.P. Paving the way to solubility through volatility: Thermodynamics of imidazolium-based ionic liquids of the type [CnC1Im][I]. Fluid Phase Equilibria 2020, 522, 112767. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Siewert, R.; Pimerzinc, A.A.; Bülow, M.; Held, C.; Loor, M.; Schulz, S.; Verevkin, S.P. From volatility to solu-bility: Thermodynamics of imidazolium-based ionic liquids containing chloride and bromide anions. J. Mol. Liq. 2021, 323, 114998. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Topp, A.; Siegesmund, A.; Päpcke, A.; Köckerling, M.; Verevkin, S.P. In the footsteps of August Michaelis: Syntheses and Thermodynamics of Extremely Low-Volatile Ionic Liquids. ChemistryOpen 2020, 10, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Bülow, M.; Greive, M.; Zaitsau, D.H.; Verevkin, S.P.; Held, C. Extremely Low Vapor-Pressure Data as Access to PC-SAFT Parameter Estimation for Ionic Liquids and Modeling of Precursor Solubility in Ionic Liquids. ChemistryOpen 2021, 10, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Verevkin, S.P.; Zherikova, K.V.; Martynenko, E.A. Molecular versus ionic liquids: Development of a thermodynamic framework for predicting vaporization thermodynamics. J. Mol. Liq. 2022, 350, 118576. [Google Scholar] [CrossRef]
- Verevkin, S.P. Imidazolium Based Ionic Liquids: Unbiased Recovering of Vaporization Enthalpies from Infinite-Dilution Activity Coefficients. Molecules 2021, 26, 5873. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Ludwig, R. Aprotic Ionic Liquids: A Framework for Predicting Vaporization Thermodynamics. Molecules 2022, 27, 2321. [Google Scholar] [CrossRef] [PubMed]
- Fetouhi, B.; Haddad, B.; Brandán, S.A.; Paolone, A.; Villemin, D.; Boumediene, M.; Rahmouni, M.; Bresson, S. Synthesis, molecular structure, and properties of DABCO bromide based ionic liquid combining spectroscopic studies with DFT calculations. J. Mol. Struct. 2021, 1233, 130102. [Google Scholar] [CrossRef]
- Boumediene, M.; Haddad, B.; Paolone, A.; Drai, M.; Villemin, D.; Rahmouni, M.; Bresson, S.; Abbas, O. Synthesis, thermal stability, vibrational spectra and conformational studies of novel dicationic meta-xylyl linked bis-1-methylimidazolium ionic liquids. J. Mol. Struct. 2019, 1186, 68–79. [Google Scholar] [CrossRef]
- Haddad, B.; Paolone, A.; Villemin, D.; Lohier, J.F.; Drai, M.; Bresson, S.; Abbas, O.; Belarbi, E.H. para Xylyl bis 1 methylimidazolium bis (trifluoromethanesulfonyl) imide: Synthesis, crystal structure, thermal stability, vibrational studies. J. Mol. Liq. 2018, 260, 391–402. [Google Scholar] [CrossRef]
- Klingshirn, M.A.; Broker, G.A.; Holbrey, J.D.; Shaughnessy, K.H.; Rogers, R.D. Polar, non-coordinating ionic liquids as solvents for the alternating copolymerization of styrene and CO catalyzed by cationic palladium catalysts. Chem. Comm. 2002, 1394–1395. [Google Scholar] [CrossRef]
- Drai, M.; Mostefai, A.; Paolone, A.; Haddad, B.; Belarbi, E.H.; Villemin, D.; Bresson, S.; Abbas, O.; Chaker, Y.; Rahmouni, M. Synthesis, experimental and theoretical vibrational studies of 1-methyl and 1,2-dimethyl, 3-propyl imidazolium bis(trifluoromethanesulfonyl) imide. J. Chem. Sci. 2017, 129, 707–719. [Google Scholar] [CrossRef]
- Assenine, M.A.; Haddad, B.; Paolone, A.; Brandán, S.A.; Goussem, M.; Villemin, D.; Boumediene, M.; Rahmouni, M.; Bresson, S. Synthesis, thermal properties, vibrational spectra and computational studies of Trioctylmethylammonium bis (trifluoromethylsulfonyl) imide ionic liquid. J. Mol. Struct. 2021, 1232, 130085. [Google Scholar] [CrossRef]
- Chancelier, L.; Boyron, O.; Gutel, T.; Santini, C. Thermal stability of imidazolium-based ionic liquids. French-Ukrainian J. Chem. 2016, 4, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef] [PubMed]
- Bittner, B.; Wrobel, R.J.; Milchert, E. Physical properties of pyridinium ionic liquids. J. Chem. Thermodyn. 2012, 55, 159–165. [Google Scholar] [CrossRef]
- Turguła, A.; Graś, M.; Gabryelczyk, A.; Lota, G.; Pernak, J. Long-Chain Ionic Liquids Based on Monoquaternary DABCO Cations and TFSI Anions: Towards Stable Electrolytes for Electrochemical Capacitors. ChemPlusChem 2020, 85, 2679–2688. [Google Scholar] [CrossRef] [PubMed]
- Haddad, B.; Brandán, S.A.; Fetouhi, B.; Boumediene, M.; Paolone, A.; Villemin, D.; Rahmouni, M.; Bresson, S. Synthesis, NMR, vibrational spectroscopy, thermal and DFT studies of new DABCO hexafluorophosphate based ionic liquid. J. Mol. Struct. 2022, 1258, 132682. [Google Scholar] [CrossRef]
- Lee, J.S.; Bae, J.J.; Lee, H.; Quan, N.D.; Kim, H.S.; Kim, H. Ionic liquids as electrolytes for Li ion batteries. J. Ind. Eng. Chem. 2004, 10, 1086–1089. [Google Scholar]
- Palumbo, O.; Trequattrini, F.; Navarra, M.A.; Brubach, J.-B.; Roy, P.; Paolone, A. Tailoring the physical properties of the mixtures of ionic liquids: A microscopic point of view. Phys. Chem. Chem. Phys. 2017, 19, 8322–8329. [Google Scholar] [CrossRef]
- Crosthwaite, J.M.; Muldoon, M.J.; Dixon, J.K.; Anderson, J.L.; Brennecke, J.F. Phase transition and decomposition temperatures, heat capacities and viscosities of pyridinium ionic liquids. J. Chem. Thermodyn. 2005, 37, 559–568. [Google Scholar] [CrossRef]
- Jeremias, S.; Carewska, M.; Conte, L.; Passerini, S.; Appetecchi, G.B. Asymmetry effect of novel per(fluoroalkylsulfonyl)imide anions in pyrrolidinium ionic liquids. RSC Adv. 2013, 3, 17755–17761. [Google Scholar] [CrossRef]



| Name of Cation and Acronym | Structure of Cation |
|---|---|
| [DABCO10+]:1-decyl-1,4-diazabicyclo[2.2.2]octan-1-ium | 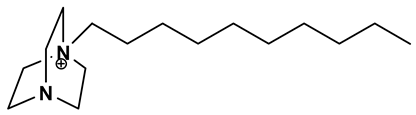 |
| [m-C6H4(CH2ImMe)+2]:3,3′-dimethyl-1,1′-(1,3-phenylenedimethylene)-bis(1H-imidazolium) | 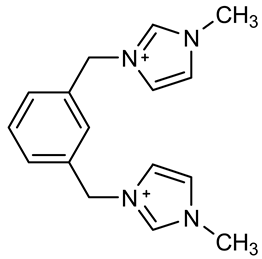 |
| [p-C6H4(CH2ImBu)2]+2:3,3’-dibutyl-1,1’-(1,4-phenylenedimethylene)-bis (1H-imidazolium) |  |
| [C6Py+]:1-hexylpyridinium | 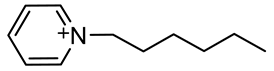 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolone, A.; Haddad, B.; Villemin, D.; Boumediene, M.; Fetouhi, B.; Assenine, M.A. Thermal Decomposition, Low Temperature Phase Transitions and Vapor Pressure of Less Common Ionic Liquids Based on the Bis(trifuoromethanesulfonyl)imide Anion. Materials 2022, 15, 5255. https://doi.org/10.3390/ma15155255
Paolone A, Haddad B, Villemin D, Boumediene M, Fetouhi B, Assenine MA. Thermal Decomposition, Low Temperature Phase Transitions and Vapor Pressure of Less Common Ionic Liquids Based on the Bis(trifuoromethanesulfonyl)imide Anion. Materials. 2022; 15(15):5255. https://doi.org/10.3390/ma15155255
Chicago/Turabian StylePaolone, Annalisa, Boumediene Haddad, Didier Villemin, Mostefa Boumediene, Bekhaled Fetouhi, and Mohammed Amin Assenine. 2022. "Thermal Decomposition, Low Temperature Phase Transitions and Vapor Pressure of Less Common Ionic Liquids Based on the Bis(trifuoromethanesulfonyl)imide Anion" Materials 15, no. 15: 5255. https://doi.org/10.3390/ma15155255
APA StylePaolone, A., Haddad, B., Villemin, D., Boumediene, M., Fetouhi, B., & Assenine, M. A. (2022). Thermal Decomposition, Low Temperature Phase Transitions and Vapor Pressure of Less Common Ionic Liquids Based on the Bis(trifuoromethanesulfonyl)imide Anion. Materials, 15(15), 5255. https://doi.org/10.3390/ma15155255







